- Characterization of electroless Ni(ZrO2) coated Ti alloy fabricated by sintering for biomedical materials
Asli Betül Yönetkena,* and Ahmet Yönetkenb
aAnkara Yıldırım Beyazıt University, Dentistry Faculty, Prosthetic dental Dept., Yayla Mahallesi Yozgat Bulvarı, 1487. Cadde No:55 Etlik-Keçiören / Ankara, Turkey
bAfyon Kocatepe University, Technology Faculty, Mechatronics Engineering Dept., 03200, Afyonkarahisar, TurkeyThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this study, It was aimed to produce Ti martis Electroless Ni coated ZrO2 ceramic powders added composites and to reveal the properties of the produced composites by characterizing them. It was preferred to use ceramic-metal powder electroless nickel plating method in sample preparation. While ZrO2, known for its biocompatibility, was chosen as the reinforcement material, Titanium (Ti) powders were preferred as the matrix powder. Different compositions were studied in the experimental phase of the Ti martis Electroless Ni coated ZrO2 ceramic added. After these compositions were prepared, a conventional (tube) oven was used for sintering processes. The sintering temperature was chosen as 1100ᵒC in the tube furnace. After sintering, the samples were examined mechanically, physically and metallographically. SEM-EDX, XRD analyzes and Vikers hardness tests were applied to characterize the produced composites. Additionally, the densities of the produced composites were calculated.
Keywords: Electroless Ni plating, Ceramic-metal composite, Powder metallurgy.
The first alloys of titanium were developed in the late 1940s. Ti-6Al4V alloy has become the most widely used titanium alloy on the market [1]. The matrix obtained by mixing elements such as Vanadium (V), molybdenum (Mo), niobium (Nb) with titanium is called titanium alloy. Titanium has comprehensive and strong properties that make it preferred. Some of these properties are: low density (4.5 g/cm3), high specific strength, fracture toughness, fatigue strength, resistance to crack propagation, high toughness at low temperatures and excellent corrosion resistance [2, 3].
Ti alloys are classified as steels, nickel alloys, etc. according to the relationship between a certain yield strength and density. They have higher mechanical strength compared to other material types. They can maintain these advantages even at temperatures of approximately 500 oC. Therefore, some titanium alloys are very suitable for the production of parts of gas turbines and jet engines [4]. The use of titanium and titanium alloys in dental and medical fields makes them popular. When we look at the research conducted only on medicine/dentistry, it shows that the studies on this subject are increasing [5, 6]. The general properties of titanium and titanium alloys, which are the most ideal metallic biomaterials used in dental and orthopedic fields, are as follows [7].
a- low density
b- little or no toxicity
c- high strength and long fatigue life
d- low modulus of elasticity
e- easy formability f-
f- mouldability
g- The use of titanium material for medical and dental implant applications has increased over the last decade.
One of the main reasons for this increase is that titanium is suitable for implant design due to its unique properties [8].
Zirconia ceramics have good mechanical properties. Due to high strength, high toughness, corrosion resistance, wear resistance and good biocompatibility, zirconia ceramic materials are most commonly used as dental restoration materials and surgical blades in the field of biomedicine. Zirconia is a material with high biocompatibility. In the biocompatibility test studies performed, no local or systemic side effects were reported [9, 10]. It has been determined that the amount of microorganisms around the restoration is lower compared to different materials [9]. They have low thermal conductivity and reduce pulp irritation [11]. All-ceramic restorations that do not contain metal alloys prevent problems such as hypersensitivity [12, 13]. In addition, zirconia substructures give a radio opaque appearance. This allows radiographic imaging and evaluation of the restoration [14, 15]. Titanium and its alloys are widely used in dentistry in the construction of dental implants, removable and fixed prostheses, thanks to their excellent biological compatibility and properties such as corrosion resistance, low elasticity values and high resistance. small or large) and is stabilized with rare earth and alkaline earth elements where it can form a solid solution. The ions of the oxides used for stabilization are replaced by Zr atoms, stabilizing the structure [16]. ZrO2 added composite materials are advantageous compared to other stabilizing materials as they have the ability to sinter at lower temperatures and have a low eutectoid transformation temperature (500 oC) [17, 18]. Metal coatings have been applied to both improve mechanical properties and prevent material loss due to corrosion, and also because ceramic powders form easier and tighter bonds with Ti powders.
