- Microwave sintering and mechanical properties of La2O3/Nb2O5 toughened Al2O3 ceramics
Jin Liu, Bingliang Liang*, Yunlong Ai, Jianjun Zhang, Fei He, Wen He and Zhiyong Liu
School of Materials Science and Engineering, Nanchang Hangkong University, No.696, South Fenghe Avenue, Nanchang 330063, Jiangxi Province, P.R. China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
To improve the toughness of Al2O3 ceramic, xLa2O3/10Nb2O5-(90–x)Al2O3 (x = 2.5-15.0 vol.%) composite ceramics were synthesized via microwave-sintering. The influences of sintering temperature (Ts) and La2O3 content on phase composition and mechanical property of xLa2O3/10Nb2O5-(90–x)Al2O3 composite ceramics were studied. It is shown in the results that the Ts of La2O3/Nb2O5 doped Al2O3 ceramic was 150 oC lower than that of Al2O3 ceramic. With the increase of x, La0.33NbO3, LaNbO4 and LaAl11O18 were formed successively. LaNbO4, columnar-Al2O3 grains and LaAl11O18 with plate-like shape were generated in-situ during the sintering period. Compared to Al2O3 ceramic, the fracture toughness of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics were at least 70% higher when 5.0 ≤ x ≤ 12.5. 7.5La2O3/10Nb2O5-82.5Al2O3 ceramic exhibited superior mechanical property: Hv = 12.0 GPa, KIC = 6.2 MPa·m1/2 (1,500 oC, 30 min)
Keywords: Al2O3 ceramics; Microwave sintering; Phase formation; Mechanical properties
Alumina (Al2O3) ceramic ranks among the most important engineering ceramics due to high hardness, high mechanical strength, high temperature insulation resistance, excellent thermal conductivity [1]. However, its potential application in engine is limited by high fragility (KIC-3.0 MPa·m1/2) [2]. Some additives or second phases such as AlCr2 [3], WC [4], TiC [5, 6], SiC [7, 8], ZrO2 [9, 10], Nb2O5 [11, 12], La2O3 [13], LaAl11O18 [14], LaNbO4 [15] were doped into the Al2O3 matrix to improve mechanical properties. In Al2O3-Nb2O5 [12] system, some equiaxed Al2O3 grains were induced to grow oriented into columnar grains. As to Al2O3-La2O3 [13] and Al2O3-LaAl11O18 [14] systems, the plate-like LaAl11O18 grains significantly improved the fracture toughness of Al2O3. Furthermore, the fracture toughness and bending strength of Al2O3 was improved by the domain conversion of LaNbO4[15, 16].
Generally, ceramics can be sintered by conventional, plasma and microwave heating methods [17-19]. Micro- wave sintering, as an efficient sintering method, leads to the volume heating of the sample through the coupling between microwave radiation and the material [20, 21]. Therefore, the temperature gradients resulted from conventional sintering process is prevented [22, 23]. In this experiment, in order to form LaNbO4 and plate like LaAl11O18 grains in-situ and improve the mechanical property, Al2O3 were doped with La2O3/Nb2O5 (xLa2O3/10Nb2O5-(90–x)Al2O3, x = 2.5-15.0 vol.%) and synthesized via microwave-sintering.
xLa2O3/10Nb2O5-(90–x)Al2O3 (x = 2.5-15.0 vol.%) composite ceramics were synthesized via microwave-sintering. High purity La2O3 (99.99 wt.%), Nb2O5 (99.99 wt.%) and Al2O3 (99.6 wt.%) were weighed according to the volume ratio of xLa2O3/10Nb2O5-(90–x) Al2O3 and then ball milled for 12 h. After dried, the mixed powders were granulated with 5 wt.% polyvinyl alcohol solution (PVA, 10 wt.%) as binder. Then granulated mixtures were pressed into regular bars by uniaxial (100 MPa) and cold-isostatic pressing (200 MPa) successively. These regular bars were placed in a conventional furnace and calcined at 600 oC for 3 h to remove binder. The sintering temperature of the samples in the microwave sintering furnace was 1,450~1,500 oC and the holding time was 30 min.
