- Regulation of polyethersulfone membrane morphology and its influence on filtration performance
Meixia Shia,b,*, Haiming Songa,b, Hao Lia,b and Yucheng Maob
aNingbo Key Laboratory of High Performance Petroleum Resin Preparation Engineering and Technology, Ningbo Polytechnic, Ningbo 315800, China
bChemical Engineering Department, Ningbo Polytechnic, Ningbo 315800, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Optimizing the filtration performance of polymer membranes, mainly through morphology manipulation, remains a crucial focus in membrane research. This study systematically examined the relationship between membrane morphology and filtration performance in polyethersulfone (PES) membranes by varying the composition of the casting solution. Scanning electron microscopy (SEM), atomic force microscopy (AFM), and Brunauer-Emmett-Teller (BET) analysis were used to investigate the membrane's microstructure thoroughly. Results showed that increasing PES concentration led to smaller pore sizes and reduced porosity, resulting in lower water permeance and higher retention rates. Additionally, analyses of the t-plot micropore area and BJH cumulative pore surface area revealed the influence of micropores and mesopores on permeance and retention. Different molecular weights and concentrations of polyethylene glycol (PEG) significantly altered the pore distribution, impacting overall membrane performance.
Keywords: Polyethersulfone, Polyethylene glycol, Membrane morphology, Filtration performance.
Membrane separation technology has been widely applied in water treatment due to its high efficiency, cost-effectiveness, and environmental benefits [1-3]. The membrane morphology is critical in determining its filtration properties during these separation processes [4-6]. Polyethersulfone (PES) membranes exhibit unique advantages due to their excellent thermal stability, mechanical strength, and chemical resistance, particularly in the fields of wastewater treatment [7, 8] and blood purification [9, 10]. However, further optimizing the pore structure of PES membranes to enhance their filtering performance remains an important topic in current research [11-13]. This is significant for membrane design optimization, improving treatment efficiency, and their wide application in environmental protection and resource recovery [14].
In order to improve the permeability and selectivity of PES membranes, researchers have made various attempts [15-19]. Modifying the PES membrane by adding polyethylene glycol (PEG) has been proven effective [20-22]. PEG, as a co-porogenic agent, not only affects the viscosity of the casting solution [23] but also exerts a strong influence on the membrane's pore structure and surface properties, affecting its overall properties.[24, 25] Specifically, the effects of different molecular weights and concentrations of PEG on the membrane's microstructure and permeation characteristics exhibit notable variation.
It is demonstrated that PEG molecular weight is crucial in determining the pore size distribution and overall morphology of the membrane [20, 26]. High molecular weight PEG can usually form larger pore structures, which can enhance the water permeance of the membrane but may also reduce the selectivity due to the increased pore size [27]. On the other hand, PEG with low molecular weight tends to form a relatively tight pore structure, which enhances the membrane selectivity but may lead to decreased permeability. By blending or copolymerizing PEG and PES, we can regulate the membrane's pore structure and optimize its mechanical properties and thermal stability, thus further improving its overall performance. In addition, the amount of PEG content on the membrane performance is different [28, 29], but if the content is too large, the mechanical strength will weaken the strength of the membrane [30].
In this study, our innovation lies in the fine regulation of the relationship between the pore structure and the filtration performance of the PES membranes by systematically adjusting the composition of the casting solution. Unlike previous studies focusing on a single variable, this study comprehensively investigated the synergistic effects of PES concentration and different molecular weights and concentrations of polyethylene glycol (PEG) on membrane properties. This multivariate experimental design allows us to understand the microstructural changes of membranes under different preparation conditions and how these changes affect membrane permeability and selectivity. We verified PEG's role in membrane pore regulation by systematically collecting and comparing the performance data of membranes prepared under different conditions. We proposed new strategies to optimize the PES membrane design. These findings provide crucial theoretical support and practical guidance for the further application of PES membranes in water treatment and other fields.
