- Synthesis of AlON powders through a polymerization template approach by spark plasma sintering
Zhihui Dinga, Wemshi Zhengb, Fei Chenc, Ik Jin Kimd, Wulong Liue, James F. Shackelfordf, Hyoung-Won Song, Tadachika Nakayamaf, Sukyoung Kimh,j, Young-Hwan Hani,* and Jooseong Kimh,j,*
aMilky-Way Sustainable Energy Materials Technology Ltd., Zhuhai, 519000, People’s Republic of China
bSchool of Architecture and Construction, Guangzhou University, 510006, China
cState Key Lab of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, People’s Republic of China
dInstitute for Processing and Application of Inorganic Materials (PAIM), Department of Materials Science and Engineering, Hanseo University, #46, Hanseo 1-ro, Haemi-myun, Seosan-si, Chungnam, 31962, Korea
eNingbo Toupu Industrial Automation Co. Ltd, No.268, Yuwangshan Road, Beilun, Ningbo, People’s Republic of China
fDepartment of Materials Science and Engineering, University of California at Davis, Davis, CA, USA
gExtreme Energy‐Density Research Institute, Nagaoka University of Technology, 940-2188 Nagaoka, Niigata, Japan
hSchool of Materials Science and Engineering, Yeungnam University, 280 Daehak-ro, Gyeongsan, Gyeongbuk 38541, Republic of Korea
iWuhan University of Technology, International School of Materials Science and Engineering, 122 Luoshi, Hongshan, Wuhan 430070, People’s Republic of China
jHudensBio Co., Ltd, Gwangju, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
As a new wet chemical method, polymer template strategy uses monomer polymerization to form a continuous carbon network. The carbon skeleton can make the carbothermal reduction reaction take place in the original, thus preventing the excessive agglomeration of grains. The Al2O3 / C ceramic precursor were obtained by polymer template strategy, and then AlON powders with 3 µm size were synthesized through a spark plasma sintering (SPS) method at 1,650 °C. We confirmed that a continuous network of carbon chains was formed by polymerization to encapsulate the alumina powder, so as to reduce the contact growth of the grains during the high-temperature carbothermal reaction. We established that, when the mass ratio of carbon source to alumina was 1.6:10, the pure AlON powder could be prepared by calcining at 1,650 °C for 20 min in a flowing nitrogen atmosphere
Keywords: SPS, AlON powders, Ceramic precursor, Carbothermal nitridation
Alumina nitride (γ-AlON) is a stable eutectic phase of Al2O3-AlN, which has a spinel-like microstructure [1]. Its excellent light transmittance [2], high hardness [3] and strength, and high chemical stability are mainly used in extreme fields, such as transparent armor [4], military aircraft lens [5], missile domes [6], infrared and laser windows [7], super hemispherical domes, and semiconductor processing. So far, the most commonly used preparation processes of transparent AlON ceramics are powder synthesis, ball milling, green body molding, and ceramic sintering [8, 9]. The synthesis methods of powders include solid state reaction, aluminothermic reduction nitridation, and carbothermal reduction nitridation (CRN) [10]. Because of its low cost, the combination of carbothermal nitriding and pressure-less sintering is the most convenient method, which makes it possible to commercialize large-scale production of high-transparency AlON ceramics.
AlON powder synthesized by the CRN method can be divided into physical dry mixing, and wet chemical mixing. The dry process is to mix raw powder by ball milling, and then calcining [11]. However, these methods are confronted with obstacles, such as high price and difficult implementation, which prompted the research and development of the wet chemical method to synthesize AlON powder [12]. The wet chemical method disperses the raw materials in the solvent using coprecipitation or gel to achieve molecular level mixing [13]. At present, the wet chemical method shows the best performance [14, 15]. Due to the high synthesis temperature (of above 1,700 °C), AlON powders synthesized by the CRN method also have large grain sizes and high agglomeration. The large size of these powders seriously affects their sintering properties, and makes it difficult to prepare high-transparency ceramics [16, 17]. In contrast, smaller particle sizes help to improve the densification rate by improving the surface diffusion. Through this diffusion, mass transfer significantly promotes densification, especially in the initial and intermediate stages of sintering [18]. Therefore, the key to preparing high-transparency AlON ceramics is to obtain high purity, small average particle size, narrow particle size distribution with good dispersion. In order to achieve this goal, a variety of methods have been used, such as the ball milling method, and the optimized CRN method with core-shell structure of Al2O3/C as raw material.