All sintered specimens were polished before the test. Their densities were evaluated by the Archimedes method. The phase analysis was carried out by X-ray diffractometry (XRD, D8ADVANCE Diffractometer, Bruker-AXS). The observation of microstructure were performed on thermal etched specimens by scanning electron microscopy (SEM, Nova Nano SEM450, FEI). The elemental analysis was conducted using energy dispersed spectroscopy (EDS). Vickers hardness (Hv) and fracture toughness (KIC) was tested by HVS-1000 Vickers tester and single edge precracked beam method (SEPB), respectively.
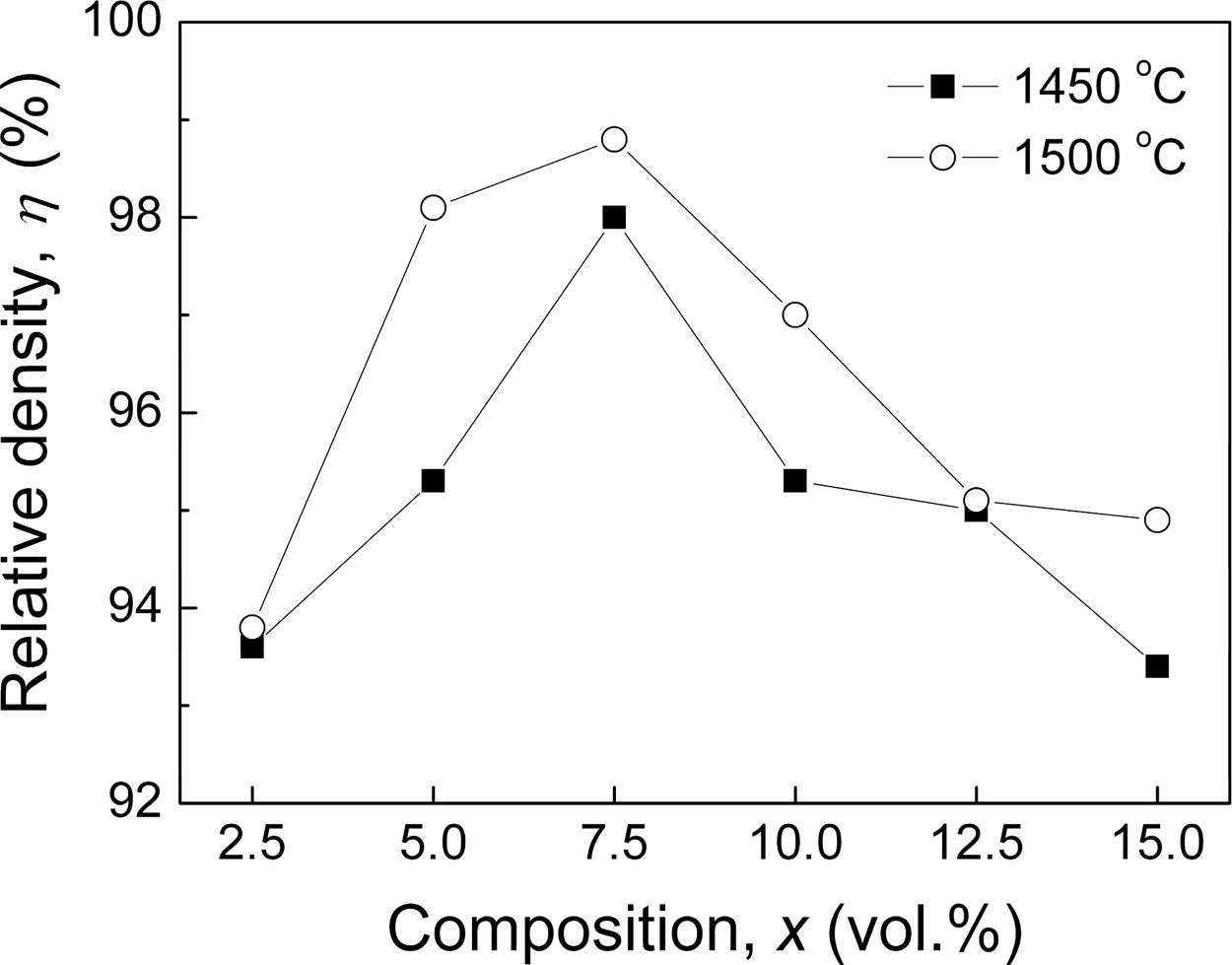
The relative density (h) of xLa2O3/10Nb2O5-(90–x) Al2O3 specimens is shown in Fig. 1. The h of specimens sintered at 1,450 oC increased firstly from 93.6% (x = 2.5) to 98.0% (x = 7.5) and then decreased to 93.4% (x = 15.0). Meanwhile, the h of specimens sintered at 1,500 oC exhibited slightly higher relative density with the same variation trend and reached the maximum of 98.8% for x = 7.5. It indicated that the sintering tem- peratures of xLa2O3/10Nb2O5-(90–x)Al2O3 were 150~ 200 oC lower than that of Al2O3 ceramic (1,650 oC) [23]. The improvement of sinterability could be attributed to two reasons. On one hand, the doping of La2O3 and Nb2O5 might result in the formation of liquid phase during the sintering period. On the other hand, the volumetric heating enables microwave sintering to allow for more rapid and uniform heating than conventional sintering, leading to noticeable decreases in sintering temperature and time [24, 25].
