- Enhancement of compressive strength of lightweight geopolymers
Yootaek Kim and Minjeong Kim*
Department of Materials Engineering, Kyonggi University, Suwon 16227, Korea
For recycling purposes, integrated gasification combined cycle (IGCC) slag and Si sludge were used as the basis for preparing lightweight panels with high compressive strength. The properties of the specimens were examined with the variation in the alkaline activator, addition of frames, addition of aluminum dihydrogen phosphate, particle-size separation of the IGCC slag, and variation in the specimen size by preparing large specimens with different W/S ratios and cutting the specimens at different positions. An optimum specimen with a low density and high compressive strength (8.1 MPa and 0.74 g cm-3, respectively) was acquired from larger specimens (300 × 300 × 100 m3) having various densities and compressive strengths by controlling the W/S ratio and particle size of the IGCC slag. Therefore, it is expected that specimens with both, a low density and high compressive strength, can be obtained by developing and controlling the process factors during the production of lightweight geopolymers.
Keywords: Integrated gasification combined cycle slag, Si sludge, Lightweight, Compressive strength
With the advances in the construction industry, buildings have become larger, lighter, taller, and more functional. Artificial lightweight concrete (ALC) has recently gained prominence and is widely used in many fields of construction because it is inexpensive, reusable, and simple to apply in the construction of buildings. Additionally, ALC acts as a thermal insulator and is fire-resistant, lightweight, soundproof, and significantly lighter than concrete, making it a promising construction material for future applications, with properties far superior to those of existing construction materials. However, ALC has disadvantages compared with existing construction materials, because it is not highly waterproof and easily factures under mechanical stress. The large energy consumption required for ALC production is an additional disadvantage, as ALC comprises mainly cement (which already requires a large amount of energy for production) and further requires a large amount of energy for steaming and heating during processing. Specifically, the autoclaving process used for bloating to produce pores requires the application of a high pressure and high temperature for at least 12 h. Therefore, studies on the development of new ecological materials that can replace ALC must be performed for energy and environmental conservation.
A fire safety standard was mandated in Korea in the first half of 2010, where the construction laws were modified and ordinances were implemented by the Ministry of Land, Transportation, and Maritime Affairs because of recent frequent large-scale fires in buildings. Moreover, the modified construction law mandating the fireproofing of the outer-wall materials as well as the interior materials of buildings became effective on December 29, 2010. Therefore, it is necessary to develop methods for enhancing the fireproof and refractory properties of wall materials, which account for the largest proportion of building materials [1]. It is thus necessary to develop and study new and ecological construction materials having fireproof and refractory properties as replacements for existing ALC panels.
The most widely used construction material is Portland cement. The production of Portland cement generates 0.7-1.0 tons of CO2 per ton of cement, which is equivalent to 7% of the total CO2 output worldwide [2]. Because of the recent surge in the demand for ecological construction materials containing no cement components as replacements for cement, studies on concrete comprising alkaline activators and fly ash, blast furnace slag, and meta-kaolin are being actively performed in Australia, the United States of America, and the European Union [3]. The Korean government has set a goal of maximizing the recycling rate, and thus aims to systemize a sustainable resource recycling society by passing “The Basic Law for Resource Recycling.” Therefore, it is imperative to initiate research on the recycling of industrial wastes and on replacements for cement for reducing the output of carbon dioxide [4]. Hence, in this study, lightweight geopolymers that can replace ALC and act as substitute materials for cement were investigated.
Integrated gasification combined cycle (IGCC) slag, which is generated during IGCC power generation, was used as a raw material in this study. IGCC slag is an industrial byproduct rich in SiO2 and Al2O3 [5]. IGCC generation is environmentally friendlier and delivers a higher efficiency than conventional thermal power generation using coal. The process of synthetic gas production for power generation creates a large amount of slag [6].
Si sludge is used as a bloating material for forming pores in geopolymers. Si sludge is also an industrial byproduct from the processing of Si wafers, as it is generated by the cutting and polishing of Si ingots. These ecofriendly materials (IGCC slag and Si sludge) were used as raw materials in the present study, and the possibility of replacing existing construction materials that require large energy consumption for production was investigated with the objective of achieving ecological sustainability.
Materials
IGCC slag
IGCC fused slag samples, which were produced as a byproduct of IGCC power plants in Korea during power generation, were used as raw materials. The analytical data for the IGCC slag samples are presented in Fig. 1 and Table 1. Fig. 1 shows the X-ray diffraction (XRD) pattern of the IGCC slag, indicating that the IGCC slag is a typical amorphous material that is suitable as a raw material for fabricating geopolymers. Table 1 presents the composition of the IGCC slag determined via X-ray fluorescence (XRF) analysis, which confirms that IGCC slag is suitable for fabricating geopolymers, because it was mainly composed of silica and alumina, which are two major necessary components for the geopolymerization reaction. Geopolymers are construction materials with a high compressive strength, comprising a gel consisting of ionized Si and Al derived from a raw material (herein, IGCC slag). Therefore, the raw materials for geopolymers should contain sufficient amounts of Si and Al and should also be amorphous, because the Si and Al components must be easily dissolved by alkaline activators to generate the corresponding ions. The surfaces of the IGCC particles in the amorphous state were coated with glass; the Si and Al atoms were dissolved to form ions through an ionization process by contact with the alkaline activator, which broke up the amorphous surface layers. The data in Fig. 1 and Table 1 indicate that the IGCC slag was completely amorphous, and the total weight of SiO2 and Al2O3 was as high as 69.1 wt.%; thus, the IGCC slag is deemed suitable as a raw material for fabricating lightweight geopolymers.
Si sludge
Bloating materials are essential for fabricating lightweight geopolymers. There are many types of bloating materials, such as Al powder, vegetable powder, and animal powder. Si sludge produced during the Si wafer slicing process was used as the bloating material in this study for the purpose of recycling, which was one of the main objectives of this study. The Si sludge was analyzed via XRD and XRF, as shown in Fig. 2 and Table 2, respectively. The Si sludge had a perfectly crystalline structure (Fig. 2) and consisted of mostly Si; 1.97 wt.% Al2O3 was detected in the XRF analysis (Table 2). This alumina component might be from abrasive materials used during the slicing of the Si ingot. This small amount of alumina may participate in the geopolymerization reaction as a raw material and possibly has no adverse effect on the reaction. The bloating reaction of the Si sludge with the alkaline activator occurs via the reaction expressed in Eq. (1); pores are formed in the ceramic paste by the H2 gas produced during the reaction.
Si + 2NaOH + H2O → Na2SiO3 + 2H2 (1)
The Gibbs free energy of the bloating reaction is 858.23 kJ mol-1, which is lower than that of the geopolymerization reaction [7]. Thus, the bloating reaction occurs first, followed by the geopolymerization reaction.
Alkaline activator
NaOH was used as an unmixed alkaline activator because it is the most widely used alkaline activator for fabricating geopolymers and has advantages regarding the price for future commercialization [8]. The compressive strengths of the mixed and unmixed counterparts were comparatively evaluated. The mixed alkaline activator was prepared with a sodium silicate solution (Na2SiO3 · nH2O; Daejung Co.) and a 98.0% NaOH solution. The mixing ratio was 1:1, and this ratio was maintained throughout the study.
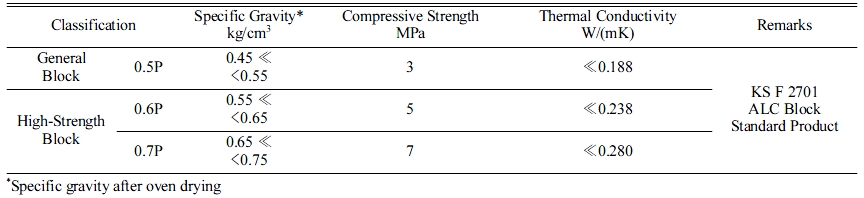
Standard ALC panel block KS (KS F 2701)
Table 3 presents the properties of the ALC blocks, as suggested by KS F 2701 [9]. The target product in this study was 0.7P, and all the specimens were designed to reach the values shown in Table 3. Some ALC panels employ internal steel wires, such as KS D 3503 (general structure purpose pressed steel), KS D 3504 (steel concrete purpose steel), and KS D 3552 (steel wire). These are employed for the frame inside geopolymers to enhance the compressive strength.
