- Synthesis of hydrophobically modified, rice husk-derived spherical silica particles
Jin Hyung Leea,*, Ji Yeon Parka,b, Jinyoung Chuna, Byoung Seung Jeona, Hye Sun Leea and Byoung-In Sangb
aKorea Institute of Ceramic Engineering and Technology, Osong 28160, Republic of Korea
bDepartment of Chemical Engineering, Hanyang University, Seoul 04763, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Rice husks are a renewable source of silicon because of their high silica content. In this study, hydrophobic spherical silica particles, which are widely used in several industries, were synthesized using rice husk-derived silica. Silica was extracted as sodium silicate using a one-pot alkali hydrothermal treatment and ball milling equipment. Spherical particles of silica were synthesized by the precipitation of sodium silicate using acetic acid and a polyethylene glycol additive; subsequently they were modified with triethoxyvinylsilane to obtain hydrophobic silica particles. The presence of C-H on the surface of the silica particles was confirmed by Fourier-transform infrared spectroscopy, which revealed the hydrophobicity of the particles. The contact angle of the modified spherical silica particles was 159°, whereas that of the unmodified silica particles was 0°. The hydrophobicity was confirmed by dispersing the particles in water. This study thus demonstrated that rice husk-derived silica can serve as an alternative to chemically derived silica; moreover, the material can be integrated into existing silica processes and used as a polymer filler.
Keywords: Rice husk-derived silica, Renewable resource, Hydrophobic silica, Lignocellulosic.
Silica plays a crucial role in various industries including rubber, plastics, coatings, and paints [1, 2]. It is a neutral filler in polymer matrices such as resins, varnishes, and silicon rubbers. The mechanical strength and thermal stability of the composites can be improved by incorporating silica into a polymer [3, 4]. The premise of its application in polymer composites is the good dispersibility of silica fillers in polymer matrices. Basically, silica contains numerous hydroxyl groups and is hydrophilic. Therefore, silica possesses a significantly different polarity compared with hydrophobic polymers and is poorly dispersed in the polymer matrix [5-7]. For application as a polymer filler, the surface of silica should be modified with appropriate substances to render it hydrophobic [8]. Organoalkoxysilanes are widely used as modifying substances in the preparation of various silica-based composites [9, 10]. After modification, silica exhibits specific physicochemical and surface properties. Its surface becomes more hydrophobic and exhibits an improved affinity to organic compounds [11], and is most suitable for use as a polymer filler.