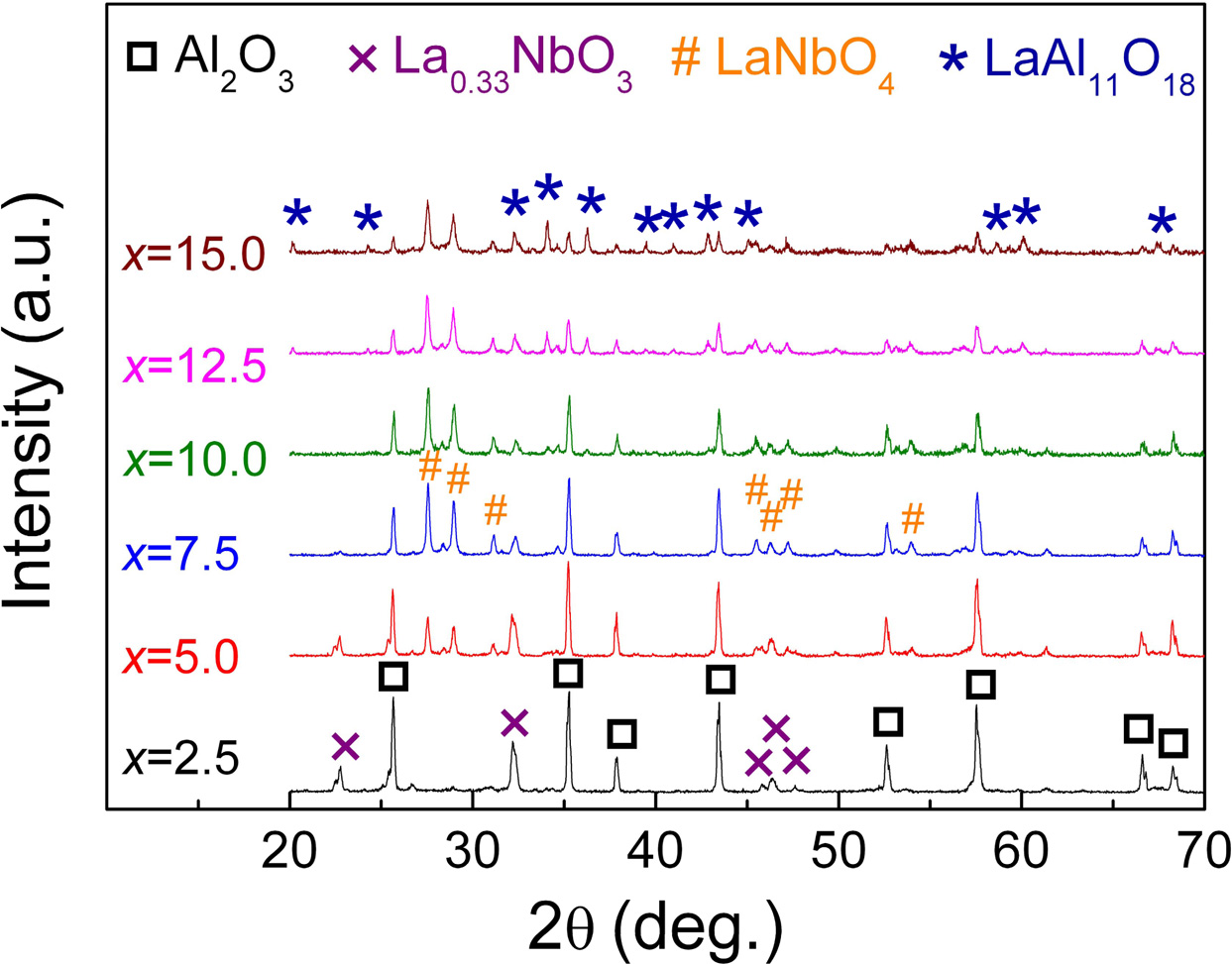
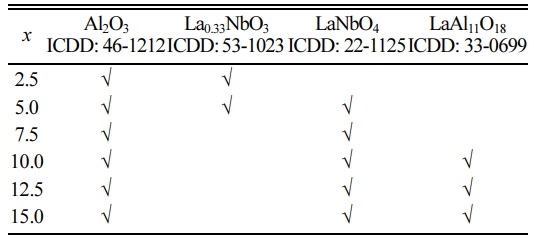
The XRD patterns of the xLa2O3/10Nb2O5-(90–x) Al2O3 ceramics are shown in Fig. 2. When x = 2.5, α-Al2O3 (ICDD: 46-1212) and La0.33NbO3 (ICDD: 53-1023) were detected. With the increase of the content of La2O3, LaNbO4 (ICDD: 22-1125) and LaAl11O18 were formed successively. No diffraction peaks corres- ponding with La2O3 and Nb2O5 were observed. It indicated that La0.33NbO3, LaNbO4 and LaAl11O18 were generated successively in the process of sintering when the mole ratio of La2O3 to Nb2O5 increased from 0.30:1 (x = 2.5) to 1.78:1 (x = 15.0). The phase compositions of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics according to XRD analysis were listed in Table 1, which is similar to previous work from our group [26].
At the same time, when x exceeded 7.5, the intensities of diffraction peaks falling within LaAl11O18 increased, i.e., the content of LaAl11O18 ascended with the increasing La2O3 content. Of course, since La2O3 reacts with Al2O3, the content of Al2O3 is continuously decreasing. When x = 15.0, the main phase transformed from Al2O3 to LaAl11O18.
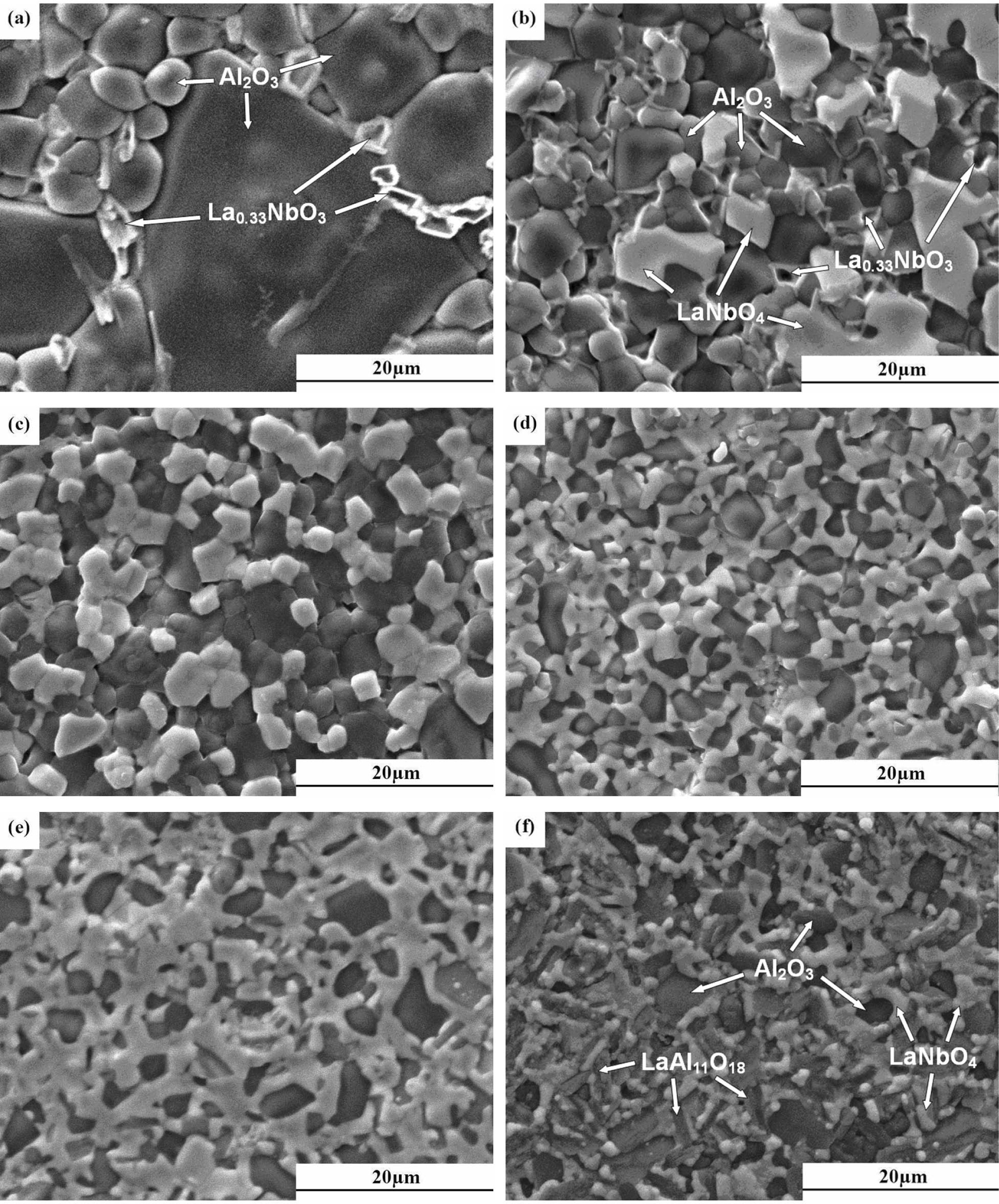
Fig. 3 is a presentation of the SEM images of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics. All the specimens exhibited a very small number of pores because the high heating rate in microwave sintering is favorable to boundary diffusion and in turn grain growth [27, 28]. When x = 2.5, the specimen was consisted of equiaxed grains and fewer grain boundary phase, which were determined to be Al2O3 and La0.33NbO3, respectively, by the EDS analysis (as shown in Fig. 3(a)). LaNbO4 