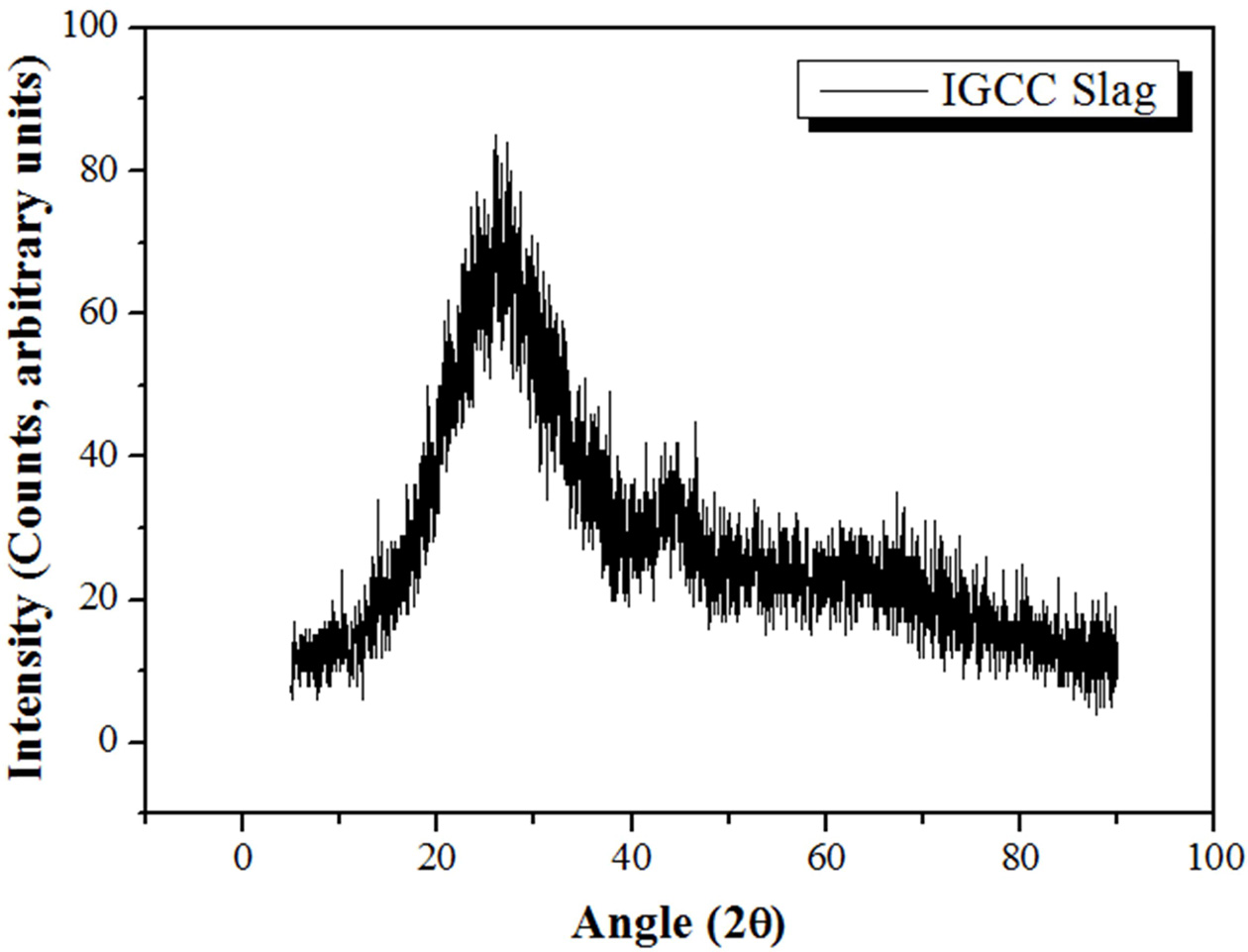
|
Fig. 1 XRD pattern of the IGCC slag. |
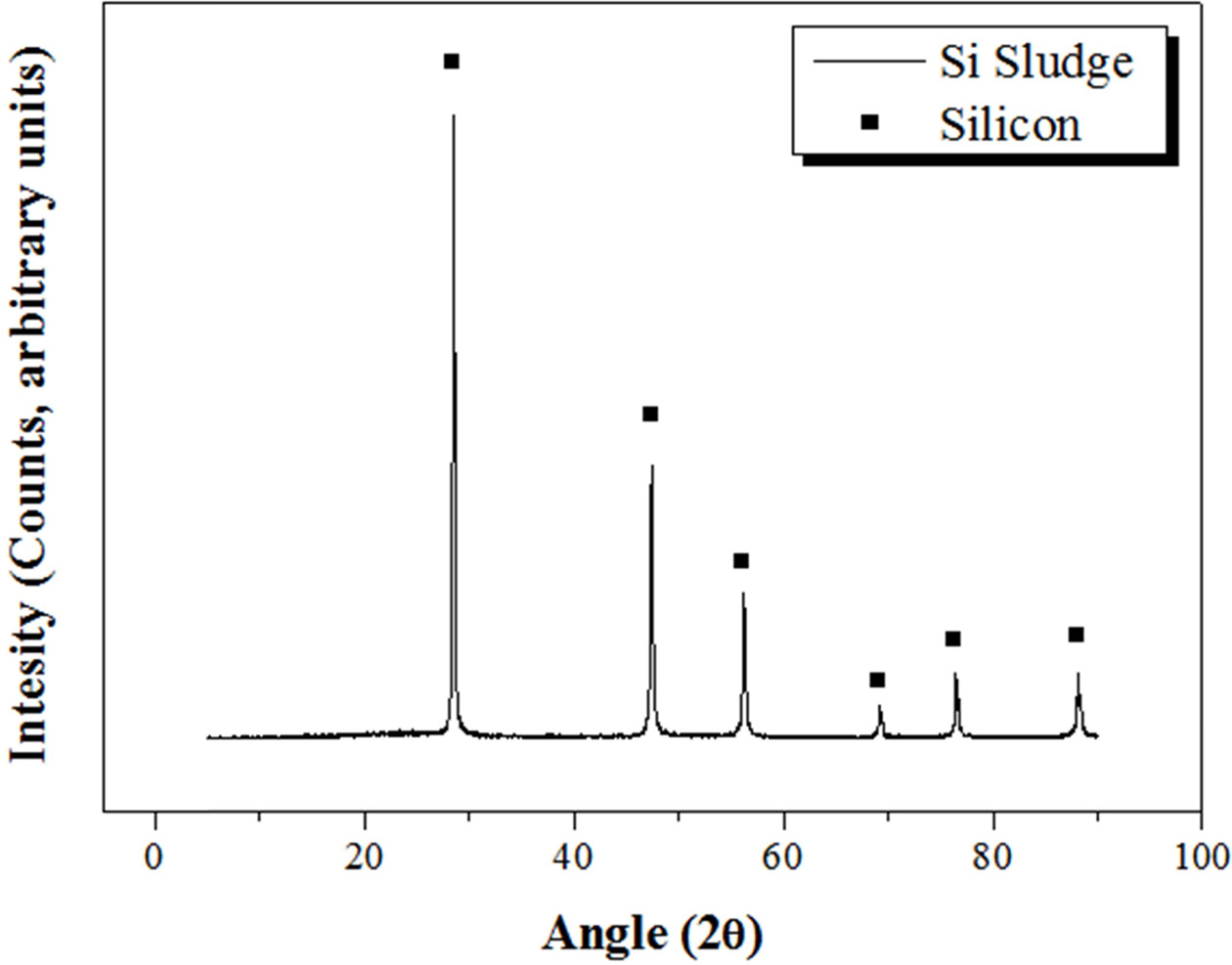
|
Fig. 2 XRD pattern of the Si sludge. |
Addition of Si sludge: comparison between mixed and unmixed alkaline activators
The compressive strength of specimens produced with mixed alkaline activators is generally higher than that of specimens prepared with unmixed activators [10]. However, because the molar weight of Na2SiO3 is three times larger than that of NaOH, the density of specimens prepared with mixed alkaline activators inevitably increases, which may be adverse for fabricating lightweight geopolymers. Because the first step of this study was to produce a lightweight geopolymer and enhance the compressive strength, an unmixed alkaline activator was used to reduce the density of the specimens, and the properties of the resulting specimens were compared with those of specimens prepared using a mixed alkaline activator.
