- Hydroxyapatite production and characterization from four pufferfish species teeth
Servet Ahmet Doğdua,b,*, Cemal Turanb, Tolga Depcic, Ersin Bahçecid, Kemal Sangüne and Deniz Ayasf
aIskenderun Technical University, Maritime Vocational School of Higher Education, Underwater Technologies, TR-31200, Hatay, Türkiye
bIskenderun Technical University, Faculty of Marine Sciences and Technology, Molecular Ecology and Fisheries Genetics Laboratory, TR-31200 Iskenderun, Hatay, Türkiye
cDepartment of Petrol and Natural Gases Engineering, Iskenderun Technical University, Iskenderun, Hatay, TR-31200, Türkiye
dMetallurgical and Material Eng Engineering Department, Iskenderun Technical University, Iskenderun, Hatay, TR-31200, Türkiye
eHatay Mustafa Kemal University, Faculty of Arts and Sciences, Department of Chemistry, Hatay, TR-31060, Türkiye
fMersin University, Fisheries Faculty, Fishing and Seafood Processing Technology Department, Mersin, TR-33010, TürkiyeThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The pufferfishes are invasive alien fish species in the Mediterranean Sea with a powerful toxin in its body called tetrodotoxin. It has no economic value and is very abundant all over the world’s marine waters. Calcium phosphate bioceramic materials especially hydroxyapatite are commonly applied as implant resources because of their close resemblance in structure with natural bone. In this study, natural hydroxyapatite was obtained from the teeth of four pufferfish, the obtained products were characterised and their potential uses in the biomedical industry were revealed. Pufferfish teeth were extracted from the fish, thoroughly rinsed using deionized water, measured, and subsequently placed within a furnace set at a temperature of 105 °C. Once dehydrated, the teeth were finely pulverized using a planetary ball mill. This powdered material was then subjected to various analyses, including elemental composition assessment, TTX analysis, and SEM-EDX examinations. Ca/P atomic ratio values of Lagocephalus suezensis, L. guentheri, Sphoeroides pachygaster and Torguigener favimaculasus teeth were obtained as 1.58, 1.64, 1.67 and 1.47, respectively. The analysis findings suggest that pufferfish teeth could serve as a promising natural alternative source for biomedical and various other industries in need of natural hydroxyapatite. This hydroxyapatite can be utilized for creating fillers, implants, bone powder, and prostheses with applications in biomedicine and various industries. However, it should not be forgotten that the necessary tests must be carried out before any human use is made available.
Keywords: Calcium phosphate bioceramic, Blue biomaterials, Teeth, Pufferfish, Hydroxyapatite.
Biomaterials are resources that have special properties that make them precise tools to be used with living tissues. Because of their applicability, they have become increasingly important role of the biomedical industry [1]. Biomaterials serve the purpose of restoring, repairing, or replacing damaged tissue by integrating with the affected body part, thereby increasing lifespan [2]. Biomaterials must have special components such as biocompatibility, serializability, functionality, and manufacturability [3]. Biomaterials are frequently employed in the realms of implants, tissue engineering, organ transplants, and drug delivery systems [4].
