- Synthesis of Ca14Zn6Al10O35:Mn4+, Nd3+ phosphor using starch as an impregnation medium
Soo-Jong Kim*
Department of Chemical Engineering, Halla University, 28 Halladaegil, Wonju, Gangwon State, 26404, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ca14Zn6Al10O35:Mn4+, Nd3+ phosphors were prepared by a liquid phase impregnation method. The results of XRD, SEM, and EDS analysis confirmed that pure CZA:Mn4+ and CZA:Mn4+, Nd3+ phosphors with single phases were synthesized. The Mn4+ doped phosphors exhibited strong red emission in the range of 600 to 850 nm when excited at a wavelength of 356 nm. The luminescence properties were investigated according to changes in the concentrations of Mn4+ and Nd3+ ions. The particle sizes of the phosphor when fired at 1200 °C for 3 hours were 100 to 500 nm
Keywords: Ca14Zn6Al10O35: Mn4+, Nd3+, Starch, Red phosphor.
Changes in the Earth's climate have become more serious in recent years. However, these changes in climate can be offset by growing eco-friendly crops in plant factories. Plant factories are operating in several places. In plant factories, LEDs with phosphors as key components are usually utilized [1-3]. Most phosphor materials emitting multiple wavelengths are manufactured in a reducing atmosphere [4]. However, this study aimed to develop Ca14Zn6Al10O35:Mn4+, Nd3+ red fluorescent materials that can be easily manufactured in air. The wavelength emitted by Ca14Zn6Al10O35:Mn4+ red phosphor is 660 nm, and its irradiation to plants increases the efficiency of photosynthesis, which greatly promotes plant growth [5, 6]. To synthesize phosphor particles, various liquid synthesis methods such as sol-gel method, hydrothermal synthesis method, microemulsion method, and precipitation method have been attempted [7-11]. In this synthesis method, a precursor precipitation process is essentially applied. This precipitation operation uses a large amount of solvent, and metal hydroxide precipitate is obtained in the form of metal hydroxide salt. Since metal oxide nanoparticles are