The experimental conditions were as follows: molar concentration of NaOH (unmixed alkaline activator), 15 M (mol/L); W/S ratio, 0.23; degree of milling, coarse.
As shown in Fig. 3, both the compressive strength and density decreased with the increasing amount of added Si sludge. The density of the specimen before the addition of the Si sludge was >2.0 g cm-3, and the density decreased by >50% compared with that of the specimen with 0.1 wt.% Si sludge. Further addition of Si sludge caused a continuous reduction in the density; the density was 0.5 g cm-3 with the addition of 0.4 wt.% Si sludge. In comparison, the reduction in the compressive strength with an increase in the amount of Si sludge was far smaller. When the amount of Si sludge exceeded 0.1 wt.%, the compressive strength was <5 MPa; therefore, the addition of Si sludge beyond 0.1 wt.% is not suitable for the production of geopolymers that are lightweight and have a high compressive strength.
Addition of Si sludge: mixed alkaline activator
The experimental conditions were as follows: molar concentration of NaOH (mixed alkaline activator), 15 M; W/S ratio, 0.22, 0.225, 0.230, 0.235, or 0.240; degree of milling, coarse; amount of Si sludge added, 0.1 wt.%.
For the specimen with a W/S ratio of 0.230 (Fig. 4), adding the same amount of Si sludge as above (0.1 wt.%) with the mixed alkaline activator produced a specimen with a compressive strength of 10 MPa and a density of 1.0 g cm-3, which is far from the expectation drawn from a reference paper. The density was similar to our expectation; however, the compressive strength was far lower than expected [11]. When a mixed alkaline activator is used (Fig. 4), it is speculated that a higher concentration of Si in the sodium silicate solution (Na2SiO3) can enhance the geopolymerization reaction, resulting in an enhanced compressive strength through the formation of three-dimensional network structures in the geopolymer.
In contrast, when an unmixed alkaline activator is used, the Si concentration is far lower than that for the mixed counterpart; therefore, the compressive strength of a specimen prepared using an unmixed activator is lower than that of a specimen prepared using a mixed activator. Hence, it is considered that the addition of 0.1 wt.% Si sludge with a mixed alkaline activator is desirable for fabricating high-compressive strength lightweight geopolymers.
Addition of frame
It is well known that concrete has a poor resistance to tensile stress, and the tensile strength of concrete can be enhanced by the incorporation of steel wires. Additionally, in commercialized ALC panels, steel wires are used to increase the tensile strength. The steel wire used in ALC was employed in this study to determine whether the insertion of the wire would enhance the compressive strength of the lightweight geopolymers. The experimental conditions were as follows: molar concentration of the mixed alkaline activator, 15 M; W/S ratio, 0.24; degree of milling, coarse; amount of Si sludge added, 0.1 wt.%. The steel wires were of a two-dimensional flat type, and three layers of steel wires were placed on the top, in the middle, and on the bottom of the lightweight geopolymer specimens.
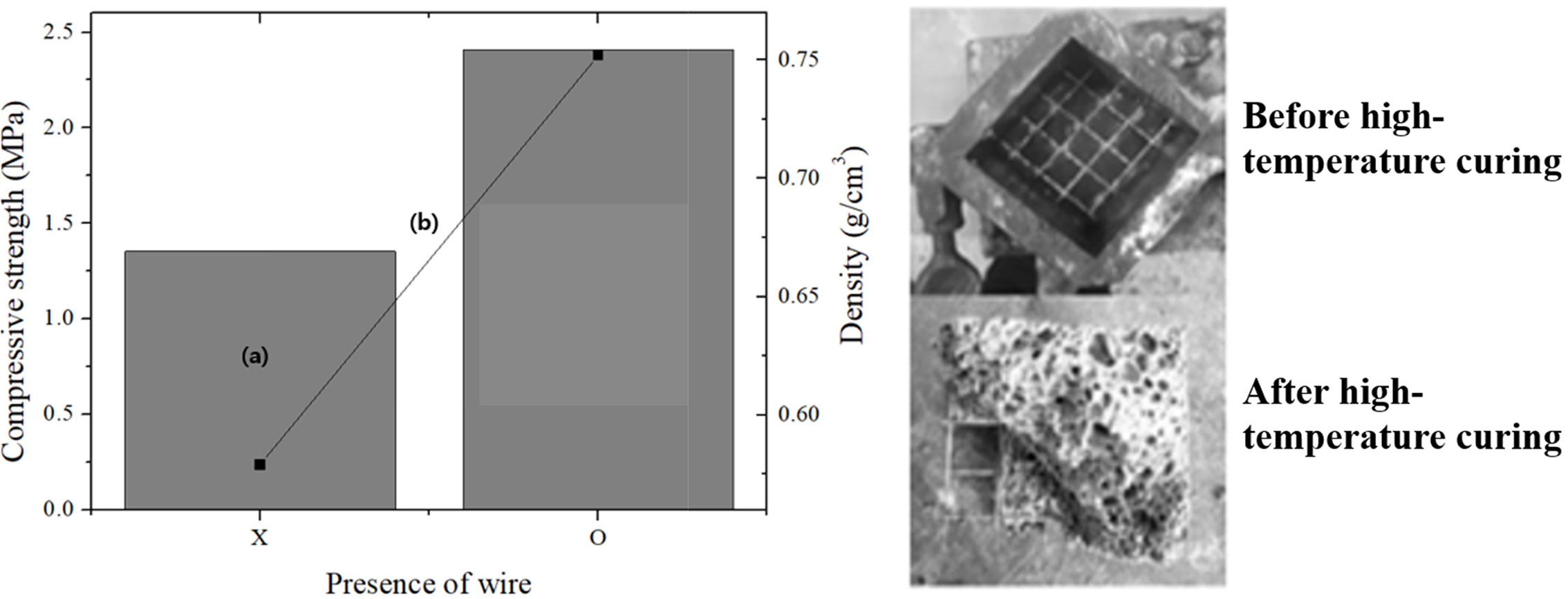
As shown in Fig. 5, the compressive strength and density of the specimen without the steel wire were 1.3 MPa and 0.58 g cm-3, respectively, and those of the specimen with the steel wire inserted were 2.4 MPa and 0.756 g cm-3, respectively. The compressive strength of the specimens with the steel wires was 1.1 MPa higher than that of the specimens without them; however, the density was 1.7 g cm-3 higher, which is adverse for fabricating lightweight geopolymers. Although it is well known that the insertion of wires or fibers does not significantly increase the compressive strength, the increase in the compressive strength of the specimens with wires was far smaller than expected, because the Si sludge used as a bloating material resulted in the formation of numerous pores with irregular shapes and sizes around the steel wires. This prevented secure adhesion between the geopolymer matrix and the steel wires; thus, the insertion of the steel wires did not significantly enhance the compressive strength of the lightweight geopolymers, in contrast to the case of steel-reinforced concrete [12].
It was speculated that the insertion of steel wire frames may increase the tensile strength of lightweight geopolymers, as indicated by the literature [13]. However, it was recognized that steel wires can have little effect on the compressive strength of lightweight geopolymers unless the adhesion between the wire frame and the geopolymer matrix can be strengthened. The tensile-strength data for the geopolymers are not provided herein, because our objective was to enhance the compressive strength of the specimens. In the future, it is necessary to study the enhancement of the adhesion between the wire frames and the matrix, particularly for a matrix of lightweight materials having many irregular pores.
