- Rapid quantification of viable spore used in healing concrete cracks by a simple spectrophotometric method
Jinlong Zhanga,b,c, Jingkun Lub, Bing Liuc,d, Qiuyue Liub, Fan Jinb, Miaojun Zhangb, Yerong Liub,c, Yujun Songb, Chenhui Dongb, Wanyi Zhangb, Ningxu Hanc, Xu Deng b,* and Feng Xinga,c,*
aKey Laboratory of Earthquake Engineering and Engineering Vibration, Institute of Engineering Mechanics, China Earthquake Administration, Harbin 150086, P.R. China
bShenzhen Key Laboratory of Marine Bioresource and Eco-environmental Science, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518060, P.R. China
cGuangdong Provincial Key Laboratory of Durability for Marine Civil Engineering; College of Civil Engineering, Shenzhen University, Shenzhen 518060, P.R. China
dShenzhen Institute of Information Technology, Shenzhen 518172, P.R. China
Quantification of viable spores is a time taking task due to the lack of rapid, efficient and accurate methods. This study presented a simple spectrophotometric method for the detection of viable spores based on spore’s property of losing refractivity during the germination process. By comparison of the results obtained by both spectrophotometric method and colony counting method, a good linear correlation (R2 = 0.99) was achieved between viable spore concentration and OD loss under appropriate conditions. To avoid interference from ungerminable spores and vegetative cells, a turbidity complementation strategy of keeping the initial concentration of spore suspensions at the same and relatively lower level was required. The calibration equation developed could be used to predict the viable spore yield produced in a series of fermentation experiments. The experimental results proved that this novel spectrophotometric method was sensitive, rapid, and easy to perform compared to conventional colony counting method.
Keywords: Self-healing concrete, Bacillus, Spore germination, Colony forming unit, OD loss, Viability
As one of the most resistant life forms on the Earth exhibiting remarkable longevity, bacterial spore (endospore) is a dormant cellular structure during the stationary phase in the life cycle of spore-forming bacteria such as Bacillus sp. [1-3]. Endospores are associated with commercial production of antibiotics, industrial chemicals, and enzymes [4-5]. On the other hand, dormant spores also contribute to food spoilage [6-7]. For example, heat resistance of thermophilic Bacillus spores is especially problematic to the dried milk industry. For either situation, the viability of endospores is a key factor, as not all dormant spores can germinate [8]. Therefore, an effective method to evaluate the viability of dormant spores is important.
Hitherto, the standard method for enumerating viable endospores is based on colony forming unit (CFU) quantification technique [9-10]. This conventional method requires serial dilutions, plating, incubation and colony counting, apparently being time-consuming and labor-intensive [11]. Furthermore, due to the existence of ‘viable but not culturable’ (VBNC) microorganisms [12], the quantity of spores determined by plate counting method is actually the number of ‘culturable’ rather than viable spores, though ‘viable’ has been widely used [13-14]. From the point of view, plate counting method most likely underestimates the number of viable spores in the samples.