In this study, the polymer used was polyethersulfone (PES) with the model number E6020P, and the solvent was N,N-dimethylformamide (DMF). Polyethylene glycol (PEG) served as the porogenic agent, with different molecular weights, including 400, 1500, and 4000. Bovine serum albumin (BSA) was used as the solute to measure the retention rate of the membrane. The specific experimental procedure followed the methods outlined in a previously published paper [4].
For membrane fabrication, different casting solutions were prepared by stirring at 60 ℃ overnight. The solutions were then allowed to stand for up to 6 hours without heating or stirring to eliminate air bubbles. The casting solutions were prepared under four conditions: (1) varying PES concentrations (12%, 13.5%, 15%, 16.5%, and 18%); (2) for 15% PES membranes, 10% PEG with varying molecular weights (PEG400, PEG1500, and PEG4000) was added; (3) for 15% PES, PEG1500 concentrations were varied (0%, 5%, 10%, and 15%); (4) combinations of PEGs with different molecular weights were tested at a fixed 10% total PEG concentration, including 10% PEG1500, 5% PEG1500 + 5% PEG400, 5% PEG1500 + 5% PEG4000, and 2.5% PEG400 + 5% PEG1500 + 2.5% PEG4000. Membranes were fabricated via the phase inversion process. The casting solution was spread onto a glass plate with a 150 µm gap, which was then immediately immersed in a deionized (DI) water coagulation bath. After the membrane was detached from the glass plate, it was thoroughly rinsed and stored in DI water.
The water permeance of the membranes was evaluated using a lab-scale dead-end ultrafiltration setup under a constant pressure of 1 bar. The permeate weight was recorded over a fixed testing duration, and the pure water flux was calculated based on the collected data. Solution permeance is measured concurrently with retention rate testing and calculated using the same method as pure water permeance. Porosity was determined using the dry-wet membrane mass method, where the wet membrane was freeze-dried to obtain the dry state.
Characterization techniques were used to analyze the membrane morphology and structure. Scanning electron microscopy (SEM) was used to observe the surface and cross-sectional structure of the membranes [31]. Atomic force microscopy (AFM) was employed to measure the surface roughness and topography [32]. BET analysis was conducted to evaluate the specific surface area, pore size distribution, and pore volume of the membranes [33].
Different PES concentrations of polyethersulfone membranes
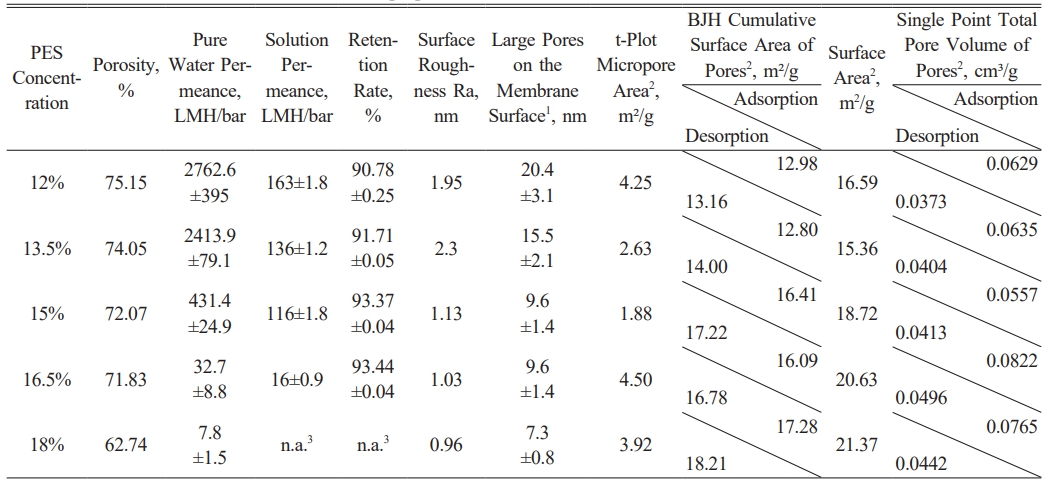
Through experimental studies on the structural characteristics and filtration properties of polyethersulfone membranes, we observed that porosity gradually decreases with increasing polymer concentration. In particular, at the highest polyethersulfone concentration (18%), porosity dropped significantly to 62.74%. This suggests that higher concentrations in the casting solution lead to a tighter polymer chain structure during membrane formation, thereby reducing pore formation. While this reduction in porosity enhances membrane retention, as indicated by the data showing an increase in retention with decreasing porosity, it also causes a notable decline in pure water permeance. At 18% concentration, the pure water permeance reaches an extremely low level, severely limiting the membrane's practical applications.
Regarding filtration performance, PES membranes with 12% and 13.5% concentrations exhibited high pure water permeance but low retention due to their larger pore sizes. In contrast, membranes with 16.5% and 18% concentrations showed lower permeance, with the 18% concentration having pores so small that the pure water permeance was extremely low, making it impossible to accurately calculate the retention rate or solution permeance. The 15% concentration membrane demonstrated a retention rate similar to the 16.5% membrane, and its surface pore size was comparable to the 16.5% and 18% membranes, though its permeance was lower than the 12% and 13.5% membranes. This suggests that the 15% concentration membrane provides adequate pure water permeance while maintaining a reasonable retention rate, making it the focus of further investigation and optimization in this study.