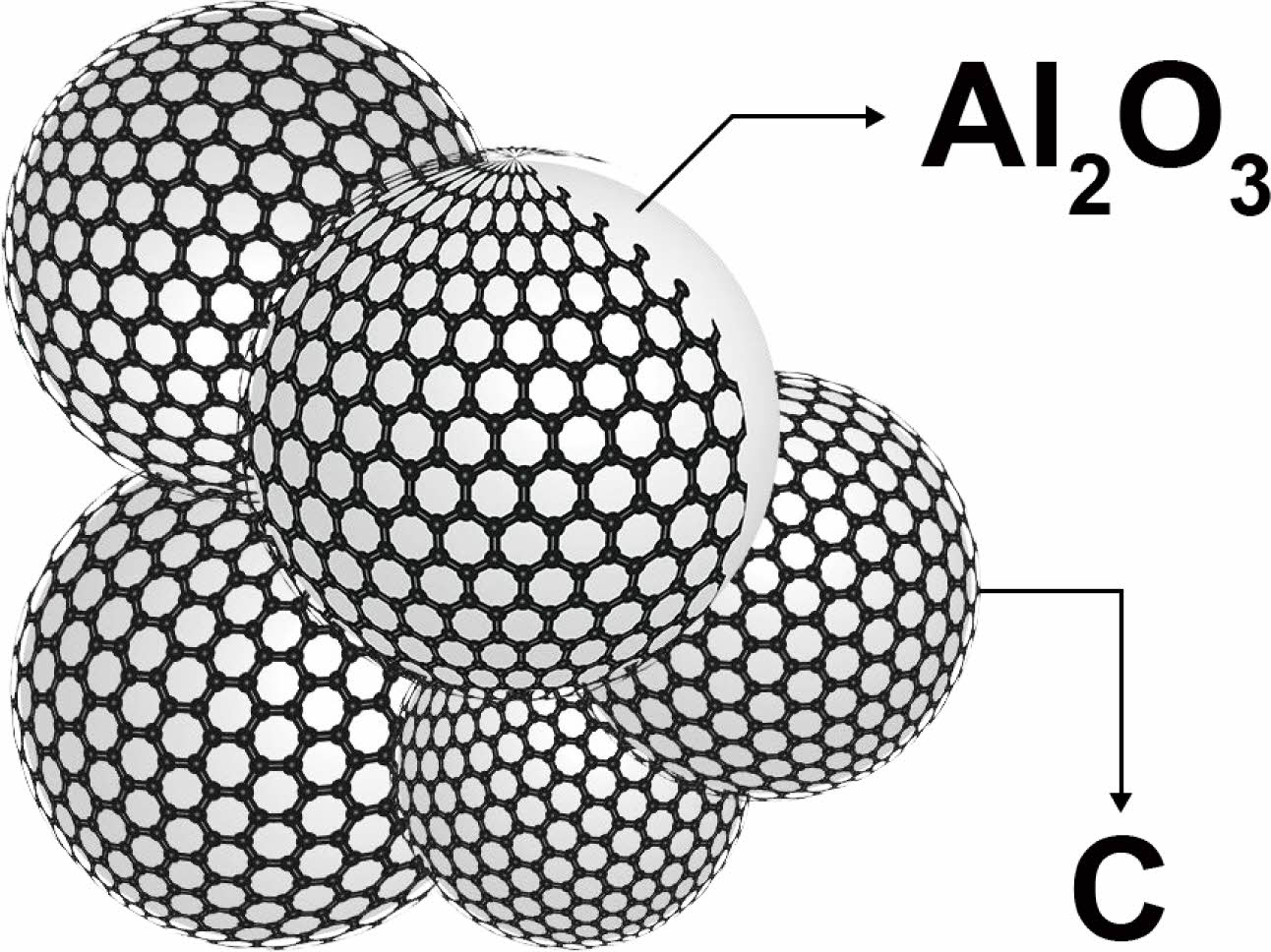
This communication shows that the Polymer-templating strategy as a new wet chemistry method is an effective method for the synthesis of ultra-fine AlON powders at low temperature. The polymer templating strategy uses monomer polymerization to form a continuous carbon network [19], and Al2O3 can be embedded in the continuous carbon network (Fig. 1 shows Al2O3/C). In the process of carbothermal nitriding, the alumina particles can be fixed in their original position to prevent the particles from agglomerating and growing in size, so as to obtain the powder with small size, low agglomeration, and uniform particle dispersion. The carbothermal nitriding reaction equation is:
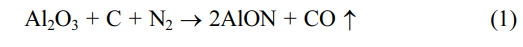
|
Fig. 1 Schematic diagram of Al2O3/C Structure. |
Al2O3 (98%) was used as the source of Al2O3. Chitosan (CS: deacetylation {{{EQUATIONS}}}95%, Viscosity (100-200) mPa·s) was used as polymerizable monomer, and the carbon source as well. Glutaraldehyde (GR, 50% in H2O) was used as a crosslinking agent.
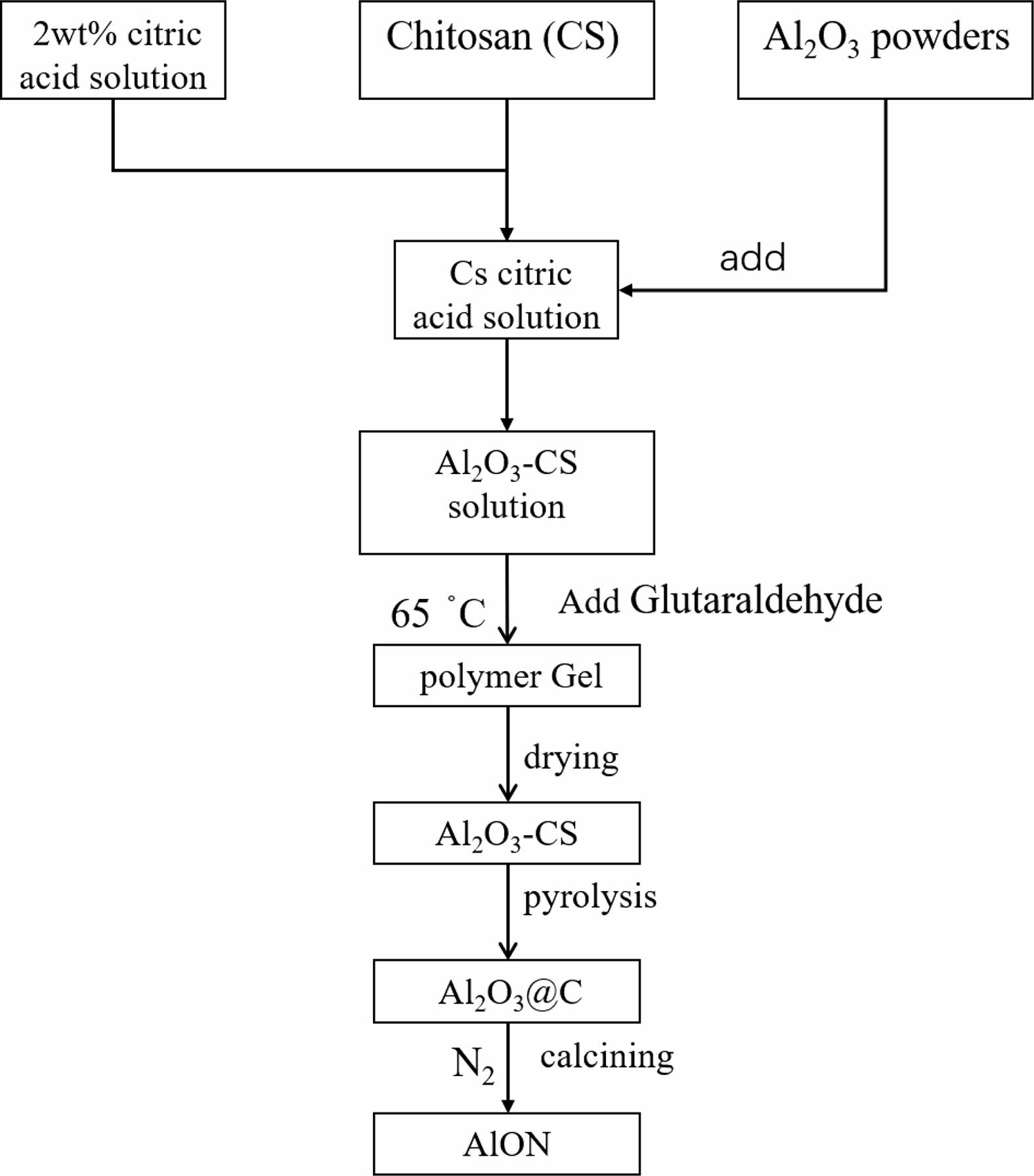
Figure 2 shows the detailed process of the synthesis of pure AlON powders, which is described below. Typically, a 1 wt.% solution of chitosan in acetic acid was prepared by dissolving chitosan in a 2 wt.