Different from vegetative cells that contain a large content of water, spores consist of a large amount of refractile materials with high-density, dehydrated core surrounded by a lower-density coat of crosslinked polypeptides. Consequently, Spores have a higher refractive index than vegetative cells. As long as the environment becomes appropriate, germination of viable spores can be initiated on a timescale of minutes [15-16]. During the germination process, refractivity of the viable spores is gradually diminished due to both the release of dipicolinic acid (DPA) from the core of the spore and water uptake, followed by subsequent cortex hydrolysis that leads to core swelling and further water uptake [17-19]. Ungerminable spores cannot activate such process, thus not leading to any change of refractivity. The decline of refractivity of the germinating spores can be detected by monitoring the optical density of spore suspension and expressed as OD loss [20-22]. Therefore, if the refractivity decline of the spore suspension during germination can be correlated to the quantity of viable (germinable) spores, then the spore viability can be simply indicated by OD loss.
In this study, we attempt to establish a rapid and simple spectrophotometric method for the first time to detect the viability of the spore samples. Firstly, we demonstrate that OD loss of spore germination process can be reliably converted to the concentration of viable spores; secondly, we investigate the influence of vegetative cells and ungerminable spores on the new method. Finally, the validation of the spectrophotometric method will be further evaluated by comparing the results of spore production detected by this new method and traditional plate counting method under different spore production conditions. This novel spectrophotometric method can hopefully be a good substitute for traditional plate counting method.
An alkaliphilic spore-forming strain (designated as H4) was isolated from the sediment samples collected from a mangrove conservation area in Shenzhen Bay, which was then identified as Bacillus species and was used in studies on bacterial self-healing concrete [23-25]. The spore of Bacillus sp. H4 can germinate at pH 10.5 and was used to establish and validate the novel assay of viable spores. The strain H4 was maintained on alkaline LB agar containing NaHCO3 (4.2 g/L) and Na2CO3 (5.3g/L) at 4 ℃, pH 9.7. A neutrophilic strain Bacillus subtilus ATCC6051, obtained from American Type Culture Collection (Rockville, MD), was used to provide “dead” or ungerminable spores in the experiment because its spores cannot germinate as pH value of the culture is more than 10.0. Bacillus subtilus ATCC6051 was maintained at 4 ℃ on LB agar.
A modified sporulating medium (MSP medium) based on that in Jonkers’ study [26] was used for spore production of strain H4, which contained (1L): NH4NO3 0.3 g, KH2PO4 0.02 g, CaCl2·2H2O 0.225 g, KCl 0.476 g, MgCl2·6H2O 0.2 g, MnSO4·2H2O 0.01 g, yeast extract 3 g, soluble starch 1 g, NaHCO3 4.2 g and Na2CO3 5.3 g. The pH of the medium was about 9.7. Strain H4 was grown overnight at 30 ℃ in alkaline LB broth containing NaHCO3 (4.2 g/L) and Na2CO3 (5.3 g/L), and 8 ml grown culture was inoculated into 100 ml fresh MSP medium. The cultivation was carried out in a series of 500 ml Erlenmeyer flasks with 100 ml working volume in a rotary incubator shaker at 30 oC, 150rpm. Bacillus subtilus ATCC6051 was point inoculated on YA agar (yeast extract 15 g/L, NaCl 5 g/L) to yield single colony. After 7 days, the spores were harvested from the incubation liquid or the plate surface and washed eight times with ice-cold deionized water. Washed spore preparations contained less than 5% vegetative cells as determined by phase contrast microscopy. The spore stock suspensions of strain H4 and strain ATCC6051 were both prepared to contain about 2.0 × 109 spores/ml (OD490 was approximately 1.7), as determined by direct spore counting with a Helber bacterial counting chamber (Hawksley, Lansing, UK) under a phase contrast microscopy. The number of viable spores in spore stock suspensions of strain H4 was about 9.27 × 108 cfu/ml as determined by traditional plate counting on alkaline LB agar after incubation at 30 ℃ for 48 hrs. The stock solution could be diluted to different concentrations according to experimental requirements. The spores were stored at -20 ℃ in deionized water.