grains were observed as x exceeded 2.5(as shown in Fig. 3(b)) and the amount of which increased with increasing x. Meanwhile, the Al2O3 grains for x > 2.5 were smaller than that for x = 2.5 and homogeneously distributed with LaNbO4. As to x = 15.0, a large quantity of plate-like grains identified as LaAl11O18 were formed (as shown in Fig. 3(f)), indicating that there were LaNbO4 and plate-like LaAl11O18 obtained in-situ during the sintering process. This phenomenon resembles what was previously reported by Brito et al. [14] and Zhang et al. [15]. As Fig. 2 demonstrates, the SEM and EDS analysis exhibited the results consistent with those of XRD analysis above.
The Vickers hardness (Hv) of 5Nb2O5/xLa2O3-(95–x) Al2O3 composite ceramics was summarized in Fig. 4. As 2.5 ≤ x ≤ 15.0, Hv of the specimens sintered at 1,500 oC was higher, to some extent, than Hv of which sintered at 1,450 oC. With the increase of x value, Hv increased firstly (2.5 ≤ x ≤ 7.5) and then decreased gradually (7.5 ≤ x ≤ 15.0), and it reached the maximum value for x = 7.5. All the samples exhibited Hv higher than 10.1 GPa. The highest value of Hv is 12.6 GPa (x = 7.5). The variation trend of Hv was similar to that of the relative density. As shown in Fig. 1, the specimens sintered at 1,500 oC were denser than which sintered at 1,450 oC. Therefore, when composition was determined, Hv of the former was higher than that of the latter [29].
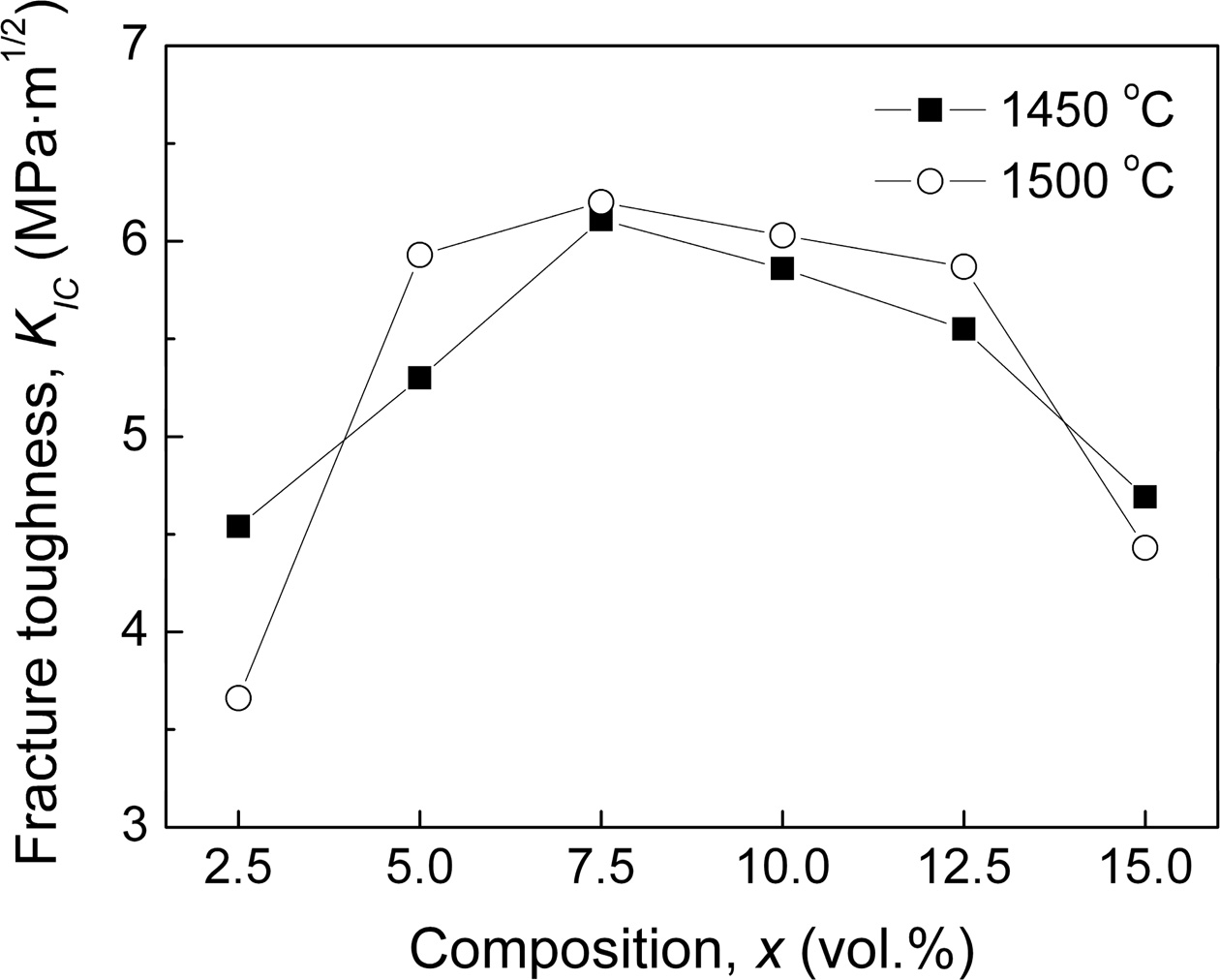
Like Vickers hardness, as shown in Fig. 5, the fracture toughness (KIC) of xLa2O3/10Nb2O5-(90–x)Al2O3 com- posite ceramics climbed to the maximum for x = 7.5 and then declined gradually with the increasing x. When 5.0 ≤ x ≤ 12.5, the xLa2O3/10Nb2O5-(90–x)Al2O3 composite ceramics exhibited fracture toughness exceeding 5.3 MPa·m1/2, over 70% higher than that of Al2O3 ceramic (~3.0 MPa·m1/2) [2].