Bioceramics of calcium phosphate are mostly used in tissue engineering and dentistry [5]. The chemical makeup of calcium phosphate bioceramics, particularly hydroxyapatite (HA), closely resembles the mineral composition found in human bones [6]. This similarity renders them well-suited for applications in orthopaedic implants and dental materials, as noted by Puad et al. [1]. These materials have been found to have excellent bioactivity, a high degree of biocompatibility and excellent osteoconduction properties [7-9].
Marine resources are an incredible natural alternative to invitro biomaterials. Because they can provide sustainability, also, it is the lack of disease transmission risks, along with the absence of religious reasons reinforces the use of marine organisms for biomaterials [10]. Over the past few years, marine organisms have gained prominence as sources for extracting biomaterials with potential biomedical applications, including therapeutic and medical diagnostic purposes [11-16]. In addition, the wastes of commercially consumed marine species such as skin, head, internal organs, fins and scales are used as a source of biomaterials [17].
Pufferfishes, categorized as invasive alien fish species within the Mediterranean Sea, possess a potent toxin called tetrodotoxin in their bodies. Despite their lack of economic significance, these species are abundantly found in marine waters globally [18]. Pufferfishes occur throughout tropical and subtropical areas of the Indian, Pacific and Atlantic Oceans and are also a good indication of the tropicalization of the Mediterranean fish fauna [19]. In Turkish marine waters, pufferfish is represented by four genera and seven species Lagocephalus lagocephalus, L. guentheri, L. sceleratus, L. suezensis, Sphoeroides pachygaster, Torquigener favimaculosus and Tylerius spinosissimus [20-22].The pufferfish species is renowned for harbouring potent paralytic toxins within its body, specifically known as tetrodotoxin (TTX) [23]. According to the current European legislative requirements, pufferfish species must not be placed on the market [24]. The high strength of pufferfish teeth makes them suitable for bioceramic applications, which could result in significant gains. Additionally, pufferfish teeth have similar characteristic properties to human teeth, making them a promising natural bioceramic for the dental industry [3, 25]. It is believed that invasive alien species such as pufferfish into the economy can help ecosystems that have been degraded by climate change to restore themselves.
The aim of study is to obtain bioceramics with natural calcium phosphate content from the teeth of four puffer fish to characterise the obtained products and reveal their potential uses in the biomedical industry.
Preparation of Teeth Samples
We collected four species of pufferfish from Iskenderun Bay on the Mediterranean coast of Türkiye, including Lagocephalus suezensis, Lagocephalus guentheri, Sphoeroides pachygaster and Torquigener flavimaculosus. We transferred the samples to the laboratory in ice bags. We removed the teeth from each fish sample and Fig. 1. shows the process we followed.
The teeth samples were placed within sterile plastic containers and submerged in a 30% H2O2 solution. The solution was stirred vigorously at a rate of approximately 0.75 ml/min for a duration of one hour. Subsequently, the solution underwent filtration, and any remaining soft tissues and blood present on the tooth samples were delicately eliminated using a combination of H2O2 solution and a brush. Following this step, the teeth samples were subjected to a thorough washing process using deionized water. Subsequently, they were dried in an oven at a temperature of 105 °C throughout the night. The resulting dried material was then subjected to grinding using a ball mill. The resulting powder, derived from the teeth, underwent multiple cycles of washing with a 30% H2O2 solution within sterile plastic containers. After each wash, the material was filtered, and then it was dried once more overnight at 105 °C.