A heat shock treatment of 60 ℃ for 30 min was applied to kill vegetative cells in spore suspensions. Serial dilutions were prepared to obtain spore suspensions containing about 102 to 103 spores/ml. One hundred μl aliquots from each dilution were plated in triplicate on alkaline LB agar, and incubated at 30 ℃ for 2 days. Colonies were counted and the mean number of CFU/ml in each original sample was calculated.
H4 spore stock suspension was diluted with ATCC6051 spore stock suspension to prepare working standard suspensions of H4 spore at concentrations ranging from 1 × 108 to 2 × 109 spores/ml. In all above spore suspensions, the total spore concentrations kept the same, while the concentrations of H4 spores exhibited gradient arrays.
For assay of spore germination, the standard spore suspensions were heat activated in water at 60 ℃ for 30 min. Six aliquots (25 μl) of various standard spore suspensions were transferred into individual well of 96-well microplate. 175 μl of alkaline germination solution (AGS, 100 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS), 200 mM NaCl, 10 mM L-inosine, pH 10.5) was added to each well and then the plate was incubated at 30 ℃ for 2 hrs. AGS was designed to contain no other nutrients except germinant (L-inosine) to assure that spores were limited to germination process. Germination was monitored by the change of optical density (OD) of samples at 490 nm in a microplate reader (Molecular device MX190 plus 384, US). The average values of OD loss obtained were plotted against viable spore concentrations obtained by plate counting method to get a linear calibration equation.
Strain H4 was inoculated in alkaline LB broth and incubated for 5 days at 30 ℃ to produce vegetative cells. The cells were centrifuged (6000 × g, 10 min) and washed 3 times with water. The stock suspension of H4 vegetative cells was prepared to contain about 2.0 × 109 cells/ml as determined by microscopic enumeration with a Helber bacterial counting chamber.
To investigate the influence of ungerminable spore and vegetative cell present in the spore suspension on the OD loss of viable spore, H4 spore stock suspensions was mixed with either ATCC 6051 spore stock suspension or H4 cell stock suspension as well as deionized water in different volume ratios: 10:1:9, 10:2:8, 10:4:6, 10:6:4, 10:8:2 and 10:10:0. For all spore mixtures, the concentrations of H4 spores kept the same, while that of ATCC 6051 spores or H4 vegetable cells was different. To further evaluate the effect of sample compositions on the OD loss of germination process at different initial concentrations of viable spores, five different sample volumes (25 μl, 37.5 μl, 50 μl, 62.5 μl, 75 μl) of each above spore mixture were added to AGS to obtain spore suspensions with initial concentrations of H4 spores being 1.25 × 108, 1.875 × 108, 2.5 × 108, 3.125 × 108 and 3.75 × 108 spores/ml, respectively. All germination processes were carried out in AGS at 30 ℃ for 2 hours. The OD loss was detected as above.