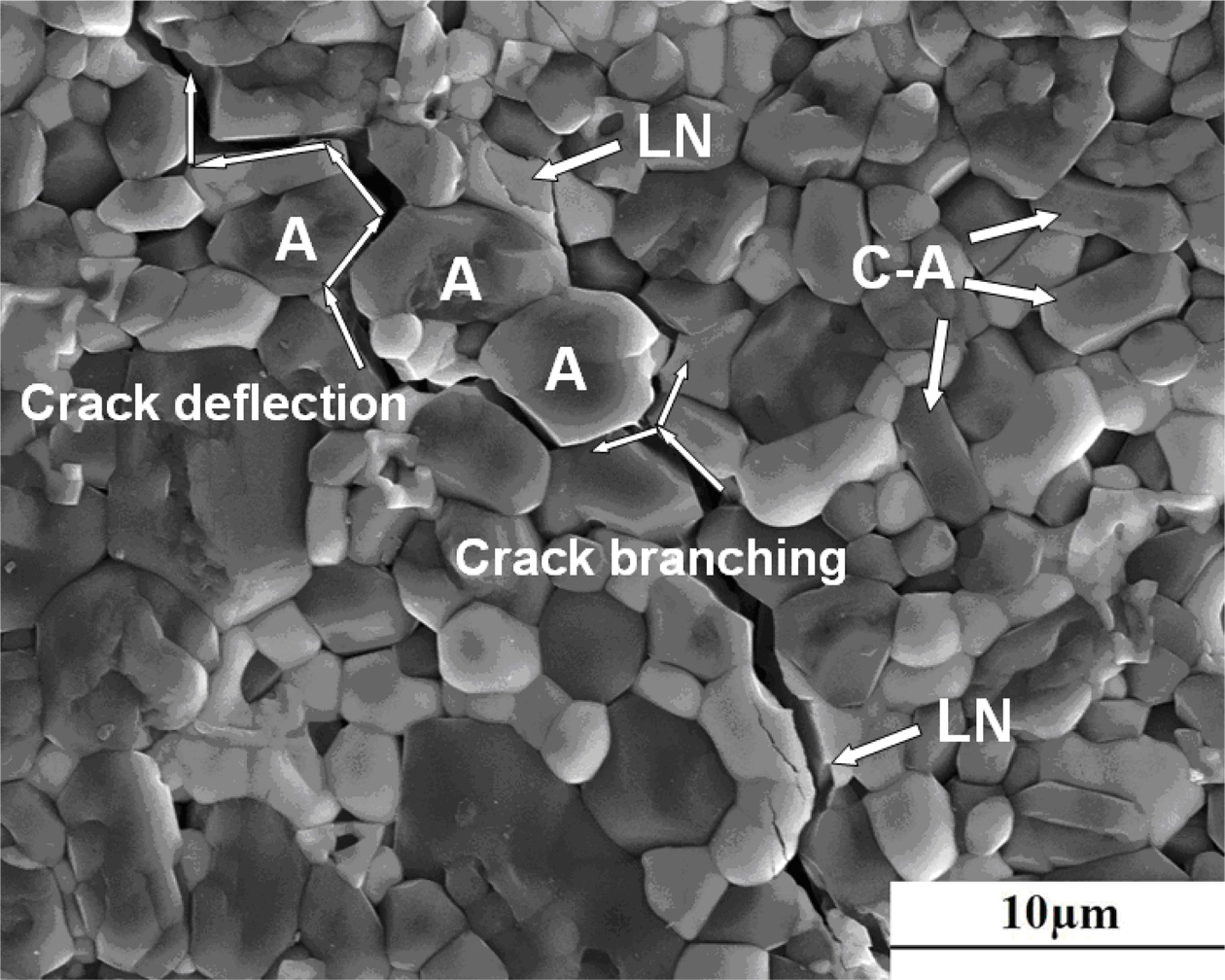
The micrographs of cracks propagation of xLa2O3/10Nb2O5-(90–x)Al2O3 (x = 7.5) ceramics, originating from the corners of Vickers indentations, are presented in Fig. 6. LaNbO4 grains reveal transgranular fracture and absorb energy through domain switch before cracking [28]. This sort of energy absorption performed by LaNbO4 grains in xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics, in some extent, is similar to ZrO2 in Al2O3 ceramics [10, 30]. Remarkably, few columnar Al2O3 grains (marked as C-A with arrows in Fig. 6) with length-diameter ratio of about 3:1 were formed, i.e., some Al2O3 grains were induced to grow oriented by the combined addition of La2O3 and Nb2O5. The existence of columnar Al2O3 grains in the path of crack propagation forced the cracks to deflect and diverge, extending the length of cracks. In general, the combined effects of domain switching of LaNbO4, crack bridging and crack deflection play an important role in the significant improvement of the fracture toughness of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics. As a result, 7.5La2O3/10Nb2O5-82.5Al2O3 ceramic exhibited high fracture toughness: 6.2 MPa·m1/2(1,500 oC, 30 min).
|
Fig. 1 Relative density (h) of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics |
|
Fig. 2 XRD patterns of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics. |
|
Fig. 3 SEM images of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics sintered at 1,500 oC for 30 min: (a)~(f), x = 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, respectively. |