Element Composition Analysis
The powdered tooth samples were weighed (0.20-0.25 g) and transferred to glass tubes using a plastic spoon. A solution containing HCl (2 mL), HNO3 (6 mL) and H2O2 (2 mL) was used to fully dissolve the teeth. Subsequently, the mixture was heated on a 150 °C hot plate until all samples had fully dissolved. Finally, we subjected the solution to analysis with an inductively coupled plasma mass spectrometer (ICP-MS) to identify the metals present in the teeth structure. The experimental settings used for ICP-MS were as follows: the radio frequency was set to 1500 W, the plasma gas flow rate was kept at 15 L min-1, the auxiliary gas flow rate was at 1 L min-1, and the carrier gas flow rate was set to 1.1 L min-1. The spray chamber temperature was set to 2 °C. The sample depth was 8.6 mm, and the sample introduction flow rate was set to 1 mL min-1. The nebuliser pump was set to 0.1 rps, and the extracted lens was set to 1.5 V. The concentrations of macroelements (Na, Mg, P, K, Ca), trace elements (Co, Cu, Zn, Mo, Ni, Se), and potentially toxic metals (Cd, Pb, As, Cr) were measured as μg metal g-1 dry weight in all tooth samples. The elements were identified using the High Purity Multi-Standard (Charleston, SC 29423). Standard solutions were used to dilute macro, trace and potentially toxic metals to obtain calibration curves. The solutions that were prepared included lead, cadmium, arsenic, and chromium, with concentrations spanning from 1 to 50 parts per billion (ppb) or equivalently from 0.001 to 0.050 milligrams per litre (mg/L) for the toxic metals. Additionally, copper, iron, and zinc were present in concentrations ranging from 1 to 50 parts per million (ppm) or 1 to 50 milligrams per litre (mg/L) for the macro and trace elements.
Tetrodotoxin Analysis
To detect the presence of TTX in tooth samples, a method was employed. Firstly, 1 gram of powdered tooth sample was mixed with a 3 mL solution of methanol containing 1% acetic acid. The mixture was then subjected to ultrasonic treatment within a plastic container. After allowing the samples to sit at room temperature for 15 minutes, they underwent centrifugation at 4500 rpm and 4 °C for 20 minutes. Following centrifugation, the upper liquid phase (supernatant) was separated. To the remaining residue, another 3 mL of methanol containing 1% acetic acid was added. The supernatant obtained from the second centrifugation was combined with the initial supernatant, resulting in a solution that was adjusted to a total volume of 7 mL. This solution was thoroughly mixed using a vortex machine. Afterwards, a volume of 1 mL from the solution was passed through a C18 cartridge (3 mL/500 mg) that had previously been primed with 6 mL of water and 6 mL of methanol, utilizing a vacuum manifold for the filtration process. Another 10 mL of methanol was then passed through the cartridge. To generate the final solution, 12 mL of methanol was utilized and agitated using a vortex mixer. The sample then underwent an evaporation process using an evaporator, resulting in a residue that was subsequently dissolved in 1 mL of methanol. This solution was then filtered through 0.45 μm membrane filters and transferred to vials for analysis, following the procedure outlined by Silva et al. [26]. The presence of TTX in the teeth of pufferfish was assessed using an LC-MS-MS system.