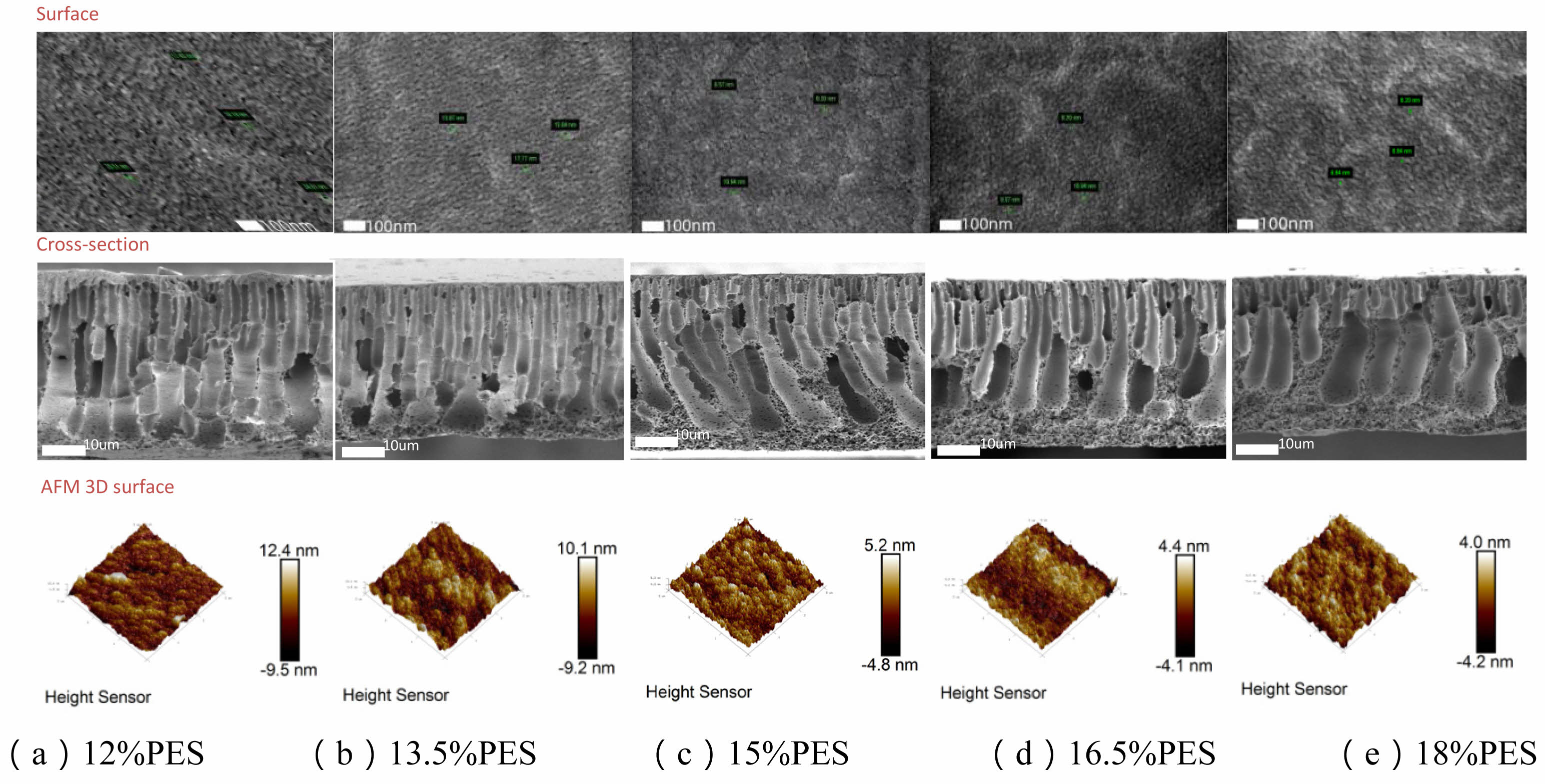
In terms of membrane surface roughness (Ra), the
surface becomes progressively smoother as the concentration increases, particularly at higher concentrations, which aligns with the decreasing surface height differences observed in the AFM images. As shown in Fig. 1 (a-e), different PES concentrations exhibit significant structural differences in surface, cross-section, and AFM 3D imaging. The 12% and 13.5% PES membranes have relatively higher surface roughness, with AFM height differences of 12.4 nm and 10.1 nm, indicating more irregular micropores and surface protrusions. As the PES concentration increases, the membrane surface gradually smoothens, particularly at 15%, 16.5%, and 18% concentrations, with surface height differences reduced to 5.2 nm, 4.4 nm, and 4.0 nm, respectively. While a smoother surface helps minimize membrane fouling and makes cleaning and maintenance easier during filtration, an overly smooth surface may compromise hydrophilicity, thus reducing the pure water and solution permeance. Therefore, although higher concentration membranes exhibit smoother surfaces, additional surface modifications may be necessary to enhance hydrophilicity and maintain permeance as shown in Table 1.
The BET analysis revealed that the membrane morphology and pore structure were significantly influenced by the PES concentration. The t-Plot Micropore Area initially decreased with increasing PES concentration, from 4.25 m²/g at 12% to 1.88 m²/g at 15%, indicating a reduction in micropore formation due to the increased solution viscosity restricting pore development. Interestingly, the micropore area increased to 4.50 m²/g at 16.5%, suggesting structural reorganization or enhanced phase separation kinetics at higher concentrations.
The BJH Cumulative Surface Area of Pores showed a consistent increase with PES concentration, rising from 12.98 m²/g at 12% to 17.28 m²/g at 18% (adsorption data). This trend indicates a higher formation of mesopores and macropores at elevated PES concentrations. Notably, desorption values were slightly higher than adsorption values, potentially due to the presence of bottle-neck or closed pores that hindered full adsorption saturation but allowed more complete desorption.
Similarly, the BET Surface Area increased from 16.59 m²/g to 21.37 m²/g, confirming that higher PES concentrations contributed to a more developed and extensive pore structure. The single point total pore volume reached its peak at 16.5% PES concentration (0.0822 cm³/g), reflecting a more open and accessible porous network. However, the consistent difference between adsorption and desorption pore volumes suggests the potential existence of closed or ink-bottle pores, which may influence fluid transport properties.
Overall, the results suggest that a PES concentration of 16.5% optimizes pore formation, combining higher surface area and pore volume with a balanced microporous structure. This concentration may provide the most favorable conditions for enhancing membrane permeability and filtration performance.
PES membranes with PEG porogens of different molecular weights
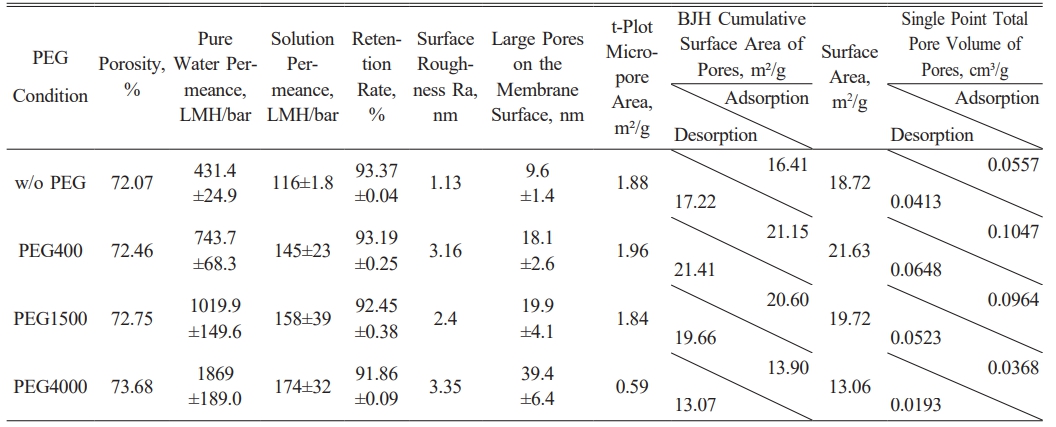
Based on a 15% PES membrane, we conducted comparative experiments by adding 10% of PEG with varying molecular weights (PEG400, PEG1500, and PEG4000) to the casting solution to explore the effects on membrane structure and filtration performance. The results showed that porosity increased from 72.07% (without PEG) to 73.68% after the addition of PEG4000, indicating that PEG effectively regulates the pore structure, with higher molecular weights leading to more pore formation and enhanced permeability. Although the trend is not perfectly linear, high molecular weight PEG significantly improves pore connectivity as shown in Table 2.
Changes in pure water and solution permeance followed a similar trend. Pure water permeance increased from 431.4 LMH/bar (without PEG) to 1869 LMH/bar with PEG4000, while solution permeance rose from 116 LMH/bar to 174 LMH/bar. This suggests that high molecular weight PEG contributes to forming larger, more open pore structures, thereby improving overall permeability, although other structural factors may also influence the membrane's performance.