% acetic acid solution. Then, Al2O3 was added into chitosan solution to form a milky Al2O3-CS precursor solution. Glutaraldehyde was added into the suspension solution, which was then kept at a temperature of 65 °C for 10 min to polymerize with chitosan, until a stable gel formed. To take advantage of the uniform distribution of Al2O3 particles in the precursor, the water was sublimed away from the freeze-drying method. Finally, the as-obtained mixture powder was pyrolyzed at a rate of 8 °C/min to 800 °C for 1 h in a tube furnace under flow of N2 gas, transforming the Al2O3-CS precursor into Al2O3/C.
High-temperature carbonitriding was carried out in a flowing nitrogen atmosphere. The prepared Al2O3/C precursor powder was put into a special graphite mold and calcined in an SPS sintering furnace in the flowing nitrogen atmosphere. First, the temperature was raised to 1,300 °C for 5 min at 50 °C/min, and then to 1,800 °C for 10 min at 20 °C/ min. In order to study the optimal synthesis parameters of AlON, the Al2O3/C precursors with different ratios were calcined at a high temperature (1,800 °C). After determining the optimal ratio, the powders were calcined at (1,400-1,700) °C to study the temperature factor.
The phase composition of the products was designated by powder X-ray diffractometry (XRD, Bruker AXS D8 Discover, Germany) with Cu-Ka radiation and Si as an internal standard. The morphology of the products was observed using scanning electron microscopy (SEM, Hitachi S-4800, Japan) equipped with an energy dispersive spectroscopy (EDS) system. Transmission electron microscopy (TEM, Tecnai F20, Phillip, Holland) was also applied to determine the phase composition.