manufactured through filtration, dehydration, and heat treatment of precipitated metal hydroxide, a large amount of waste solvent generated during this process must be recovered or treated, and the cost incurred at this time becomes a problem [12]. Therefore, the most important point in these various liquid synthesis methods is to reduce manufacturing costs by using a method that is simple in the process and does not generate waste water solutions. In this study, Ca14Zn6Al10O35:Mn4+, Nd3+ phosphor precursors were prepared by impregnating starch, which is a natural polymer with a microfibril structure, with an aqueous solution of metal salts such as Ca, Zn, Al, Mn, and Eu. This precursor was dried and calcined to synthesize phosphor particles. Since the shape of the generated Ca14Zn6Al10O35:Mn4+, Nd3+ phosphor particles is determined by the microstructure of starch, the crystal shape and particle size can be controlled [13]. This synthesis method does not involve using large amounts of solvents, such as precipitation processes, nor does it involve alkaline aqueous solutions. In addition, phosphors can be manufactured at firing temperatures lower than the solid phase method [14, 15]. Therefore, there is no need for solvent recovery or treatment, and it is possible to obtain uniform phosphor particles at a relatively low temperature [16]. By synthesis method used in this study is heat treated by impregnating a hydrate of a metal salt or metal salt in a polymer matrix to produce the ceramic fine particles, it is possible to easily manufacture the ceramic multi-component particles [17]. The starch, pulp and crystalline cellulose used as a cellulose source in the impregnation operation and there are microfibrillar structure and molecular fine structure of a size below few hundreds nm. In the impregnation process, metal salt aqueous solution ions enter and settle in an empty space between these molecular chains. Therefore, the shape of the particles produced after the heat treatment is a powder which particle size is