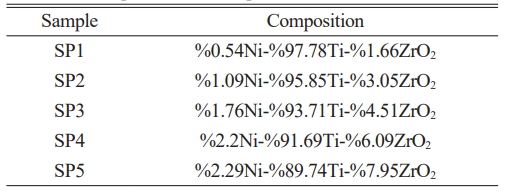
In the study (%0.54Ni-97.78%Ti-1.66%ZrO2, 1.09%Ni-95.85%Ti-3.05%ZrO2, 1.76%Ni-93.71%Ti-4.51%ZrO2, 2.2%Ni-91.69%Ti-6.09%ZrO2 and 2.29%Ni-89.74%Ti-7.95%ZrO2) compositions were selected. This study covers the general characterization of electroless Ni coated Zirconia powders by producing them in determined compositions on Titanium material.
Ceramic materials
ZrO2, a ceramic material, was used in the study. ZrO2 powders were purchased. from Sigma aldrich company with -325 mesh grain size and 97% purity.
Metallic Materials
Titanium (Ti), was chosen as the metal powder in the study. Ti powders with 10 μm grain size and 99% purity were supplied by Sigma Aldrich. Nickel chloride (NiCl2∙6H2O) chemical, which is used to obtain pure nickel in the chemical plating bath, was preferred.
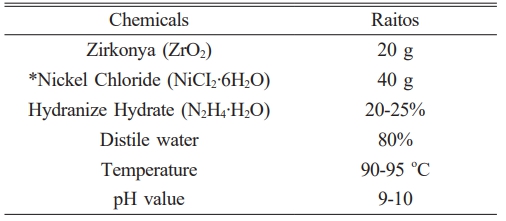
In the study, ZrO2 powders were coated in a hydrazine hydrate (N2H4∙H2O) chemical nickel plating bath by electroless plating method. Ni coated ZrO2 powders and Ti powders were prepared in different compositions and the powders required for ceramic-metal composite production were prepared in different compositions. Electroless nickel plating occurs as a result of the reaction of chemicals with each other without electric current. In this study, Hydrazine bath, a method that yields pure nickel, was used. The most important advantage of electroless coating is that all surfaces of the coated powders are covered equally. It is difficult to obtain a complete and homogeneous coating in electrolytic plating [19-24]. Electroless Ni coated powders and Ti powders were mixed in the determined compositions (Table 1). It has high thermal expansion, excellent thermal insulation/low thermal conductivity, very high resistance to crack propagation, and high fracture toughness [25-34]. Among other properties of titanium, titanium and Preference of alloys in dentistry applications The most important reason for their adoption is biological compatibility. Biocompatibility of titanium and its alloys Its properties stem from its superior corrosion resistance. Corrosion resistance is the effect that occurs on the surface and passive oxide that protects against electrochemical attacks It is a result of the layer. The modulus of elasticity is closer to the bone titanium and its alloys other restorative materials makes it more advantageous compared to alternatives. As a biomaterial, especially hard tissue Titanium alloys to be used as prosthesis, high resistance - low modulus of elasticity, high fatigue It must be resistant and easy to operate [35-40].
Ceramic metal powders were cold formed in a uniaxial hydraulic press under 400bar pressure. The raw samples shaped in the press were sintered in a tube furnace at 1100 oC in an argon atmosphere. The metallographic properties of the produced ceramic-metal composite samples were examined and they were subjected to mechanical tests and the results were given. Table 1 shows the chemicals and their ratios used in the Ni plating bath.
It is prepared in the proportions in Table 1, and ceramic or ceramic-metal powders are mixed into the plating bath at 90-95 ºC, according to the composition we determine. During the coating reaction, the bath temperature and pH value were measured at 5-minute intervals and kept constant at 10 with a tolerance of ±0.5 throughout the reaction. The reaction is completed with the complete precipitation of Nickel. It can be understood that the reaction has precipitated when the plating bath turns from blue to transparent and the mixing effect ends. On average, it is completed in 1-1.5 hours. At the end of the reaction, the coated powders are washed and filtered. In particular, the washing process was repeated so that no chemical residue remained on the coated powders. After washing and filtering, it was dried in an oven at 105 ºC for 24 hours. After the drying process, cold forming was done in the press under 400 bar. In addition, without coating, in the specified compositions, the powders are heated at 25 dv/min. It was mixed in a high speed rotating mixer for 24 hours to obtain a homogeneous mixture.
During electroless nickel plating, the temperature of the plating bath was measured with an optical pyrometer at 5-minute intervals. Fig. 1 shows the mixing effect of the coated powders in the bath as a result of the chemical reaction. During the plating reaction, the plating bath was stirred with a glass rod. When the reaction ends, the color of the plating bath changes from blue to clear and the mixing effect ends. The coated powders settle at the bottom of the beaker. Moving the particles into the bath by mixing them during coating allows Ni to precipitate on the particles. Even if there are no particles in the bath, Ni precipitation will occur on the surface of the beaker.

