- Calcium phosphate porous bioceramics prepared by direct foaming
Kamrun Nahar Fatemaa, Md Rokon Ud Dowla Biswasa, Kee Sung Leeb and Ik Jin Kima,c,*
aInstitute of Processing and Applications of Inorganic Materials (PAIM), Department of Materials Science and Engineering, Hanseo University, 46, Hanseo 1-ro, Haemi-myeon, Seosan-si, Chungcheongnam-do, 31962, Republic of Korea
bSchool of Mechanical Engineering, Kookmin University, 77, Jeongneung-ro, Seongbuk-gu, Seoul 02707, Republic of Korea
cFaculty of Mechanical and Electrical Engineering, German-Mongolian Institute for Resources and Technology, GMIT, 2nd Khoroo, Nalaikh district, Ulaanbaatar, MongoliaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Calcium Phosphate ceramics are widely recognized as promising candidates for bone substitute materials due to their excellent biocompatibility, bioactivity, and osteoconductive properties. This study investigates the fabrication of interconnected porous hydroxyapatite (HA) ceramics via the direct foaming method, focusing on the optimization of synthesis parameters and the evaluation of mechanical performance using the Hertzian indentation method. By systematically varying the solid content, the microstructural and mechanical properties of the resulting ceramics were tailored to achieve an optimal balance between porosity and strength. Comprehensive microstructural analysis using scanning electron microscopy (SEM) confirmed the formation of highly interconnected porous networks. Mechanical testing validated the structural integrity of the foamed HA ceramics, demonstrating their potential for load-bearing applications in bone tissue engineering. This study provides critical insights into the direct foaming process, highlighting its potential for scalable and reproducible fabrication of porous HA scaffolds.
Keywords: Colloidal suspension, Calcium phosphate, Direct foaming, Wet foam stability, Porous bioceramics, Interconnected pore.
The development of biomaterials for bone tissue engineering has gained significant attention in recent years, with a focus on achieving both mechanical integrity and biological compatibility with host tissue. Biodegradable materials featuring interconnected porous microstructures have been widely explored due to their ability to support cell adhesion, migration, and proliferation, facilitating effective bone regeneration [1, 2]. Among these materials, hydroxyapatite (HA) has been extensively studied due to its chemical and structural similarity to the mineral phase of natural bone, as well as its excellent biocompatibility and osteoconductivity [3]. As a calcium phosphate ceramic, HA is a fundamental component of bone and dental tissues, making it an attractive candidate for orthopedic and dental applications [4]. The porous architecture of HA ceramics plays a crucial role in nutrient exchange, vascularization, and extracellular matrix deposition, essential for osteointegration and bone remodeling. The presence of interconnected pores enhances the surface area for cellular attachment and proliferation, while also facilitating the migration of osteoblasts and mesenchymal stem cells, thereby accelerating bone tissue regeneration [5, 6]. Our study focuses on evaluating the mechanical behavior of porous HA ceramics fabricated via the direct foaming method [13-26], with a particular emphasis on damage resistance under constrained loading conditions using the Hertzian indentation method. This approach provides a detailed assessment of deformation, crack initiation, and failure mechanisms in porous HA scaffolds [13-16].