Recently, lignocellulosic biomass has been recognized as a renewable resource that can replenish the portion depleted by consumption through natural reproduction [12]. It contains numerous biopolymers such as cellulose, hemicellulose, and lignin. In addition to carbonaceous materials, biomass-derived silica has attracted increasing interest as a renewable source of silicon-based materials [13]. Among plants, rice husk is the highest SiO2 containing plant, maximally 20% [14]. Rice husk-derived silica can be used as a low-cost precursor for Si-based materials [15-18], Rice husks are an attractive industrial resource because they are produced in large quantities as a byproduct of rice mill plants and are already dried, reducing the transportation and drying costs [19]. Recently, we developed a one-pot alkali hydrothermal and ball-milling silica extraction method from rice husks [19]. This process solubilizes silica in an alkali solution and separates it from the other components. Soluble silica can be used as a precursor for Si-based materials [20-22].
Therefore, in this study, we used rice husk as a source of silica and synthesized hydrophobic silica particles that are widely used in various industries. Silica was extracted in the form of sodium silicate from the rice husk. Notably, the traditional method for synthesizing sodium silicate involves high energy consumption and greenhouse gas emission, as well as environmental waste generation [23]. By contrast, the current process using rice husk-derived silica is a green process and may improve sustainability in silica industries.
Materials
The rice husks used in this study were supplied by a rice processing facility located in Chengju, Republic of Korea; they were harvested in 2021. Sodium hydroxide powder, acetic acid (99.5%), triethoxyvinylsilane (98.0%), and ethanol (99.5%) were purchased from Deajung Chemicals and Metals Inc. (Siheung, Republic of Korea). Polyethylene glycol (PEG) (MW 4,000) was purchased from Sigma-Aldrich (Darmstadt, Germany). Sodium hydroxide powder was dissolved in distilled water before use. The other materials were used as received without further purification.
Preparation and modification of rice husk-derived silica particles