The validity of this spectrophotometric method was confirmed by accuracy of the estimation of viable spore production under different medium and fermentation conditions. In the experiment, some components of MSP medium including soluble starch (0, 2.5, 5,10 and 20 g/l), yeast extract (0, 0.5, 1, 2, 3, 4, 6, 8, 10 g/l) and sodium chloride (0, 1, 2, 4, 8, 16, 32, 64, 128 g/l), and cultivation conditions such as initial pH (11.0, 10.5, 10.0, 9.5, 9.0) and working volumes (25, 50, 75, 100, 125, 150 ml in 250 ml Erlenmeyer flasks) were separately adjusted to various levels.
Strain H4 was cultivated under different conditions at 30 ℃ for 7 days, and the spores were centrifuged (6000 × g, 10 min) and resuspended in equal volumes of deionized water. 1.5 ml of such spore suspension was transferred into a 5 mm light-path cuvette and the OD490 value was recorded with a spectrophotometer (Molecular device MX190 plus 384, US). Added adequate aliquots of ATCC6051 spore suspension (containing about 4.0 × 1010 spores/ml in water) into the cuvette, mixed the whole suspension thoroughly by pipetting back and forth several times and then read the OD490 value, and repeated the operation until the OD490 value reached approximately 1.7 (total spore concentration was about 2 × 109 spores/ml). Six aliquots (25 μl) of the spore mixture were transferred into individual well of 96-well microplate. 175 μl of AGS was added to each well and the plate was incubated at 30 ℃ for 2 hrs. The OD490 reduction value was detected by the spectrophotometric method and converted to the number of cfu/ml by means of the calibration equation. The achieved value of cfu/ml, representing viable spore concentration of the suspension, was further compared with the result obtained by the plate counting method.
All results were presented as mean ± standard deviation (S.D). Student's t-test was used to find any statistically significant difference in the results. P-values less than 0.05 were considered to represent statistically significant difference in spore concentration.
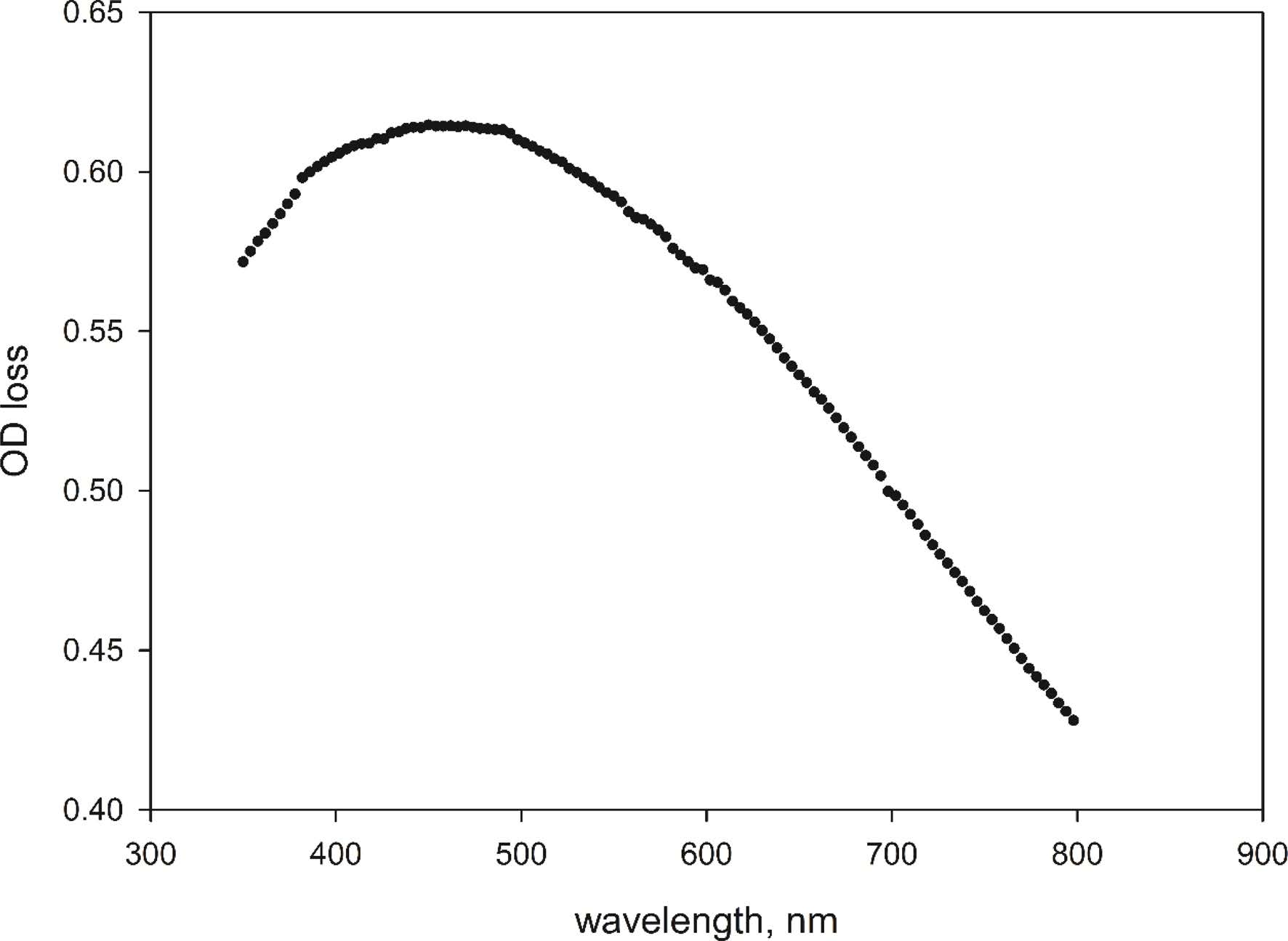
The suitable wavelength for detection of OD loss induced by spore germination was selected from the OD loss spectrum (Fig. 1). A flat peak appeared at about 450-500 nm and 490nm was selected to be the suitable wavelength accordingly.
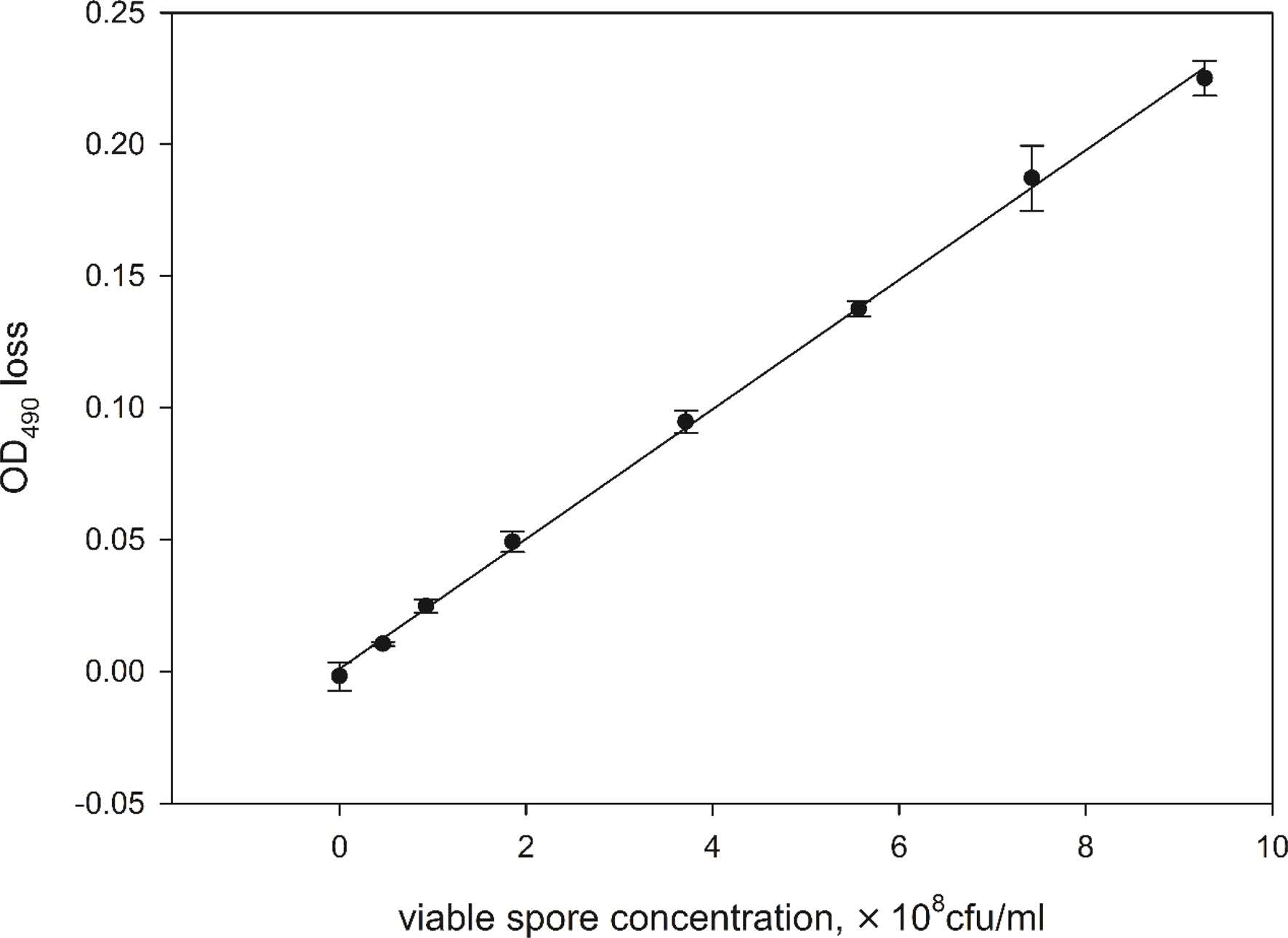
As the total spore concentrations of all standard spore samples kept constant, A linear correlation(R2 = 0.9989) of OD loss to CFU-forming spore concentration was determined in the range of viable spore concentrations from 0.5 × 108 to 9 × 108 cfu/ml. The calibration curve in Fig. 2 can be expressed by a linear equation:
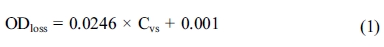
Where Cvs is viable spore concentration of the sample (×108 cfu/ml), and ODloss is the OD loss of spore suspension in AGS after 2 hrs.
|
Fig. 1 The spectrum of OD loss induced by spore germination. |
|
Fig. 2 Calibration curve of OD loss to viable spore concentration. Error bars represent 1 standard deviation. |
The spore samples to be tested likely contain ungerminable spores and/or vegetative cells. It is known that the ungerminable spore and vegetative cell cannot germinate and therefore do not contribute to OD loss. However, whether the presence of ungerminable spores and vegetative cells in the sample influences the determination of OD loss of viable spores, and consequently decreases the accuracy of the assay of viable spores, has not been investigated in previous studies.
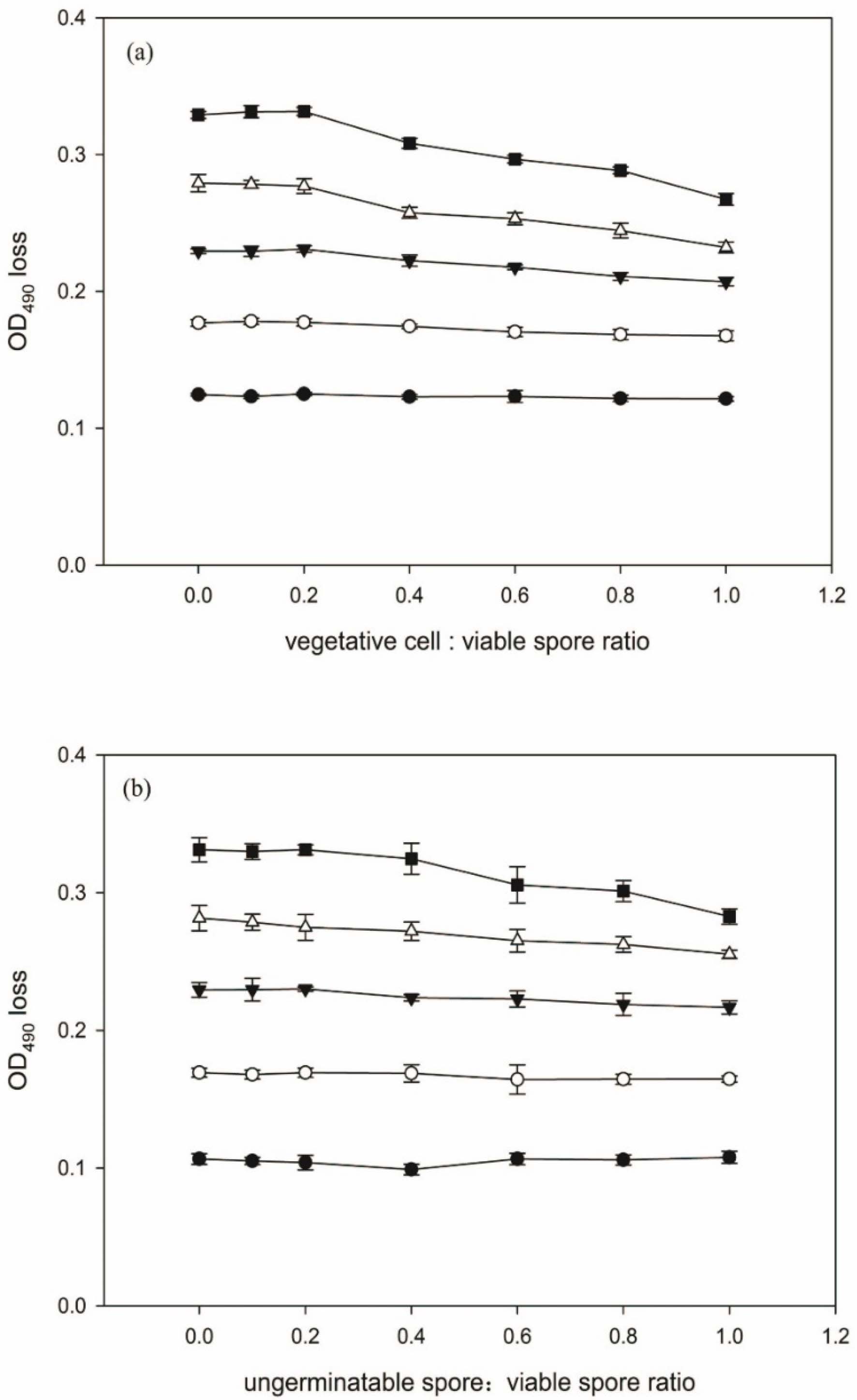