The Hertzian indentation test was performed to analyze load-displacement behavior during compressive loading, offering insights into the contact damage resistance and mechanical integrity of the foamed HA ceramics. By systematically varying the solid content and propyl gallate concentration, we investigated their influence on pore morphology, mechanical stability, and failure mechanisms. The results highlight the critical role of porosity and microstructural connectivity in determining the load-bearing capacity and damage tolerance of the scaffolds [15-17, 23, 25-27].
This study provides a comprehensive understanding of how Hertzian indentation testing can be utilized to assess the mechanical performance of porous bone substitute materials, contributing to the optimization of next-generation biomaterials for bone tissue engineering.
Raw materials
Hydroxyapatite (HA) powder (KC, South Korea), with an average particle diameter, Propyl gallate (Fluka Analytical, Germany). Further chemicals used for this study were 10 (M) HCl (Yakuri Pure Chemicals, Osaka, Japan), Distilled water.
Preparation of colloidal HA suspension
HA powder was dispersed in distilled water and homogenized at 80 rpm for 1 h. Propyl gallate (2.5 wt.%) was then added dropwise to the HA suspension (16–32 vol.% solid content) under 400 rpm stirring to hydrophobized the HA particles. The pH was adjusted to 3.0 using HCl, and the final solid content was set by adding water.
Wet foaming of hydroxyapatite by direct foaming method
The direct foaming process involves incorporating air into a liquid polymer matrix through physical mixing, chemical reactions, or nucleation. As bubbles form and rise due to buoyancy, a thin liquid film stabilizes the foam structure. However, bubble rupture and coalescence influence pore size, drainage rate, viscosity, and surface tension, ultimately affecting foam morphology and stability. Closed-cell structures exhibit lower density and improved mechanical properties. Fig. 1 illustrates the direct foaming method.
Drying and sintering
Wet foams were dried at 25 °C for 48 hours. Specimens were then sintered at 1300 °C for 1 hour in a Super Kanthal furnace, with heating and cooling rates of 1 °C/min and 3 °C/min, respectively.
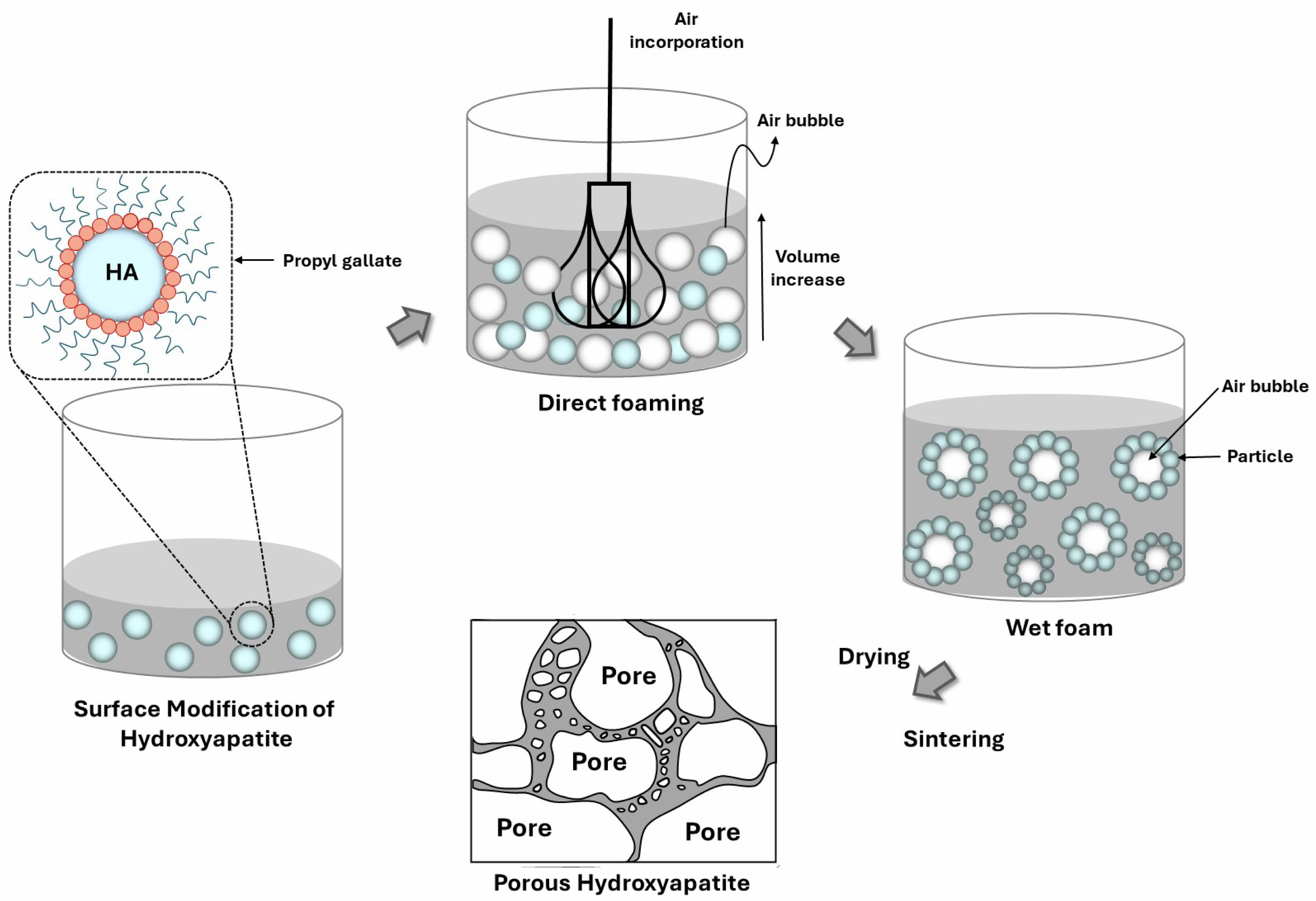
|
Fig. 1 Direct foaming method for producing porous hydroxyapatite ceramics. |
Rheological properties
The rheological behavior of HA slurry with different wt.% of propyl gallate (PG) was evaluated with a rheometer (MCR502, Anton Paar, Germany) a room temperature. The rheometer was used with a cone-cup type (CC 27, 27 mmcone diameter) at a temperature of 25 °C, and for the PG binder, which has relatively low viscosity, the double-gap cup type (DG26.7, 26.7 mm cup diameter) was used. The flow curves of the slurries were measured for shear rates from 1/s to 1000/s with a variable duration from 10 to 1 s for the overall 120 s.
Air content and wet foam stability
100 ml suspensions were foamed using a 150 W household mixer (Super Mix, France) at full power for 30 min. Bubble size distribution was analyzed via an optical microscope (Somtech Vision, South Korea). Air content, indicating wet foam stability, was quantified as the percentage volume increase before and after foaming, calculated using Eq. [13, 15, 20].
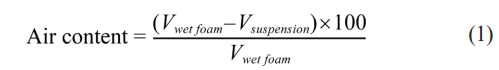