Addition of aluminum dihydrogen phosphate (ADP)
The phosphate ions of aluminum dihydrogen triphosphate (ATP) increase the compressive strength of geopolymers by enhancing the geopolymerization reaction with Si4+ and Al3+ ions [14]. In accordance with a previous report, ADP, which has similar chemical composition to ATP and is widely available, was used to enhance the compressive strength of the lightweight geopolymers. ADP was used in the aqueous state; it has more H2O molecules than ATP. The experimental conditions were as follows: molar concentration of the mixed alkaline activator, 15 M; W/S ratio, 0.24; degree of milling, coarse; amount of Si sludge added, 0.1 wt.%.
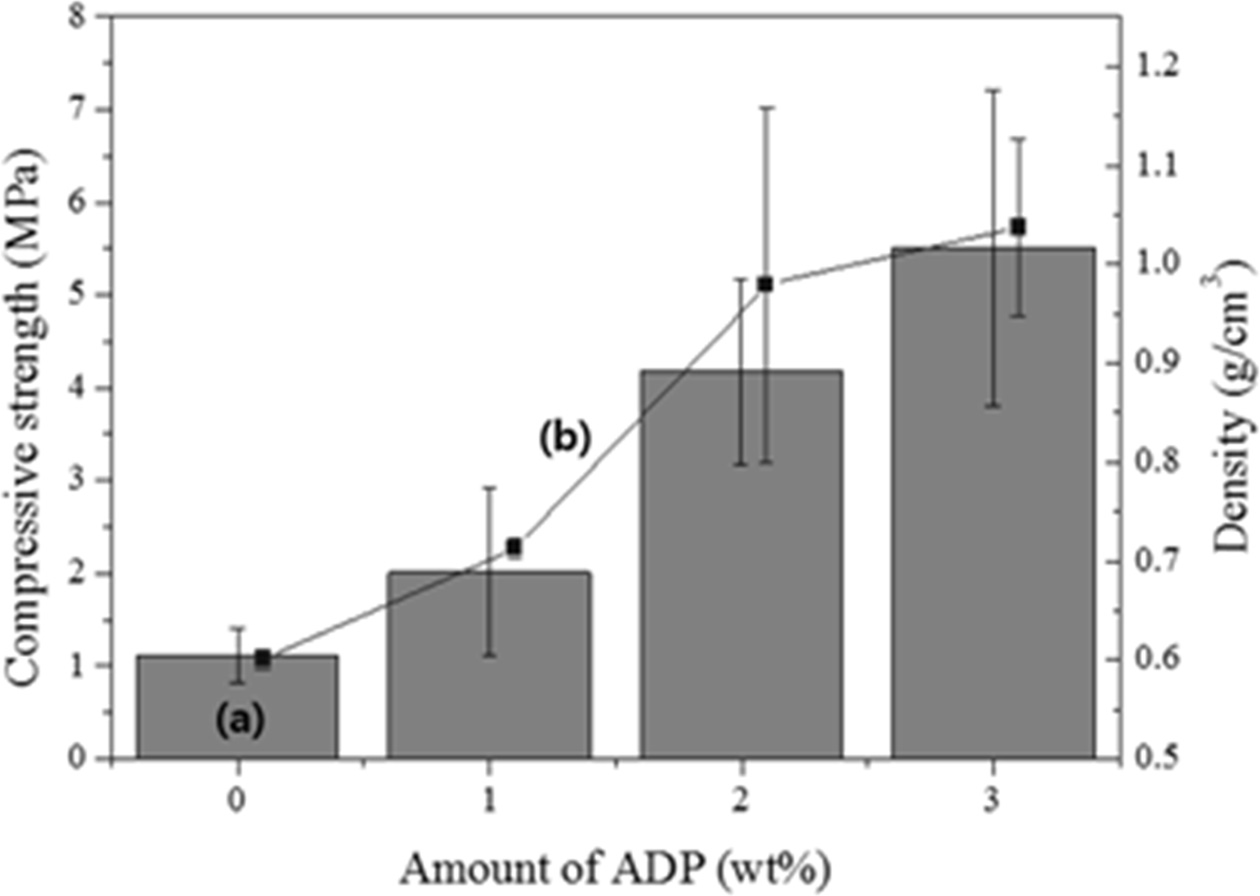
As shown in Fig. 6, the compressive strength and density of the specimen before the addition of ADP were 1.1 MPa and 0.6 g cm-3, respectively, and those after the addition of 1 wt.% ADP were 2 MPa and 0.7 g cm-3, respectively. In general, both the compressive strength and density increased with the addition of ADP; however, the increase in the compressive strength with the addition of ADP was 1/80 of that observed in a previous study, where ATP was used instead of ADP [12]. It is speculated that this difference was caused by the large pores in the specimens because of the high flow rate used in the formation of the geopolymer paste with the bloating material and the high W/S ratio, which were not the same as the experimental conditions employed in the previous report. Therefore, additional studies on controlling the flow rate of the paste and the pore size/distribution may be needed to enhance the compressive strength of lightweight geopolymers with the addition of ADP. A high flow rate of the geopolymer paste and a small and homogenous pore distribution are both necessary to obtain lightweight geopolymers with a high compressive strength, because the workability of geopolymer specimens mainly depends on the flow rate of the geopolymer pastes, resulting pore size, pore distribution, and pore homogeneity. Overall, the addition of ADP had a slight positive effect on the compressive strength of the lightweight geopolymers; however, the density also increased with an increase in the amount of ADP. Thus, ADP is not suitable as an additive for both enhancing the compressive strength and reducing the weight of geopolymers.
Effect of particle-size separation
It was reported that the compressive strength of geopolymers could be enhanced by particle-size separation of the raw materials [15]. In accordance with that study, the IGCC slag was separated into three different sizes via sieving, and lightweight geopolymer specimens were then formed with the addition of Si sludge. The experimental conditions were as follows: molar concentration of the mixed alkaline activator, 15 M; W/S ratio, 0.24; degree of milling, coarse; amount of Si sludge added, 0.1 wt.%; sieving size, smaller than 75, 106, and 180 μm. “Reference specimen” refers to the raw IGCC slag before sieving.