|
Fig. 1 Reaction image in electroless nickel plating. |
Physical Properties
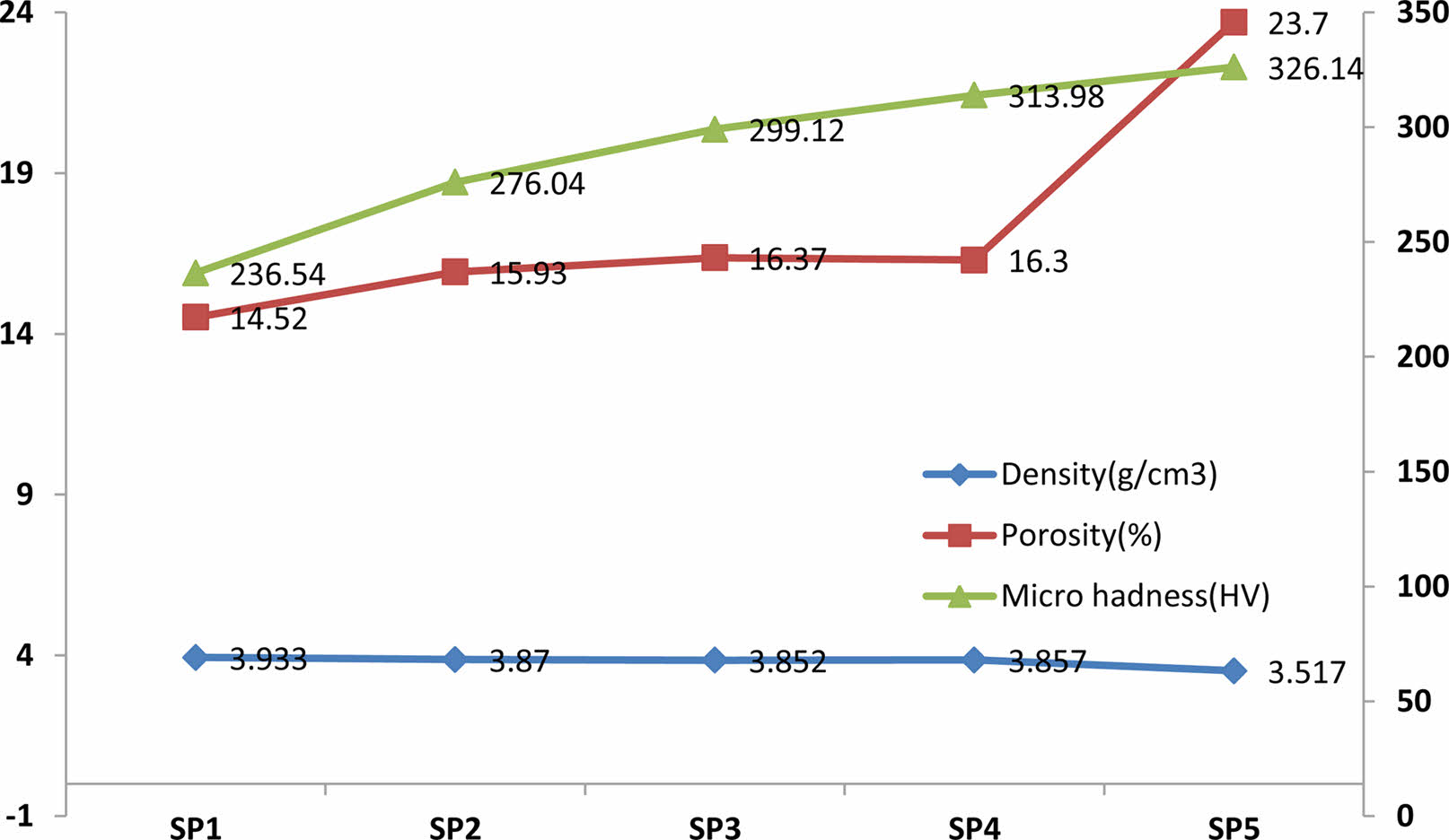
Figure 2 shows the density, porosity and microhardness curves of the produced samples. In the density curve (Fig. 2), a higher density was obtained in the SP1 sample compared to the other samples due to the use of a low volumetric amount of ZrO2. In the SP5 sample, there was a decrease in the total density due to the use of volumetrically higher ZrO2 compared to the SP1, SP2, SP3, and SP4 samples. Additionally, the highest % porosity calculated was obtained in this sample. When the measured microhardness values of SP1, SP2, SP3, SP4 and SP5 samples were compared, the highest hardness value was measured in the SP5 sample, which contained the highest volumetric ZrO2, compared to the other samples. Different compositions (SP1, SP2, SP3, SP4 and SP5) were used in the experimental phase. As the ceramic contribution increased, it was measured that there was an increase in the vikers hardness value in the hardness measurements. The increase in ceramic phase also increased porosity. SEM photographs provide information about grain coarsening, neck formation and grain size in the samples produced. EDX elemental analysis performed on the samples shows that the electroless Ni coating process used in the study is realized.
Microstructural Investigation
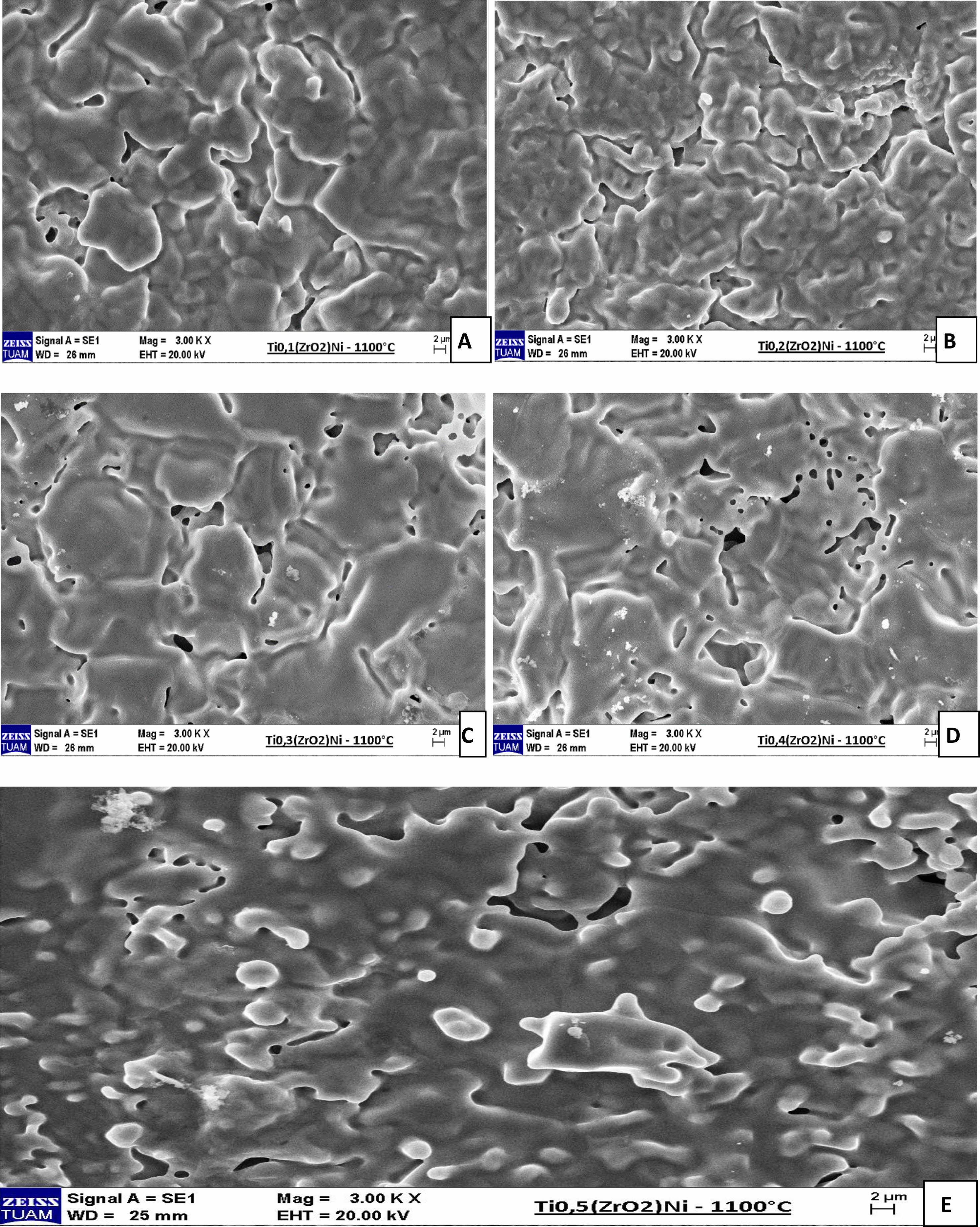