However, retention exhibited a slight decrease with increasing PEG molecular weight, dropping from 93.37% (without PEG) to 91.86% with PEG4000. This decline is likely due to the formation of larger pores, allowing solutes to pass more easily and slightly reducing retention efficiency. Despite this, the reduction in retention was relatively minor, indicating that high molecular weight PEG does not significantly compromise membrane selectivity.
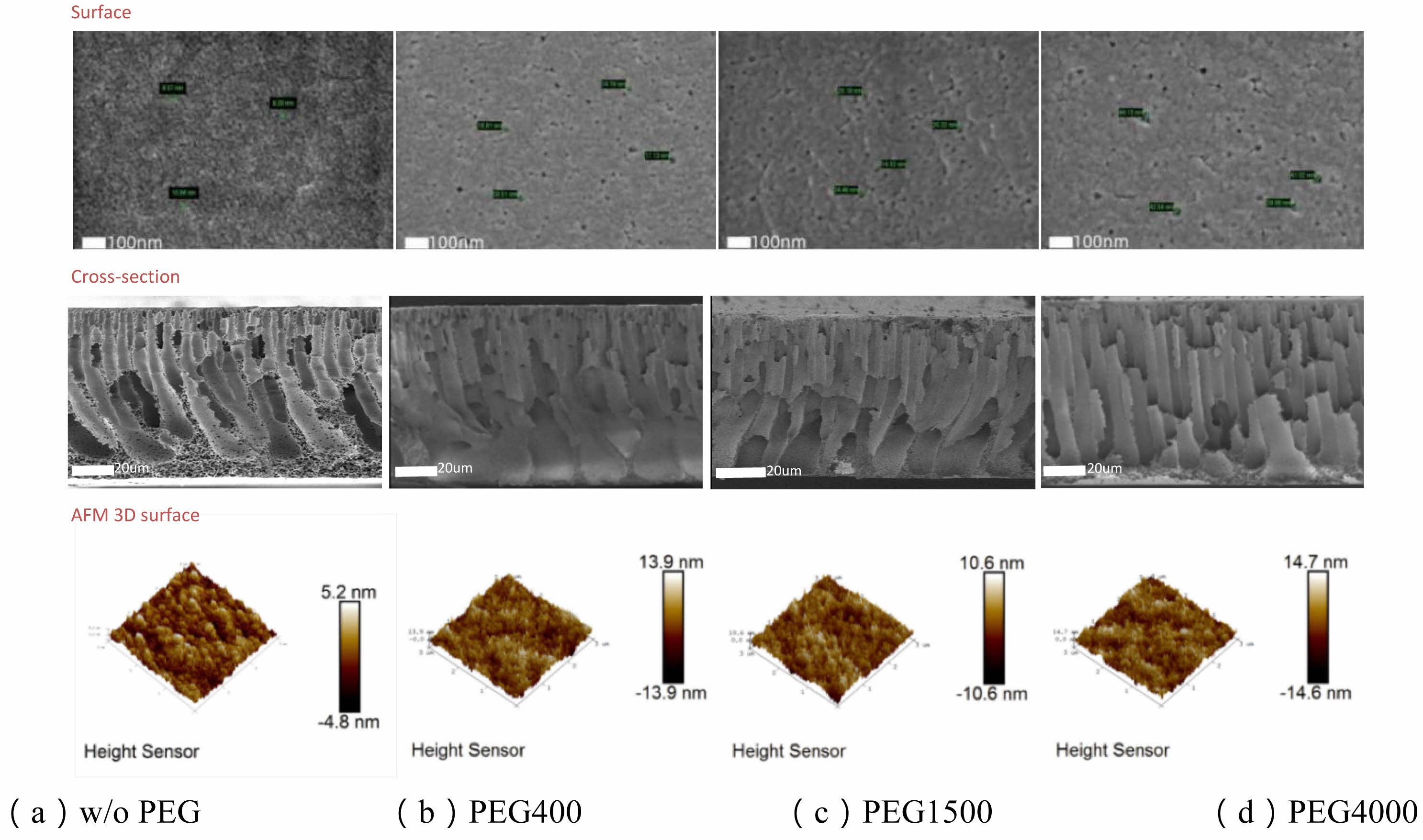
Membrane surface roughness (Ra) generally increased with the addition of PEG, rising from 1.13 nm (without PEG) to 3.35 nm with PEG4000. However, an exception was observed with PEG400, where surface roughness increased to 3.16 nm, higher than expected for its lower molecular weight. AFM images (Fig. 2) confirmed that, in most cases, the surface became progressively rougher as PEG molecular weight increased, with greater height differences between surface protrusions and depressions. This supports the notion that higher molecular weight PEG typically introduces more pores and irregularities into the membrane structure, although this trend is not strictly linear.
Additionally, analysis of the t-Plot micropore area and BJH surface area revealed that, as PEG molecular weight increased, the proportion of macropores also rose. SEM images corroborated these findings, showing a significant increase in pore size with increasing PEG molecular weight, particularly after PEG4000, where the average pore size reached 39.4 nm. These larger pores enhance permeability but may negatively impact retention efficiency.
Cross-sectional images showed that high molecular weight PEG promoted the formation of more pronounced finger-like structures, further enhancing permeability. While PEG of different molecular weights exhibited some nonlinear trends, high molecular weight PEG generally favored the development of larger pores and improved permeance.
Overall, the addition of PEG with varying molecular weights had a significant impact on membrane structure and performance, with the results demonstrating a clear pattern. High molecular weight PEG improved pure water and solution permeance, though retention decreased slightly. Changes in surface roughness and pore structure suggest that the molecular weight and concentration of PEG must be carefully balanced according to specific process requirements to achieve optimal membrane performance.
PES membranes with Different PEG concentrations
On the basis of a 15% PES membrane, we conducted comparative experiments by adding various concentrations of PEG1500 (0%, 5%, 10%, and 15%), labeled as PEG-0, PEG-5%, PEG-10%, and PEG-15%, to the casting solution. The experimental results revealed some notable trends regarding the impact of different PEG concentrations on the structural characteristics and filtration properties of polyethersulfone membranes.
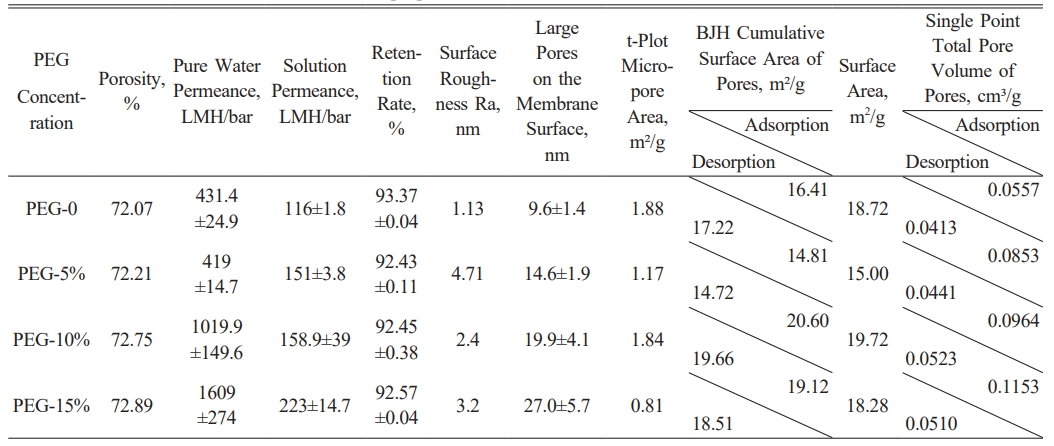
First, the porosity of the membranes slightly increased with higher PEG concentrations, from 72.07% without PEG to 72.89% with the addition of 15% PEG. Although this change was relatively small, it indicates that higher PEG concentrations can fine-tune the pore structure and slightly increase porosity. However, the magnitude of this change is limited, suggesting that PEG’s main influence may lie in other structural and performance aspects.
In terms of pure water permeance, the permeance increased significantly with higher PEG concentrations, rising from 431.4 LMH/bar without PEG to 1609 LMH/bar after adding 15% PEG. This suggests that higher PEG concentrations promote the formation of larger pores and more open channels within the membrane, thereby substantially enhancing water permeability. Solution permeance also increased, from 116 LMH/bar to 223 LMH/bar, further validating PEG’s positive role in improving filtration performance.
However, despite the improvements in pure water and solution permeance, there was a slight reduction in retention. Without PEG, the membrane's retention was 93.37%, but this decreased to 92.57% with 15% PEG. This decline may be attributed to the formation of larger, more open pores at higher PEG concentrations, allowing solutes to pass through more easily and slightly reducing interception efficiency. Nonetheless, the decrease in retention was minor, indicating that PEG has little impact on the membrane's overall selectivity as shown in Table 3.