Where Vwet foam is wet foam volume after foaming and Vsuspension is the volume of suspension before foaming.
Wet foam stability was assessed by placing samples in cylindrical molds for 48 hours and measuring volume loss as an indicator of stability. Foam shrinkage and rupture occurred due to drainage and bubble collisions. Stability was defined by the volume reduction after drying at 20–25 °C and expressed as [15, 20]:
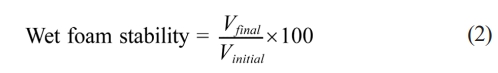
Where Vfinal is the volume of wet foam after 48 hours and Vinitial is the volume of wet foam before 48 hours.
Pore analysis
The morphology of porous hydroxyapatite ceramics was analyzed using SEM (Hitachi S-4800, Japan, 10 kV) after Pt sputtering. Pore size was measured from SEM images using ImageJ software. Porosity was determined via Archimedes’ principle and Micromeritics mercury intrusion porosimetry. Samples were weighed dry, then soaked in kerosene inside a desiccator for 30 min, and both suspended and soaked weights were recorded.
Mechanical behaviors by Hertzian method
The mechanical behavior of sintered porous HA ceramics was studied using Hertzian indentation tests in air with a universal testing machine (Instron 5567, MA). Tests were conducted at a 0.2 mm/min cross-head speed over a 5–200 N load range using tungsten carbide spheres (r = 7.93 mm). Load-displacement curves were recorded during loading and unloading, with displacements processed via amplifiers, converters, and a digital signal processor.
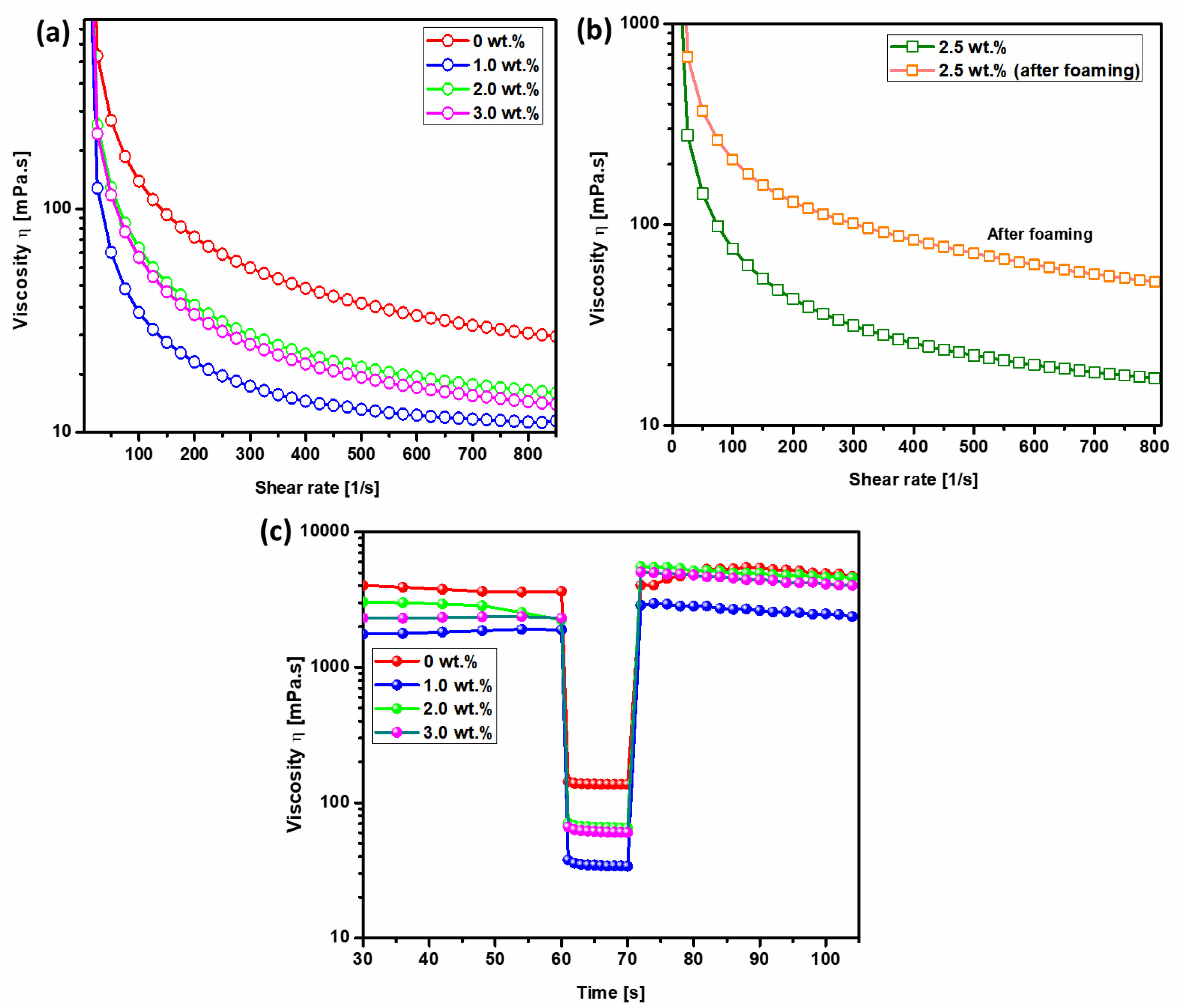
Fig. 2(a) presents the flow curves of hydroxyapatite (HA) slurries with varying surfactant concentrations, exhibiting characteristic shear-thinning behavior, where viscosity decreases with increasing shear rate. This phenomenon arises as the shear rate increases, facilitating the displacement of the liquid surrounding the particles and subsequently reducing the apparent viscosity of the colloidal suspension. The introduction of a surfactant into the HA colloidal system results in its adsorption onto particle surfaces, altering their surface properties and diminishing interparticle attractive forces, thereby enhancing dispersion and lowering viscosity compared to the surfactant-free suspension. The elevated viscosity in the absence of surfactant is primarily attributed to Van der Waals forces, which dominate colloidal interactions. An initial increase in viscosity is observed as the surfactant content rises to 3.0 wt.%, likely due to the adsorption-free energy effect, a common occurrence in colloidal systems [25]. Enhanced surfactant adsorption at elevated concentrations intensifies interparticle interactions, leading to significant viscosity augmentation. This is attributed to the screening of the particle surface charge, which occurs as amphiphilic molecules adsorb onto the particles or remain freely dispersed in the liquid medium. The resulting reduction in the electrical double layer thickness weakens repulsive forces, amplifying Van der Waals attractions and increasing foam viscosity. Overall, the flow curves confirm the shear-thinning nature of all suspensions, particularly at low shear rates, where surface forces predominantly govern rheological behavior [28].