In the experiment, the effect of vegetative cells and ungerminable spores on the OD loss of spore suspensions at different initial H4 spore concentrations was evaluated and the results were shown in Fig. 3. From Fig. 3(a), it could be noted that the presence of vegetative cells generally led to decline of OD loss, but the extent of decline varied with the initial concentration of viable spores. As the initial H4 spore concentration was lower than 2.5 × 108 spores/ml, the decline of OD loss was almost negligible (less than 5%). With the increase of initial H4 spore concentration, the decline of OD loss became visible, but not remarkable. Even at 3.75 × 108 spores/ml of H4 spore concentration, the maximum decline of OD loss was less than 20% as the ratio of vegetative cell to viable spore reached 1.0. The effect of ungerminable spores on OD loss of spore suspensions was similar to that of vegetative cells (Fig. 3(b)).
In summary, ungerminable spores and vegetative cells present in spore samples interfere with the measurement of OD in varying degrees. The degree depends on both the initial concentration of viable spores and the concentration of interfering substance. From the experimental results, it can be concluded that the interfering influence of vegetative cells or ungerminable spores on OD loss can be reduced by keeping the initial viable spore concentration in germination solution at a relatively low level.
|
Fig. 3 OD loss of spore suspension in the presence of different concentrations of vegetative cells (a) or ungerminable spores (b). The initial concentrations of H4 spore in AGS were 1.25 × 108(●), 1.875 × 108(○), 2.5 × 108(▼), 3.125 × 108(△) and 3.75×108 spores/ml (■), respectively. Error bars represent 1 standard deviation. |
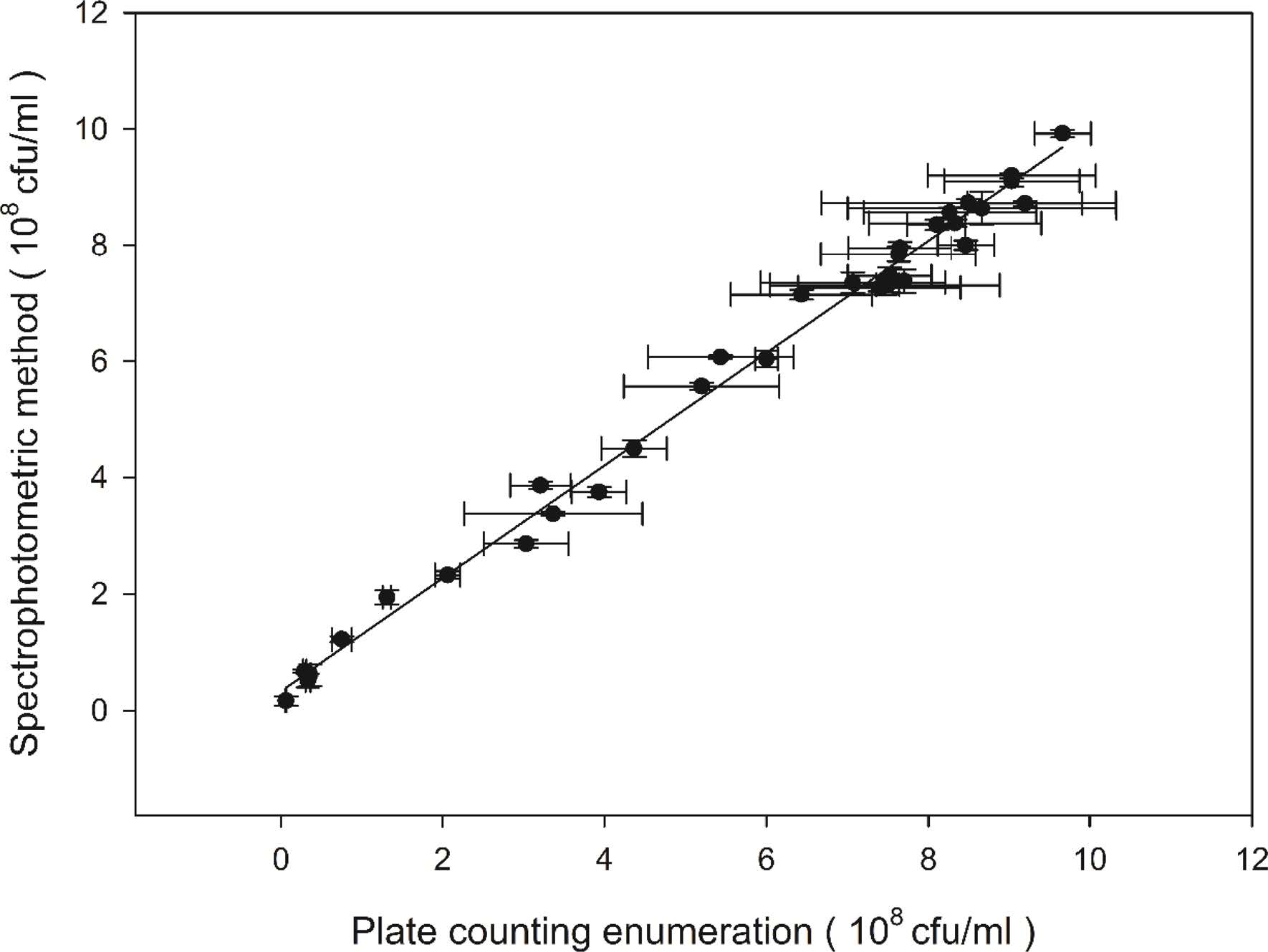
The validation of the present spectrophotometric method was carried out with a series of fermentation experiments as described earlier in Materials and methods section. The viable spore concentration was measured both by spectrophotometric method and the traditional plate counting method. According to the experimental results, the viable spore concentration values obtained by the spectrophotometric method were in close agreement with the plate count measurements. Student's t-test indicated that these differences were statistically insignificant. The plate count values and the detected viable spore yields by spectrophotometric measurements displayed a good linear correlation (y = 0.9679x + 0.3363, r2 = 0.9981, n = 34; Fig. 4 and table 1).The slope for the correlation was 0.9697. From the results, it can be concluded that the novel spectrophotometric method developed in this study can be an effective alternative to traditional plate counting method for the determination of viable spores.
|
Fig. 4 Correlation between plate counting enumeration and the spectrophotometric method for quantification of viable spore concentration. Each point represents a different culture. The solid line is the least square fit with r2 being 0.9918. The line shown indicates a 1:1 correspondence. Error bars represent 1 standard deviation. |
|
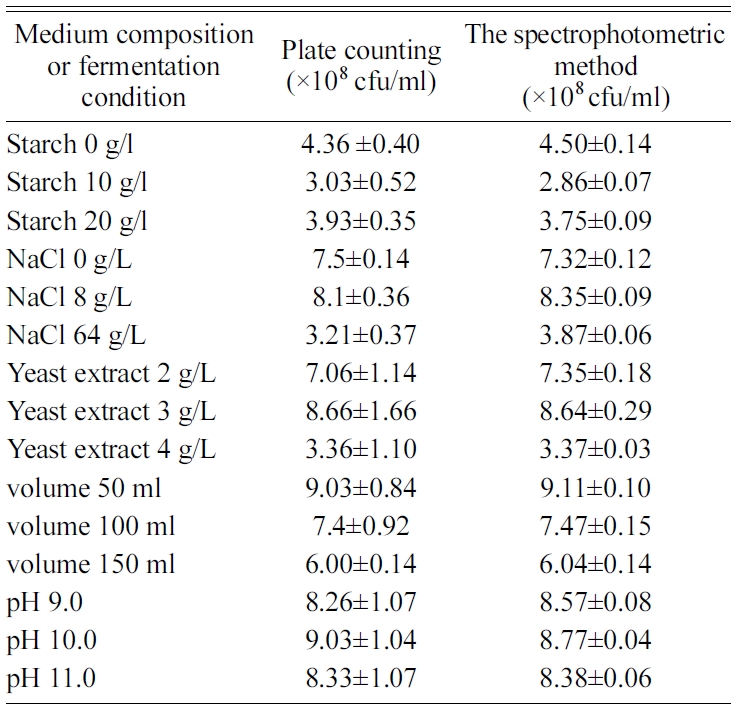
Table 1 Viable spore yield with different medium composition and fermentation conditions measured by plate counting method and spectrophotometric method. |
conditions measured by plate counting method and spectrophotometric method. |
The water content in the spore core is as low as 25-50% of wet weight. Due to the existence of a large amount of refractile materials, spore has a high refractive index. During spore germination, a series of biophysical events are triggered by germinants. These include small molecules (monovalent cations like H+, Na+, and K+; DPA-Ca) release and their replacement by water (stage I), cortex hydrolysis and full core rehydration (stage II), metabolism initiation and spore outgrowth (stage III) followed elongation and finally cell division. Among these stages, Stage I and II lead to a large decrease in the core’s refractive index. [21-22].