Figure 3 shows SEM images of SP1, SP2, SP3, SP4, and SP5 samples sintered at 1100 oC. When looking at the pictures, it can be seen that the sintering is good as the neck and grain become larger and the grain boundaries become clear. The (ZrO2)Ni contribution rate was increased in the samples, respectively. The lowest solid belongs to the SP1 sample and the highest contribution rate belongs to the SP5 sample. When the SEM images are examined, it is seen that intergranular neck formation and grain coarsening occur due to the increase in the ZrO2 contribution rate. Grain boundaries are visible in the samples. It was calculated that the porosity increased from 14.52% to 23.7% with the increase of ZrO2 contribution rate. It can be seen that the theoretical density of 76.29% has been approached in the SP5 sample. When the sample densities were compared, it was calculated that the density of the SP5 sample decreased compared to the SP1 sample as the ceramic solid ratio increased. The fact that the ZrO2 density is lower than the Titanium density and that the SP5 sample contains the highest ZrO2 contribution explains the increase in hardness and low density in this sample. It is seen that neck formation and grain coarsening are better in SP5 sample than in other samples. It is also known that porosity in implant materials is necessary to facilitate bond formation between the implant material and tissues.
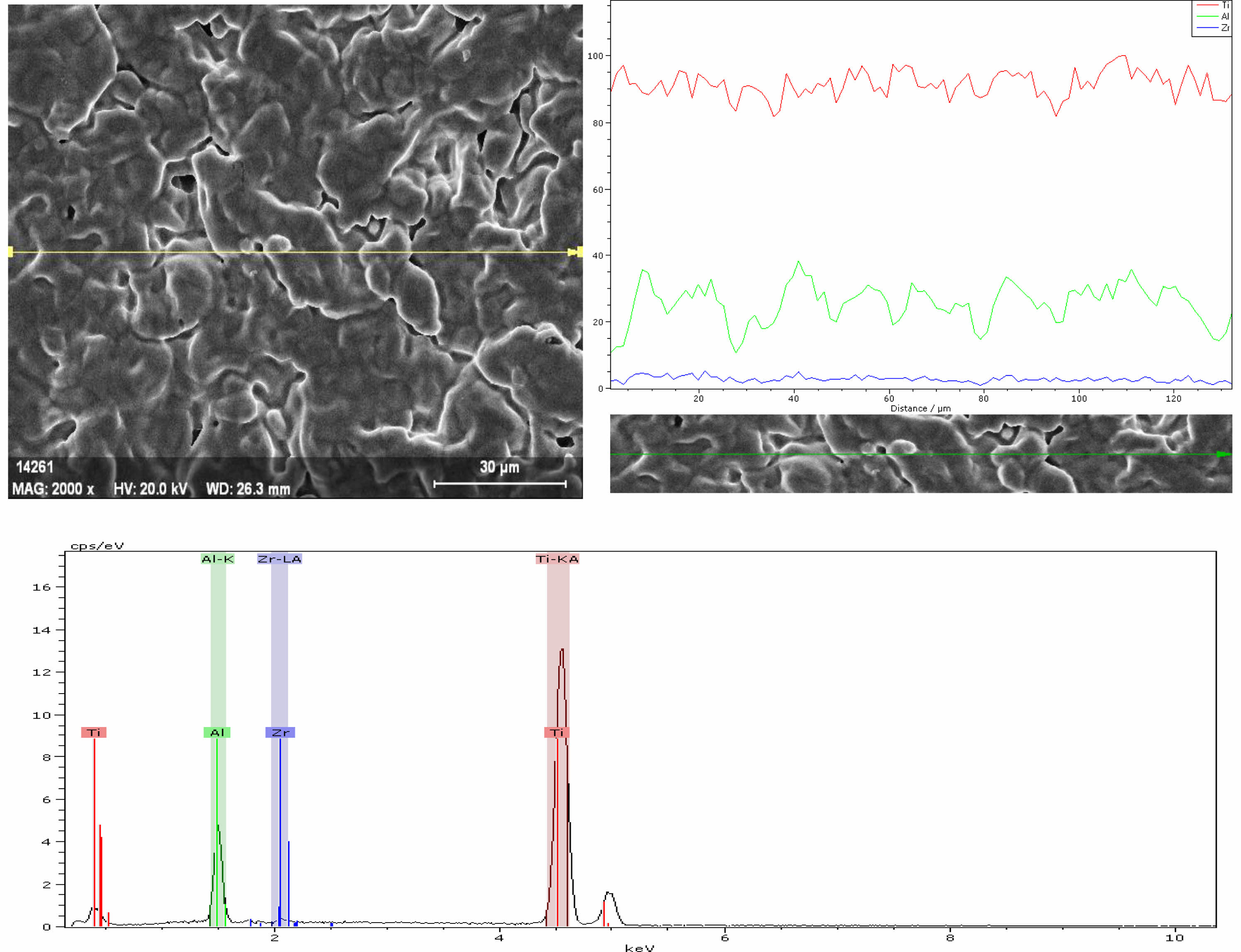