Rice husk-derived silica particles were prepared using a method reported previously [19]. Silica was extracted from rice husks using a one-pot alkali hydrothermal treatment and ball milling. Rice husk was continuously supplied via a screw and, simultaneously, a 0.5 M NaOH solution was continuously supplied to the reactor. The rice husk was reacted at 80 ℃ with the resident time of 150 min. Silicon was extracted from the rice husks in the form of sodium silicate. The silica particles were synthesized according to the method described in our previous study [21]. Initially, PEG was dissolved in a sodium silicate solution with 0.2%(w/v), and acetic acid (2.5 mL) was added to the mixture. The mixture was stirred at 300 rpm at room temperature for 2 h. The precipitated silica particles were filtered and washed with distilled water, followed by calcination at 550 ℃ for 2 h.
Triethoxyvinylsilane and ethanol with the ratio of 1:1 were mixed at room temperature for 15 min by stirring them at 300 rpm. Silica particles were dispersed in the mixture with 2%(w/v) and stirred at 300 rpm at room temperature for 3 h using an electronic overhead stirrer (MD 3060D; MTOPS, Yangju, Korea). The modified silica particles obtained were filtered and washed with distilled water three times. The washed silica particles were then dried at 80 ℃ for 24 h. The preparation of the hydrophobic rice-husk-derived spherical silica particles is illustrated in Fig. 1.
Material characterization
Fourier-transform infrared (FT-IR) spectra for 25 scans were recorded using an FT-IR-460 Plus spectrometer (Perkin Elmer, Massachusetts, USA) in the range between 4000 cm–1 and 500 cm–1 by the KBr method. Field-emission scanning electron microscopy (FE-SEM; JEOL, JSM-7000F, Tokyo, Japan; acceleration voltage: 10.0 kV) and transmission electron microscopy (TEM; JEOL, JEM-2000EX, Tokyo, Japan; acceleration voltage: 200 kV) were used to analyze the morphology of the spherical silica particles. The water contact angles before and after the modification of the spherical silica particles were measured using a contact angle analyzer (GITSoft Co., Ansan, Republic of Korea). The spherical silica particles were deposited over a cylindrical glass holder (diameter: 1.7 cm) using a hydraulic press (Crushir digital hydraulic press; Pike technologies, Madison, USA) to obtain silica coating layers. The contact angles of the silica coating layers were determined using a contact angle analyzer connected to a personal computer. Each water contact angle measurement was conducted three times with 10 μL of distilled water. Images during the measurements were captured using an optical imaging camera (Navitar Inc., New York, USA). The contact angles were calculated from the drop images after shape accentuation, from which radius, and string readings were determined. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to study the formation of spherical particles.