|
Fig. 4 Vickers hardness (Hv) of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics. |
|
Fig. 5 Fracture toughness (KIC) of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics. |
|
Fig. 6 SEM image of the crack propagation paths in xLa2O3/ 10Nb2O5 -(90–x)Al2O3 ceramics(A: Al2O3, LN: LaNbO4, C-A: Columnar Al2O3): x = 7.5, 1,500 oC, 30 min. |
xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics were synthesized via microwave sintering at 1,450~1,500 oC for 30 min. The relative densities of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics are higher than 93%. The sintering temperature for preparing the composite ceramic was 150~200 oC lower than that of Al2O3 ceramic. LaNbO4, columnar-Al2O3 grains and LaAl11O18 with plate-like shape were generated in-situ during the sintering period. The fracture toughness of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics were enhanced by the synergistic effect of columnar-Al2O3 grains and domain-switched LaNbO4 grains. Compared to Al2O3 ceramic, the fracture toughness of xLa2O3/10Nb2O5-(90–x)Al2O3 ceramics were at least 70% higher when 5.0 ≤ x ≤ 12.5. The 7.5La2O3/10Nb2O5-82.5Al2O3 ceramic exhibited superior mechanical property: Hv = 12.0 GPa, KIC = 6.2 MPa·m1/2(1,500 oC, 30 min).
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No.51664043, No. 51802140 and No.51064022) and Natural Science Foundation of Jiangxi Province (20192BAB206007)
- 1. Q.B. Tian, J.S. Dai, Z.J. Lv, and T.G. Zhai, J. Ceram. Process. Res. 17[7] (2016) 676-680.
- 2. R. Danzer, T. Lube, P. Supancic, and R. Damani, Adv. Eng. Mater. 10[4] (2008) 275-298.
-