|
Fig. 2 Flowchart for the synthesis of AlON precursor powders using a polymer-templating strategy. |
The Properties of precursors
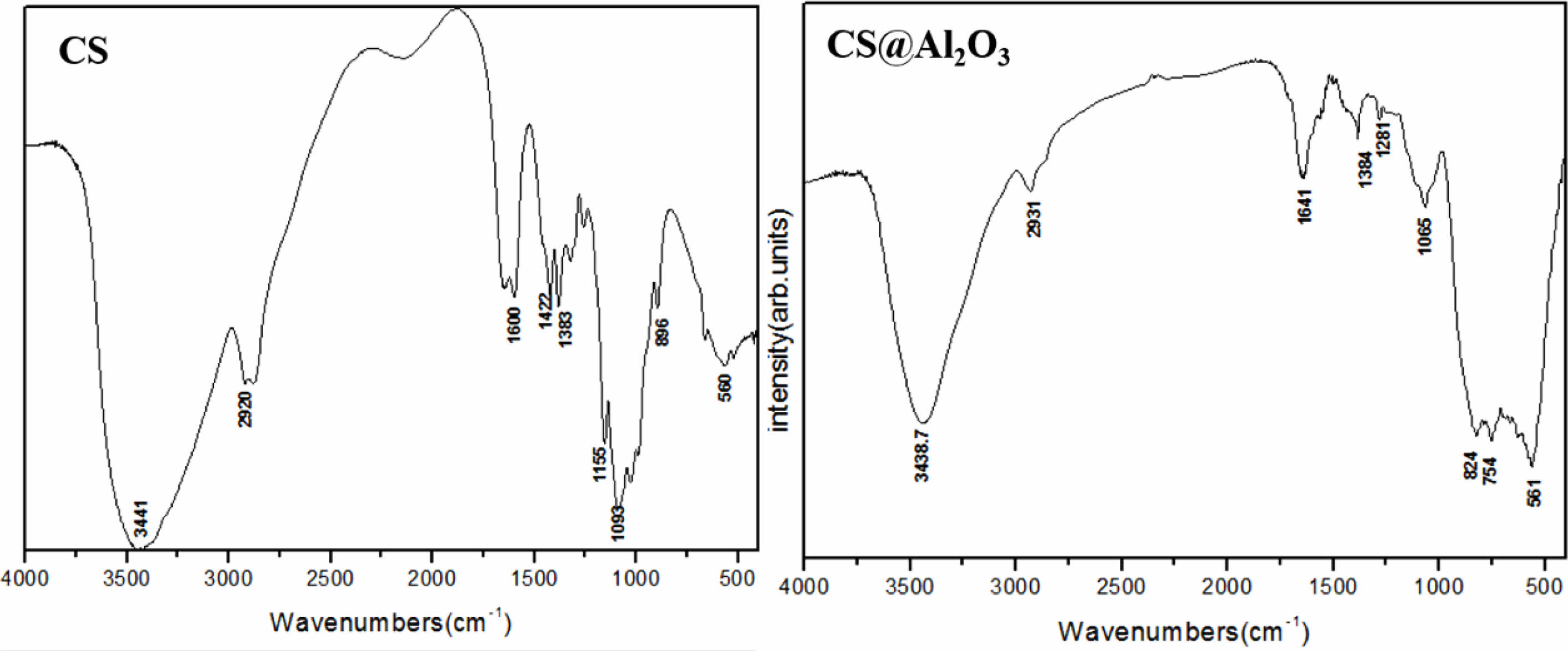
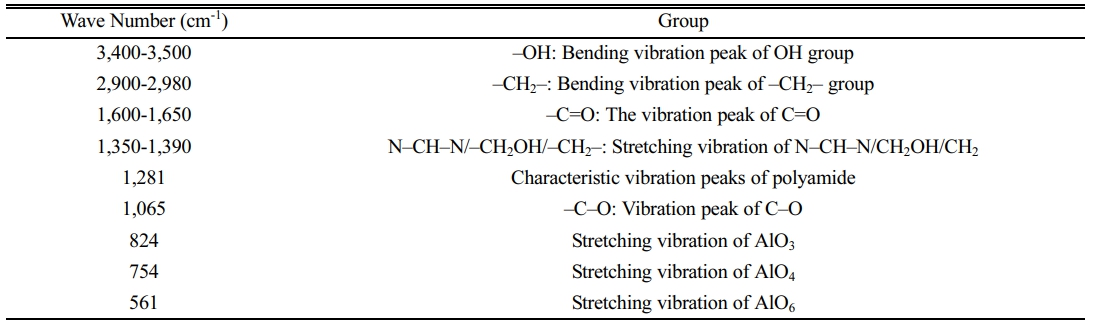
Chitosan has a large number of –OH groups, which can form –O–Al– bonds with Al in alumina. 20 In this way, alumina can be well encapsulated in chitosan after crosslinking. Figure 3 shows the infrared absorption spectra of chitosan and chitosan alumina precursor. Table 1 shows the groups corresponding to the characteristic absorption peaks. Compared to the pure CS, the infrared absorption spectra of Al2O3/CS with characteristic absorption peak of –O–Al– appeared in the range (500-900) cm-1, which indicated that alumina was successfully combined with chitosan. At the same time, due to the formation of the –O–Al– bond between –OH and Al and the cross-linking reaction of –NH, the absorption peak of –OH and –NH was weakened.
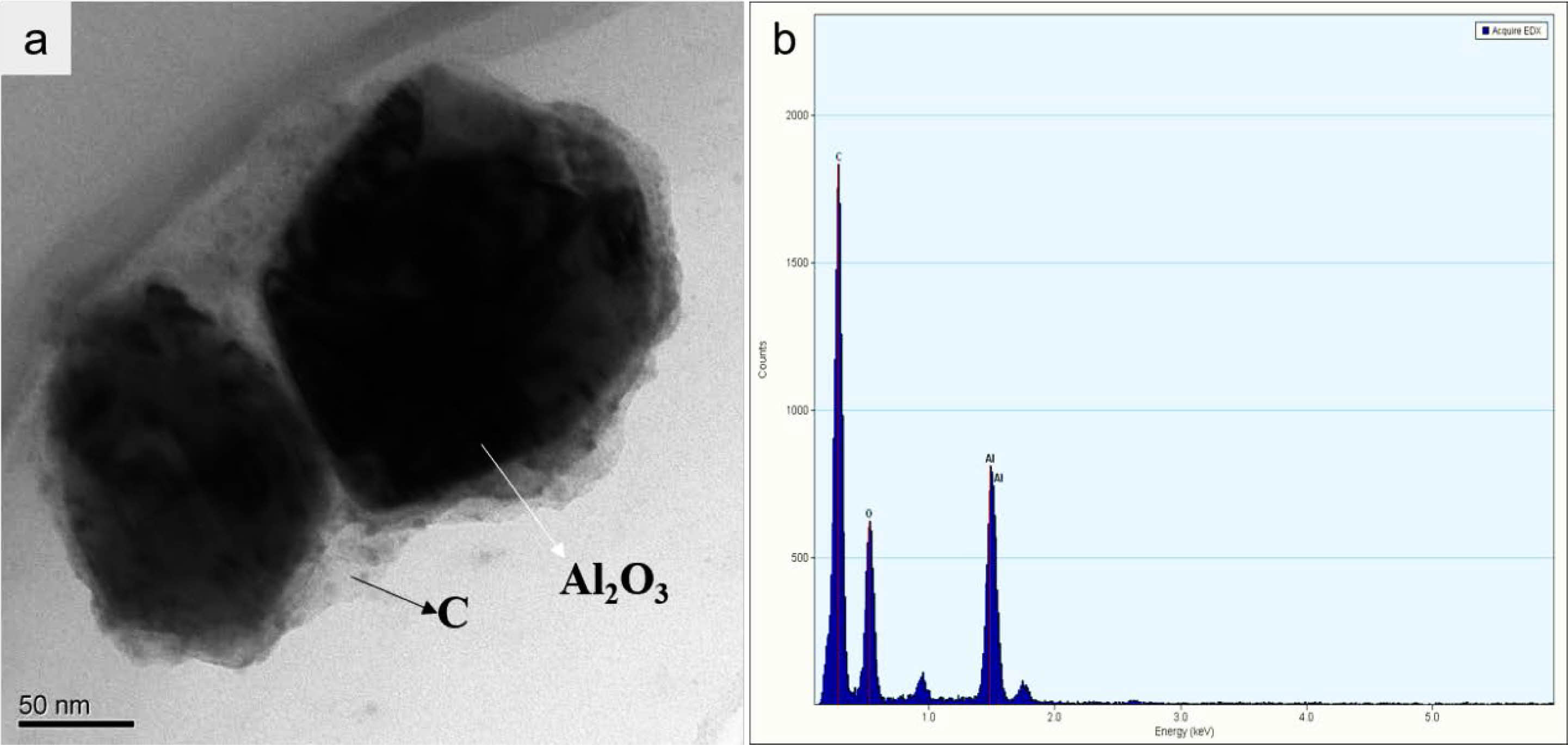
The precursor powders underwent pyrolysis at 800 °C for 1 h by a tube furnace with nitrogen. Figure 4 shows the results of the morphology and microstructure of the pyrolysis powders that were observed by TEM and mass spectrum. As Fig. 