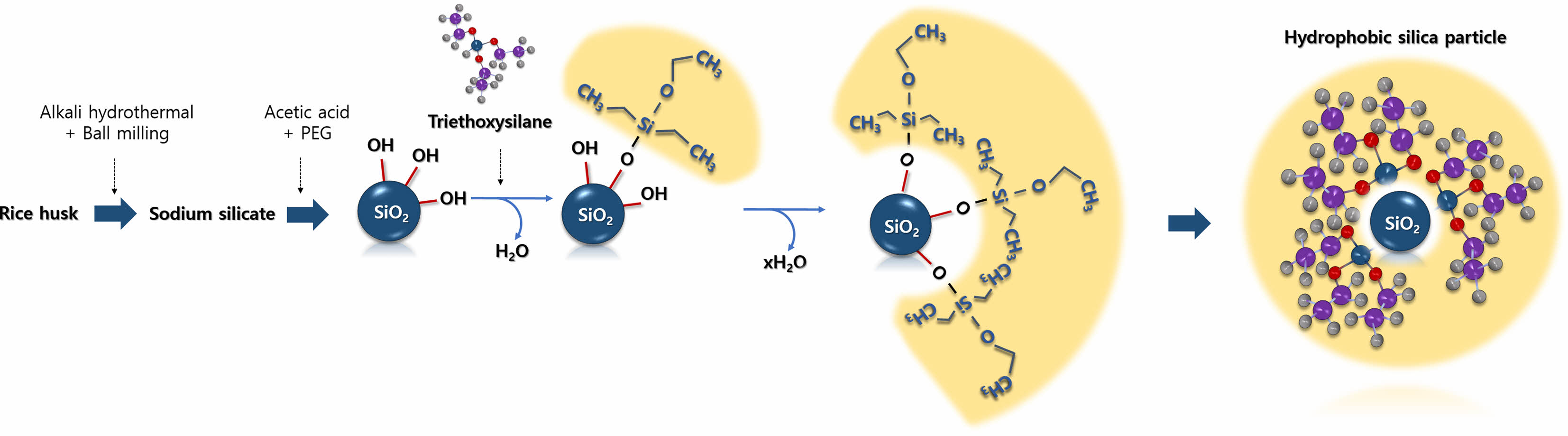
|
Fig. 1 Schematic for synthesizing hydrophobic silica particles. |
Sodium silicate extracted using a one-pot alkali hydrothermal treatment and ball milling process was used to synthesize silica particles [19]. The silica extraction yield in the one-pot alkali hydrothermal treatment and ball milling process was 86.0% at a residence time of 150 min, reaction temperature of 80 ℃ and solid content of 13% during long-term operation. Spherical silica microparticles were obtained by the precipitation of sodium silicate using acetic acid and a PEG additive. The SEM and TEM images show spherical particles with an average diameter of 780 µm (Fig. 2). The pores were not clearly visible in the TEM image (Fig. 2d), but the nitrogen adsorption isotherms indicated that mesopores that were 20 nm in size were partially developed (data not shown). The aim of this study was to develop hydrophobic silica particles using rice husk-derived silica; however, the size of the silica particles and their pore characteristics could also be controlled by adjusting the reaction temperature during precipitation [21]. When the reaction temperature was lowered, the average silica particle size increased. For instance, at a precipitation temperature of 60 ℃, the average particle size was 250 nm. Conversely, under a lower precipitation temperature of 5 ℃, the average particle size increased to 1.4 μm. The precipitation temperature, a factor influencing the nucleation rate, plays a crucial role in determining particle size. Additionally, the precipitation temperature is related to the change in the hydrophilicity of PEG, impacting pore size. Increasing the precipitation temperature from 5 to 60 ℃ resulted in an enlargement of the pore size of silica particles, ranging from less than 2 nm to approximately 10 nm.
Before modifying the surface of the spherical silica particles, the high-intensity broad absorption peak of silica at 3438 cm–1 was attributed to the stretching vibration of the hydroxyl groups of SiO2 (Fig. 3). Silica was initially hydrophilic because of the presence of hydrogen bonds between SiO2 and H2O via surface hydroxyl groups. The IR spectrum of the unmodified silica particles shows a distinct peak at 1632 cm–1 which is attributed to the bending vibration of the OH groups of the adsorbed water. After modifying the surface of the silica spherical particles with triethoxyvinylsilane, the peak at 1632 cm–1 disappeared in the modified silica particles, thus indicating change in particle polarity. In the modified silica particles, the primary C-H stretching peaks were