Scanning electron microscope (SEM) and X-ray spectroscopy (EDX)
The scanning electron microscope was employed to examine the morphology and chemical composition of hydroxyapatite (HA) derived from the pufferfish teeth powder. The microscope operated with an accelerating voltage of 10 KV and was equipped with an energy-dispersive X-ray (EDX) spectrometer.
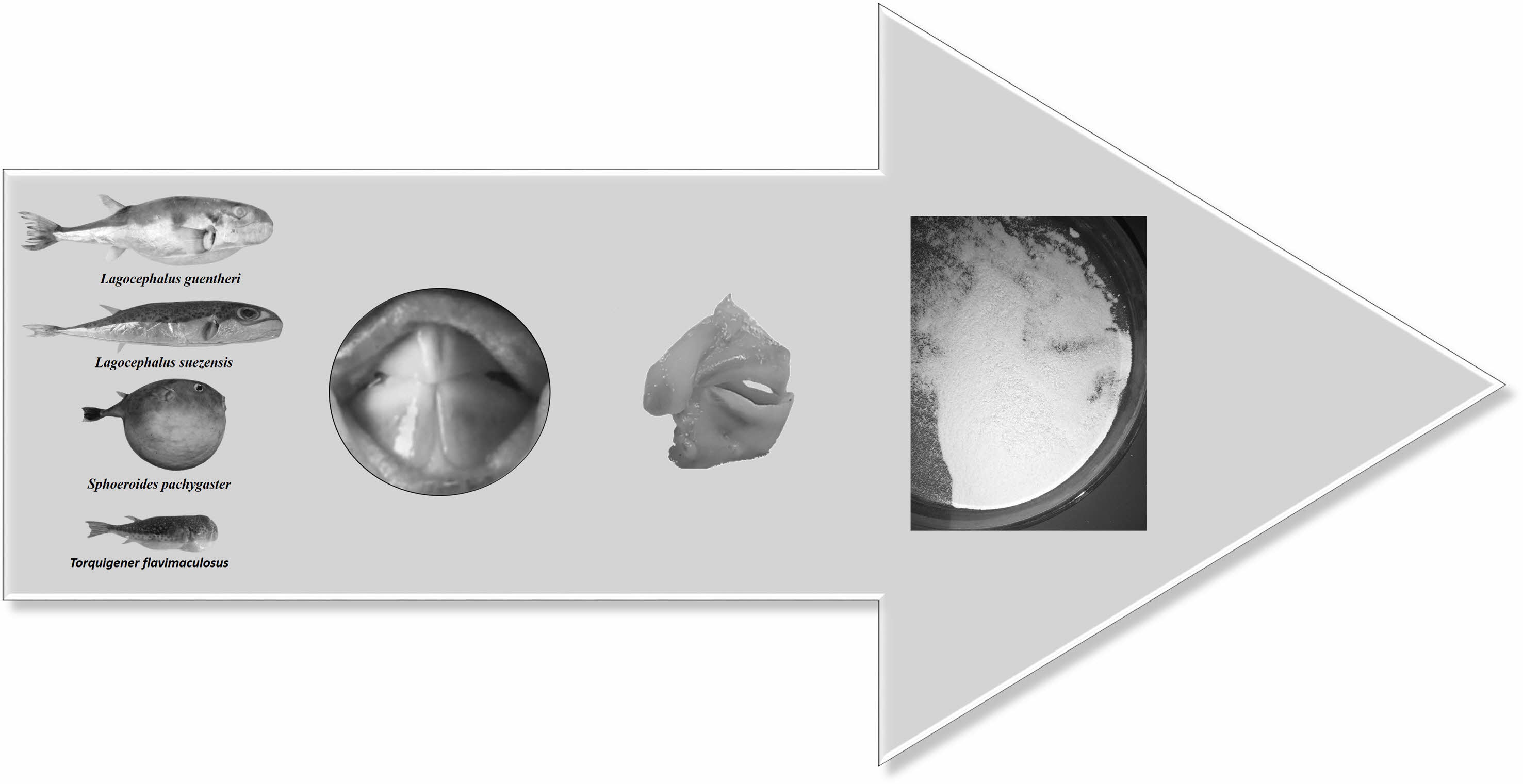
|
Fig. 1 Preparation of pufferfish teeth samples. |
Table 1 shows the elemental composition of HA extracted from the teeth of four pufferfish species Lagocephalus suezensis, L. guentheri, Sphoeroides pachygaster and Torquigener flavimaculosus. The elemental composition of the HA has minor differences between the species. The main structure of HA elemental composition includes an average of four species; 57-60% calcium, 36-38% phosphate, along with 1-1.13% magnesium, and trace amounts of other elements.
Hydroxyapatite can be produced through the utilization of either inorganic constituents or naturally derived organic materials. Hydroxyapatite derived from natural organic sources often exhibits non-stoichiometric properties due to the trace presence of ions like Na, Zn, Mg, K, Si, and Ba inherent to these materials. Despite this disparity, both categories of hydroxyapatite manifest bioactivity and biocompatibility, making them equally valuable for in vitro applications. However, a significant challenge associated with biomaterials synthesized from inorganic sources primarily composed of “Ca” and “P” is the substantial cost linked to the synthetic process. Traditional chemical methods commonly yield hydroxyapatite (HA) lacking the advantageous elements including Na, Zn, Mg, K, Si, and Ba. Nevertheless, studies have demonstrated that the presence of these ions has a direct impact on various biochemical reactions associated with bone metabolism [27]. The elemental composition results of hydroxyapatite from the teeth of pufferfish showed Ca/P ratios of 1.58, 1.64, 1.67 and 1.47 in L. suezensis, L. guentheri, S. pachygaster, and T. flavimaculosus, respectively. The hydroxyapatite discovered in pufferfish teeth exhibits characteristics typical of the mineral phase found in biological apatites. Within this phase, the presence of carbonates can significantly impact the calcium-to-phosphorus (Ca/P) ratio [28]. Lee et al. [29] reported that the Ca/P ratio of HA from cuttlefish bone was between 1.64 and 1.70. Bahrololoom et al. [30] investigated the Ca/P ratio of natural HA from various animal bones and reported a range of 1.46-2.01. Nazarpak et al. [31] found the Ca/P ratio of synthetic HA to be 1.62. Latif et al. [32] extracted natural HA from Thunnus thynnus and found the Ca/P ratio to be 1.60. Doğdu et al. [3] extracted and characterized HA from the Lagocephalus sceleratus and reported the Ca/P ratios as 1.32. Dermawan et al. [33] extracted and characterized natural hydroxyapatite from Black Tilapia fish bone and reported the Ca/P ratio as higher than 1.67. Kim et al. [34] extracted carbonated HA from the Paralichthys olivaceus bone and found the Ca/P ratio as 1.96. Deb et al. [35] extracted HA from the fish scales of Catla catla and detected the Ca/P ratio as 1.58. Silveira et al. [36] reported Ca/P ratio of HA from Arapaima gigas scales as 2.00. Rohan et al. [37] extracted HA from the crab shell of Portunus sanguinolentus and found the Ca/P ratio to be 1.67. Niakan et al. [38] found the Ca/P ratio of HA from