4 shows, the precursor powders have an obvious mosaic structure, and the Al2O3 powder was firmly fixed in the carbon matrix. The result shows that the amorphous carbon wrapped Al2O3, like a carbon skeleton, could prevent aggregation of the particles in the heating process.
The influence of the ratio of CS and Al2O3
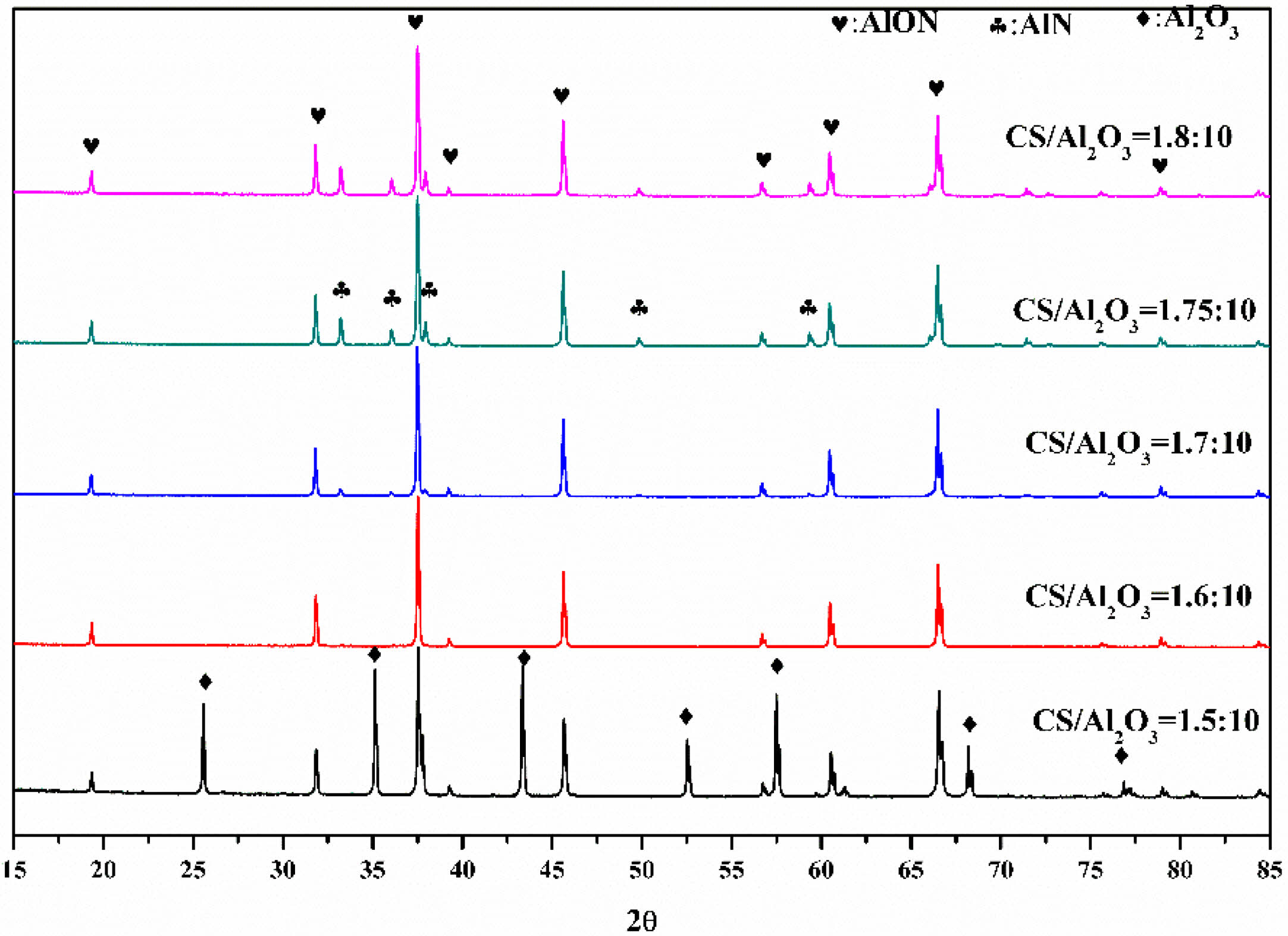
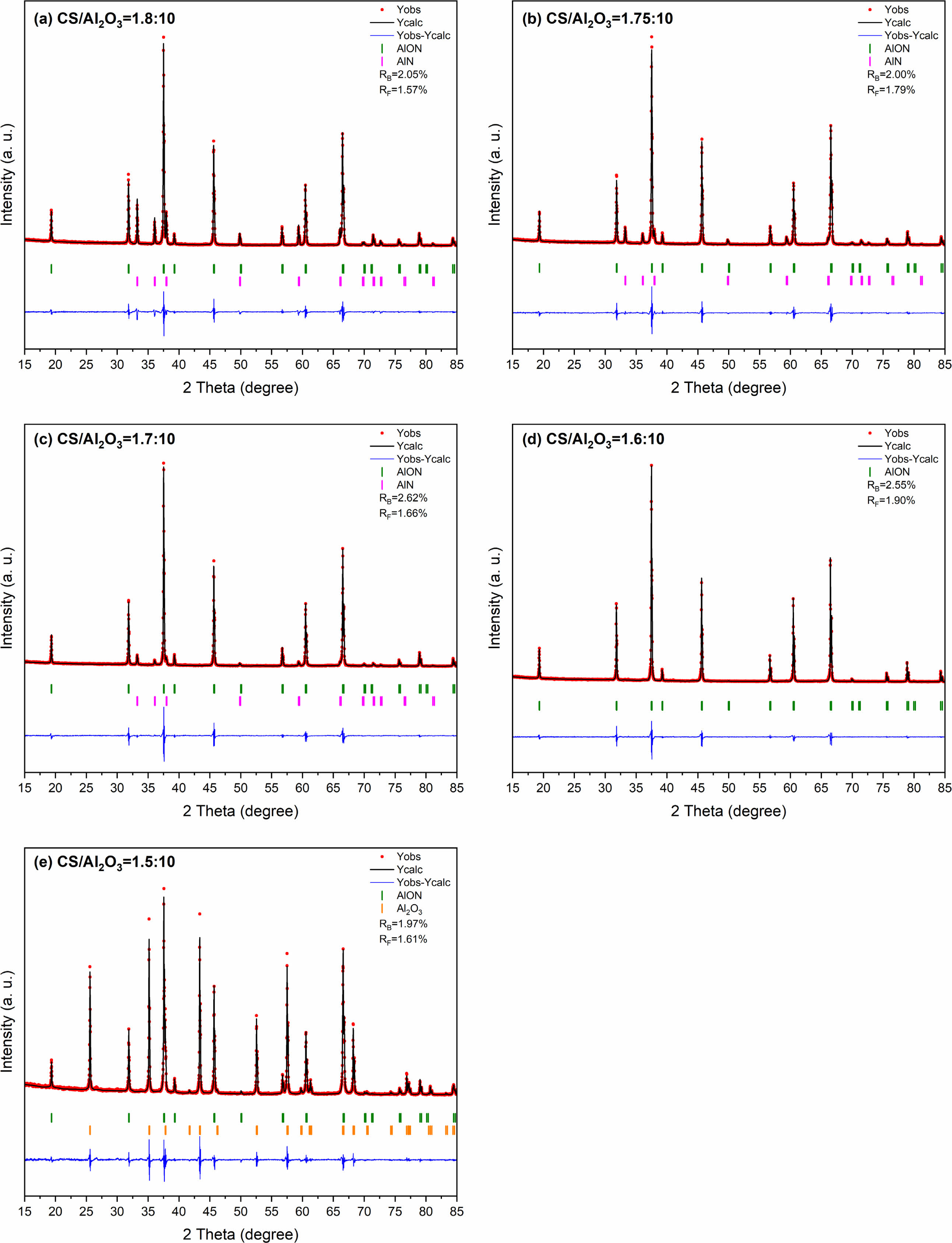
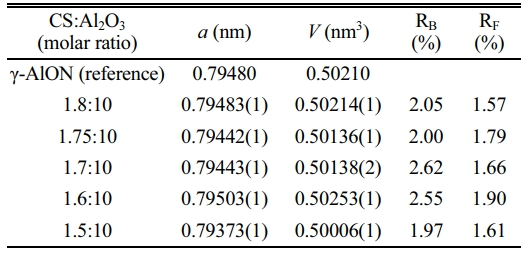
In this study, AlON powders were prepared by the carbothermal nitridation reaction in one step (as reaction Eq. (1)), so the appropriate C content was very important for the whole reaction. The content of C was adjusted by adjusting the content of chitosan. To determine the best carbon content, the precursors with CS/Al2O3 ratio of (1.5:10, 1.6:10, 1.7:10, 1.75:10, and 1.8:10) were prepared. After pyrolysis at 800 °C, the Al2O3/C precursors were calcined at high temperature (1,800 °C) in SPS for 20 min in flowing N2 atmosphere. Figure 5 shows the XRD results. The results show that after calcination at 1,800 °C, the main phase of CS/Al2O3=1.5:10 is alumina. With the increase of carbon content, the alumina phase decreases, while the AlN phase increases. Pure AlON powder is obtained when CS/Al2O3=1.6:10, after calcination at 1,800 °C. The lattice parameter and volume of each sample synthesized at 1,800 °C were estimated via profile matching within the