The elemental analysis was performed along the linear analysis method line (Fig. 4). Ti, Zr and Al elements were detected in the SP1 sample. It is thought to be due to the fact that the purity level of the powders used in Al is not 100%. In addition, due to the very low Ni content in the SP1 sample, the resulting peak could not be observed on the curve due to its low intensity.
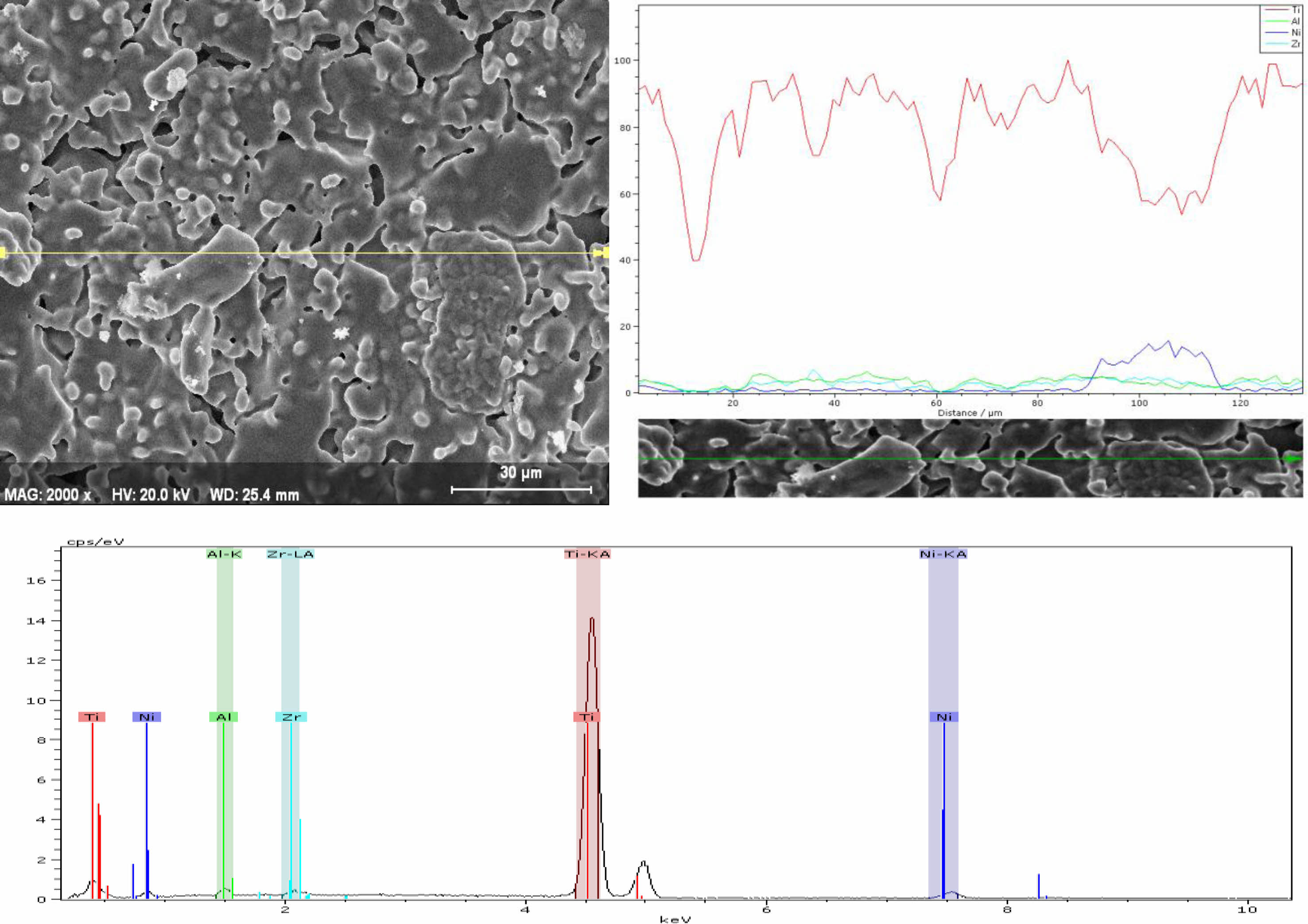
In Fig. 5, elemental analysis was performed along the line using the linear analysis method. Ti, Zr, Al and Ni elements were detected in the SP5 sample. Since the Ni ratio in the SP5 sample was much higher than in the SP1 sample, the resulting peak was observed on the curve, although its intensity was lower.
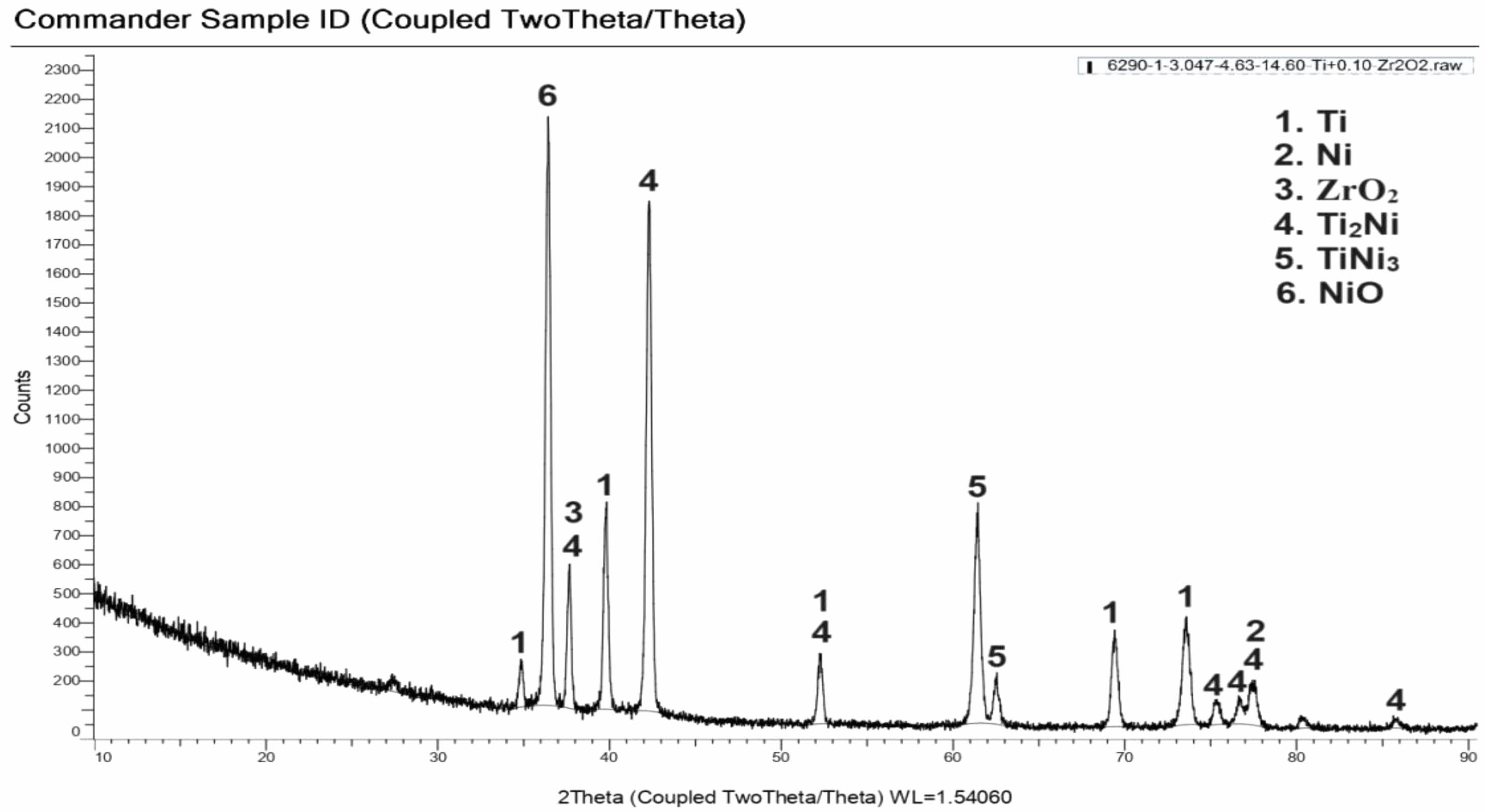
Electroless Ni coated ZrO2 powders were mixed homogeneously with Ti powders in the determined compositions and then shaped in a uniaxial cold press. Samples sintered in a tube furnace in argon atmosphere at 1100 ºC were examined and characterized by XRD analysis. The XRD analysis graph of the composite sample of the SP1 sample is given in Fig. 4. According to the analysis results, it was determined that Ti, Ni ZrO2, Ti2Ni, TiNi3 and NiO phases were present.
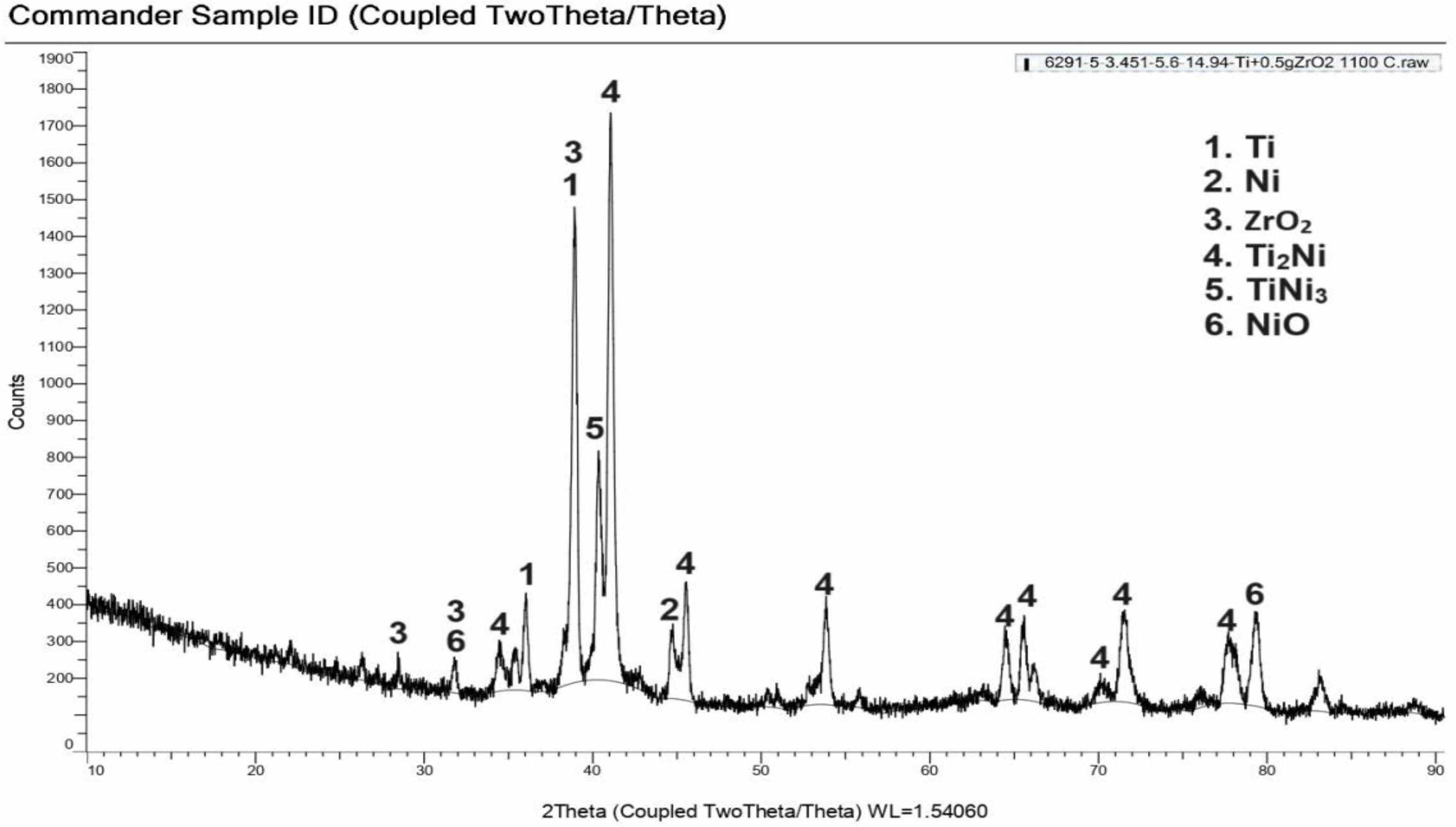