uniform, depending on the microstructure of the starch, pulp etc. This synthesis method can easily control the particle size from different types of the matrices such as starch, pulp and crystalline cellulose, or by changing the firing conditions [18]. And it has the advantage that you do not use strong alkaline organic solvents used in other synthesis methods, such as co-precipitation method. Ca14Zn6Al10O35:Mn4+, Nd3+ phosphors can generate wavelengths of 650 to 770 nm and emit far-infrared rays that can match the absorption spectrum of plant pigments, so they are applied to indoor plant cultivation [19, 20]. Ca14Zn6Al10O35: Mn3+, Nd3+ phosphors produced by the synthesis method used in this study emit wavelengths similar to those of sunlight. Therefore, they can provide an environment suitable for plant growth.
The samples were prepared by the liquid phase impregnation method. The raw materials were Ca(NO3)2·4H2O, Mn(NO3)2·6H2O, Al2(NO3)3·9H2O, Zn(NO3)2·6H2O and Nd(NO3)3·6H2O. All reagents were analytically pure and used without further purification. Nitrates were used as metal salt starting materials, and aqueous solutions were prepared to have a predetermined concentration. The % concentration of the aqueous solutions of Al2(NO3)3·9H2O and Mn(NO3)2·6H2O was 15%, Ca(NO3)2·4H2O and Zn(NO3)2·6H2O was 20%, and Nd(NO3)3·6H2O was 2.25%. All the solutions were added to beakers and mixed for 2 hours while stirring with a magnetic stirrer. Then each mixed aqueous solution was poured into starch powder. The starch and each precursor solution were impregnated at a weight ratio of 1:1.3. After standing for 2 h, the impregnated mixtures were dried at 120 °C for 3 hours to obtain precursors. The dried precursors were calcined in an electric furnace at 500 °C for 1 hour to remove the organic components in the precursor and the starch. The calcined samples were pulverized with agate and then calcined in an electric furnace at a temperature range of 600 °C to 1,200 °C (heating rate: 5 °C/min) for 1 to 3 hours. The calcined samples