Further analysis of the t-Plot micropore area and BET results revealed that increasing PEG concentrations significantly affected the ratio of micro- to macropores. Higher PEG concentrations facilitated the formation of macropores, which, while enhancing permeance, may have contributed to the slight reduction in retention efficiency. This indicates that PEG affects both the macroscopic pore structure and the microscopic pore distribution, particularly increasing the proportion of larger pores.
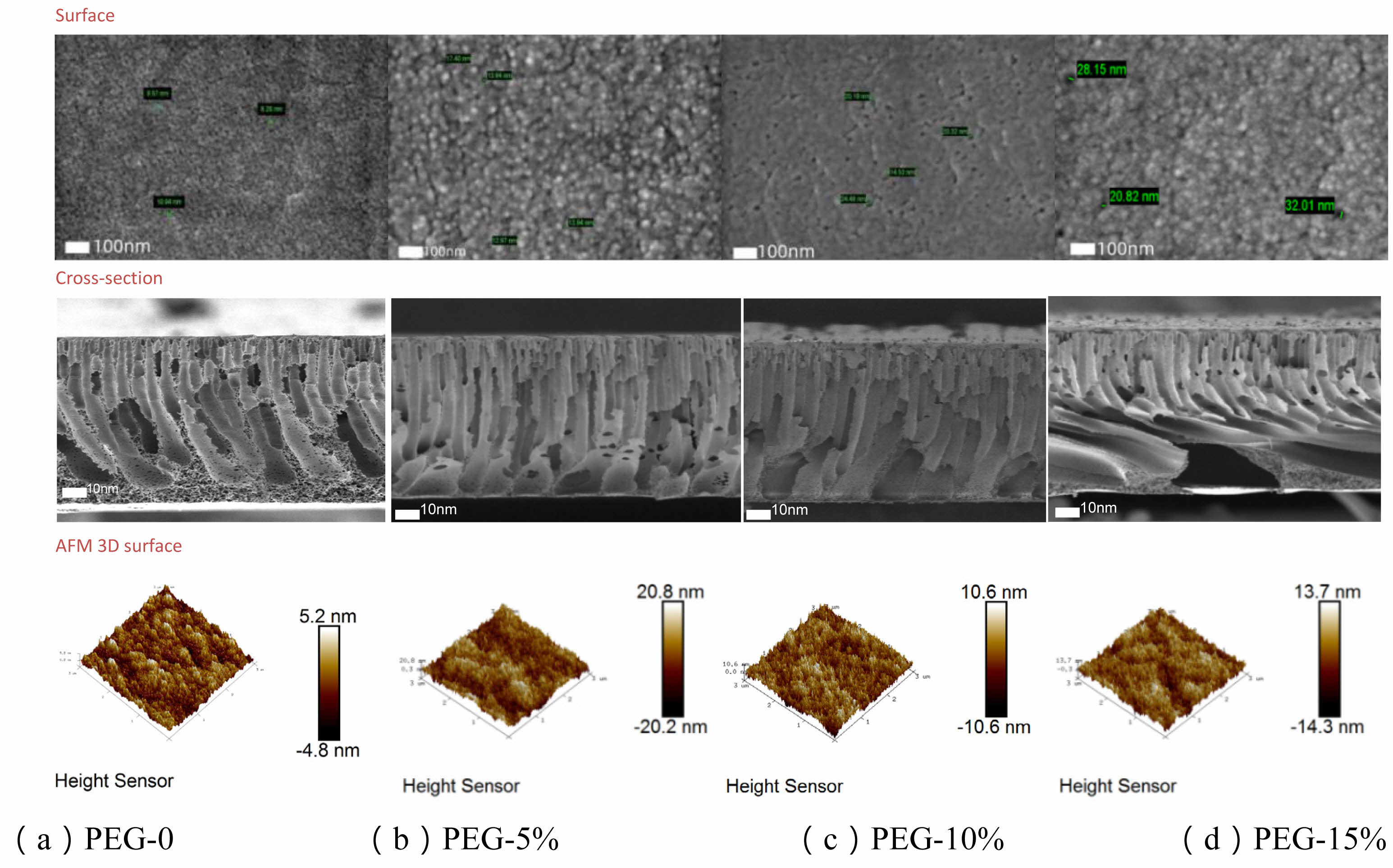
Surface roughness (Ra) generally increased with higher PEG concentrations, with one exception. Without PEG, roughness was 1.13 nm, rising to 2.4 nm and 3.2 nm at 10% and 15% PEG, respectively. However, at 5% PEG, roughness peaked unexpectedly at 4.71 nm, indicating that PEG’s effect on surface texture may be more pronounced at lower concentrations. AFM images confirmed these trends, showing a consistent increase in surface height difference with rising PEG concentrations, while highlighting the sharp increase at 5% as a localized peak in surface roughness.
SEM images showed that pore sizes on the membrane surface increased significantly with higher PEG concentrations, especially after the addition of 10% and 15% PEG, where average pore sizes reached 23.55 nm and 24.25 nm, respectively. These larger pores contributed to higher pure water and solution permeance but were accompanied by a slight decrease in retention.
In conclusion, different concentrations of PEG significantly influenced the structure and performance of polyethersulfone membranes. Higher PEG concentrations effectively improved pure water and solution permeance but also caused a slight reduction in retention and changes in surface roughness. These findings suggest that the appropriate PEG concentration should be selected based on specific filtration requirements to achieve an optimal balance between filtration performance and retention efficiency in practical applications.
Based on the previous experimental results and data analysis, it can be inferred that under the same PEG molecular weight, adding different concentrations of PEG to the casting solution significantly increases the membrane’s permeance, slightly raises porosity, and slightly decreases retention. The pore size on the membrane surface increased significantly, and both specific surface area and pore volume rose. Meanwhile, surface roughness decreased, resulting in a smoother membrane surface. Going forward, this study will further explore mixing PEG with different molecular weights in the casting solution to optimize the membrane's pore structure and filtration performance as shown in Fig. 3.
PES membranes with different PEG combinations
Based on 15% PES membranes, we performed comparative experiments with different PEG combinations. We added the same concentration of 10% PEG to the casting solution, consisting of PEG1500 mixed with PEG of other molecular weights. The combinations included 10% PEG1500, 5% PEG1500 + 5% PEG400, 5% PEG1500 + 5% PEG4000, and 2.5% PEG400 + 5% PEG1500 + 2.5% PEG4000, labeled as PEG1500, PEG low mix, PEG high mix, and PEG triple mix, respectively. The results showed that different PEG combinations significantly affected the structural characteristics and filtration performance of polyethersulfone membranes.
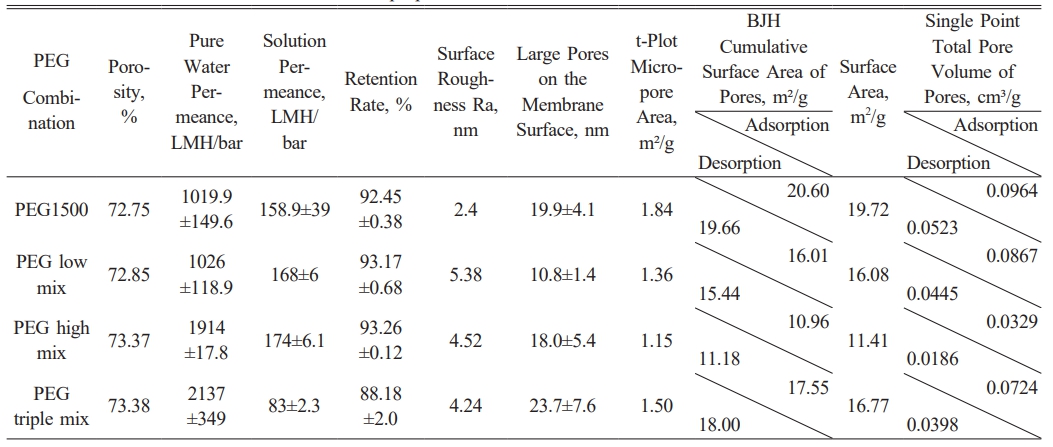
First, in terms of porosity, the membrane's porosity slightly increased with varying PEG combinations, rising from 72.75% for PEG1500 alone to 73.38% with multiple PEG combinations. Although the change in porosity was modest, this subtle difference could influence the overall membrane performance, particularly in terms of permeance and retention efficiency.
In terms of pure water permeance and solution permeance, adding different PEG combinations significantly enhanced membrane permeability. With PEG1500 alone, the pure water permeance was 1019.9 LMH/bar, and the solution permeance was 158 LMH/bar. However, when using various PEG combinations (such as PEG high mix and PEG triple mix), the pure water permeance increased to 1914 LMH/bar and 2137 LMH/bar, respectively, and the solution permeance increased accordingly. Interestingly, in the PEG triple mix, the solution permeance decreased to 83 LMH/bar. This may be attributed to the structural complexity (changes in the porous structure), flow channel inhomogeneity, or changes in surface characteristics (such as hydrophilicity or surface roughness), which may have negatively impacted the solution flow, counteracting the benefits of larger pore sizes.