the bovine bone as 1.67-1.83. Hydroxyapatite as a calcium phosphate-based biomaterial is a group of compounds with a Ca/P molar ratio in the range of 0.5-2 and is the most sought-after biomaterial for its construction especially in the dental and orthopaedic field [39, 40]. Synthetic HA with a Ca/P ratio is 1.67 [7]. Our results show that HA, which is extracted from pufferfishes, and Ca/P ratios were detected in the range of the acceptable limits [41] that it can be used as a natural HA source in biomedical applications. Also, the TTX in the HA samples were below the detectable limit (0.1 µg/g).
To confirm the calcium-phosphorus ratio, EDX spectroscopy was utilized. The EDX spectra depicting the hydroxyapatite extracted from pufferfishes are presented in Fig. 2. The established chemical formula for standard HA indicates a Ca/P atomic ratio of 1.67 [7, 42]. The Ca/P atomic ratios obtained by our study from the L. suezensis, L. guentheri, S. pachygaster and T. flavimaculosus were 1.58, 1.64, 1.67 and 1.47, respectively. These results are consistent with the elemental composition analysis results. The Ca/P ratio was found to be proportionally dependent on the reaction mixture from the EDX results.
The SEM image of the HA sample in Fig. 3. shows the presence of oval and plate-shaped particles in the structure. First, the teeth obtained from pufferfishes were light off-white and yellow in colour, indicating the presence of other organic fragments such as collagen in the HA. When the teeth are processed by the alkaline method, the yellow colour disappears, indicating the definite exclusion of collagen and other organic parts. The oval and plate-shaped structure and size of natural HA obtained from the teeth of puffer fish indicate that it can provide important properties such as good bioactivity and flexibility [43].
In conclusion, in previous studies for HA extraction, the sources were land-based animals such as pigs’ bones and bonive bones [44-46]. In recent years, many studies have been carried out using marine organisms as alternative sources [34, 47-49]. Natural or synthetic hydroxyapatite is a frequently used material for bone tissue engineering due to its close composition similarity with natural bone. It has been extensively studied as an implant material for orthopaedic and dental applications, demonstrating excellent bioactivity, adequate mechanical rigidity and structure, osteoconductivity, and angiogenic properties, as well as being non-toxic and not causing inflammatory or antigenic reactions [50-53]. In this study, we employed the alkaline hydrolysis method to extract hydroxyapatite from the teeth of four distinct pufferfish species: L. suezensis, L. guentheri, S. pachygaster, and T. flavimaculosus. This endeavour aimed to identify a potential natural marine source of HA. It was revealed that the extracted HA can be used as a natural source of HA in dental applications. The study has unveiled that pufferfish, despite lacking economic significance in many regions (except Asia), represent a natural, unique, and alternative source of hydroxyapatite. This hydroxyapatite can be utilized for creating fillers, implants, bone powder, and prostheses with applications in biomedicine and various industries. However, it should not be forgotten that the necessary tests must be carried out before any human use is made available.
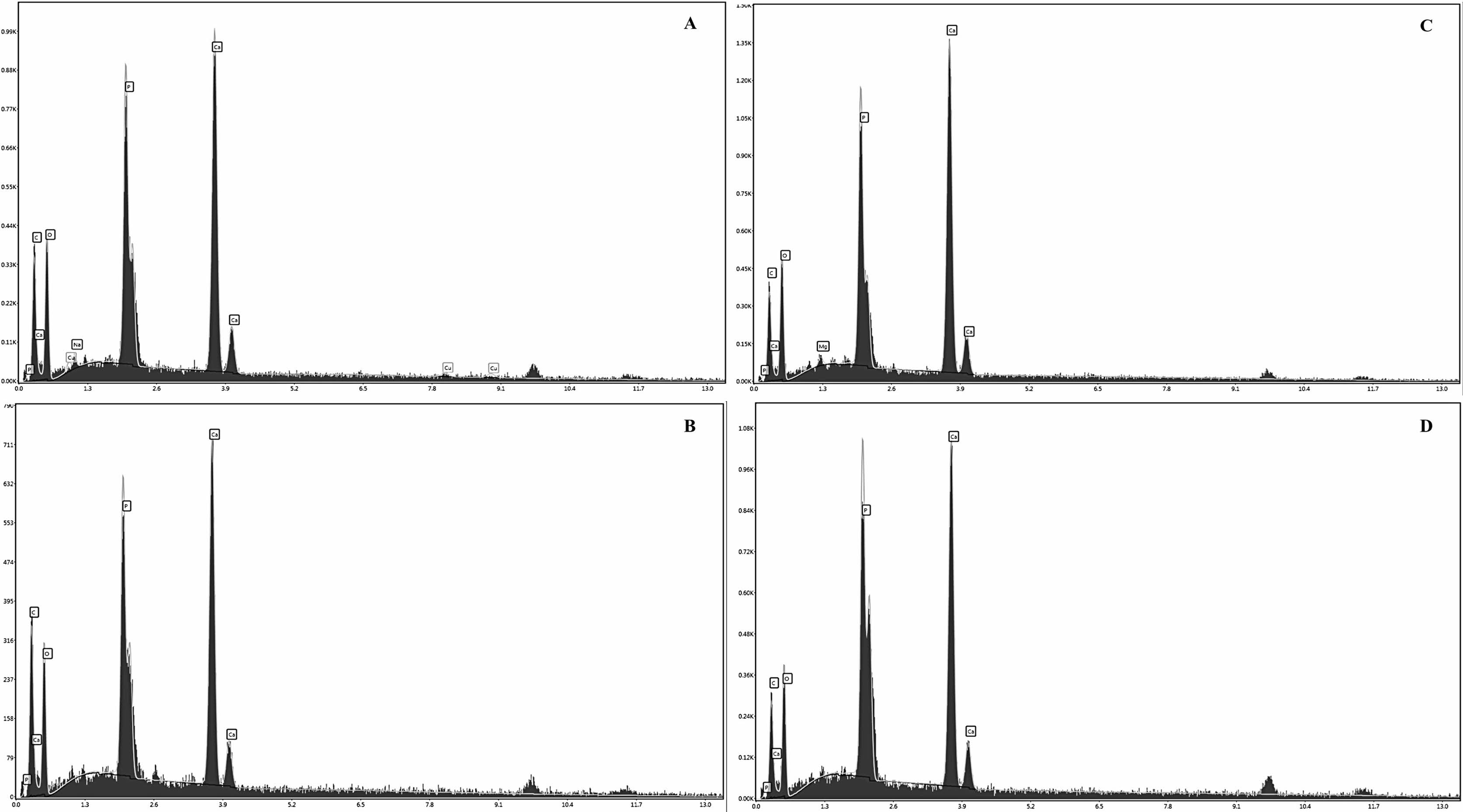
|
Fig. 2 The EDX spectra of the HA extracted from the pufferfish species as A) L. suezensis, B) L. guentheri, C) S. pachygaster, D) T. flavimaculosus. |