were pulverized to obtain the final Ca14Zn6Al10O35: Mn3+, Nd3+ powder. The x-ray diffraction pattern was investigated using the PANalytical X'Pert Pro X-ray diffractometer at UC Davis. Cu-Kα with a Ni filter was used to measure at a scanning speed of 5°/min, an acceleration voltage of 40 kV, and an acceleration current of 30 mA at a diffraction angle (2θ) range of 10 to 80°. FE-SEM (JEOL:JSM-7610F) was used to analyze the surface structure of the prepared Ca14Zn6Al10O35: Mn3+, Nd3+ powder.
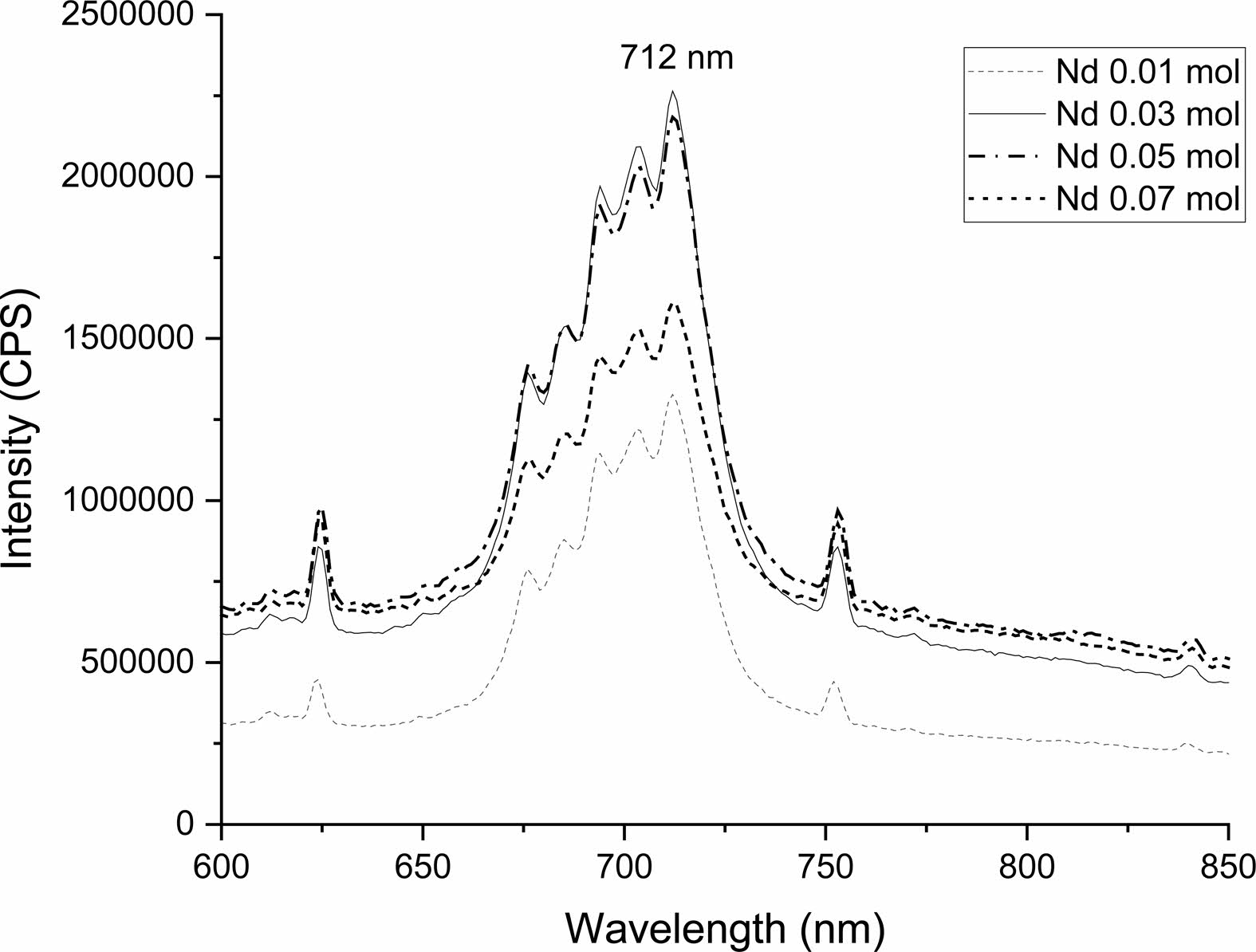
The crystal structure of Ca14Zn6Al10O35 is a cubic symmetrical lattice structure, a = 1.4868 nm, and the spatial group is F23 [21]. Fig. 1 shows the XRD patterns of the Ca14Zn6Al10O35:Mn samples obtained by firing at 1,000 °C and 1,200 °C for 3 hours. The XRD pattern is shown according to the change in concentration (0.03, 0.05, 0.07, and 0.1 mol%) of the Mn ions used as an activator. It has been reported that Ca14Zn6Al10O35 synthesized by the solid phase method has calcium oxide (CaO) as an impurity [19]. However, it can be seen from the XRD pattern that pure Ca14Zn6Al10O35 without calcium oxide was synthesized using the liquid precursor method in this study. All diffraction peaks coincide with the pattern of Ca14Zn6Al10O35 (JCPDS 50-0426), and there is no change in the crystal structure according to the concentration change of Mn [22]. In general, the phase purity of both the host lattice and the activated ion affects the emission characteristics of the phosphor. Mn4+ affects optical absorption behavior, and CZA host lattice affects both the chemical stability and optical properties of the phosphor. CZA:Mn4+ and CZA:Mn4+, Nd3+ phosphors can be used as phosphors emitting wavelengths in the red region in LED lighting for plant factories. Since the wavelength in the 650-750 nm region emitted by CZA:Mn4+ and CZA:Mn4+, Nd3+ phosphors is close to the maximum absorption wavelength of chlorophyll, the main photoreceptive pigment of plants, it is efficiently absorbed by plants to promote