found in the 2830-3078 cm–1 region and at 1391, 1365, and 956 cm–1. The intensity of the peak of the hydroxyl groups at 3438 cm–1 decreased considerably in the modified silica particles, compared with the unmodified silica particles, owing to the replacement of the -OH group with the Si-O linkage. The presence of C-H on the surface of the particles led to hydrophobicity of the particles. Surface modification was confirmed using the FT-IR spectroscopy.
The contact angle measured for the modified spherical silica particles was 159°, whereas that of the unmodified silica particles was 0°, as water drops spread instantly when placed on the surface of the substrate (Fig. 4a). The hydrophilicity of unmodified silica particles is due to their hydroxyl groups, which render their surfaces hydrophilic. By contrast, the-CH3 groups on the surface of the modified silica particles render them hydrophobic. Therefore, surface modification with triethoxyvinylsilane clearly changed the hydrophobicity of the silica surface. Because unmodified silica particles are attracted to water owing to their affinity for water molecules, they were dispersed in water (Fig. 4b). Some of unmodified particles, because of their weight, settled at the bottom. However, the modified silica particles were not attracted to water and tended to aggregate to minimize contact with water molecules. No modified silica settled or dispersed in the water, indicating the uniform and effective surface modification of silica particles (Fig 4b). Therefore, it can be concluded that the modified silica particles form aggregates and are suspended on the surface of the water. Increasing the hydrophobicity of silica results in improved dispersion, reduced moisture absorption, and enhanced compatibility in non-polar matrices [8]. These changes made silica applicable in industries such as paints, adhesives and rubber manufacturing [1, 2].
Sodium silicate is used in a broad range of products such as detergents, and chemical feedstocks, as well as in industrial applications pertaining to paper manufacturing, civil engineering, and adhesives [23]. Sodium silicate is generally produced through the reaction of sodium carbonate (soda ash) and silica (sand) at high temperatures in the rage of 1400-1500 ℃. This process expends 10,953 MJ of energy and releases 1,066 kg of CO2 to produce 1,000 kg of sodium silicate [23], i.e., it consumes high amount of energy, produces greenhouse gas emissions, and generates a lot of environmental waste. Sodium silicate synthesis used in this study was at a reaction temperature of 80 ℃ and the chemical reaction did not generate any greenhouse