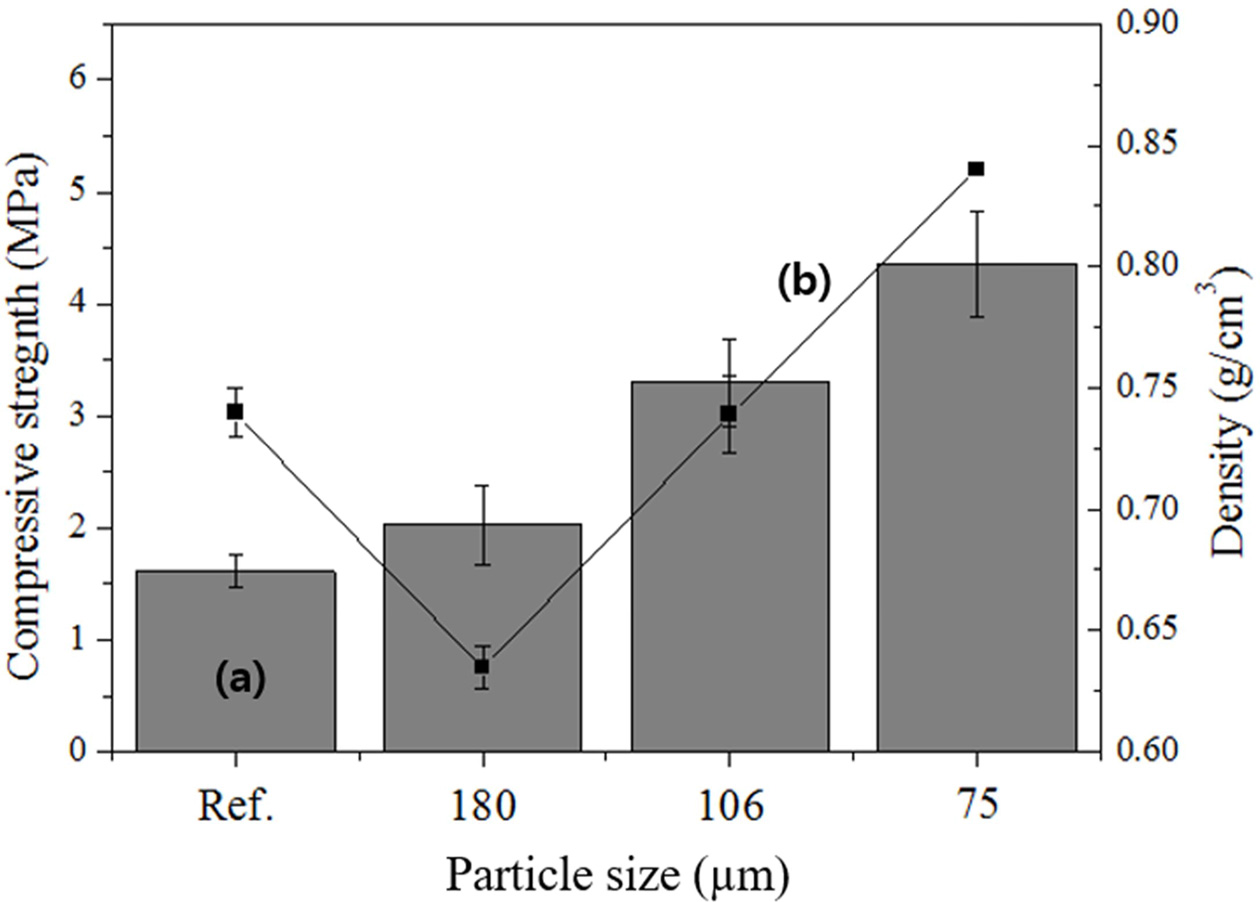
As shown in Fig. 7, the compressive strength and density of the reference specimen were 1.5 MPa and 0.74 g cm-3, respectively. For the samples prepared with particle sizes smaller than 180, 106, and 75 μm, the compressive strengths were 2.0, 3.2, and 4.1 MPa, respectively, and the corresponding densities were 0.63, 0.74, and 0.84 g cm-3. Notably, the compressive strength of the specimen prepared with a particle size of <180 μm increased by 0.5 MPa and the density decreased by 0.1 g cm-3 compared with those of the reference specimen. The compressive strength of the specimen with a particle size of <106 μm increased by 1.5 MPa compared with that of the reference specimen, although the densities were the same. Generally, a smaller particle size yielded a higher compressive strength. Therefore, size separation (reducing the particle size) of the raw material was effective for increasing the compressive strength of the lightweight geopolymers by enhancing the geopolymerization reaction, indicating that reducing the particle size enhances the reaction with the alkaline activator. However, a substantial increase in the compressive strength of lightweight geopolymers cannot be expected solely on the basis of particle-size separation.
Effect of position in large specimen
According to previous research, the pore size and distributions differ significantly at different heights in lightweight geopolymer specimens; thus, the compressive strength and density of the specimens may change according to the specimen height. The general size of the specimens in this study was 50 × 50 × 50 mm3 (denoted as “standard size specimens”); however, a significantly larger specimen (400 × 100 × 200 mm3, i.e., six times larger than the standard size specimen) was fabricated and cut to the standard size, and the differences in the properties were investigated according to the position from which the standard sample was cut from the larger specimen. The experimental conditions were as follows: molar concentration of the mixed alkaline activator, 15 M; W/S ratio, 0.20; degree of milling, coarse; amount of Si sludge added, 1 wt.%; sieving size: <106 μm.
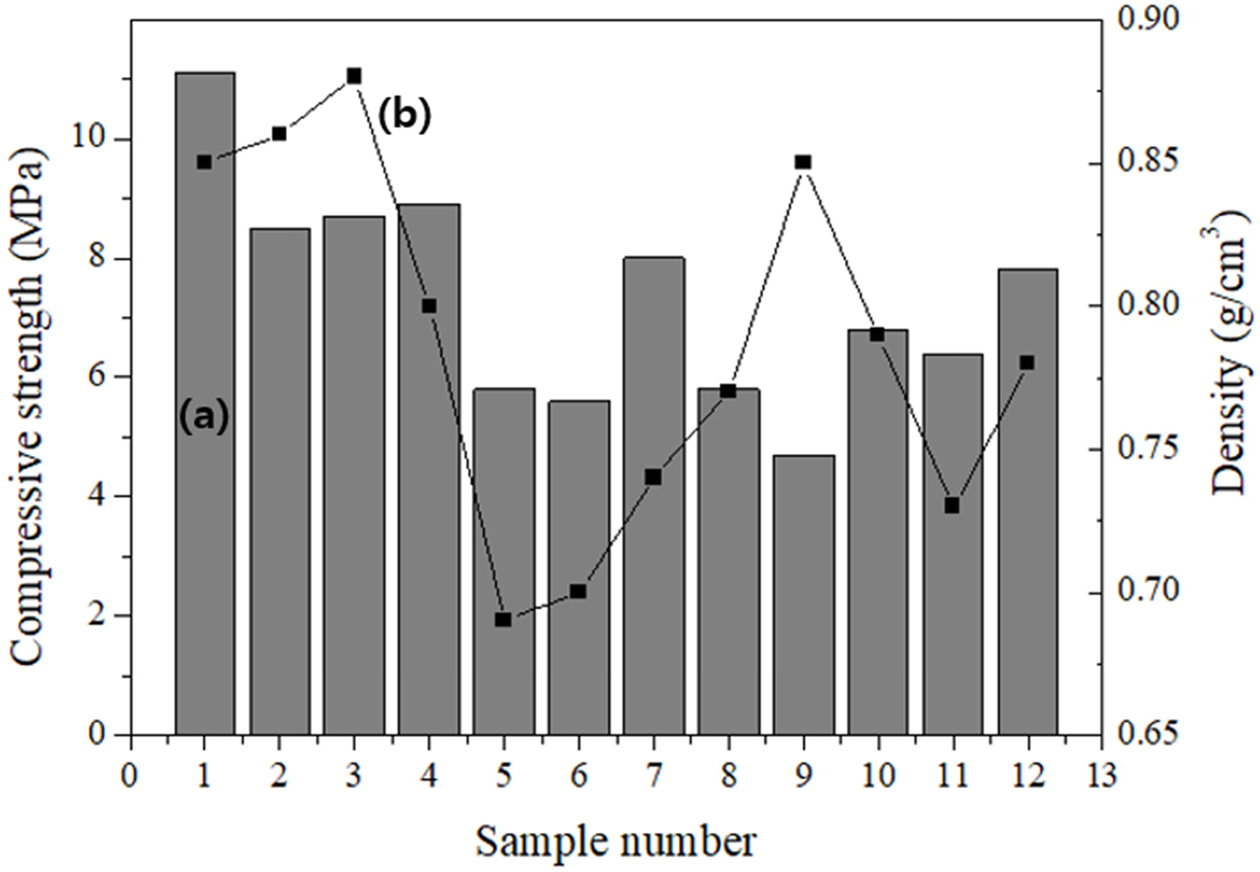
Standard size specimens were randomly cut from various positions of the large specimen. The compressive strength of the specimens varied over a wide range (4.5-11.0 MPa) depending on the position, with an average value of 7.4 MPa. The density of the specimens also varied over a wide range (0.69-0.88 g cm-3) depending on the position, with an average value of 0.76 g cm-3.
Among the specimens, specimen #7 had both a low density and a high compressive strength, as shown in Fig. 8. The compressive strength and density of specimen #7 were 8.1 MPa and 0.74 g cm-3, respectively, which are close to the properties of the target material of this study. As the size of the specimen increased, the pores were localized mainly in the top part of the large specimen; thus, the specimens from the top part had the lowest density and compressive strength, and the specimens from the middle of the large specimen had a relatively lower density and higher compressive strength, which were close to the target properties of the lightweight geopolymer in this study. This confirms that specimens having both a low density and a high compressive strength can be obtained by designing the production process of lightweight geopolymers.
A cross-sectional image of the large specimen after cutting is shown in Fig. 9. A dense structure with fewer and smaller pores was observed for the bottom part of the specimen. Because the pores produced during the bloating reaction were localized in the top part of the specimen and large pores were formed, more and larger pores were observed in the top part of the specimen. Considering the pore size and distribution in the specimen, as shown in the cross-sectional image of Fig. 9, it was expected that a specimen cut from the top part of the large specimen would have a low density and compressive strength and that a specimen from the bottom part would have a higher density and compressive strength. This expectation is in accordance with the results in Fig. 8.