photosynthesis. Fig. 2 shows each XRD pattern according to the change in concentration (0.03, 0.05, 0.07, and 0.1 mol%) of Nd, a co-activator, while the Mn concentration is fixed at 0.07 mol. No changes in the diffraction peaks were observed according to the change in the concentration of Nd. Fig. 3 shows SEM photographs according to the change in the amount of Mn and Nd added. It was found that the change in the mol ratio of Mn and Nd did not affect the shape or size of the particles. Fig. 4 shows the EDS spectrum, electron image, and element analysis results of the Ca14Zn6Al9.86O35:Mn0.074+, Nd0.073+ samples fired at 1,200 °C for 3 hours. In the electron image, nanometer-sized phosphor particles in the form of flakes were observed. In the EDS spectrum, peaks of all elements constituting the phosphor compound were observed. Peaks specifying a sharp and strong intensity of Zn, Al, Ca, and O atoms and peaks of Mn and Nd were also observed, indicating that pure Ca14Zn6Al9.86O35:Mn0.074+, Nd0.073+ nanoparticles were generated. When used in optical devices such as LEDs, the uniformity of particle size and shape of the phosphor affects the optical properties [23, 24]. In the case of YAG:Ce phosphor, it was reported that the more uniform the particle size and shape, the higher the brightness. And particle size plays an important role in the packaging performance of phosphor-converted white LEDs [25]. The weight ratio of the phosphor particle components was 11.27 wt% of Ca, 34.72 wt% of O, 6.48 wt% of Al, and 30.9 wt% of Zn. In EDS analysis, if C is about 5.0%, the sample is considered to not contain carbon at all. In the EDS analysis, C was usually about 15%. Fig. 5 shows the results of the photoluminescence (PL) measurement of the Ca14Zn6Al10O35:Mn4+, Nd3+ powder fired at 1,200 °C. The powder was excited with an Xe discharge lamp. The Mn4+ ion used as an activator serves as a luminescent center, and the Nd3- ion used as a co-activator serves to compensate for the charge of the Mn4+ ion. Among the phosphors used as LED