Fig. 2(b) illustrates the flow curves of foamed HA slurries containing 2.5 wt.% surfactant, which also exhibit shear-thinning characteristics. During foaming, the introduction of air bubbles increases the liquid’s surface area, creating additional interfaces for surfactant adsorption. This expanded surface area enhances surfactant-particle interactions, further modifying the surface chemistry and interparticle forces within the colloidal system. Consequently, the presence of surfactant in the foamed suspension exerts a more pronounced influence on rheological behavior, particularly viscosity, due to the amplified effect of surfactant adsorption at the newly formed interfaces. We observed that the process of foaming, which inherently increases the surface area of the liquid, led to a decrease in viscosity (Fig. 2). Specifically, the data shows a clear trend where increasing the surfactant concentration from 1.0 wt.% to 3.0 wt.% correspondingly decreases the viscosity both before and after foaming. This decrease in viscosity can be attributed to the reduced intermolecular forces as the surfactant molecules align at the air-liquid interface, effectively lowering the resistance to flow within the fluid matrix. To substantiate these observations, we conducted detailed analyses of both surface tension and contact angle, which are directly influenced by the presence and concentration of surfactants. Our findings (Fig. S2) indicate that as the surfactant concentration increases, the contact angle also increases from 54° at 1.0 wt.% to 87° at 3.0 wt.%. This increase suggests a higher degree of hydrophobicity at the surface, which correlates with the observed rise in surface tension across the same range of surfactant concentrations. These surface property measurements support our claim regarding the influence of surfactant concentration on viscosity through changes in surface tension and wettability. The increased surface tension and contact angle reflect a more hydrophobic surface which is less conducive to spreading, a factor that, combined with the structural changes in the foam, contributes to the overall reduction in viscosity.
Fig. 2(c) presents an investigation into the thixotropic recovery behavior of hydroxyapatite (HA) colloidal suspensions under controlled shear rate conditions [28, 29]. The study employs a three-interval shear rate protocol: Interval 1 (shear rate of 1 s⁻¹ for 60 s), followed by Interval 2 (shear rate of 500 s⁻¹ for 10 s), and concluding with Interval 3 (shear rate of 1 s⁻¹ for 30 s). The flow curves corresponding to Intervals 1 and 3 are presented on a linear scale, with Interval 3 providing insight into the extent of structural recovery over time relative to the initial state in Interval 1. The results indicate that recovery is more rapid in the absence of surfactant during Interval 2, with viscosity in Interval 3 increasing significantly compared to Interval 1. This behavior is attributed to the dispersive effect of surfactant under high shear conditions in Interval 2, which subsequently influences the rheological response during Interval 3. The presence of surfactant modifies the colloidal interactions, leading to an overall increase in viscosity in Interval 3. Here are the specific findings for the surfactant-free suspension, the recovery rate was approximately 90%, indicating a significant but not complete recovery. Suspensions containing 1 wt.% of surfactants exhibited a recovery rate of 93%, showing slightly better thixotropic behavior compared to the surfactant-free system. At 2 wt.% surfactant concentration, the recovery rate further increased to 96%, demonstrating enhanced structural regeneration. Notably, suspensions containing 2 wt.% and 3 wt.