The XRD analysis graph of the composite sample of the SP5 sample is given in Fig. 5. According to the analysis results, it was determined that Ti, Ni ZrO2, Ti2Ni, TiNi3 and NiO phases were present. Unlike the SP5 sample, the peaks belonging to the Ti2Ni phases have higher numerical values than the SP1 sample, indicating that the microstructure properties have changed. Sem photographs and the increase in hardness confirm this situation. Fig. 6 Fig. 7 Table 2
|
Fig. 2 Density, Porosity and Microhadness curves |
|
Fig. 3 SEM photographs of sintered samples. |
|
Fig. 4 EDX analysis of the SP1 sample sintered at 1100 oC. |
|
Fig. 5 EDX analysis of the SP5 sample sintered at 1100 oC. |
|
Fig. 6 XRD analysis of sample (%0,54Ni-%97,78Ti-%1,66ZrO2) sintered at 1100 ºC |
|
Fig. 7 XRD analysis of sample (%2,29Ni-%89,74Ti-%7,95ZrO2) sintered at 1100 ºC |
In this study, certain proportions of Ni-coated ZrO2 powders were added to the Titanium element as an additive. In addition, the zirconium element added to titanium with 989% purity had an increasing effect on the hardness of the alloy. Laboratory test data have shown that Zr alloys can show better mechanical behavior when placed in the mouth as a biomaterial compared to pure titanium [8]. In order to increase the wetting of ZrO2 powders used in the study by Ti, the electroless Ni coating method of ceramic powders was used. Increasing the contribution rate of ZrO2 powders also increased the hardness and porosity. Porosity is a desirable feature because it strengthens bond formation with body tissue. Sintering at 1100 oC gave positive results in the Ti-(ZrO2)Ni system. Since titanium alloys will be used in a corrosive environment within the body, performing abrasion and corrosive environment test experiments will contribute to the determination of the mechanical behavior of the biomaterial on living tissue. Different compositions (SP1, SP2, SP3, SP4 and SP5) produced in the experimental study were used. The Ni coating process enabled the ceramic materials to sinter more easily and at lower temperatures and to wet the ceramic powders. It contributed positively to the physical and mechanical properties of the produced material. When looking at the calculated densities of the produced composites (SP1, SP2, SP3, SP4 and SP5), there are differences between different compositions. It increased the porosity by decreasing the density of the ceramic phase additive. Lightening has occurred in the material produced. By increasing porosity, it increases the formation of stronger bonds with biological tissues. It improved the physical and mechanical properties of Ti-ZrO2 duo used as implant material. The mechanical, physical and metallographic properties of the composites (SP1, SP2, SP3, SP4 and SP5) produced as biomaterials at this temperature were significantly improved by looking at the data obtained. The electroless Ni plating process improved its mechanical properties by increasing the bond formation between ceramic-metal particles.
- 1. C. Leyens and M. Peters, Titanium and titanium alloys 1st ed. (John Wiley & Sons, Ltd, 2003) p. 231.
-