light sources in plant factories, ZnGa2O4:0.02Cr3+ has been reported to emit wavelengths in the 650-750 nm range [26]. Phosphors that emit wavelengths in the 650-750 nm range have a significant effect on the chlorophyll of plants and promote growth [27]. The excitation spectrum of Ca14Zn6Al10O35:Mn4+, Nd3+ was obtained by fixing the emission wavelength at 712 nm, and the emission spectrum was measured by fixing the excitation wavelength at 356 nm. Both red emission from Mn⁴⁺ and near infrared (NIR) emission from Nd3⁺ were observed from the Ca14Zn6Al10O35:Mn4+, Nd3+ phosphors. The emission spectrum of Ca14Zn6Al10O35:Mn4+, Nd3+ appeared in a wavelength range between 600 and 850 nm, and the maximum emission peak was 712 nm. Mn4+ ions are excited into their charge transfer or 4T1, 4T2 states by light irradiation. The excited Mn4+ ions rapidly relax to the 2E state in the metastable state. In addition, energy transfer occurs for Mn4+ ions from the 2E state to 4A2 (698-712 nm), and for Nd3+ ions, energy transfer occurs from the 4I9/2 state to 4F9/2, 4F7/2, and 4S3/2, respectively [28-30]. Spin-forbidden transitions are transitions that occur in the triplet state. Spin-forbidden transitions emit light of lower energy than the wavelength of light emitted by spin-allowed transitions. Excitation into the absorption band at 356 nm gives an intense broad red emission around 712 nm originating from the 2E →4A2 spin-forbidden transition of Mn4+ ions in Ca14Zn6Al10O35:Mn4+, Nd3+ phosphors. The wavelength of light emitted from the spin-forbidden transition from the 2E state to the 4A2 state of the Mn ion in Ca14Zn6Al10O35:Mn4+, Nd3+ phosphors is 650-750 nm. Ca14Zn6Al10O35:Mn4+, Nd3+ phosphor have a spectral range of 650 to 750 nm that allows efficient cultivation of indoor plants, so they are applied to artificial lighting for plant factories.
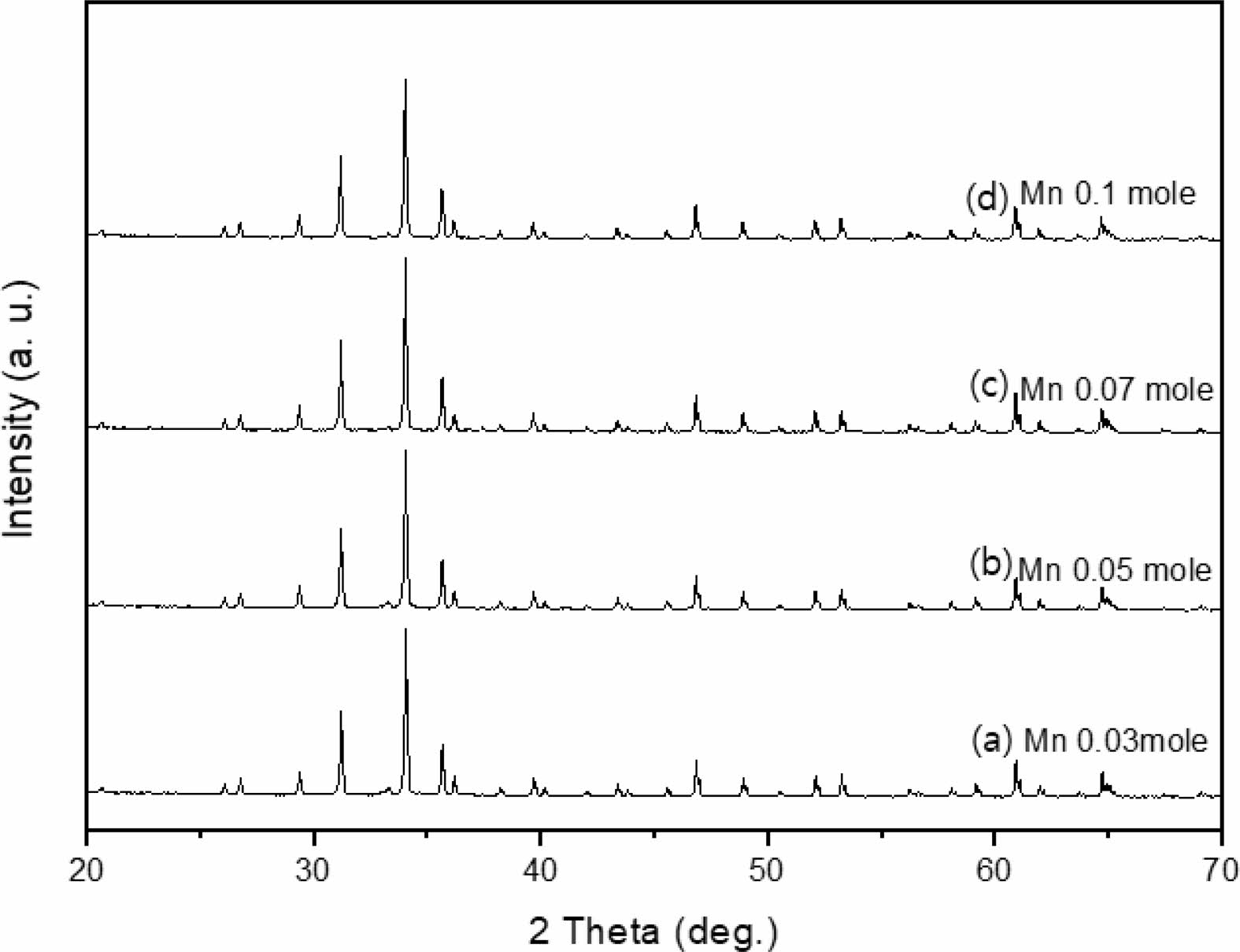
|
Fig. 1 XRD patterns of Ca14Zn6Al9.92O35:Mn0.073+ phosphors with various Mn content, (a) 0.03 mole, (b) 0.05 mole, (c) 0.07 mole, and (d) 0.1 mole. |