Spore germination is initiated by specific germinants binding to cognate receptors located in the spore’s inner membrane. As a result, some signals are transduced to downstream effectors [19, 22, 27]. The decrease of refractivity of spores during germination actually reflects the signal reception and transduction in the spores. As the decrease in degree of refractivity could usually be measured as a reduction in the optical density (OD) of the spore suspension [20-22], OD loss of a spore suspension likely indicates the amount of spores with intact signal channel for completing germination stage I and II. However, even germination is initiated and spore’s refractivity decreases, the spore is still not committed to the resumption of vegetative growth. The stage III and the following cell division involve a much more complicated and huge metabolism with extensive demands for nutrient and energy compared to stage I and II. Some spores can go through stage I and II but cannot keep growing due to defective metabolism.
In our experiment, spore germination solution was designed to contain only germinant with no other nutrients. Therefore, OD loss detected by the spectrophotometric method only represented the number of viable spores that go through first two stages. Traditional plate counting method, however, is known to be used to determine viable (actually culturable) spores that go through all three stages. From this point of view, the establishment of a calibration relation between OD loss of spore germination process and the number of viable spores measured by plate counting method is crucial to make the novel spectrophotometric method practicable. Encouragingly, our experimental result confirmed this correlation as the total spore concentrations of all samples kept equivalent (Fig. 2), suggesting a quantified connection really existing between the number of OD-reducing spores and cfu-forming spores.
The spore samples to be tested likely contain ungerminable spores and/or vegetative cells. Although the presence of ungerminable spores and vegetative cells in AGS do not lead to any decline of OD during germination process, the measurement of OD loss of viable spores can possibly be interfered. This interfering effect on OD loss of germination process has been proved in our experiment, though the effect is not remarkable (Fig. 3). Furthermore, the interfering effect tended to rise with the increase of ungerminable spore and/or vegetative cell concentration, leading to increasing extent of decline of OD loss. This difference in OD loss can be associated with the turbidness difference caused by the amount of ungerminable spore and/or vegetative cells present in the spore samples. Such turbidness effect is similar to masking effects. The increased turbidness attenuates the effective OD response to spore germination by either scattering or absorbance, consequently resulting in the decrease or weakness of OD loss.
Two steps can be performed to eliminate this interference. Firstly, a sample pretreatment procedure was proposed to adjust turbidity of all samples to an equal level. For example, by adding ungerminable spores into the samples before germination process to make the OD490 values of all spore samples equivalent, all samples will have same levels of turbidness effect and therefore the same levels of OD loss readings. Secondly, keeping the initial concentration of spore suspension at a relatively low level in the assay procedure can also weaken the masking effect of turbidity caused by ungerminable and/or vegetative cells. Our study proved that the strategy of turbidity complementation together with low initial concentration of spores was effective to ensure the accurate determination of OD loss for spore germination in the presence of ungerminable spores and vegetative cells (Fig. 3).