|
Fig. 3 SEM images of HA from the pufferfish species A) L. suezensis, B) L. guentheri, C) S. pachygaster, D) T. flavimaculosus. |
|
Table 1 The elemental composition of HA extracted from teeth of four pufferfish species (µg g-1 dw). |
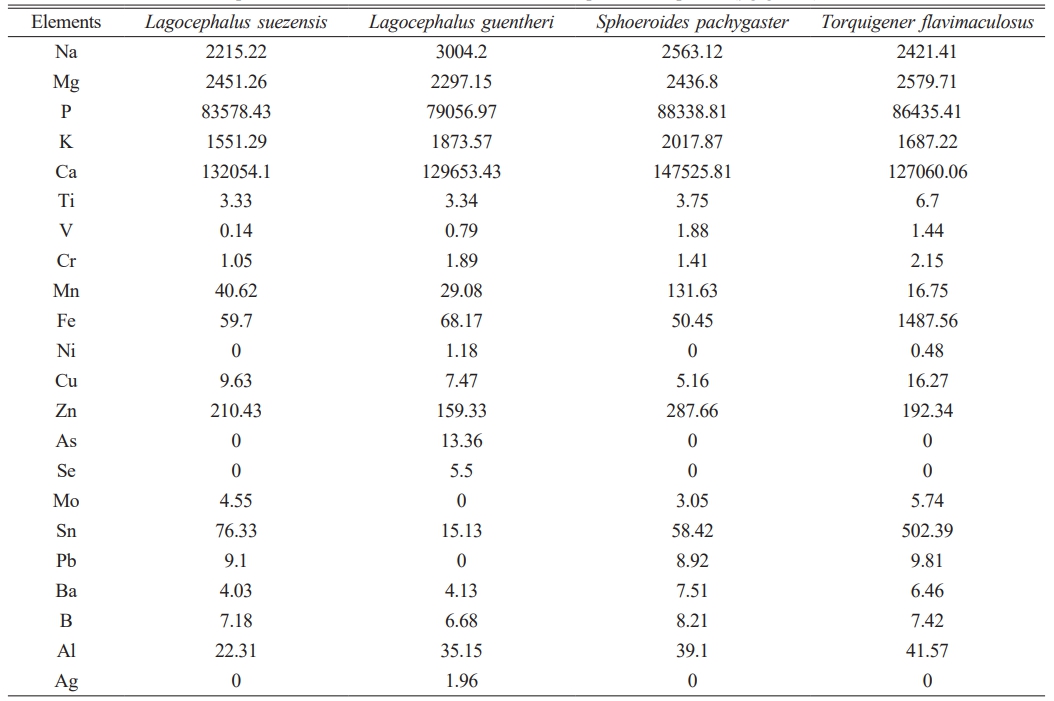
dw: a dry weight |
- 1. N.M. Puad, P. Koshy, H.Z. Abdullah, M.I. Idris, and T.C. Lee, Heliyon. 5[5] (2019) 1-14.
-