% surfactant exhibit higher thixotropic behavior than the surfactant-free system. A 98% recovery rate is observed when the shear rate in Interval 2 is maintained at 500 s⁻¹, indicating a high degree of structural regeneration. As demonstrated in our data, particularly evident from the viscosity recovery curves shown in the top right graph of our submission, the viscosity recovery post-shear indicates almost complete reversibility. This confirms the thixotropic nature of our hydroxyapatite foam, as the structure that contributes to increased viscosity can reform after the cessation of shear or dilution. Furthermore, the surfactant concentrations ranging from 2.0 to 3.0 wt.% resulted in contact angles of 82°, 86°, and 87°, respectively. These measurements, displayed in Figure S1, indicate typical behavior of partially hydrophobized colloidal solutions. The partial hydrophobization facilitates reversible inter-particle interactions, which are crucial for the thixotropic properties observed. Under shear, these interactions are temporarily disrupted, reducing the viscosity, and are re-established upon removal of shear, allowing the viscosity to recover. This pronounced thixotropic response is crucial for enhancing the stability of wet foams during the foaming process. These findings underscore the critical role of surfactant concentration and shear history in dictating the thixotropic properties of HA colloidal suspensions, with direct implications for optimizing foam stability in processing applications. Particle size distribution was assessed using SEM images (Fig. S3). HA particles displayed a broad size range, with shapes varying from elongated to irregular. Using Image J software, we calculated an average diameter of approximately 106 nm. The wide distribution in particle size can contribute to different packing densities in the slurry, affecting the viscosity and the thixotropic behavior observed.
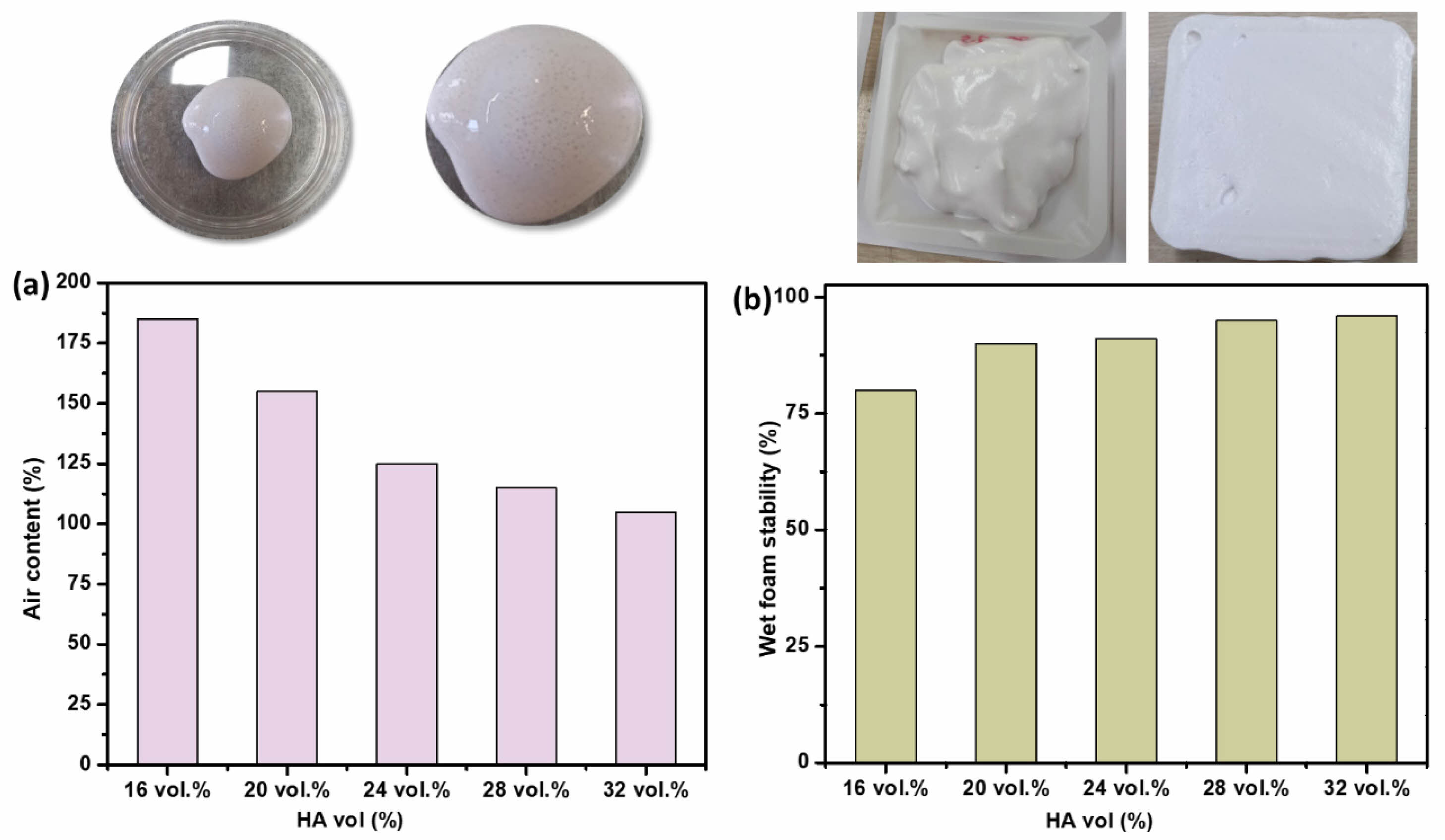
Fig. 3(a-b) illustrates the variation in air content of hydroxyapatite (HA) suspensions as a function of solid content. The results indicate a gradual increase in air content with rising HA concentration, reaching approximately 120% at 24 vol.% HA, where the wet foam stability is maximized at ~93%. The observed trend is attributed to the interplay between suspension viscosity and particle packing density. As the solid content increases, the interstitial spaces between particles decrease, impeding air penetration and consequently reducing air entrapment. However, a sufficiently high air content contributes to foam stability, as entrapped air bubbles provide mechanical support, preventing particle sedimentation. Beyond a critical solid content, excessive viscosity compromises foam integrity, hindering the formation of a stable structure.
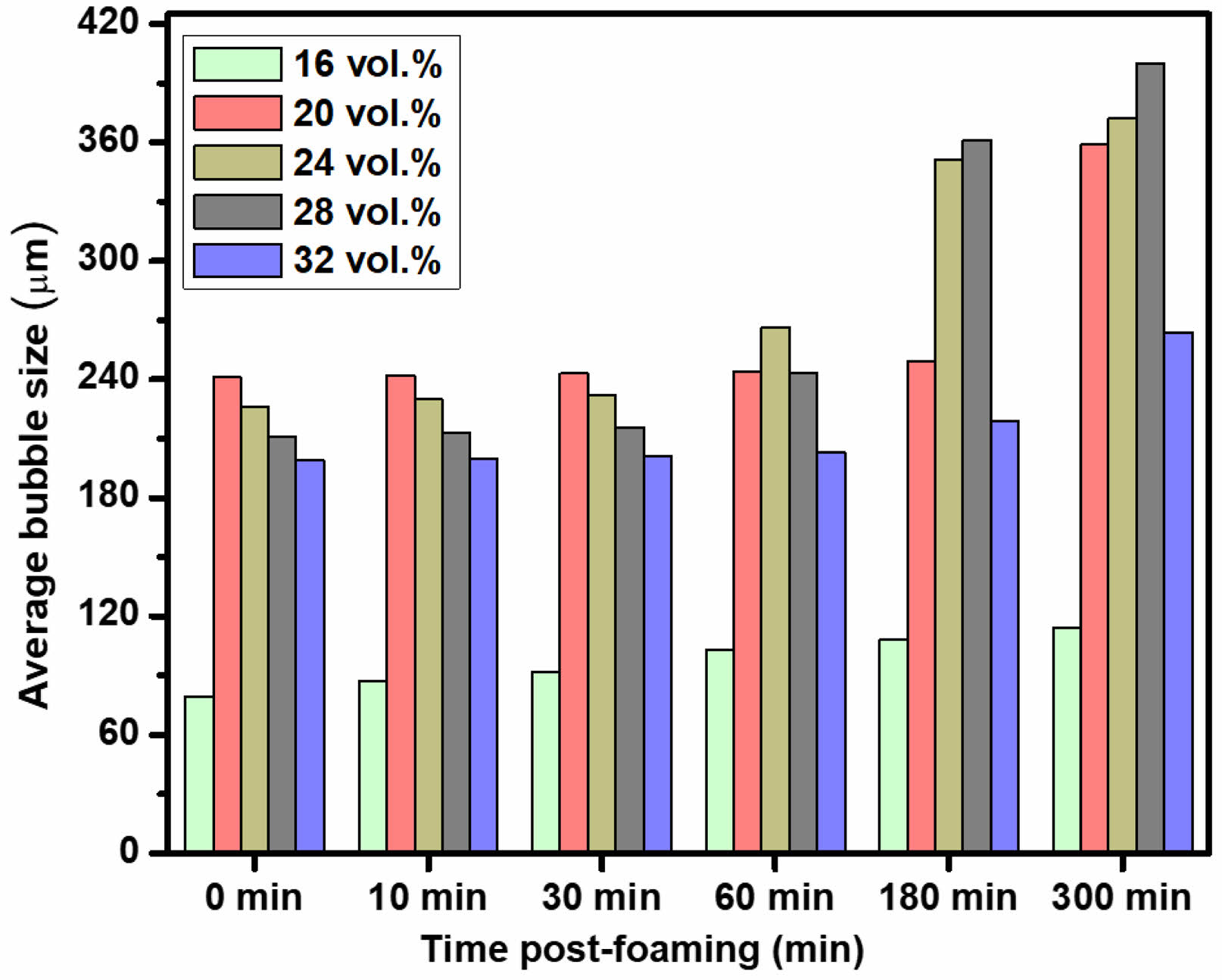
Fig. 4 presents the evolution of average bubble size over time (0–320 min), illustrating the impact of Ostwald ripening on bubble stability. A gradual increase in bubble size is observed due to gas diffusion from smaller to larger bubbles, leading to bubble coarsening. Among the tested compositions, the 28 vol.% hydroxyapatite (HA) suspension exhibits the greatest increase in average bubble size over time. Conversely, suspensions containing 24 vol.% HA with 2.5 wt.% propyl gallate (PG) demonstrate superior bubble stability, attributed to the high wet foam stability conferred by optimized particle-surfactant interactions. In contrast, 32 vol.% HA suspensions exhibit a drastic reduction in average bubble size over time. This phenomenon is attributed to excessive HA particle concentration, which leads to increased foam viscosity and impedes bubble growth, ultimately destabilizing the foam structure. These findings underscore the critical role of HA solid content in governing the stability and longevity of wet foams.