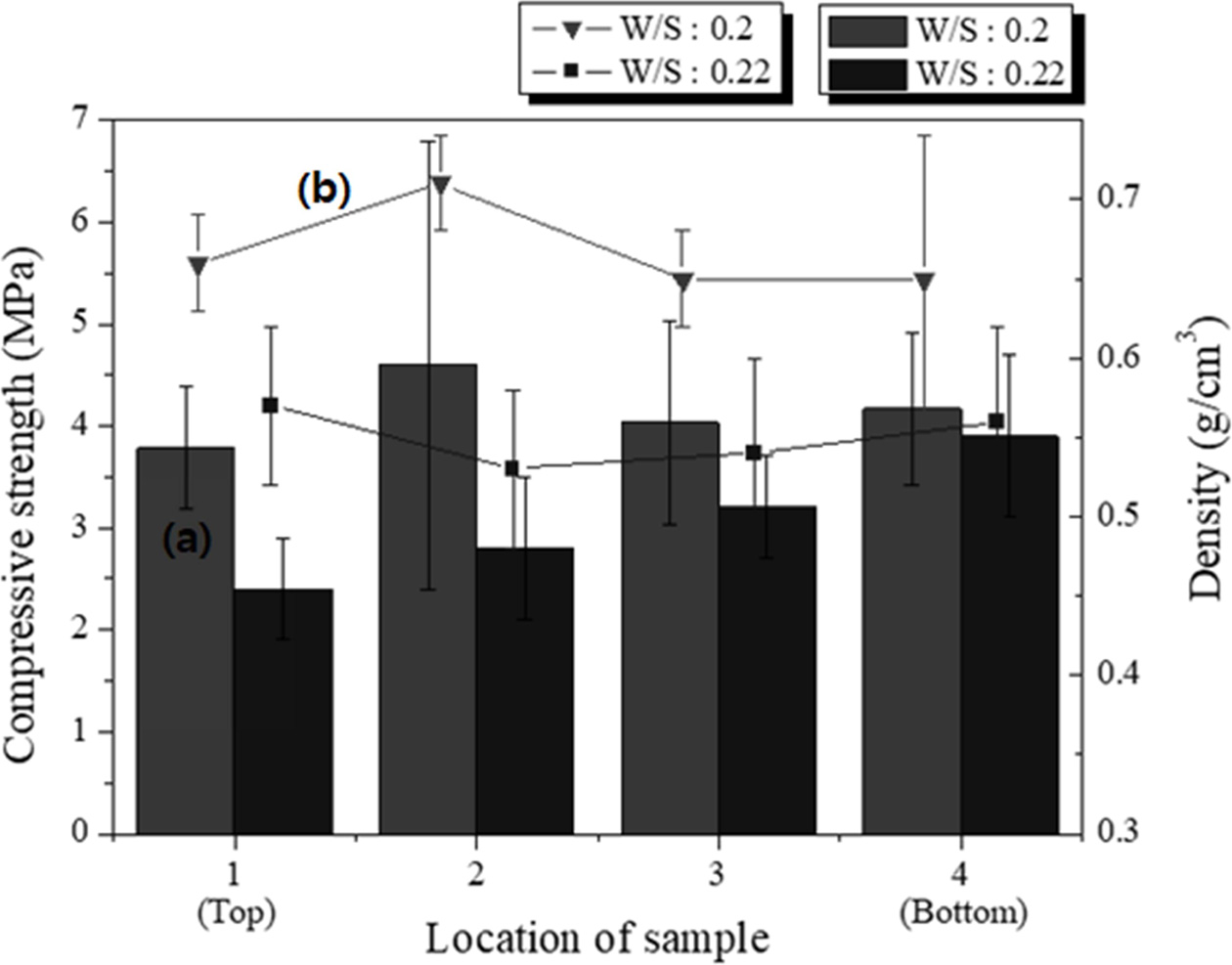
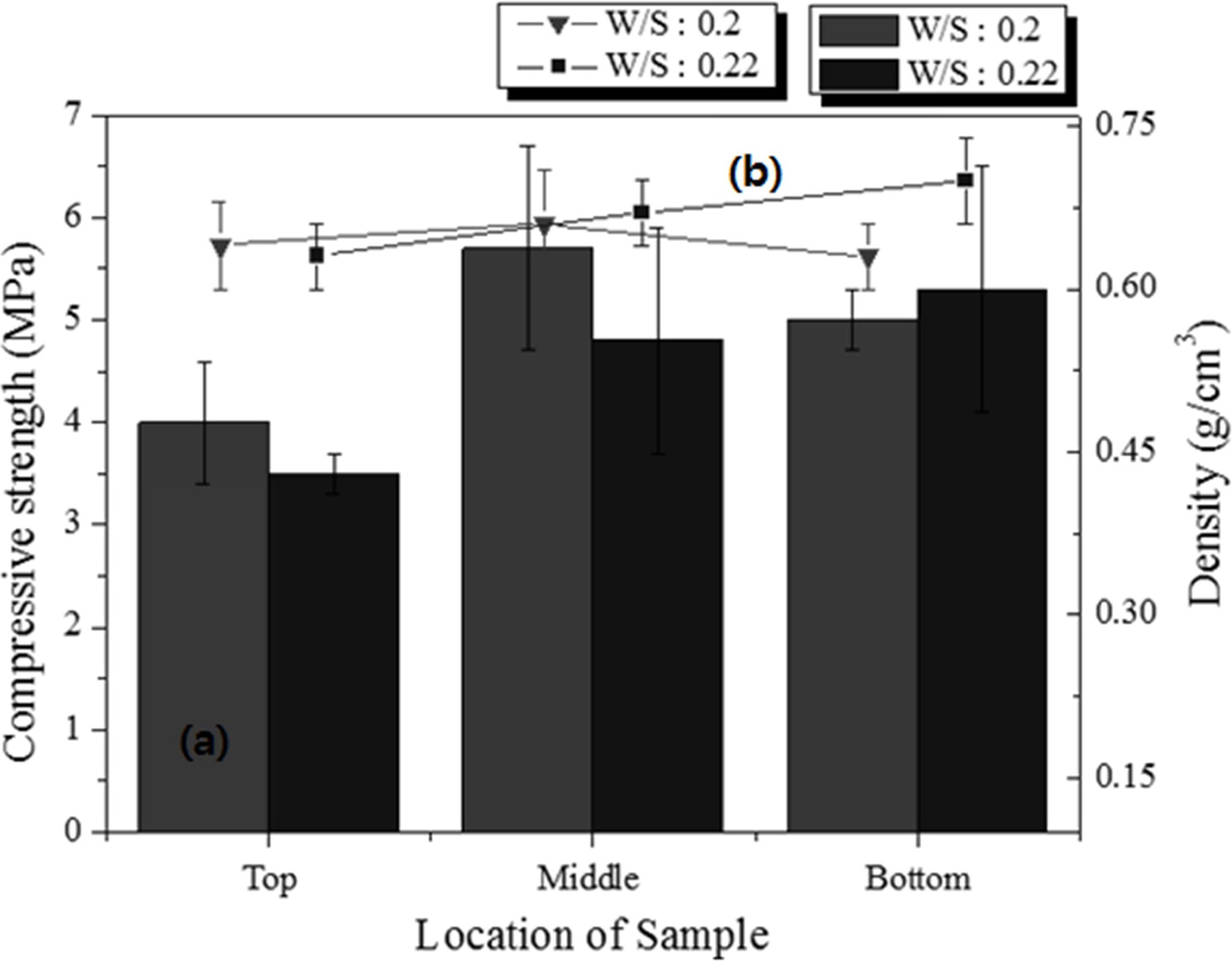
Specimens with dimensions of 250 × 250 × 250 mm3 having two different W/S ratios were prepared, and samples with dimensions of 50 × 50 × 50 mm3 were cut from the top, middle, and bottom parts of the large specimens to compare the properties according to the height of the specimen. The pore size and distribution differed among the different positions of the specimen, as indicated by microscopic observation of the cross sections. The experimental conditions were as follows: molar concentration of the mixed alkaline activator, 15 M; W/S ratios, 0.20 and 0.22; degree of milling, coarse; amount of Si sludge added, 1 wt.%; sieving size: <106 μm.