Assay of viable spore yield under different nutrients and cultivation conditions
To validate this spectrophotometric method, we performed parallel germination experiments with spores obtained under different cultural conditions. The spore yield was quite different with the change in the concentration of medium components such as yeast extract, soluble starch and sodium chloride, and in cultivation conditions including pH and working volume. For all the samples, the viable spore concentrations measured with the spectrophotometric method were consistent with that obtained by plate counting method (Fig. 4 and Table 1). Compared to the plate counting method, the spectrophotometric assay doesn’t require time-consuming and labor-intensive procedures such as serial dilution and colony formation, and accordingly is sensitive, rapid and easy to perform. Consequently, this rapid method can show the potential in biotechnological application, for example, facilitating the optimization of operational conditions in spore production.
Endospore’s property of losing refractivity during germination has been extensively focused. Based on this property, the influence of environmental factors on spore germination process and the role of particular gene such as gerA, gerD and gerAB in germination have been studied [28-30]. The OD loss of spore suspension caused by germination of viable spores can be easily quantified. Firstly, the spectrophotometric method is a high-throughput procedure that allows semi-automatic detection of OD loss because germination can be carried out in large scale, for example, in 96-well plate; Secondly, the spectrophotometric method needs no sterilization or aseptic operation due to poor nutrition in germination solution; Finally and most importantly, the spectrophotometric method is rapid as the germination process is very short (i.e., 2 hours in our experiment), while in colony counting method, incubation for colony forming usually needs at least 12 hrs and even several days for some particular species.
Besides applications in rapid measurement of viable spore yield in fermentation system and the fermentation condition optimization, this novel method can also be employed to evaluate the effect of physical and chemical factors on the germination, aging, super-dormancy and sterilization of spores, for example, the screening of germination-inactivating or spore-killing agent, the mechanism of how the germination is affected by spore age, etc.
Based on endospore’s property of losing refractivity during germination process, a novel spectrophotometric method was developed for rapid detection of viable spores. Our experimental results showed that under suitable conditions, the viable spore concentrations measured by the spectrophotometric method were in good agreement with that obtained by plate counting method. Furthermore, without serial dilution and colony formation process, this novel method is sensitive, rapid and easy to perform. Therefore, this spectrophotometric method is an effective alternative to conventional colony counting method in viable spore determination, especially for large-scale detection of spore samples.
The authors acknowledge the financial support provided by National Natural Science Foundation of China (No.51120185002, No. 51578339, No. 5150 8338).
- 1. W.L. Nicholson, N. Munakata, G. Horneck, H.J. Melosh and P. Setlow, Microbiol. Mol. Biol. Rev. 64[3] (2000) 548-572.
-