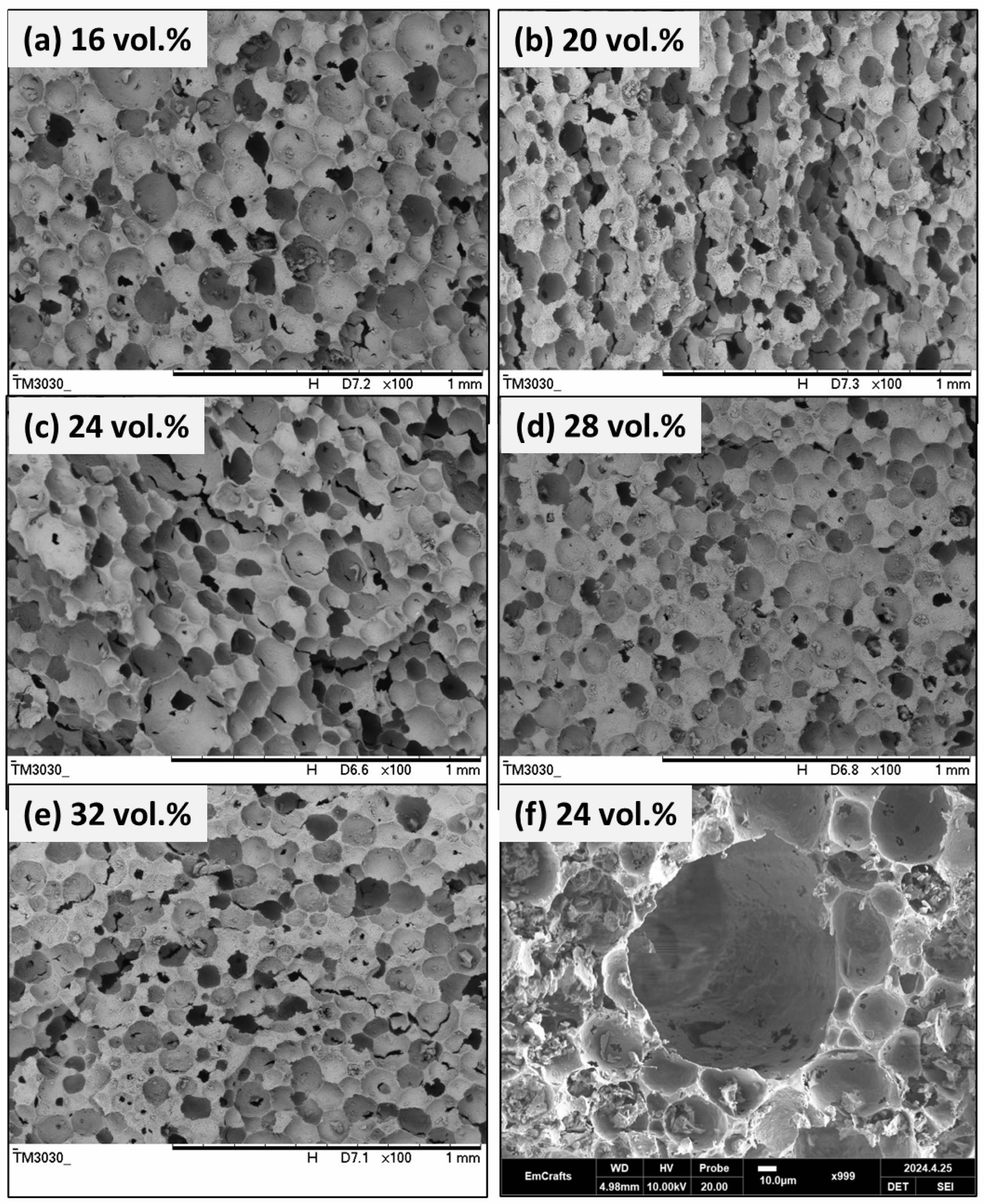
Fig. 5 presents the microstructure of porous hydroxyapatite (HA) ceramics prepared using 2.5 wt.% propyl gallate (PG) as a pore-forming agent. The solid content of the ceramic suspension is systematically varied from 16 vol.% to 32 vol.%, with the corresponding microstructures depicted in panels (a–e). At 16 vol.% HA (Fig. 5a), the microstructure exhibits a relatively uniform distribution of interconnected pores, forming a well-integrated porous network. As the solid content increases to 20 vol.% HA (Fig. 5b), the microstructure becomes coarser, characterized by larger pores and thicker struts. At 24 vol.% HA (Fig. 5c), the microstructure remains highly porous, with interconnected pores of varying sizes. The incorporation of PG facilitates pore formation while simultaneously stabilizing the pore walls, preventing their collapse during processing. At higher solid contents of 28 vol.% and 32 vol.% HA (Fig. 5d–e), the microstructure becomes increasingly coarse, with larger, more regularly shaped single pores and thicker struts (Fig. 5f). Despite these morphological changes, the interconnected pore network remains intact, ensuring the structural integrity of the porous HA ceramics.
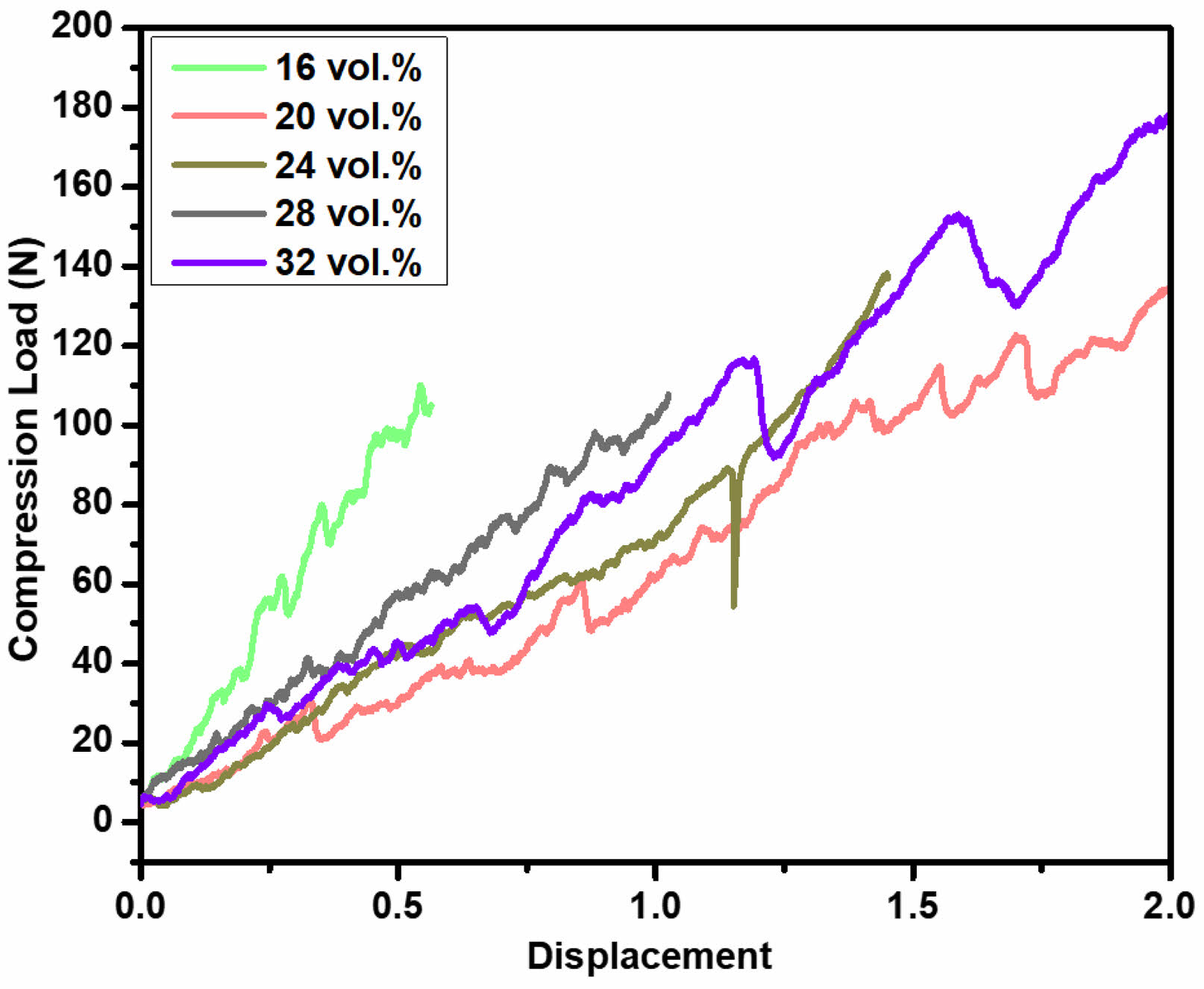
Fig. 6 presents the load-displacement curves for porous hydroxyapatite (HA) ceramics with varying solid contents of 16 vol.%, 20 vol.%, 24 vol.%, 28 vol.%, and 32 vol.%, prepared using 2.5 wt.% propyl gallate (PG). The compression load-strain behavior exhibits a typical response for open-cell porous ceramics, where the compression load increases linearly with increasing strain in the quasi-elastic region. During this phase, single strut fractures occur, predominantly at contact points within the sample fixation, a phenomenon characteristic of porous ceramics [30]. The load-displacement graph shown here depicts different samples labeled 16, 20, 24, 28, and 32 vol.%. As can be seen, post-yield, the samples exhibit a variety of behaviors, particularly in how they handle continued stress application (Fig. S4). Notably, some samples (such as 16, 20 and 32 vol.%) show a relatively smooth decline in load-bearing capacity post-yield, which suggests a gradual deterioration of structural integrity. This variance in behavior can be attributed to differences in microstructural characteristics such as particle bonding, porosity, and grain size distribution, which affect how cracks initiate and propagate through the material. Upon reaching the yield strength, catastrophic failure due to macroscopic crack propagation across the sample's cross-section, typically associated with a sudden load drop, would be expected. However, all tested samples remained structurally intact beyond the yield point, preserving their mechanical integrity. The load-displacement curves for 20 vol.% and 24 vol.% HA exhibit a lower and flatter profile compared to those of 16 vol.%, 28 vol.%, and 32 vol.