Regarding retention, membrane retention exhibited a polarized trend with changing PEG combinations. With PEG1500 alone, retention was 92.45%. In the PEG low mix and high mix combinations, retention increased to 93.17% and 93.26%, respectively. However, the more pronounced solute accumulation on the membrane surface in these combinations likely enhanced the concentration polarization effect, resulting in lower solution permeance. Despite this, these combinations maintained relatively high permeance due to the improved membrane surface structure and uniform pore distribution. In contrast, retention dropped significantly to 88.18% in the PEG triple mix. This decrease in retention may be attributed to the larger pore sizes and more complex circulation paths, allowing solute molecules to pass through more easily as shown in Table 4.
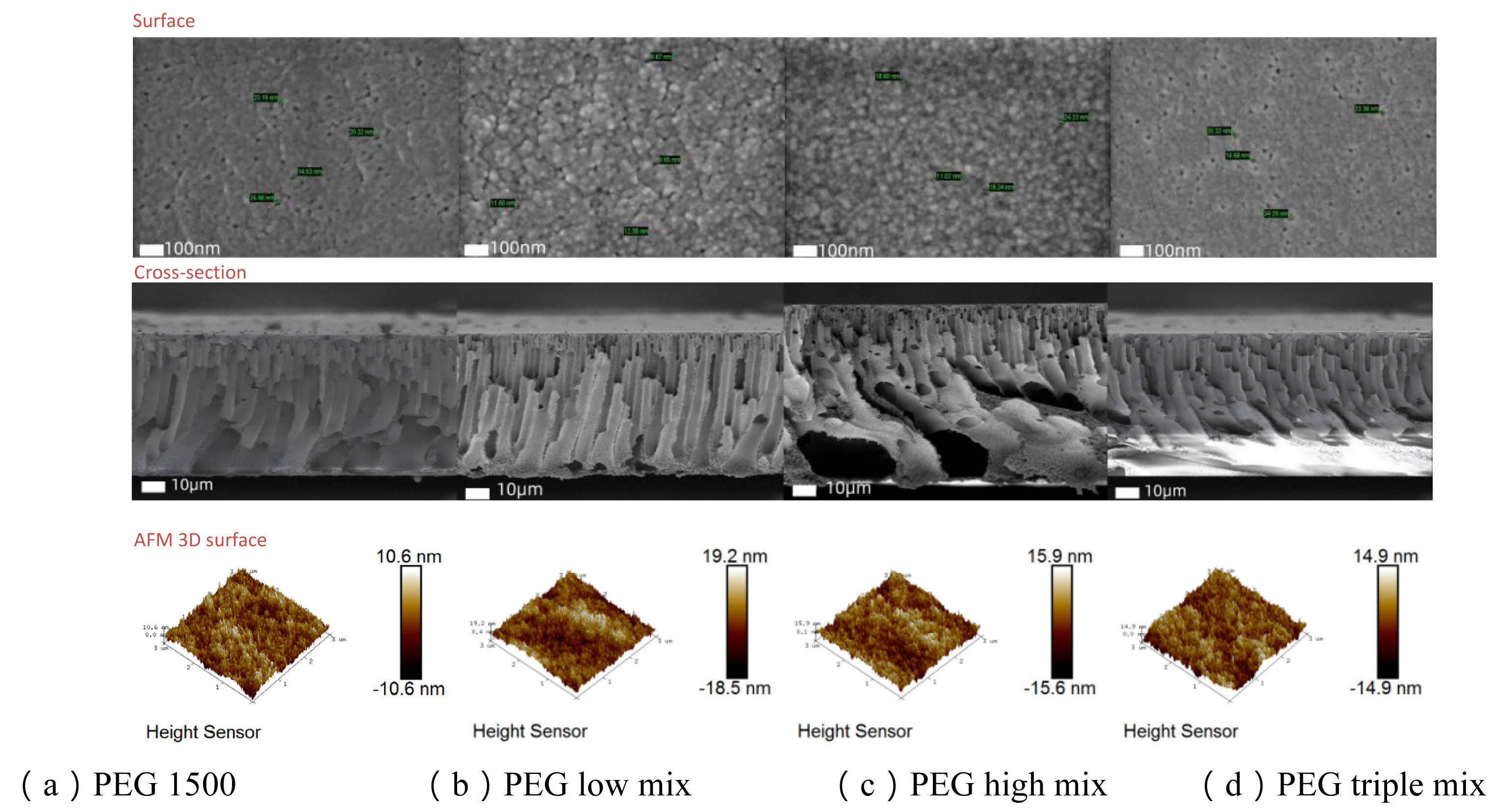
Membrane surface roughness (Ra) varied significantly with different PEG combinations. AFM images showed a notable increase in surface height differences with varying PEG combinations. In particular, the PEG high mix and PEG triple mix exhibited roughness values of 4.52 nm and 4.24 nm, respectively. In contrast, the surface roughness of PEG1500 alone was 2.4 nm, indicating a relatively smoother surface. The increased surface roughness may enhance the membrane's antifouling properties but could also increase the contact angle of water molecules, potentially impacting hydrophilicity and filtration performance.
The BET analysis of PES membranes with different PEG combinations reveals significant variations in pore structure and surface area. The t-Plot Micropore Area decreases from 1.84 m²/g for PEG1500 to 1.36 m²/g for the PEG low mix, suggesting that the combination of PEG1500 with PEG400 results in a more uniform pore structure, with a reduction in microporosity compared to PEG1500 alone.The PEG high mix further decreases the micropore area to 1.15 m²/g, suggesting that the larger PEG molecules, such as PEG4000, promote the formation of larger pores, resulting in a more open structure with fewer micropores. The PEG triple mix shows a slight decrease in micropore area compared to PEG1500, but still maintains a higher micropore area than the PEG low mix and PEG high mix, suggesting that the combination of different PEGs promotes a more balanced pore structure, retaining relatively favorable microporosity.
The BJH Cumulative Surface Area of Pores during adsorption is highest for PEG1500 at 20.60 m²/g, followed by PEG triple mix at 17.55 m²/g, PEG low mix at 16.01 m²/g, and lowest for PEG high mix at 10.96 m²/g. This trend reflects the structural changes in the membrane with increasing PEG molecular weight, where larger PEG molecules reduce the number of micropores and promote the formation of larger pores. The PEG triple mix retains relatively high surface area, indicating that a combination of PEGs maintains more open pores compared to PEG4000 alone. Similar trends are observed in the desorption data, with PEG1500 showing the highest desorption surface area (19.66 m²/g), followed by PEG high mix at 18.00 m²/g, PEG low mix at 15.44 m²/g, and lowest for PEG high mix at 11.18 m²/g.
The Single Point Total Pore Volume for PEG1500 during adsorption is 0.0964 cm³/g, the highest among the combinations, indicating a more open pore structure. The PEG low mix shows a decrease to 0.0867 cm³/g, and PEG high mix further decreases to 0.0329 cm³/g, reflecting a reduction in pore volume and a shift towards a denser pore structure with fewer and larger pores. The PEG triple mix retains a higher pore volume (0.0724 cm³/g), suggesting that the combination of PEGs helps preserve a favorable pore structure, though slightly reduced compared to PEG1500. Desorption pore volumes follow a similar pattern, with PEG1500 having the highest volume (0.0523 cm³/g) and PEG high mix the lowest (0.0186 cm³/g).
In summary, the BET analysis shows that the combination of PEG molecular weights plays a critical role in controlling the pore structure of PES membranes. PEG1500 alone promotes a more porous membrane with higher microporosity, surface area, and pore volume, while the addition of higher molecular weight PEGs, such as PEG4000, reduces these properties, resulting in a denser structure with fewer and larger pores. The PEG low mix (PEG1500 + PEG400) results in a more uniform pore structure, slightly reducing microporosity while maintaining relatively high surface area. The PEG triple mix offers a balanced membrane structure, retaining moderate pore volume and surface area compared to PEG high mix, optimizing pore formation and improving membrane performance for filtration applications.