- 2. O.M. Ivasyshyn and A.V. Aleksandrov, Mater. Sci. 44[3] (2008) 311-327.
-

- 3. C. Cui, B.M. Hu, L. Zhao, and S. Liu, Mater. Des. 32 (2011) 1684-1691.
-

- 4. D. Ulutan and T. Ozel, Int. J. Mach. Tools Manuf. 51 (2011) 250-280.
-

- 5. Y. Oshida, Bioscience and Bioengineering of Titanium Materials 2nd ed. (Elsevier, 2007).
- 6. G. Wnek and G. Bowlin, Encyclopedia of Biomaterials and Biomedical Engineering, 2nd ed. (CRC Press, 2008) p. 87.
- 7. D.L Wise, D.J. Trantolo, D.E. Altobelli, M.J. Yaszemski, J.D. Gresser, and E.R. Schwartz, New York (Marcel Dekker, 1995) 1325-1430.
- 8. G. He, J. Eckert, Q.L. Dai, M.L. Sui, W. Löser, M. Hagiwara, and E. Ma, Biomater. 24[5] (2003) 115-200.
-

- 9. V. Covacci, N. Bruzzese, G. Maccauro, C. Andreassi, G.A. Ricci, C. Piconi, E. Marmo, W. Burger, and A. Cittadini, Biomater. 20 (1999) 371-376.
-

- 10. L. Rimondini, L. Cerroni, A. Carrassi, and P. Torricelli, Int. J. Oral. Maxillofac. 17 (2002) 793-798.
- 11. A.J. Raigrodski and G.J. Chiche, J. Prosthet. Dent. 86 (2001) 520-525.
-

- 12. J.P. Moffa, A.D. Guckes, M.T. Okawa, and G.E. Lilly, J. Prosthet. Dent. 30 (1973) 432-441.
-

- 13. P.A. Hansen and L.A. West, J. Prosthet. Dent. 6[2] (1997) 144-148.
-

- 14. A.J. Raigrodski, Dent. Clin. N. Am. 48[2] (2004) 531-544.
-

- 15. Ö. Kırmalı and A.K. Özdemir, Annals of Health Scie. Res. 1[2] (2012) 15-18.
-

- 16. P. Southon, Structural Evolution During the Preparation and Heating of Nanophase Zirconia Gels, (University of Technology, Sydney, 2000) p. 32.
- 17. M.H. Bocanegre-Bernal and S. Diaz De La Torre, J. Mater. Sci. 37 (2002) 4947-4971.
-

- 18. E.R. Andrievskaya, J. Eur. Ceram. 28 (2008) 2363-2388.
-

- 19. A. Yönetken and A. Erol, Sci. Eng. Compos. 18[3] (2011) 145-149.
- 20. A. Erol, A. Yönetken, and M. Erdoğan, Met. Powder Rep. 65[6] (2010) 40-43.
-

- 21. A. Yönetken, Trans. Indian Inst. Met. 68[5] (2015) 675-681.
-

- 22. A. Erol and A.Yönetken, GU J. Sci. 22[3] (2009) 227-233.
- 23. A. Yönetken and A. Erol, Sci. Eng. Compos. 17[3] (2011) 191-198.
-

- 24. A. Yönetken and A. Erol, Met. Powder Rep. 25[2] (2009) 18-23.
- 25. G. Gyawali, D.R. Dhakal, B. Joshi, J.-H.Choi, and S.W. Lee, J. Ceram. Process. Res. 20[1] (2019) 84-89.
-

- 26. W. Dong, Y. Liang, Q. Bao, X. Gu, and J.-W. Yang, J. Ceram. Process. Res. 22[3] (2021). 317-322.
-

- 27. K.-H. Lee, S.-H. Ahn, and K.-W. Namc, J. Ceram. Process. Res. 19[3] (2018) 183-188.
-

- 28. S.-C. Jeong, S.-H. Ahn, and K.-W. Nam, J. Ceram. Process. Res. 17[10] (2016) 1088-1094.
-

- 29. X. Wang, T. Yu, X. Sun, Z. Wang, and W. Wang, J. Ceram. Process. Res. 17[9] (2016) 969-973.
-

- 30. Q. Yang, Y. You, B. Cheng, L. Chen, J. Cheng, D. Lou, Y. Wang, and D. Liu, J. Ceram. Process. Res. 24[2] (2023) 230-236.
-

- 31. A. Rittidech, S. Wantrong, and S. Chommi, J. Ceram. Process. Res. 22[2] (2021) 221-225.
-

- 32. K.-H. Lee and K.-W. Nam, J. Ceram. Process. Res. 19[1] (2018) 54-64.
-

- 33. K.-W. Nam, J. Ceram. Process. Res. 17[6] (2016) 543-549.
-

- 34. E.R. Rangel and H.B. Ramíreza, J. Ceram. Process. Res. 5[1] (2004) 58-63.
- 35. E.P. Lautenschlager and P. Monaghan, Int Dent J. 43 (1993) 245-253.
- 36. A.C.L. Faria, R.C.S. Rodrigues, A.L. Rosa, and R.F. Ribeiro, J. Prosthet Dent. 112 (2014) 1448-1460.
-

- 37. X. Wang, T. Yu, X. Sun, Z. Wang, and W. Wang, J. Ceram. Process. Res. 7[9] (2016) 969-973.
-

- 38. Y.C. Lina, J. C. Hungb, H.M. Chowc, and A.C. Wangd, J. Ceram. Process. Res. 16[2] (2015) 249-257.
-

- 39. Q. Yang, Y. You, B. Cheng, L. Chen, J. Cheng, D. Lou, Y. Wang, and D. Liu, J. Ceram. Process. Res. 24[2] (2023) 230-236.
-

- 40. I.J. Shon and N.R. Park, J. Ceram. Process. Res. 14[3] (2013) 380-384.
-

 This Article
This Article
-
2024; 25(1): 97-103
Published on Feb 29, 2024
- 10.36410/jcpr.2024.25.1.97
- Received on Dec 11, 2023
- Revised on Jan 22, 2024
- Accepted on Jan 24, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- Asli Betül Yönetken
-
Ankara Yıldırım Beyazıt University, Dentistry Faculty, Prosthetic dental Dept., Yayla Mahallesi Yozgat Bulvarı, 1487. Cadde No:55 Etlik-Keçiören / Ankara, Turkey
Tel : +90 312 906 1000 Fax: +90 312 906 2950 - E-mail: abyonetken@aybu.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.