%, suggesting a more quasi-plastic deformation behavior. According to the study by Young Min Byun et al. (2021), Bhaskar, S., et al. (2015) [31, 32], evident that porous ceramics maintain their mechanical integrity even after surpassing their apparent yield strength. To further support this, we have included Table 1, which summarizes the physical properties of porous HA ceramics for various solid contents. The data demonstrate that the 24 vol.% sample has a lower porosity compared to the 16 vol.% and 20 vol.% samples, resulting in higher compressive load values (138 N). Additionally, the sample exhibits a relative modulus of 27.82%, which is comparable to the 20 vol.% sample. These properties suggest that the 24 vol.% sample is a promising candidate for applications requiring both strength and damage tolerance. The 24 vol.% sample's unique combination of quasi-plastic behavior, high damage tolerance, and competitive compressive strength makes it well-suited for applications where mechanical performance under stress is critical. Notably, the 24 vol.% HA sample with 2.5 wt.% PG demonstrates enhanced mechanical performance, indicating its suitability for applications requiring improved mechanical properties [24, 25].
|
Fig. 2 (a) Variation in viscosity of hydroxyapatite suspension with increasing surfactant content, (b) Changes in viscosity of hydroxyapatite suspension containing 2.5 wt.% surfactant before and after the foaming process, and (c) Thixotropic behavior and 3ITT flow curve analysis of hydroxyapatite suspension at varying surfactant concentrations. |
|
Fig. 3 (a) Air content of hydroxyapatite foam as a function of solid content, and (b) wet foam stability of hydroxyapatite foam as a function of solid content. |
|
Fig. 4 Average bubble size over time as a function of solid content. |
|
Fig. 5 Microstructure of porous hydroxyapatite ceramics prepared with 2.5 wt.% propyl gallate at different solid contents: (a) 16 vol.%, (b) 20 vol.%, (c) 24 vol.%, (d) 28 vol.%, and (e) 32 vol.%, (f) single pore structure of 24 vol.%. |
|
Fig. 6 Compressive load vs displacement curve of porous hydroxyapatite ceramics as a function of solid content. |
This study successfully developed interconnected porous hydroxyapatite (HA) ceramics using the direct foaming method, demonstrating significant potential as an effective bone substitute. The research primarily focused on the synthesis of porous HA ceramics from particle-stabilized wet foams, with propyl gallate (PG) serving as a surface-modifying agent to impart partial hydrophobicity and enhance foam stability. The results indicate that increasing the solid content led to smaller average bubble sizes, attributed to reduced surface tension and enhanced particle hydrophobicity. The compressive load of the resulting ceramics ranged from 60 to 180 N, highlighting their favorable mechanical performance. The primary objective of this study was to develop a cost-effective and scalable approach for fabricating interconnected porous HA ceramics as bone substitutes. Future research should investigate there in vivo performance for bone regeneration applications. In conclusion, the findings demonstrate that the direct foaming method is a promising technique for producing highly porous, mechanically robust HA ceramics, with broad applications in bone tissue engineering. This approach offers a novel and efficient strategy for developing next-generation bone graft materials.
This study was finally supported by Hanseo University.
- 1. P. Dec, A. Modrzejewski, and A. Pawlik, Int. J. Mol. Sci. 24[1] (2022) 529.
-