SEM images further supported these observations, showing the effects of different PEG combinations on membrane pore size. In the PEG triple mix, the surface pore size increased significantly to 23.7 nm. This increase in pore size likely explains the substantial improvement in pure water permeance, although it also led to a significant reduction in retention.
In conclusion, different PEG combinations had a significant impact on the structure and performance of polyethersulfone membranes. Carefully selected PEG combinations can greatly improve pure water permeance and solution permeance, but the potential trade-offs in retention and surface roughness must also be considered. In practical applications, selecting the appropriate PEG combination is crucial for achieving an optimal balance between filtration performance and retention efficiency as shown in Fig. 4.
|
Fig. 1 Surface, cross-section, and AFM 3D height maps of PES membranes at different concentrations. |
|
Fig. 2 Surface, cross-section, and AFM 3D height maps of PES membranes with different PEG molecular weights. |
|
Fig. 3 Surface, cross-section, and AFM 3D height maps of PES membranes with different PEG concentrations. |
|
Fig. 4 Surface, cross-section, and AFM 3D height maps of PES membranes with different PEG combinations. |
|
Table 1 Structural characteristics and filtration properties of PES membranes at different concentrations. |
|
Table 2 Structural characteristics and filtration properties of PES membranes with different PEG molecular weights. |
|
Table 3 Structural characteristics and filtration properties of PES membranes with different PEG concentrations. |
|
Table 4 Structural characteristics and filtration properties of PES membranes with different PEG combinations. |
This study systematically explored the effects of different PEG molecular weights and concentrations, along with varying PES concentrations, on membrane pore structure and filtration performance. The results show that increasing PES concentration reduces porosity and permeance due to the more compact polymer structure, especially at higher concentrations (e.g., 18% PES), where both pure water and solution permeance significantly decreased. At the same time, higher PES concentrations enhanced membrane retention, particularly for smaller particles and solutes.
In contrast, increasing PEG molecular weight and concentration improved porosity and permeance. High molecular weight PEG (e.g., PEG-4000) significantly boosted permeance at higher concentrations, but this increase was accompanied by a slight reduction in retention. This trend was most prominent in the PEG triple-mix combination, where increased pore size and surface roughness, along with concentration polarization effects, led to a decrease in both retention and solution permeance.
Membrane surface roughness generally increased with rising PEG molecular weight and concentration, though some deviations were observed at specific concentrations and molecular weights. AFM imaging confirmed this overall trend, showing increased surface height differences with higher PEG concentrations and molecular weights. These variations suggest that PEG’s role in influencing membrane surface characteristics is complex, with certain molecular weight and concentration combinations exhibiting more pronounced effects.
Overall, this study demonstrates that adjusting PEG and PES concentrations can effectively control pore structure, permeance, and retention in polyethersulfone membranes. For water treatment applications, optimizing the balance between permeability and retention requires careful selection of PEG and PES types and concentrations.
These findings provide valuable insights into membrane material design, offering practical guidance for developing high-performance membranes with improved filtration properties for specific applications.
The authors acknowledge Zhenwei Dong for his valuable technical support on the paper.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ21B060003 and Ningbo Polytechnic National Research Project Cultivation Topic under Grant No. NZ22GJ005.
- 1. B.F. Li, B. Qi, Z.Y. Guo, D.X. Wang, and T.F. Jiao, Chemosphere 327 (2023) 138528.
-