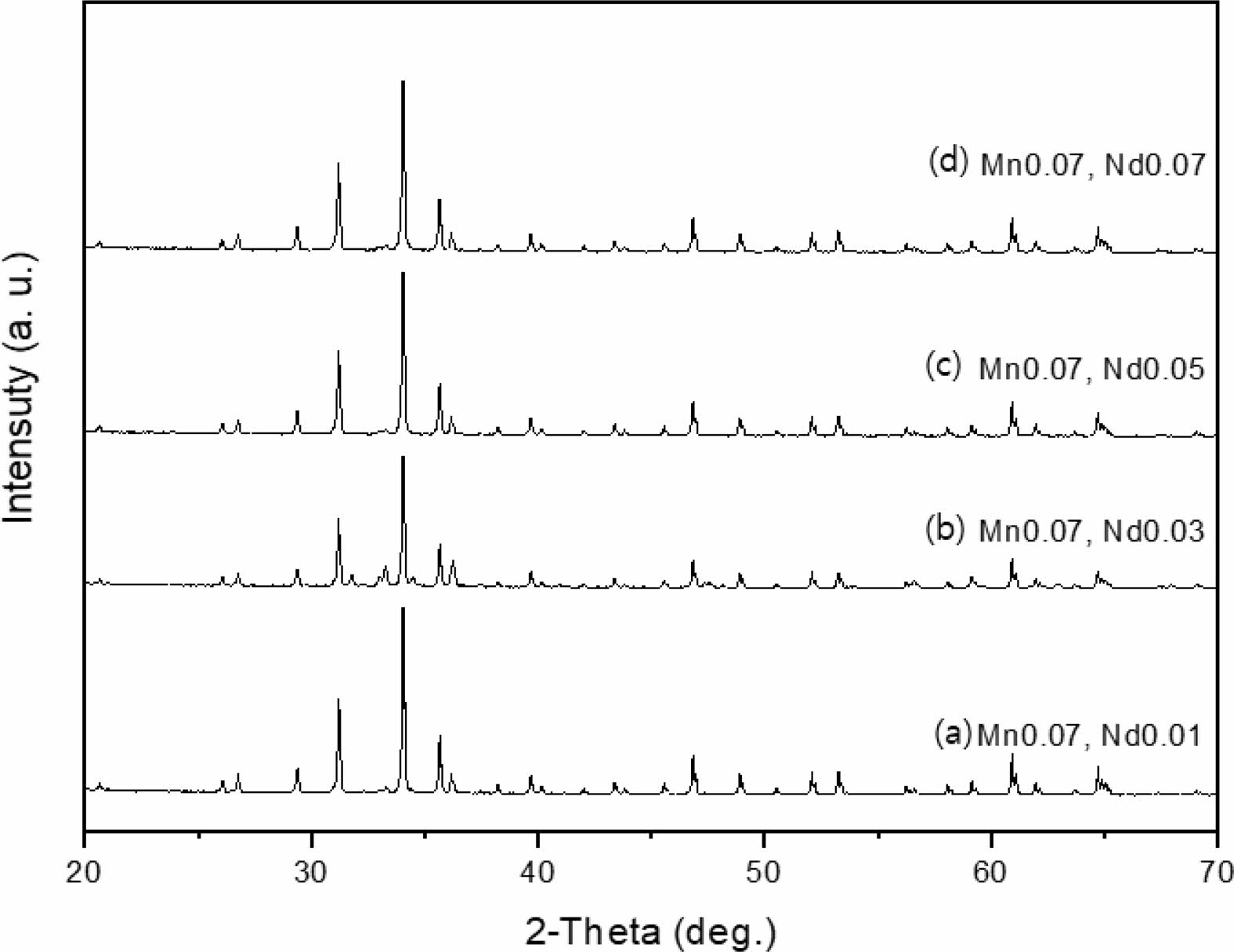
|
Fig. 2 XRD patterns of Ca14Zn6Al9.92O35:Mn0.073+, Nd0.013+ phosphor powders with various Nd content, (a) 0.03 mole, (b) 0.05 mole, (c) 0.07 mole, and (d) 0.1 mole, Mn was fixed 0.07 mole for each case. |
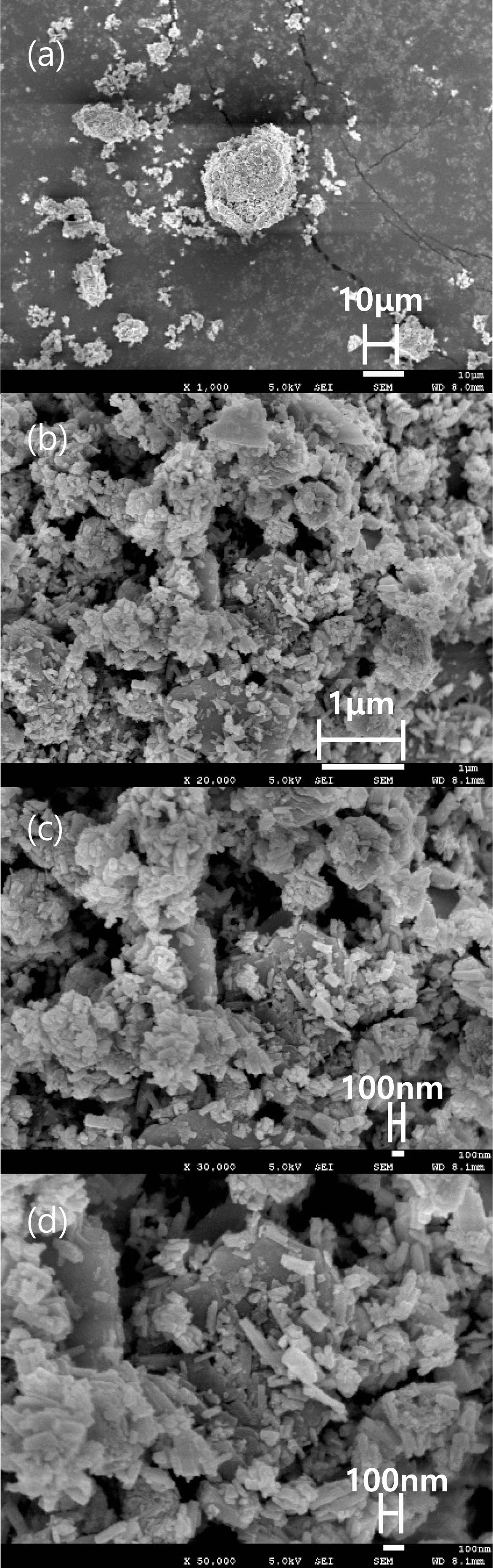
|
Fig. 3 SEM photograph of a Ca14Zn6Al9.93O35: Mn0.073+, Nd0.073+ phosphor fired at 1200 °C for 3 hours, multiplied by each (a)1,000, (b) 20,000, (c) 30,000, and (d) 50,000. |
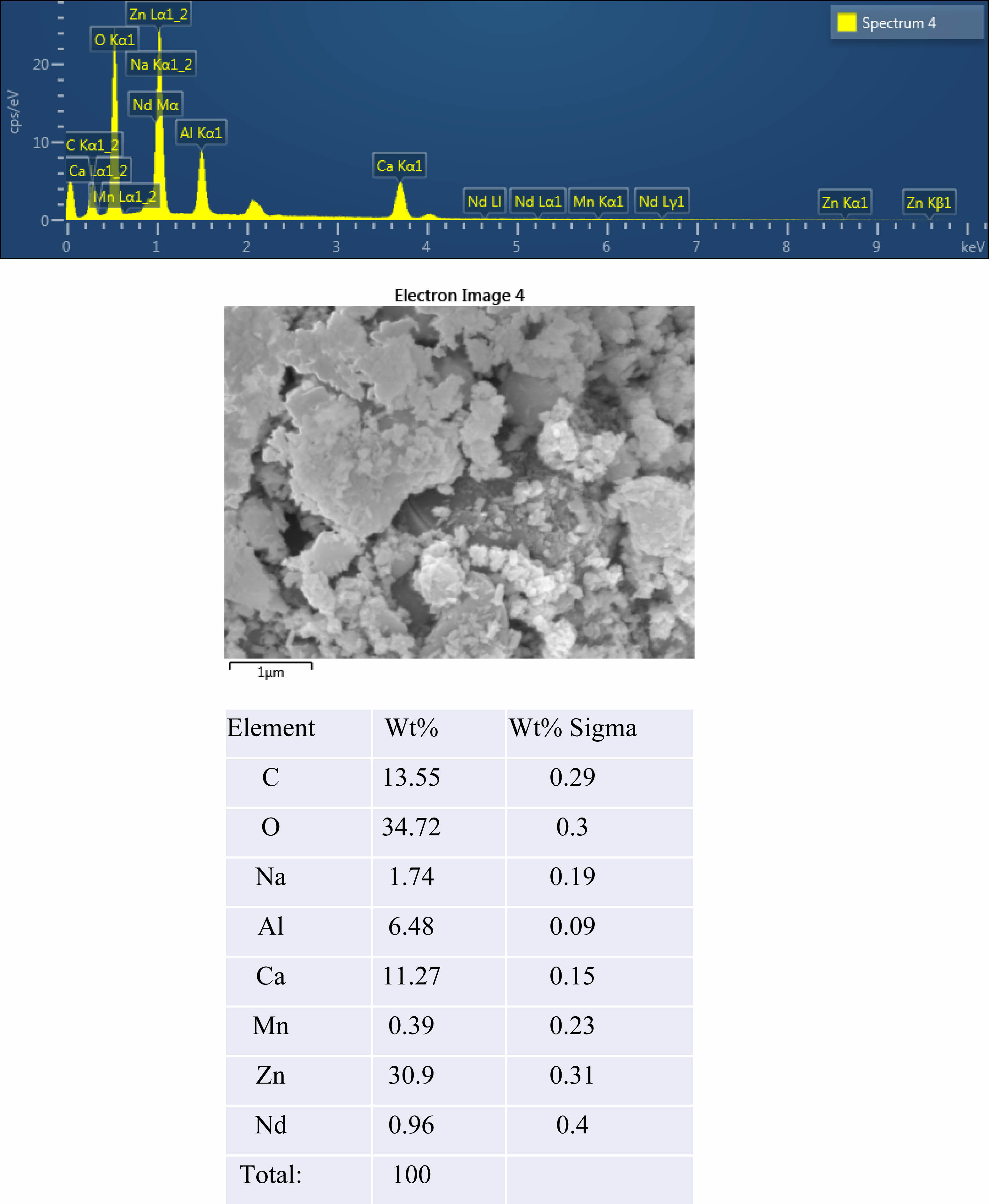
|
Fig. 4 EDS spectrum, SEM image and atomic Wt% of prepared Ca14Zn6Al9.93O35:Mn0.073+, Nd0.013+ phosphors at 1,200 ℃ |
|
Fig. 5 EDS spectrum, SEM image and atomic Wt% of prepared Ca14Zn6Al9.93O35:Mn0.073+, Nd0.013+ phosphors at 1,200 ℃. |
A precursor aqueous solution was prepared using Ca, Zn, Al, Mn, and Nd metal nitrates, with Mn and Nd as activators. This solution was used to synthesize a CZA:Mn4+, Nd3+ phosphor by the liquid precursor method. Water-soluble starch (C6H10O5)n was used as an impregnation medium. The surface structure, crystallinity, and luminescence characteristics of the synthesized CZA:Mn4+,Nd3+ phosphor were analyzed. The results of the PL measurement showed that a deep red phosphor with an emission wavelength in the near-infrared region of 600 to 850 nm was successfully synthesized. The Ca14Al10Zn6O35:Mn4+, Nd3+phosphors can be excited by light irradiation and exhibit far‐red emission due to the Mn4+ spin‐forbidden 2E to 4A2 transition. The XRD analysis results confirmed that pure CZA:Mn4+ and CZA:Mn4+,Nd3+ phosphor with a single phase were synthesized. The results of SEM and EDS analysis showed that the synthesized Ca14Zn6Al9.93O35:Mn4+0.07,Nd3+0.07 phosphor powder had a uniform particle size with flake-shaped particles ranging from 100 to 500 nm. It was also confirmed that Mn and Nd ions used as activators were present.
This work was supported by a research grant from 2023 Halla University.
- 1. S.R. Bhelave, A.R. Kadam, A.N. Yerpude, and S.J. Dhoble, Luminescence. 38[4] (2023) 279-388.
-