gases. Climate change has emerged as one of the most significant environmental threats facing the world. It is directly related to the increased emission of greenhouse gases, particularly carbon dioxide. A key challenge in the sustainable advance of the sodium silicate industry is the substantial generation of CO2 resulting from high energy consumption. The utilization of renewable sources and reduced energy consumption, as proposed in this study, will directly affect the environment and enhance the sustainability of the sodium silicate industry. Therefore, using rice-husk-derived silica will mitigate the environmental burden and improve sustainability of using silica. This study demonstrates that rice-husk-derived silica can be used as a raw material for industrial silica materials, similar to that of conventional silica.
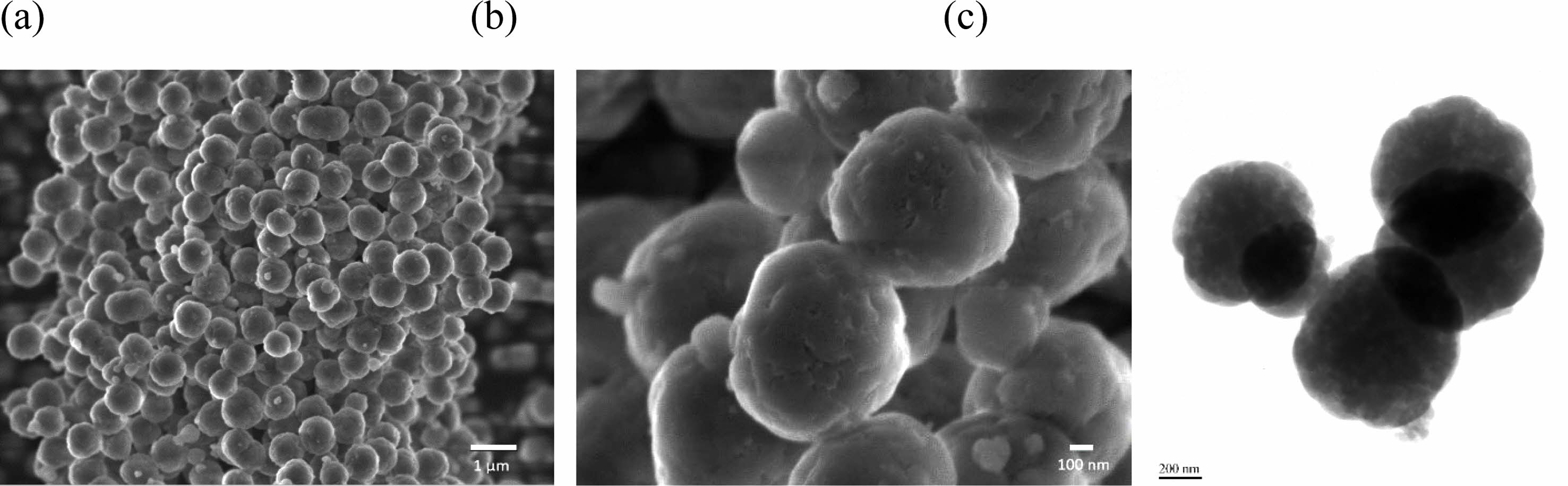
|
Fig. 2 (a), (b) SEM and (c) TEM images of precipitated silica spherical particles. |
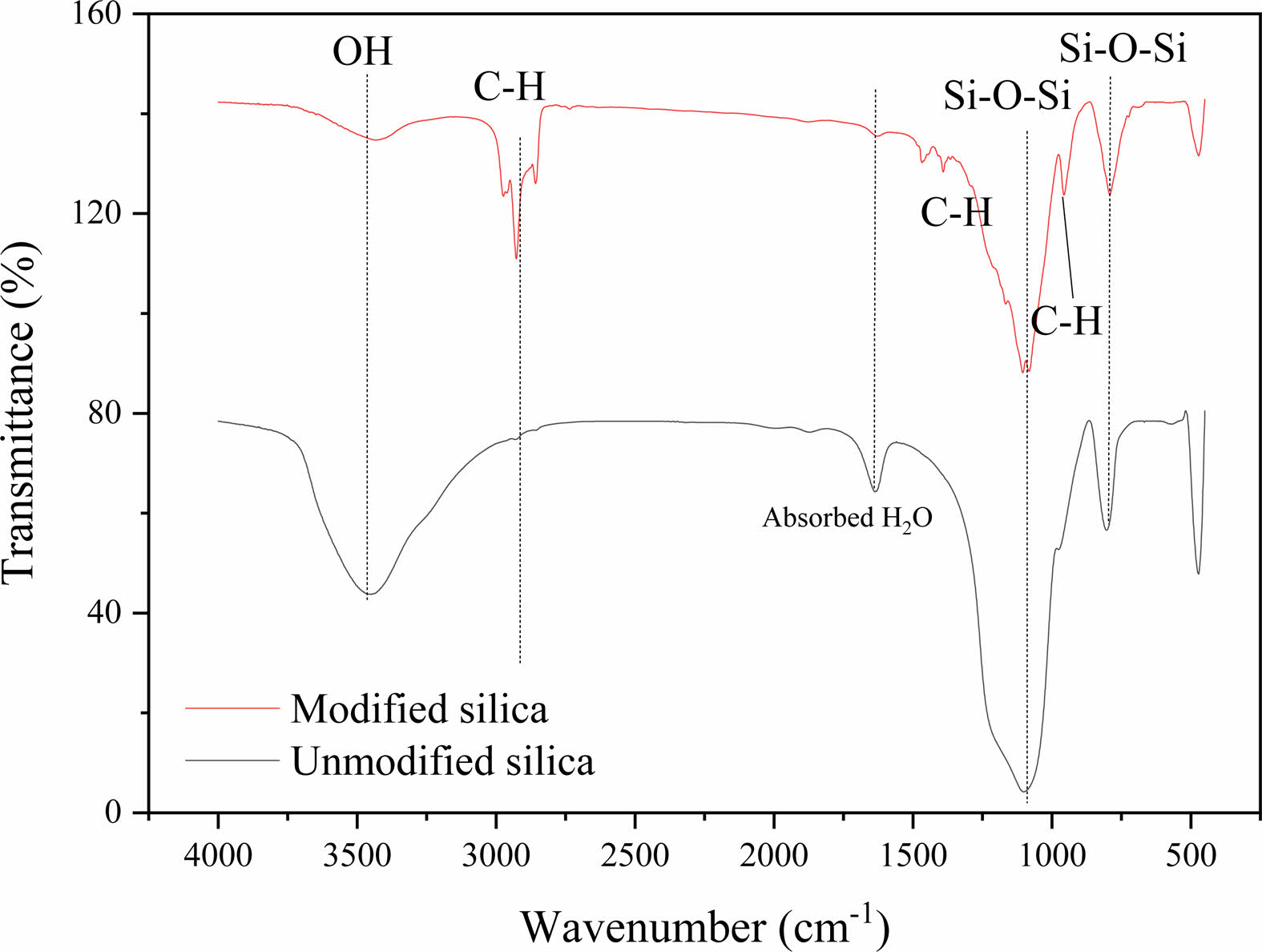
|
Fig. 3 FT-IR spectrum of surface modified and unmodified silica. |
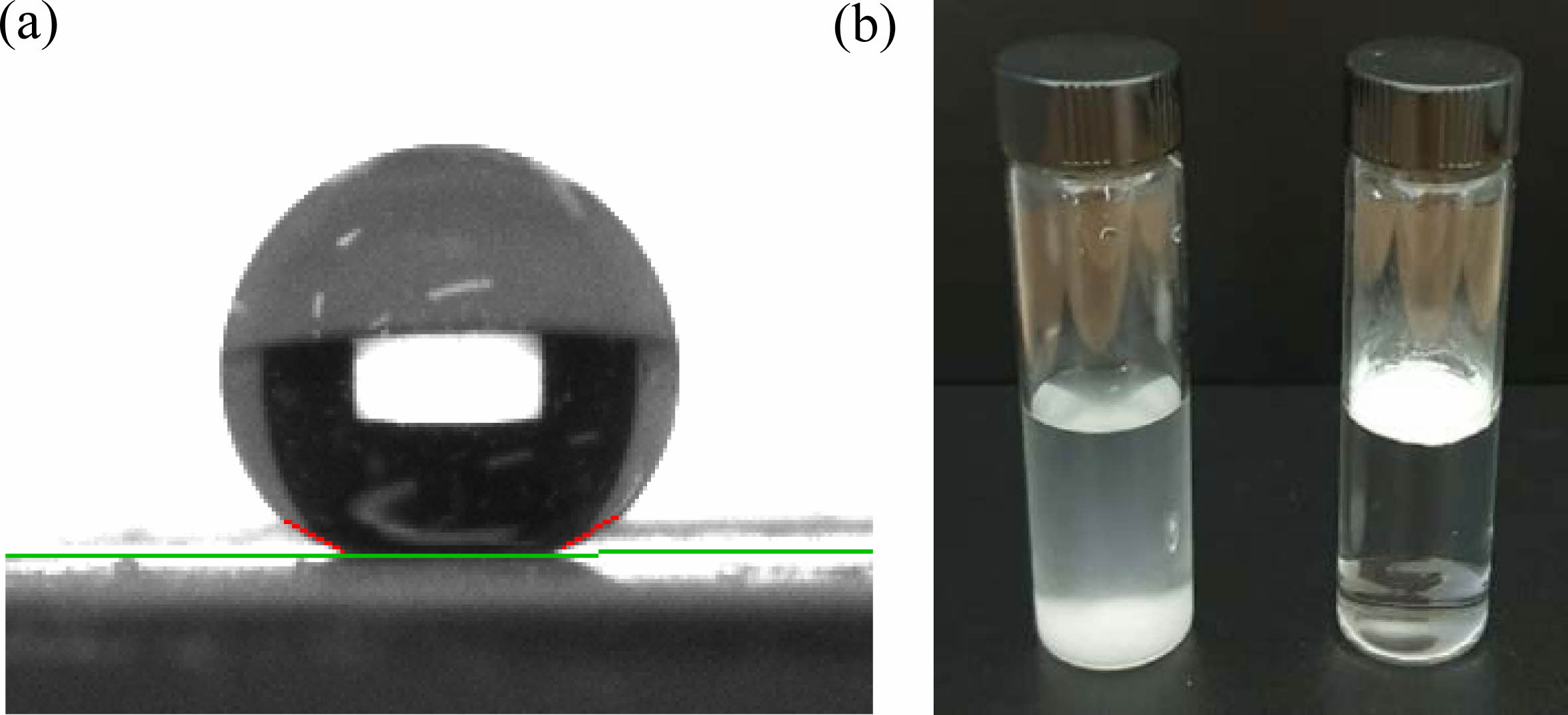
|
Fig. 4 Pictures of a water droplet deposited on a hydrophobic silica spherical particle surface obtained by powder compaction (a) and dispersions of unmodified (left) and modified (right) (b) silica particles in water. |
Herein, rice husk-derived silica was employed as the raw material to successfully produce spherical hydrophobic silica particles. Silicon in rice husks was extracted by ball milling and alkali hydrothermal processes to form sodium silicate, which was used as a precursor for hydrophobic silica particles. Sodium silicate has been used in spherical particle synthesis and surface modification processes to synthesize hydrophobic spherical silica particles. The hydrophobic spherical silica particles had a water contact angle of 159° and could be suspended in water. We believe that the results of this study underscore the feasibility of using rice husk-derived silica in industrial applications. Further studies could investigate the applicability of rice husk-derived silica to extended industrial uses.
This study was financially supported by the Korea Agency for infrastructure Technology Advancement (KAIA) through the Overseas demonstration and business model development of 6 MWth class tri-generation plant with unused renewable fuel based on modularization, which was funded by the Ministry of Land, Infrastructure and Transport in Korea (grant no. RS-2021-KA161883).
- 1. I.K. Ulfah, R. Fidyaningsih, S. Rahayu, D.A. Fitriani, D.A. Saputra, D.A. Winarto, and L.A. Wisojodharmo, Procedia Chem. 16 (2015) 258-264.
-