Fullprof program [22]. The fitted plots for each sample are shown in Fig. 6(a)-(e), and the estimated lattice parameter (a), lattice volume (V), and reliability factors are listed in Table 2. The reliability factors R-Bragg factor (RB) and RF-factor (RF) for all the refinement results are less than 3%, which indicate that the estimated parameters are reasonably acceptable. The lattice parameters and lattice volumes of all the samples were in the range (0.79373-0.79503) nm and (0.50006-0.50253) nm3, respectively. These values are in a good accordance with lattice parameter and volume of the γ-AlON (PDF#04-006-5680), 0.79480 nm and 0.50210 nm3, respectively. During the process of high-temperature calcination, carbon reacts with the nitrogen in alumina to form AlN, and then AlN reacts with alumina to form AlON [13]. Therefore, when the carbon content is insufficient, only part of the alumina is transformed into AlN, and AlON is then formed. For example, when CS/Al2O3=1.5:10, the main phase is alumina, with only a small amount of AlON. When the carbon content is excessive, AlN will form. Therefore, when CS/Al2O3=1.7/1.75/1.8:10, AlN will remain in excess. The intensity of the characteristic peak increases with the increase of carbon content. The results show that pure AlON powder can be obtained by calcining in SPS for 20 min in a nitrogen atmosphere with CS/Al2O3=1.6:10 as the optimum ratio.