- 2. H. Gest, and J. Mandelstam, Microbiol. Sci. 4[3] (1987) 69-71.
- 3. M. Potts, Microbiol. Rev. 58[4] (1994) 755-805.
- 4. M. Schallmey, A. Singh and O.P. Ward, Can. J. Microbiol. 50[1] (2004) 1-17.
-

- 5. P. Setlow, E.A. Johnson, M.P. Doyle and L.R. Beuchat, In “Food microbiology: fundamentals and frontiers” (ASM press) p35-67.
-

- 6. A.S. Levine and C.R. Fellers, J. Bacteriol. 39[5] (1940) 499-515.
- 7. M.C.T. Giffel, R.R. Beumer, S. Leijendekkers and F.M. Rombouts, Food Microbiol. 13[1] (1996) 53-58.
-

- 8. H.S. Shafaat and A. Ponce, Appl. Environ. Microbiol. 72[10] (2006) 6808-6814.
-

- 9. P.C. Turnbull, D.A. Frawley, and R.L Bull, J. Microbiol. Methods 68[2] (2007) 353-357.
-

- 10. C.A. Lenz and R.F. Vogel, Food Microbiol. 46 (2014) 434-442.
-

- 11. S. Sieuwerts, F.A. Debok, E. Mols, W.M. Devos and J.E. Vlieg, Lett. Appl. Microbiol. 47[4] (2008) 275-278.
-

- 12. R. Colwell and D. Grimes, in “Non-culturable microorganisms in the environment” (ASM Press) p10-19
-

- 13. L.J. Rose, L. Hodges, H. O'Connell and J.N. Wang, Appl. Environ. Microbiol. 77[23] (2011) 8355-8359.
-

- 14. S.E. Diomandé, S. Chamot, V. Antolinos, F. Vasai, M.H. Guinebretière, I. Bornard, C. Nguyenthe,V. Broussolle and J. Brillard, Appl. Environ. Microbiol. 80[8] (2014) 2493-2503.
-

- 15. G.M. Hills, Biochem. J. 45[3] (1949) 363-370.
-

- 16. P. Setlow, Curr. Opin. Microbiol. 6[6] (2003) 550-556.
-

- 17. P. Zhang, L. Kong, G. Wang, M. Scotland, S. Ghosh, P. Setlow and Y.Q. Li, J. Appl. Microbiol. 112[3] (2012) 526-536.
-

- 18. L. Kong, P. Zhang, G. Wang, J. Yu, P. Setlow and Y.Q. Li, Nat. Protoc. 6[5] (2011) 625-639.
-

- 19. D. Paredes-Sabja, P. Setlow and M.R. Sarker, Trends Microbiol. 19[2] (2011) 85-94.
-

- 20. I.E. Young and P.C. James, J. Cell. Biol. 12[1] (1962) 115-133.
-

- 21. T. Kodama, H. Takamatsu, K. Asai, N. Ogasawara, Y. Sadaie and K. Watabe, J. Biochem. 128[4] (2000) 655-663.
-

- 22. M.T. McKevitt, K.M. Bryant, S.M. Shakir, J.L. Larabee, S.R. Blanke, J. Lovchil, C.R. Lyons and J.D. Ballard, Infect. Immun. 75[12] (2007) 5726-5734.
-

- 23. J.L. Zhang, R.S. Wu, Y.M. Li, J.Y. Zhong, X. Deng, B. Liu, N.X. Han and F. Xing, App. Microbiol. Biotech. 100[15] (2016) 6661-6670.
-

- 24. J.L. Zhang, C.G. Wang, Q.L. Wang, J.L. Feng, W. Pan, X.C. Zheng, B. Liu, N.X. Han, F. Xing and X. Deng, App. Microbiol. Biotech. 100[24] (2016) 10295-10306.
-

- 25. J.L. Zhang, B.X. Mai, T.W. Cai, J.Y. Luo, W.H. Wu, B. Liu, N.X. Han, F. Xing and X. Deng, Mater. 10[2] (2017) 19116-19232.
- 26. H.M. Jonkers, A. Thijssen, G. Muyzer, O. Copuroglu and E. Schlangen, Ecol. Eng. 36[2] (2010) 230-235.
-

- 27. P. Setlow, Cell 135[3] (2008) 410-412.
-

- 28. P.L. Pelczar, T. Igarashi, B. Setlow and P. Setlow, J. Bacteriol 189[3] (2007) 1090-1098.
-

- 29. G.R. Cooper and A. Moir, J. Bacteriol. 193[9] (2011) 2261-2267.
-

- 30. I.S. Løvdal, C. From, E.H. Madslien, K.C. Romundset, E. Klufterud, J.T. Rosnes and P.E. Granum, BMC Microbiol. 12[1] (2012) 1134-1168.
 This Article
This Article
-
2019; 20(S1): 63-69
Published on Jul 20, 2019
- Received on Dec 9, 2018
- Revised on Jan 11, 2019
- Accepted on Jan 11, 2019
 Services
Services
- Abstract
introduction
materials and methods
results
discussions
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Xu Deng b,* and Feng Xinga,c,*
-
bShenzhen Key Laboratory of Marine Bioresource and Eco-environmental Science, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518060, P.R. China
cGuangdong Provincial Key Laboratory of Durability for Marine Civil Engineering; College of Civil Engineering, Shenzhen University, Shenzhen 518060, P.R. China
Tel : +86-755-2653-5435 - E-mail: dengxu@szu.edu.cn, xingf@szu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.