- 2. G. Gautam, S. Kumar, and K. Kumar, Mater. Today Proc. 50 (2022) 2206-2217.
-

- 3. N.A. Abdul Halim, M.Z. Hussein, and M.K. Kandar, Int. J. Nanomedicine 16 (2021) 6477-6496.
-

- 4. I. Ielo, G. Calabrese, G. De Luca, and S. Conoci, Int. J. Mol. Sci. 23[17] (2022) 9721.
-

- 5. R. Shao, R. Quan, L. Zhang, X. Wei, D. Yang, and S. Xie, J. Ceram. Soc. Jpn. 123[1433] (2015) 17-20.
-

- 6. I. Ielo, G. Calabrese, G. De Luca, and S. Conoci, Int. J. Mol. Sci. 23[17] (2022) 9721.
-

- 7. L.A.F. Vieira, I.B.D.C.J. Meireles, and E.M.B. Sousa, J. Ceram. Process. Res. 23[5] (2022) 725-736.
-

- 8. J. Partini, S.M. Bilqis, F.A. Salimy, I. Aziz, M. Sari, N. Cahyati, and Y. Yusuf, J. Ceram. Process. Res. 25[4] (2024) 599-606.
-

- 9. J.P.N. Marinho, M.F. Cipreste, A. Krohling, and E.M.B. Sousa, J. Ceram. Process. Res. 24[6] (2023) 983-991.
-

- 10. K.W. Goh, Y.H. Wong, R.S.K. Singh, H. Chandran, S.K. Wong, and K.S. Lee, J. Ceram. Process. Res. 23[2] (2022) 158-164.
-

- 11. A.A. Al-Allaq, J.S. Kashan, M.T. El-Wakad, and A.M. Soliman, J. Ceram. Process. Res. 22[4] (2021) 446-454.
-

- 12. J.H. Lee, H.J. Choi, S.Y. Yoon, B.K. Kim, and H.C. Park, J. Ceram. Process. Res. 14[4] (2013) 544-548.
-

- 13. A. Pokhrel, D.N. Seo, S.T. Lee, and I.J. Kim, J. Korean Ceram. Soc. 50[2] (2013) 93-102.
-

- 14. U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler, Langmuir 23[3] (2007) 1025-1032.
-

- 15. B. Basnet, N. Sarkar, J.G. Park, S. Mazumder, and I.J. Kim, J. Adv. Ceram. 6 (2017) 129-138.
-

- 16. W.Y. Jang, J.G. Park, B. Basnet, K.T. Woo, I.S. Han, and I.J. Kim, J. Aust. Ceram. Soc. 53 (2017) 657-665.
-

- 17. U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler, J. Am. Ceram. Soc. 90[1] (2007) 16-22.
-

- 18. U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler, Angew. Chem. Int. Ed. 45[21] (2006) 3526-3530.
-

- 19. U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler, Langmuir 22[26] (2006) 10983-10988.
-

- 20. B. Basnet, H.M. Lim, K.S. Lee, and I.J. Kim, J. Korean Ceram. Soc. 56[5] (2019) 513-520.
-

- 21. U.T. Gonzenbach, A.R. Studart, D. Steinlin, E. Tervoort, and L.J. Gauckler, J. Am. Ceram. Soc. 90[11] (2007) 3407-3414.
-

- 22. N. Sarkar, J.G. Park, S. Mazumder, A. Pokhrel, C.G. Aneziris, and I.J. Kim, Ceram. Int. 41[5] (2015) 6306-6311.
-

- 23. F.K. Juillerat, U.T. Gonzenbach, A.R. Studart, and L.J. Gauckler, Mater. Lett. 64[13] (2010) 1468-1470.
-

- 24. F. Krauss Juillerat, U.T. Gonzenbach, P. Elser, A.R. Studart, and L.J. Gauckler, J. Am. Ceram. Soc. 94[1] (2011) 77-83.
-

- 25. I.J. Kim, J.G. Park, Y.H. Han, S.Y. Kim, and J.F. Shackelford, J. Korean Ceram. Soc. 56[3] (2019) 211-232.
-

- 26. A.R. Studart, U.T. Gonzenbach, E. Tervoort, and L.J. Gauckler, J. Am. Ceram. Soc. 89[6] (2006) 1771-1789.
-

- 27. K.N. Fatema, H.M. Lim, J.S. Hong, K.S. Lee, and I.J. Kim, J. Ceram. Process. Res. 24[1] (2023) 197-204.
-

- 28. A.M.O. Zapata, S. Rodríguez-Barona, and G.I.G. Gómez, Braz. J. Food Technol. 18 (2015) 23-30.
-

- 29. A. Franck, Book of TA Instruments (2004) 1-17.
- 30. B.S.M. Seeber, U.T. Gonzenbach, and L.J. Gauckler, J. Mater. Res. 28[17] (2013) 2281-2287.
-

- 31. Y.M. Byun, G.W. Lee, K.S. Lee, J.G. Park, and I.J. Kim, 58 (2021) 269-275.
-

- 32. S. Bhaskar, J.G. Park, I.S. Han, M.J. Lee, T.Y. Lim, and I.J. Kim, J. Korean Ceram. Soc. 52[6] (2015) 455-461.
-

 This Article
This Article
-
2025; 26(3): 432-439
Published on Jun 30, 2025
- 10.36410/jcpr.2025.26.3.432
- Received on Feb 22, 2025
- Revised on May 19, 2025
- Accepted on May 26, 2025
 Services
Services
- Abstract
introduction
experimental
characterization
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Ik Jin Kim
-
aInstitute of Processing and Applications of Inorganic Materials (PAIM), Department of Materials Science and Engineering, Hanseo University, 46, Hanseo 1-ro, Haemi-myeon, Seosan-si, Chungcheongnam-do, 31962, Republic of Korea
cFaculty of Mechanical and Electrical Engineering, German-Mongolian Institute for Resources and Technology, GMIT, 2nd Khoroo, Nalaikh district, Ulaanbaatar, Mongolia
Tel : +82-10-8405-1441 - E-mail: ikjin@gmit.edu.mn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.