- 2. T. Billiet, M. Vandenhaute, J. Schelfhout, S. Van Vlierberghe, and P. Dubruel, Biomaterials. 33[26] (2002) 6020-6041.
-

- 3. S.A. Doğdu, C. Turan, T. Depci, and D. Ayas, J. Ceram. Process. Res. 22[3] (2021) 356-361.
-

- 4. S. Gundu, N. Varshney, A.K. Sahi, and S.K. Mahto, J. Polym. Res. 29[3] (2022) 1-23.
-

- 5. M. Supová, J. Aust. Ceram. Soc. 58 (2022) 227-247.
-

- 6. M. Demirel, B. Aksakal. J. Ceram. Process. Res. 19[1] (2018) 5-10.
-

- 7. J. Venkatesan, B. Lowe, P. Manivasagan, K.H. Kang, E.P. Chalisserry, S. Anil, and S.K. Kim, Materials. 8[8] (2015) 5426-5439.
-

- 8. N. Ramesh, S.C. Moratti, and G.J. Dias, J. Biomed. Mater. Res. Part B. 106[5] (2018) 2046-2057.
-

- 9. M. Mojahedian, F. Fahimipour, K.L. Larsen, M. Kalantar, F. Bastami, and N. Omatali. J. Ceram. Process. Res. 17[11] (2016) 1138-1142.
-

- 10. S.A. Doğdu, C. Turan, and T. Depci, NESciences. 6[2] (2021) 96-101.
-

- 11. H. Ehrlich, in “Biological Materials of Marine Origin” (Springer, 2010) p. 1-436.
-

- 12. A. Srivastava, A. Srivastava, A. Srivastava, and P. Chandra, in “Marine Biomaterials in Therapeutics and Diagnostic” (Springer, 2015) p.1247-1263.
- 13. Y.S. Lim, Y.J. Ok, S.Y. Hwang, J.Y. Kwak, and S. Yoon, Mar. Drugs. 17[8] (2019) 1-32.
-

- 14. S.A. Doğdu, C. Turan, and D. Ayas, NESciences. 4[3] (2019) 308-314.
-

- 15. M.C. Wan, W. Qin, C. Lei, Q.H. Li, M. Meng, M. Fang, and L.N. Niu, Bioact. Mater. 6[12] (2021) 4255-4285.
-

- 16. S.A. Doğdu, C. Turan, T. Depci, E. Bahçeci, and F. Turan, Pak. J. Mar. Sci. 32[1] (2023) 83-88.
- 17. D. Coppola, C. Lauritano, F. Palma Esposito, G. Riccio, C. Rizzo, and D. de Pascale, Mar. Drugs. 19[2] (2021) 116.
-

- 18. Katikou, P., Gokbulut, C., Kosker, A.R., Campàs, M., and F. Ozogul, Mar. Drugs. 20[1] (2022) 1-48.
-

- 19. C. Turan and S.A. Doğdu, Pak. J. Mar. Sci. 31[2] (2022) 93-111.
- 20. D. Erguden, F. Kabaklı, A. Uyan, S.A. Doğdu, S. Karan, M. Gurlek, and C. Turan, NESciences. 2[3] (2017) 67-73.
-

- 21. C. Turan, M. Gürlek, N. Başusta, A. Uyan, S.A. Doğdu, and S. Karan, NESciences. 3[3] (2018) 333-358.
-

- 22. A. Karataş, H. Filiz, K. Erciyas-Yavuz, S.C. Özeren, and C.V. Tok, in “The vertebrate biodiversity of Turkey” (Springer International Publishing, 2021) p. 175-274.
-

- 23. A.R. Kosker, F. Özogul, D. Ayas, M. Durmus, Y. Ucar, J.M. Regenstein, and Y. Özogul, Chemosphere. 209 (2019) 95-99.
-

- 24. European Commission, EU Report 29 April 2004 854/2004/EC (2004).
- 25. A.P. Thiery, T. Shono, D. Kurokawa, R. Britz, Z. Johanson, and G.J. Fraser, Proc. Natl. Acad. Sci. U. S. A. 114[22] (2017) 4425-4434.
-

- 26. M. Silva, J. Azevedo, P. Rodriguez, A. Alfonso, L.M. Botana, and V. Vasconcelos, Mar. Drugs. 10[4] (2012) 712-726.
-

- 27. M. Akram, R. Ahmed, I. Shakir, W.A.W. Ibrahim, and R. Hussain, J. Mater. Sci. 49 (2014) 1461-1475.
-

- 28. A. Antonakos, E. Liarokapis, and T. Leventouri, Biomaterials. 28[19] (2007) 3043-3054.
-

- 29. S. Lee, Y. Lee, and Y. Yoon, J. Ceram. Process. Res. 8[6] (2007) 427-430.
-

- 30. M.E. Bahrololoom, M. Javidi, S. Javadpour, and J. Ma, J. Ceram. Process. Res. 10[2] (2009) 129-138.
-

- 31. M.H. Nazarpak, M. Solati-Hashjin, and F. Moztarzadeh, J. Ceram. Proc. Res. 10[1] (2009) 54-57.
-

- 32. A.F.A. Latif, N.A.S. Mohd Pu’ad, N.A.A. Ramli, M.S. Muhamad, H.Z. Abdullah, M.I. Idris, and T.C. Lee, Mater. Sci. Forum. 1010 (2020) 584-589.
-

- 33. S.K. Dermawan, Z.M.M. Ismail, M.Z. Jaffri, and H.Z. Abdullah, Key Eng. Mater. 908 (2022) 159-164.
-