For the samples with the same W/S ratio, the density differed slightly with a few standard deviations, in contrast to the trends observed in Fig. 8. The average density of the lightweight geopolymers was 0.72 g cm-3 at the W/S ratio of 0.20 and 0.55 g cm-3 at the W/S ratio of 0.22. As shown in Fig. 10, the density and compressive strength of the specimen with a W/S ratio of 0.20 tended to be higher than those of the specimen with a W/S ratio of 0.22. For the sample with a W/S ratio of 0.20, the properties did not differ with respect to the height at which the specimen was removed; however, for the sample with a W/S ratio of 0.22, the compressive strength increased with the height. In particular, for specimen #4, the density was similar to those of the other specimens, but the compressive strength was 1 MPa higher. The compressive strength of the specimens with a W/S ratio of 0.20 exhibited an irregular trend compared with that of the specimens with a W/S ratio of 0.22; however, it must be noted that the specimen having the highest compressive strength was taken from the middle part of the large specimen. Because the paste with a W/S ratio of 0.20 has a lower flow rate than that with a W/S ratio of 0.22, the pores produced in the bloating reaction did not travel far to the top of the former specimen and were captured in the middle part of the specimen. This resulted in an ideal structure of the geopolymer, which was lightweight and had a high compressive strength, with many small and homogenous pores in the middle region. This unexpected result implies that the W/S ratio is one of the most important factors for producing lightweight/high-compressive strength geopolymers.
The amount of Si sludge added was reduced by 0.5 wt.% to enhance the compressive strength of the specimens under the same conditions as above. As shown in Fig. 11, the compressive strength of all the specimens increased by >1 MPa when the amount of Si sludge was reduced by half, and the densities were almost the same or slightly higher than those of the samples with 1.0 wt.% Si sludge. Notably, the sample with the highest compressive strength was that taken from the middle part of the specimen with a W/S ratio of 0.20, which is similar to the case of the sample with 1.0 wt.% Si sludge, as shown in Fig. 10. The compressive strength of the specimen from the bottom part of the large specimen was higher than those of the specimens from the middle and top parts in the case of the sample with a W/S ratio of 0.22. Therefore, both the compressive strength and density increased with a decrease in the amount of Si sludge. The results indicate that controlling the addition of Si sludge is not important for enhancing the compressive strength of lightweight geopolymers, because the relationship between the amount of Si sludge added and the homogeneity of the pore distribution appears to be insignificant.
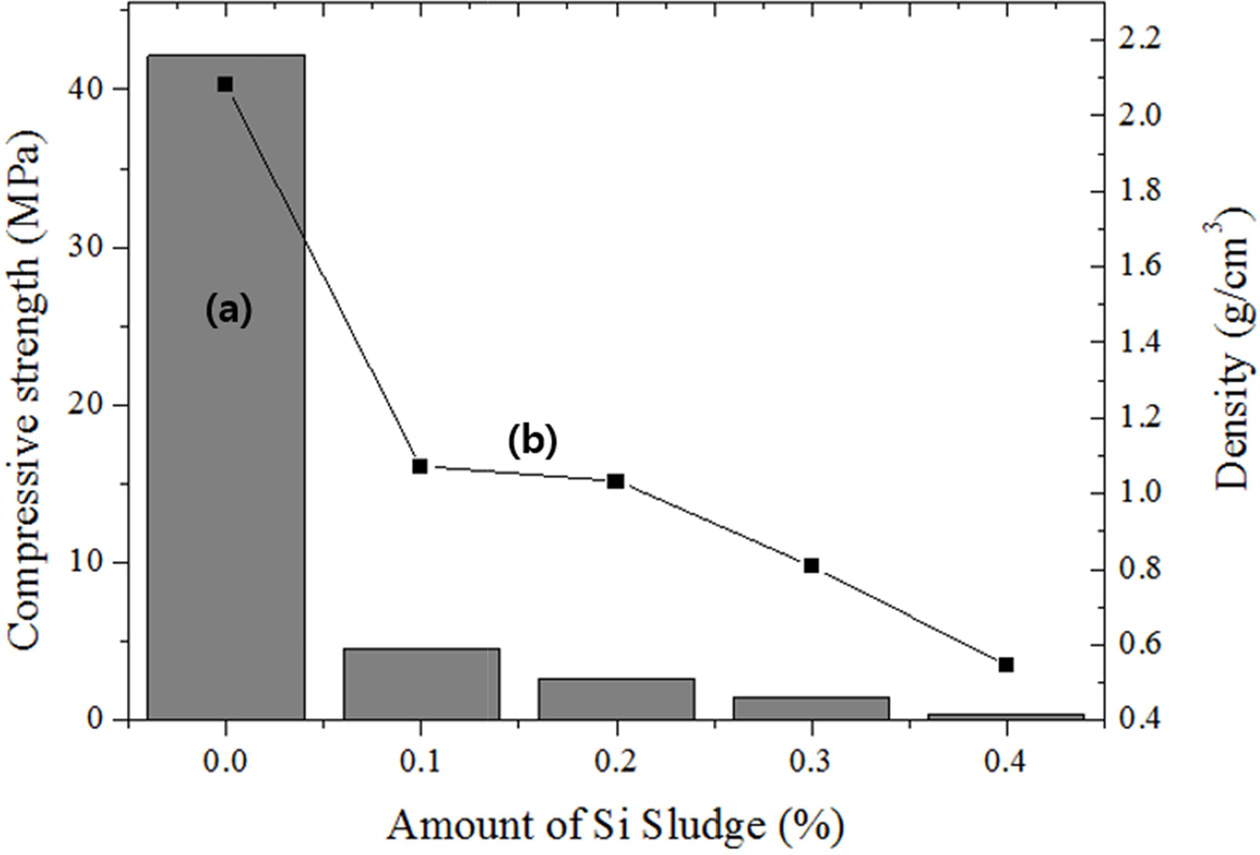
|
Fig. 3 Variations of the (a) compressive strength and (b) density with the addition of Si sludge. |
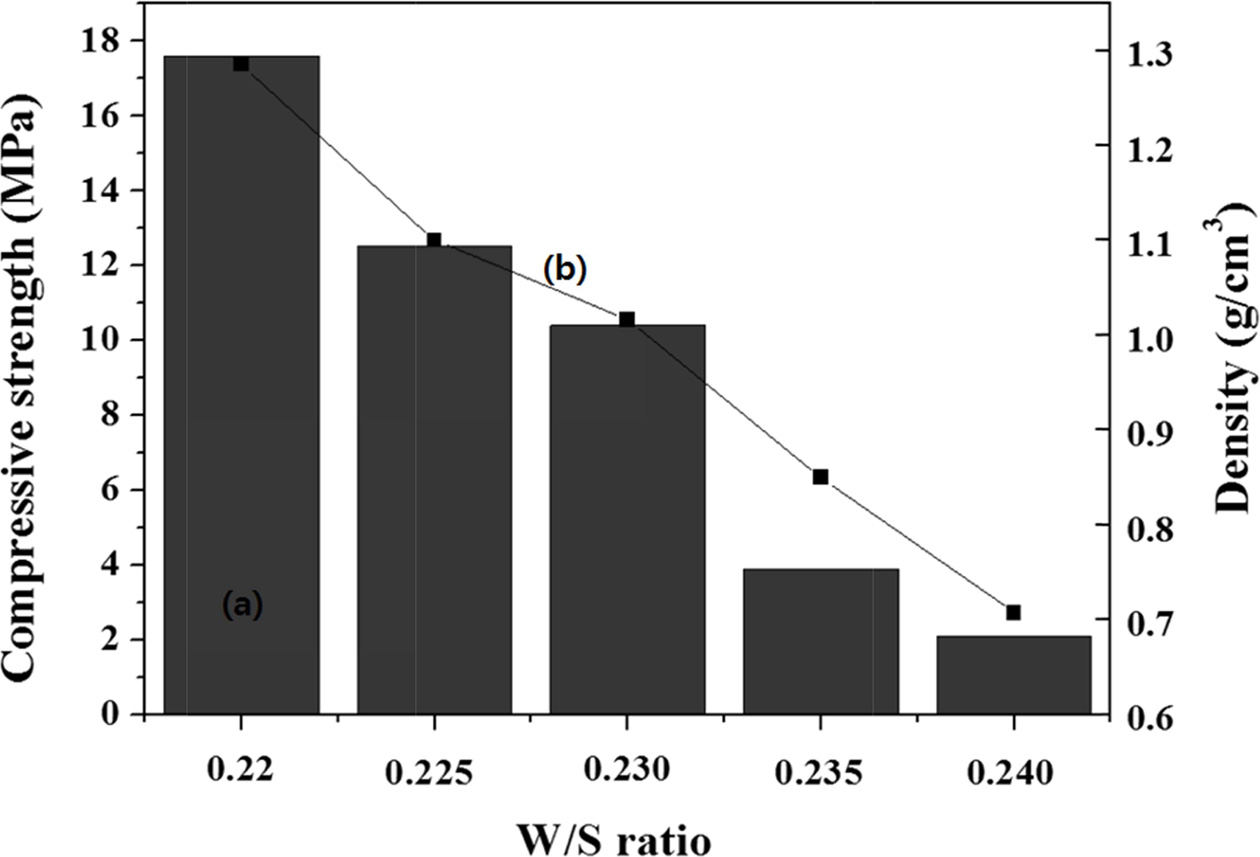
|
Fig. 4 Variations of the (a) compressive strength and (b) density of the specimens containing 0.1 wt.% Si sludge with respect to the W/S ratio. |
|
Fig. 5 Comparisons of the compressive strength and density of the lightweight geopolymers before and after the insertion of steel frames. Left: (a) compressive strength and (b) density. “X” represents “no frame,” and “O” represents “with steel frame.” Right: images of specimens before and after high-temperature curing. |
|
Fig. 6 Variations of the (a) compressive strength and (b) density of geopolymers with respect to the added amount of ADP. |
|
Fig. 7 Effects of the particle size on the (a) compressive strength and (b) density before and after particle separation. |
|
Fig. 8 (a) Compressive strength and (b) density of specimens cut to 50 × 50 × 50 mm3 from randomly selected positions of the 400 × 100 × 200 mm3 specimen. |

|
Fig. 9 Cross-sectional image of the large (400 × 100 × 200 mm3) specimen. |
|
Fig. 10 (a) Compressive strength and (b) density of the specimens sliced to 50 × 50 × 50 mm3 from different positions of the 250 × 250 × 250 mm3 specimens. |
|
Fig. 11 (a) Compressive strength and (b) density of the 50 × 50 × 50 mm3 specimens cut from different positions of the 250 × 250 × 100 mm3 specimens with 0.5 wt.% Si sludge. |