- 2. Z. Feng, J. Zhong, W. Guan, R. Tian, C. Lu, and C. Ding, Analyst 143 (2018) 2090-2095.
-

- 3. F. Yang, Y. Ou, and Z. Yu, J. Appl. Polym. Sci. 69 (1998) 355-361.
-

- 4. N. Suzuki, M.B. Zakaria, Y.D. Chiang, K.C.W. Wu, and Y. Yamauchi, Phys. Chem. Chem. Phys. 14 (2012) 7427-7432.
-

- 5. B.C. Guo, F. Chen, Y.D. Lei, and W.W. Chen, Polym. J. 42 (2010) 319-326.
-

- 6. S.W. Ha, C.E. Camalier, G.R.B. Jr, and J.K. Lee, Chem. Commun. 20 (2009) 2881-2883.
-

- 7. I. Mora-Barrantes, J.L. Valentín, A. Rodríguez, I. QuijadaGarrido, and R. Paris, J. Mater. Chem. 22 (2012) 1403-1410.
-

- 8. W.A. Daoud, J.H. Xin, and X. Tao, Appl. Surf. Sci. 252[15] (2006) 5368-5371.
-

- 9. E.N. Ngouangna, M.A. Manan, J.O. Oseh, M.N.A.M. Norddin, A. Agi, and A.O. Gbadamosi, J. Mol. Liq. 315 (2020) 113740.
-

- 10. B.J. Jeon, H.J. Hah, and S.M. Koo, J. Ceram. Process. Res. 3[3] (2002) 216-221.
- 11. T. Jesionowski and A. Krysztafkiewicz, Colloids Surf. A: Physicochem. Eng. Aspects, 207[1-3] (2002) 49-58.
-

- 12. Biomass research and development technical advisory committee Washington DC; Roadmap for bioenergy and biobased products in the United States, 2007.
- 13. J. Chun and J.H. Lee, Sustainability 12 (2020) 10683.
-

- 14. J. Zhou, L. Si, H. Xiao, Y. Sun, Q. Ma, M. Liu, M. Xu, J. Wang, L. Ni, and L. Wu, Agron J. 114[1] (2022) 555-564.
-

- 15. Y. Shen, Renew. Sustain. Energy Rev. 80 (2017) 453-466.
-

- 16. Y. Yun, J. Ceram. Process. Res. 23[3] (2022) 247-251.
-

- 17. D.Q. Minh, T.V. Khai, H.N. Minh, N.V.U. Nhi, and K.D.T. Kien, J. Ceram. Process. Res. 22[2] (2021) 246-251.
-

- 18. A.A.M. Fazli, S.K. Zakaria, N.I.N.A. Rahman, S.Z. Salleh, A.H. Yusoff, N.A. Salleh, M.A.A. Taib, F. Budiman, A. Ali, and P.T. Teo, J. Ceram. Process. Res. 21[6] (2020) 667-682.
-

- 19. J.Y. Park, Y.M. Gu, J. Chun, B. Sang, and J.H. Lee, Chem. Biol. Technol. Agric. 10 (2023) 102.
-

- 20. J. Chun, Y.M. Gu, J. Hwang, K.K. Oh, and J.H. Lee, J. Ind. Eng. Chem. 81 (2020) 135-143.
-

- 21. S. Kim, J.Y. Park, Y.M. Gu, I.-S. Jang, H. Park, K.K. Oh, J.H. Lee, and J. Chun, Nanoscale Adv. 3 (2021) 6965-6973.
-

- 22. I.-S. Jang, J.Y. Park, Y.M. Gu, J.H. Lee, and J. Chun, Chem. Eng. & Technol. 45 (2022) 381-384.
-

- 23. M. Fawer, M. Concannon, and W. Rieber, Int. J. LCA. 4 (1999) 207-212.
-

 This Article
This Article
-
2023; 24(6): 1066-1070
Published on Dec 31, 2023
- 10.36410/jcpr.2023.24.6.1066
- Received on Oct 18, 2023
- Revised on Nov 30, 2023
- Accepted on Dec 5, 2023
 Services
Services
- Abstract
introduction
experiment
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jin Hyung Lee
-
Korea Institute of Ceramic Engineering and Technology, Osong 28160, Republic of Korea
Tel : +82-43-913-1502 Fax: +82-43-913-1597 - E-mail: leejinh1@kicet.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.