- 3. J.K. Yoon and I.J. Shon, J. Ceram. Process. Res. 17[8] (2016) 876-880.
- 4. W. Acchar, C.A. Cairo, and A.M. Segadães, Mater. Sci. Eng. A 406[1-2] (2005) 74-77.
-

- 5. Z.B. Yin, C.Z. Huang, B. Zou, H.L. Liu, H.T. Zhu, and J. Wang, Mater. Sci. Eng. A 577 (2013) 9-15.
-

- 6. E.M. Sharifi, F. Karimzadeh, and M.H. Enayati, J. Alloys Compd. 491[1-2] (2010) 411-415.
-

- 7. M. Parchovianský, D. Galusek, J. Sedláček, P. Švančárek, M. Kašiarová, J. Dusza, and P. Šajgalík, J. Eur. Ceram. Soc. 33[12] (2013) 2291-2298.
-

- 8. S.C. Jeong, S.H. Ahn, and K.W. Nam, J. Ceram. Process. Res. 17[10] (2016) 1088-1094.
- 9. N.A. Rejab, A.Z.A. Azhar, M.M. Ratnam, and Z.A. Ahmad, Int. J. Refract. Met. Hard Mater. 36 (2013) 162-166.
-

- 10. V. Naglieri, P. Palmero, L. Montanaro, and J. Chevalier, Materials 6[5] (2013) 2090-2102.
-

- 11. Y.L. Ai, F. He, B.L. Liang, W. He, and W.H. Chen, Adv. Mater. Res. 338 (2011) 120-123.
-

- 12. Y.F. Hsu, Mater. Sci. Eng. A 399[1-2] (2005) 232-237.
-

- 13. Y.L. Ai, F. He, B.L. Liang, W. He, and W.H. Chen, Key Eng. Mater. 519 (2012) 265-268.
-

- 14. Y.Q. Wu, Y.F. Zhang, X.X. Huang, B.S. Li, and J.K. Guo, J. Mater. Sci. 36[17] (2001) 4195-4199.
-

- 15. Z.L. Zhang, L. Zhou, Y.G. Hu, and L. Jiang, Scripta Mater. 47[9] (2002) 637-641.
-

- 16. T. Takagi, Y.H. Choa, T. Sekino, and K. Nihara, Key Eng. Mater. 161-163 (1998) 181-184.
-

- 17. D.L. Johnson, Ceram. Int. 17[5] (1991) 295-300.
-

- 18. J.D. Katz, Annu. Rev. Mater. Sci. 22[1] (1992) 153-170.
-

- 19. A. Chatterjee, T. Basak, and K.G. Ayappa, AIChE J. 44[10] (1998) 2302-2311.
-

- 20. W. Liu, F. Xu, Y. Li, X. Hu, B. Dong, and Y. Xiao, Materials 9[3] (2016) 120.
-

- 21. K.H. Brosnan, G.L. Messing, and D.K. Agrawal, J. Am. Ceram. Soc. 86[8] (2003) 1307-1312.
-

- 22. Y. Bykov, S. Egorov, A. Eremeev, V. Kholoptsev, I. Plotnikov, K. Rybakov, and A. Sorokin, Materials 9[8] (2016) 684.
-

- 23. J.P. Cheng, D. Agrawal, Y.J. Zhang, and R. Roy, Mater. Lett. 56[4] (2002) 587-592.
-

- 24. C.Y. Fang, C.P. Wang, A.V. Polotai, D.K. Agrawal, and M.T. Lanagan, Mater. Lett. 62[17-18] (2008) 2551-2553.
-

- 25. H.S. Hao, L.H. Xu, Y. Huang, X.M. Zhang, and Z. P. Xie, Sci. China Ser. E 52[9] (2009) 2727-2731.
-

- 26. W. He, Y.L. Ai, B.L. Liang, W.H. Chen, and C.H. Liu, Mater. Sci. Eng. A 723 (2018) 134-140.
-

- 27. D. Zymelka, S. Saunier, D. Goeuriot, and J. Molimard, Ceram. Int. 39[3] (2013) 3269-3277.
-

- 28. Y. Liu, F.F. Min, J. B. Zhu, and M.X. Zhang, Mater. Sci. Eng. A 546 (2012) 328-331.
-

- 29. P. Figiel, M. Rozmus, and B. Smuk, J. Achi. Mater. Manu. Eng. 48[1] (2011) 29-34.
- 30. S. Abbas, S. Maleksaeedi, E. Kolos, and A. Ruys, Materials 8[7] (2015) 4344-4362.
-

 This Article
This Article
-
2021; 22(3): 296-300
Published on Jun 30, 2021
- 10.36410/jcpr.2021.22.3.296
- Received on Jun 18, 2020
- Revised on Dec 11, 2020
- Accepted on Dec 29, 2020
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Bingliang Liang
-
School of Materials Science and Engineering, Nanchang Hangkong University, No.696, South Fenghe Avenue, Nanchang 330063, Jiangxi Province, P.R. China
Tel : +86 791 8386 3034 Fax: +86 791 8645 3203 - E-mail: lbl@nchu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.