- 34. S.C. Kim, S.Y. Heo, G.W. Oh, M. Yi, and W.K. Jung, Mar. Drugs. 20[6] (2022) 1-17.
-

- 35. P. Deb, S. Das Lala, E. Barua, and A.B. Deoghare, Arabian J. Sci. Eng. 48[3] (2023) 27-41.
-

- 36. E.D.D. Silveira, J.C.S. Andrade, R.D.A. Rodrigues, C.C. Silva, F.D.S. Miranda, and J.C.D. Macedo Neto, Mater. Res. 26 (2023) 1-10.
-

- 37. J. Rohan, D. Thenmuhil, R. Umapriya, and D. Varatharajan, J. Ceram. Process. Res. 23[1] (2022) 22-28.
-

- 38. A. Niakan, S. Ramesh, C.Y. Tan, J. Purbolaksono, H. Chandran, and W.D. Teng, J. Ceram. Process. Res. 16[2] (2015) 223-226.
-

- 39. M. Gutierres, A.G. Dias, M.A. Lopes, N.S. Hussain, A.T. Cabral, L. Almeida, and J.D. Santos, J. Mater. Sci. Mater. Med. 18 (2007) 2377-2382.
-

- 40. S. Sakka, J. Bouaziz, and F.B. Ayed, in “Advances in Biomaterials Science and Biomedical Applications” (Springer, 2013).
-

- 41. A. Shanaghi, B. Mehrjou, Z. Ahmadian, A.R. Souri, and P.K. Chu, Mater. Sci. Eng. C. 118 (2021) 1-16.
-

- 42. S. Koutsopoulos, J. Biomed. Mater. Res. Part A. 62[4] (2002) 600-612.
-

- 43. J. Chevalier and L. Gremillard, J. Eur. Ceram. Soc. 29[7] (2009) 1245-1255.
-

- 44. C.Y. Ooi, M. Hamdi, and S. Ramesh, Ceram. Int. 33[7] (2007) 1171-1177.
-

- 45. N.A. Barakat, M.S. Khil, A.M. Omran, F.A. Sheikh, and H.Y. Kim, J. Mater. Process. Technol. 209[7] (2009) 3408-3415.
-

- 46. A. Raksujarit, K. Pengpat, G. Rujijanagul, and T. Tunkasiri, Mater. Des. 31[4] (2010) 1658-1660.
-

- 47. Y. Chai and M. Tagaya, M. Mater. Lett. 222 (2018) 156-159.
-

- 48. K.L. Hernández-Ruiz, J. López-Cervantes, D.L. Sánchez-Machado, M. del Rosario Martínez-Macias, M.A. Correa-Murrieta, and A. Sanches-Silva, Sustainable Chem. Pharm. 28 (2022) 1-14.
-

- 49. F. Fendi, B. Abdullah, S. Suryani, I. Raya, A.N. Usman, and D. Tahir, AIP Conf. Proc. 2719[1] (2023).
-

- 50. M.D. Chaves, L.S. de Souza Nunes, R.V. de Oliveira, L.A. Holgado, H. Nary Filho, M.A. Matsumoto, and D.A. Ribeiro, J. Cra. Max. Surg. 40[8] (2012) 315-320.
-

- 51. A.M. Parizi, A. Oryan, Z. Shafiei-Sarvestani, and A. Bigham-Sadegh, J. Orthop. Traumatol. 14[4] 2013 259-268.
-

- 52. M.R. Appleford, S. Oh, N. Oh, and J.L. Ong, J. Biomed. Mater. Res. Part A. 89[4] (2009) 1019-1027.
-

- 53. R.N. Granito, A.C.M. Renno, H. Yamamura, M.C. de Almeida, P.L.M. Ruiz, and D.A. Ribeiro, Int. J. Mol. and Cel. Med. 7[2] (2018) 80-90.
-

 This Article
This Article
-
2024; 25(1): 85-91
Published on Feb 29, 2024
- 10.36410/jcpr.2024.25.1.85
- Received on Nov 19, 2023
- Revised on Jan 2, 2024
- Accepted on Jan 18, 2024
 Services
Services
Shared
 Correspondence to
Correspondence to
- Servet Ahmet Doğdu
-
aIskenderun Technical University, Maritime Vocational School of Higher Education, Underwater Technologies, TR-31200, Hatay, Türkiye
bIskenderun Technical University, Faculty of Marine Sciences and Technology, Molecular Ecology and Fisheries Genetics Laboratory, TR-31200 Iskenderun, Hatay, Türkiye
Tel : +90-542-311-11-05 Fax: +90-326-613-56-13 - E-mail: servet.dogdu@iste.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.