IGCC slag and Si sludge were used as raw materials to fabricate lightweight geopolymers having a high compressive strength. A mixed alkaline activator (Na2SiO3 and 98.0% NaOH) was more effective for producing lightweight/high-compressive strength geopolymers than its unmixed counterpart (NaOH only). The insertion of steel frames into the geopolymer was ineffective for increasing the compressive strength; it only increased the density. The addition of ADP increased the compressive strength; however, the density also increased. Therefore, the use of ADP is not recommended for obtaining a lightweight and high-strength specimen.
Size separation of the crushed IGCC slag had favorable effects on both the density and the compressive strength of the lightweight geopolymer specimens. The use of IGCC slag with a particle size of <180 μm led to the lowest density (0.63 g cm-3) and a compressive strength that was 1 MPa higher than that of the reference specimen prepared with unseparated IGCC slag. Therefore, size control of IGCC slag can improve the geopolymerization reaction, yielding a slight increase in the compressive strength and a reduction in the density.
For large specimens, the density and compressive strength differed according to the height of the specimen and the W/S ratio. Specimens having a higher compressive strength and lower density could be obtained by using a W/S ratio of 0.2 and cutting the sample from the middle part of the large specimen. Therefore, it is concluded that the W/S ratio influences the mobility of pores in the paste and is one of the main factors affecting the properties of lightweight/high-compressive strength geopolymers.
Evaluation of the effects of various factors on the properties of lightweight geopolymers indicated that the compressive strength and density are affected by many factors, such as the W/S ratio, particle size of the raw material, addition of bloating material, and amount of additive. Notably, control of the pore size and distribution is the most important factor for obtaining specimens having both a low density and a high compressive strength. Therefore, it is considered that the development of processing and production technology for fabricating lightweight geopolymers is essential, because there is a limit to the extent of enhancement of the properties of lightweight geopolymers that can be achieved by controlling the mixing ratio of the raw materials, bloating materials, additives, and alkaline activators. Additionally, it is speculated that the flow rate of the slurry may be one of the most important factors affecting the pore size, distribution, and homogeneity in the specimen. Therefore, additional systematic research must be performed for producing ultra-lightweight/high-compressive strength geopolymers by varying the pore size and distribution through mobility control of the slurry during the production process.
This work was supported by a Kyonggi University Research Grant (2018).
- 1. K.P. Lee, S.S. Song and H. Young, J. Kor. Arch. Inst. 27[11] (2011) 111-118.
- 2. K.T. Koh, G.S. Ryu and J.H. Lee, J. Kor. Rec. Consrt. Res. Inst. 6[1] (2011) 63-71.
- 3. Y.T. Kim, S.Y. Kim, and T.S. Chae, J. Ceram. Proc. Res. 18[3] (2017) 214-219.
- 4. G.H. Lim, J.B. Lee, H.K. Jeong, and S.S. Kim, J. Rec. Const. Res. 4[4] (2016) 418-424.
-

- 5. M. Aineto, A. Acosta, J. Ma. Rinc?n and M. Romero, Fuel 85 (2006) 2352-2358.
-

- 6. Arman, A. Okada, and H. Takebe, Fuel 182 (2016) 304-313.
-

- 7. Y.T. Kim, S.Y. Kim, and C.S. Jang, J. Ceram. Proc. Res. 17[11] (2016) 1202-1207.
- 8. J. Devent, J. Provis, and P. Duxson, Min. Eng. 29 (2012) 89-104.
-

- 9. Korean Standards, No. KS F-2701 (2017) p. 2-5.
- 10. H.J. Kim and Y.T. Kim, Int. Proc. Che. Bio. Env. Eng. 43[32] (2012) 154-159.
- 11. J.Y. Kim, J. Kor. Ceram. Soc. 52[2] (2015) 150-153.
-

- 12. K. Ugurlu, C. Demir and A. Ilki, Second Conference on Smart Monitoring, Assessment and Rehabilitation of Civil Structures, September 2013, edited by A. Ilkin. Motavalli, C. Goksu and B. Havranek (Istanbul Technical University) p. 363.
- 13. V. Jadhao and P. Pajgade, Int. J. Eng. Adv. Technol. 2[2] (2013) 148-153.
-

- 14. Y.G. Wu, S.S. Xie, Y.F. Zhang, F.P. Du, and C. Cheng, Ceram. Int. 44[2] (2018) 2578-2583.
-

- 15. S.B. Park, K.D. Kim and S.G. Kang, J. Kor. Cry. Gro. 27[6] (2017) 319-326.
 This Article
This Article
-
2019; 20(5): 522-529
Published on Oct 31, 2019
- 10.36410/jcpr.2019.20.5.522
- Received on Apr 17, 2019
- Revised on Aug 5, 2019
- Accepted on Aug 14, 2019
 Services
Services
- Abstract
introduction
experimental procedures
results
conclusions
acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Minjeong Kim
-
Department of Materials Engineering, Kyonggi University, Suwon 16227, Korea
Tel : +82-70-4024-9765
Fax: +82-31-249-9774 - E-mail: ytkim@kgu.ac.kr








 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.