- 2. Y.C. An, X.X. Gao, W.L. Jiang, J.L. Han, Y. Ye, T.M. Chen, R.Y. Ren, J.H. Zhang, B. Liang, and Z.L. Li, Environ. Res. 223 (2023) 115409.
-

- 3. K. Suvigya, S. Lalita, and K. Gopinadhan, Sep. Purif. Technol. 325 (2023) 124693.
-

- 4. M. Shi, G. Printsypar, O. Iliev, V.M. Calo, G.L. Amy, and S. P. Nunes, J. Membr. Sci. 487 (2015) 19-31.
-

- 5. L. Liu, Y. Liu, X. Chen, S. Feng, Y. Wan, H. Lu, and J. Luo, J. Membr. Sci. 668 (2023) 121205.
-

- 6. Z. Qiu, H. Han, T. Wang, R. Dai, and Z. Wang, Desalination 552 (2023) 116457.
-

- 7. D.D. Al-Araji, F.H. Al-Ani, and Q.F. Alsalhy, Environ. Technol. 44[20] (2023) 3033-3049.
-

- 8. C. Liu, M. Yan, K. Guo, Y. Gao, F. Liu, and B. Gao, Sep. Purif. Technol. 346 (2024) 127506.
-

- 9. X. Song, H. Ji, W. Zhao, S. Sun, and C. Zhao, Adv. Membr. 1 (2021) 100013.
-

- 10. R. Dehghan and J. Barzin, Polym. Test. 85 (2020) 106438.
-

- 11. A. Fionah, K. McLarney, A. Judd, and I.C. Escobar, Membranes 13[7] (2023) 675.
-

- 12. C.N. Matindi, M. Hu, S. Kadanyo, Q.V. Ly, N.N. Gumbi, D.S. Dlamini, J. Li, Y. Hu, Z. Cui, and J. Li, J. Membr. Sci. 620 (2021) 118868.
-

- 13. Z. Liao, Y. Wu, S. Cao, S. Zhao, X. Yan, S. Yuan, K. Dong, J. Qin, C. Ou, and J. Zhu, Sep. Purif. Technol. 308 (2023) 122911.
-

- 14. H. Wang, J. Yang, H. Zhang, J. Zhao, H. Liu, J. Wang, G. Li, and H. Liang, Sci. Total Environ. 908 (2024) 168277.
-

- 15. P. Kallem, Y. Ibrahim, S.W. Hasan, P.L. Show, and F. Banat, Sep. Purif. Technol. 261 (2021) 118311.
-

- 16. S. Zheng, S. Yang, Z. Ouyang, and Y. Zhang, Appl. Surf. Sci. 614 (2023) 156157.
-

- 17. H. Rezania, V. Vatanpour, A. Arabpour, A. Shockravi, and M. Ehsani, J. Appl. Polym. Sci. 137[20] (2020) 48690.
-

- 18. P. Kallem, G. Bharath, K. Rambabu, C. Srinivasakannan, and F. Banat, J. Appl. Polym. Sci. 268 (2021) 129306.
-

- 19. T. Sriani, B. Arifvianto, A.S. Baskoro, Y. Whulanza, F. Yusof, G.S. Prihandana, and M. Mahardika, Appl. Sci. 14[16] (2024) 7290.
-

- 20. Y. Luo, Z. Liu, Y. Liu, J. Huang, and M. Zhang, J. Appl. Polym. Sci. 140[36] (2023) e54364.
-

- 21. A. Adeniyi, G.O. Odo, D. Gonzalez-Ortiz, C. Pochat-Bohatier, S. Mbakop, and M.S. Onyango, Polymers 15[12] (2023) 2636.
-

- 22. A. Idris, N.M. Zain, and M.Y. Noordin, Desalination 207[1-3] (2007) 324-339.
-

- 23. Y. Liu, G.H. Koops, and H. Strathmann, J. Membr. Sci. 223[1] (2003) 187-199.
-

- 24. N. Nasrollahi, L. Ghalamchi, V. Vatanpour, A. Khataee, and M. Yousefpoor, J. Ind. Eng. Chem. 109 (2022) 100-124.
-

- 25. S. Mei, C. Xiao, and X. Hu, J. Appl. Polym. Sci. 120[1] (2011) 557-562.
-

- 26. Y.C. Lin, H.H. Tseng, and D.K. Wang, J. Membr. Sci. 618 (2021) 118729.
-

- 27. B. Chakrabarty, A.K. Ghoshal, and M.K. Purkait, J. Membr. Sci. 309[1] (2008) 209-221.
-

- 28. M. Farjami, V. Vatanpour, and A. Moghadassi, Chem. Eng. Res. Des. 156 (2020) 371-383.
-

- 29. B.R. Lentz, Eur. Biophys. J. 36[4] (2007) 315-326.
-

- 30. J.H. Kim and K.H. Lee, J. Membr. Sci. 138[2] (1998) 153-163.
-

- 31. M. Bugdayci and G. Baran, J. Ceram. Process. Res. 22[5] (2021) 510-516.
-

- 32. A.A. Al-Allaq, J.S. Kashan, M.T. El-Wakad, and A.M. Soliman, J. Ceram. Process. Res. 22[4] (2021) 446-454.
-

- 33. Y. Lara-López, G. García-Rosales, and J. Jiménez-Becerril, J. Ceram. Process. Res. 20[1] (2019) 24-29.
-

 This Article
This Article
-
2025; 26(2): 342-351
Published on Apr 30, 2025
- 10.36410/jcpr.2025.26.2.342
- Received on Jan 21, 2025
- Revised on Mar 17, 2025
- Accepted on Apr 3, 2025
 Services
Services
- Abstract
introduction
materials and methods
results and discussion
conclusion
- Acknowledgements
- Data availability statement
- Conflict of Interest
- Funding
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Meixia Shi
-
aNingbo Key Laboratory of High Performance Petroleum Resin Preparation Engineering and Technology, Ningbo Polytechnic, Ningbo 315800, China
bChemical Engineering Department, Ningbo Polytechnic, Ningbo 315800, China
Tel : +86 0574 86894532 Fax: +86 0574 86894532 - E-mail: meixia.shi@nbpt.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.