- 2. W. Lü, W. Lv, Q. Zhao, M. Jiao, B. Shao, and H. You, Inorg. Chem. 53[22] (2014) 11985-11990.
-

- 3. S. Fang, T. Lang, M. Cai, and T. Han, J. Alloys Compd. 902 (2022) 163825.
-

- 4. K. Mori, Y. Kojima, and N. Nishimiya, Funct. Mater. Lett. 5[2] (2012) 1260012.
-

- 5. Z. Liu, G. Shao, W. Chen, G. Hu, L. Shen, Y. Cheng, X. Liang, and W. Xiang, Opt. Mater. Express. 8[9] (2018) 2532-2541.
-

- 6. Y. Zhong, S. Gai, M. Xia, S. Gu, Y. Zhang, X. Wu, J. Wang, N. Zhou, and Z. Zhou, Chem. Eng. J. 374 (2019) 381-391.
-

- 7. J. Lian, P. Liang, B. Wang, and F. Liu, J. Ceram. Process. Res. 15[6] (2014) 382-388.
-

- 8. H. Terraschke, M.F.T. Meier, Y. Voss, H. Schonherr, and C. Wickleder, J. Ceram. Process. Res. 16[1] (2015) 59-63.
-

- 9. X. Niu, J. Xu, Y. Zhang, Y. Chu, B. Yang, and C. Zhang, J. Ceram. Process. Res. 16[5] (2015) 609-613.
-

- 10. B.S. Kim, M.J. Lee, T. Abe, T. Masaki, Y.H. Song, K. Toda, and D.H. Yoon, J. Ceram. Process. Res. 17[4] (2016) 286-289.
-

- 11. Y.A. Roh, Y.H. song, T. Masaki, and D.H. Yoon, J. Ceram. Process. Res. 17[4] (2016) 300-303.
-

- 12. S.J. Kim and H.S. Kwon, J. Korean Inst. Electr. Electron. Mater. Eng. 20[8] (2007) 671-679.
-

- 13. Y.L. Song, S.H. Choi, S.J. Kim, Y.H. Song, T. Masaki, and D.H. Yoon, J. Ceram. Process. Res. 17[3] (2016) 202-204.
-

- 14. S.J. Kim and C.H. Han, J. Ceram. Process. Res. 19[2] (2018) 130-133.
-

- 15. G. Du, W. Guo, J.M.K. Al-zyadi, Y. Han, P. Liu, and Z. Liu, J. Nanopart. Res. 15 (2013) 1-8.
-

- 16. S.J. Kim, T. Masaki, S.H. Choi, and D.H. Yoon, J. Ceram. Process. Res. 17[4] (2016) 338-343.
-

- 17. S.J. Kim, J. Ceram. Process. Res. 22[1] (2021) 74-78.
-

- 18. S.J. Kim and C.H. Han, J. Ceram. Proc. Res. 19[2] (2018) 130-133.
-

- 19. N. Zhou, L. Liu, Z. Zhou, and Y. Zhang, J. Am. Ceram. Soc. 103[3] (2020) 1798-1808.
-

- 20. L. Li, H. Li, Z. Wu, G. Tian, Y. Wang, F. Ling, S. Jiang, G. Xiang, X. Zhou, and J. Xue, J. Lumin. 238 (2021) 118286.
-

- 21. K. Seki, K, Uematsu, K, Toda, and M. Sato, Chem. Lett. 43[8] (2014) 1213-1215.
-

- 22. T. Hasegawa, S.W. Kim, T. Abe, S. Kumagai, R. Yamanashi, K. Seki, K. Uematsu, K. Toda, and M. Sato, Chem. Lett. 45[9] (2016) 1096-1098.
-

- 23. W.N. Wang, W. Widiyastuti, T. Ogi, and W. Lenggoroe, Chem. Mater. 19[7] (2007) 1723-1730.
-

- 24. Y.J. Hua, H.P. Ma, C. Zhang, D.G. Deng, S.L. Zhao, L.H. Huang, et al. Chin. J. Lumin. 34[4] (2013) 427-432.
-

- 25. H.I. Won, H.H. Nersisyan, C.W. Won, and K.H. Lee, Mater. Chem. Phys. 129[3] (2011) 955-960.
-

- 26. C. Zhou, L. Peng, Z. Kong, M. Wu, M.S. Molokeev, Z. Zhou, J. Wang, and M. Xia, J. Mater. Chem. C 10 (2022) 5829-5839.
-

- 27. X. Kang, W. Yang, D. Ling, C. Jia, and W. Lü, Mater. Res. Bull. 140 (2021) 111301.
-

- 28. X. Gao, W. Xia, T. Chen, X. Yang, X. Jin, and S. Xiao, RSC Adv. 6 (2016) 7544-7552.
-

- 29. M.G. Hur, S.H. Choi, S.B. Kwon, T. Masaki, K. Toda, and D.H. Yoon, Ceram. Int. 44 (2018) 15868-15872.
-

- 30. Z. Liao, H. Xu, W. Zhao, H. Yang, J. Zhong, H. Zhang, Z. Ni, and Z.K. Zhou, Chem. Eng. J. 395 (2020) 125060.
-

 This Article
This Article
-
2023; 24(6): 963-967
Published on Dec 31, 2023
- 10.36410/jcpr.2023.24.6.963
- Received on Aug 16, 2023
- Revised on Oct 18, 2023
- Accepted on Oct 28, 2023
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Soo-Jong Kim
-
Department of Chemical Engineering, Halla University, 28 Halladaegil, Wonju, Gangwon State, 26404, Korea
Tel : +82-33-760-1237 Fax: +82-33-760-1290 - E-mail: sjkim@halla.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.