The influence of temperature factors
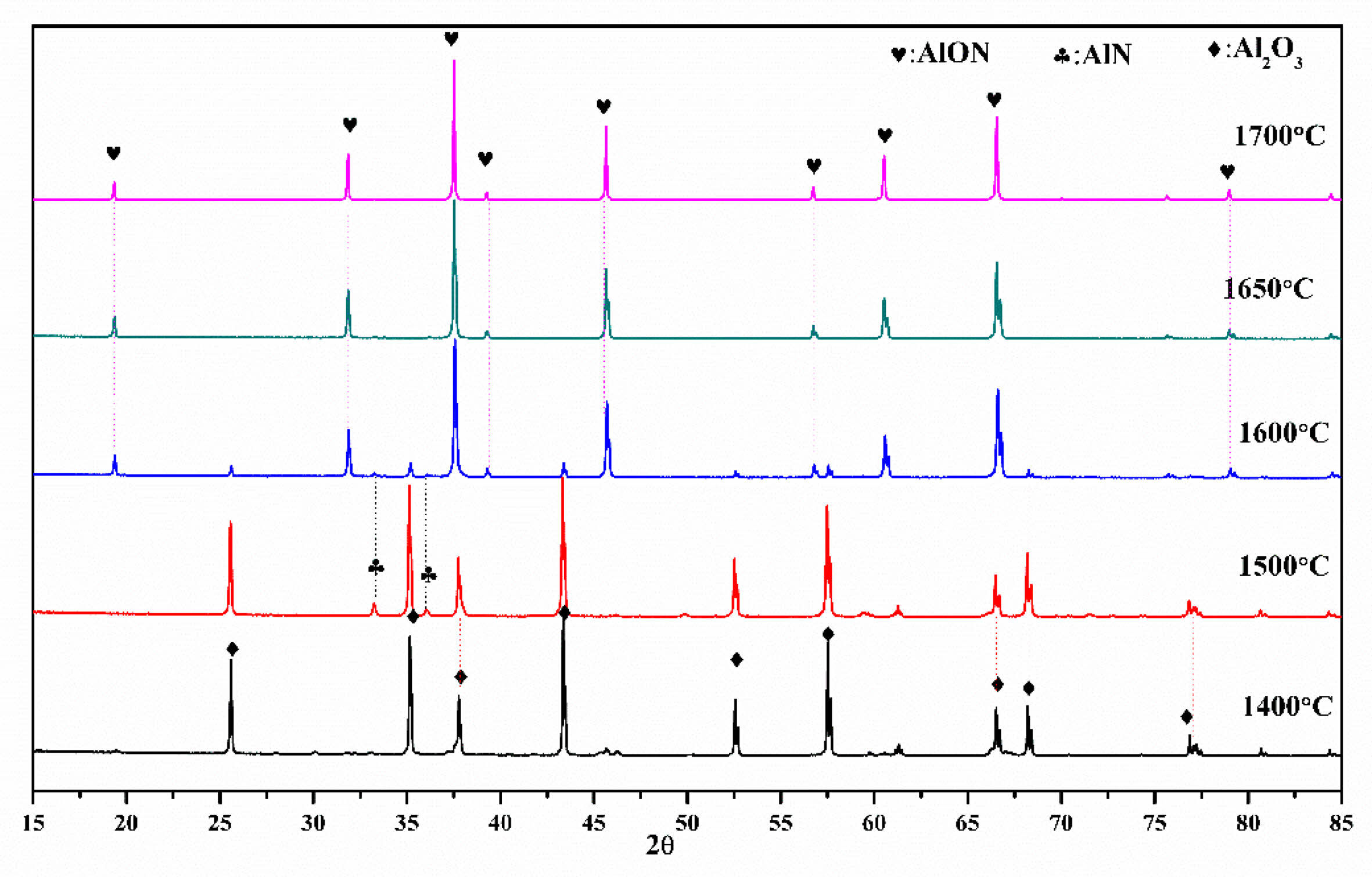
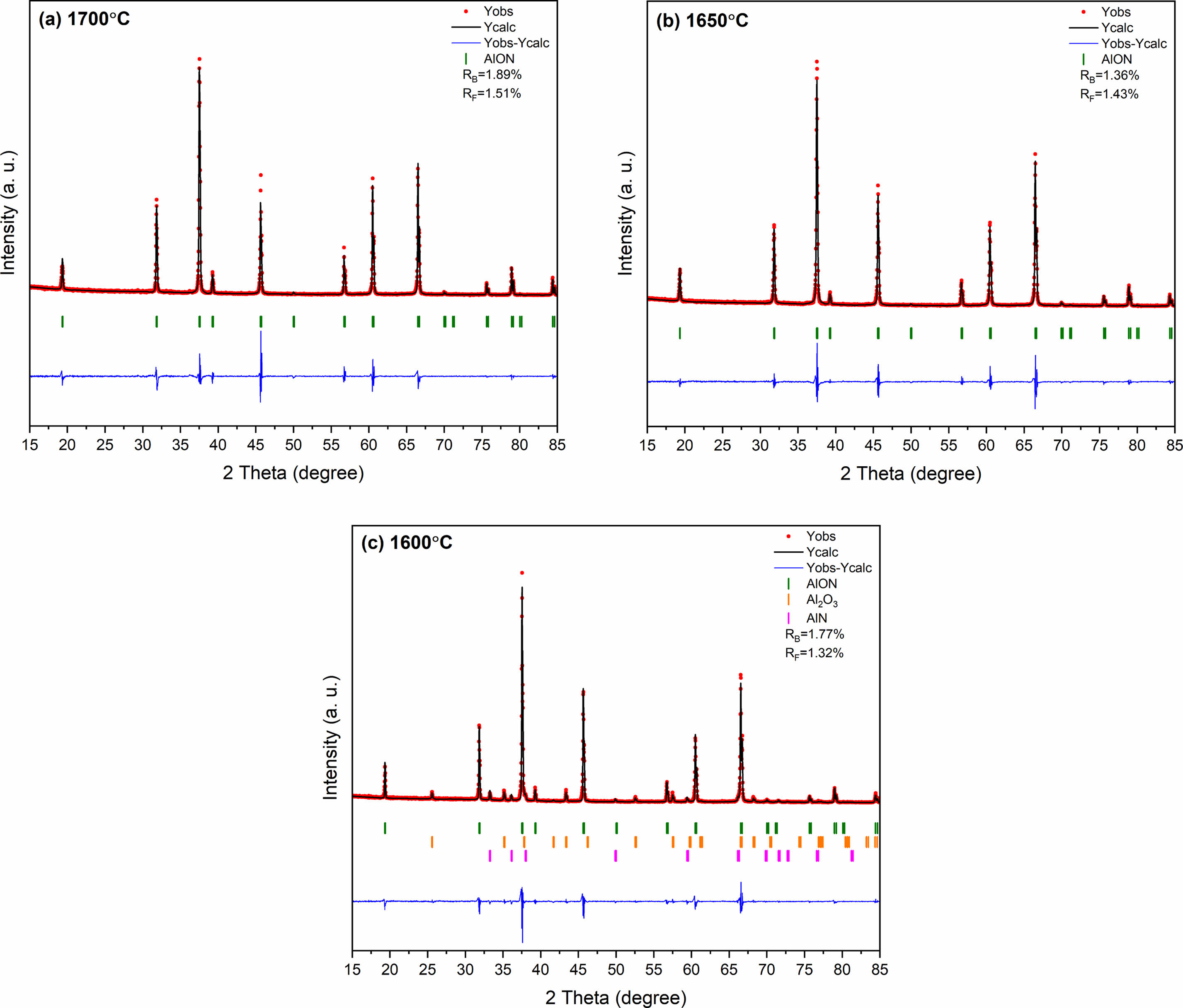
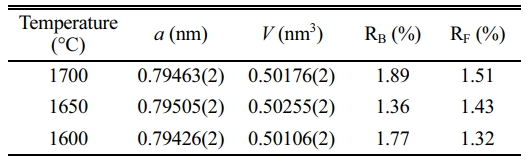
The synthesis temperature of AlON plays a decisive role in the properties of AlON powders. High synthesis temperatures are costly and also lead to the agglomeration and growth of particles, which reduces the sintering performance of powders. Therefore, the best ratio of CS/Al2O3=1.6:10 was used in the experiment, calcined at (1,400-1,700) °C, and the effect of temperature on the powder was studied. Figure 7 shows the results, which reveal that there was no carbothermal nitridation reaction at 1,400 °C, and the main phase was alumina. At 1,500 °C, part of AlN was formed, but not converted to AlON. With the increase of temperature, AlN began to react with alumina to form AlON at 1,600 °C. Therefore at 1,600 °C, the main phase of AlON and a small amount of Al2O3 and AlN phase could be observed. The results show that the pure AlON powder is obtained by calcining with SPS for 20 min at (1,650 and 1,700) °C under flowing nitrogen atmosphere. The 1,650 ℃ temperature is much lower than the general synthesis temperature of AlON (of over 1,750 °C) and only takes a very short time (20 min) for the reaction to go to completion. This method can obtain fine powder with uniform dispersion. The lattice parameter and volume of each sample synthesized with ratio of CS/Al2O3=1.6:10 at 1,600-1,700 °C were also estimated. The fitted plots for each sample are shown in Fig. 8(a)-(c), and the estimated lattice parameter, lattice volume, and reliability factors are listed in Table 3. The lattice parameters and lattice volumes of all the samples were in the range (0.79426-0.79505) nm and (0.50106-0.50255) nm3, respectively. These values also correspond well to those of the γ-AlON (PDF#04-006-5680) with acceptable reliability factors.
The microstructure of the powders
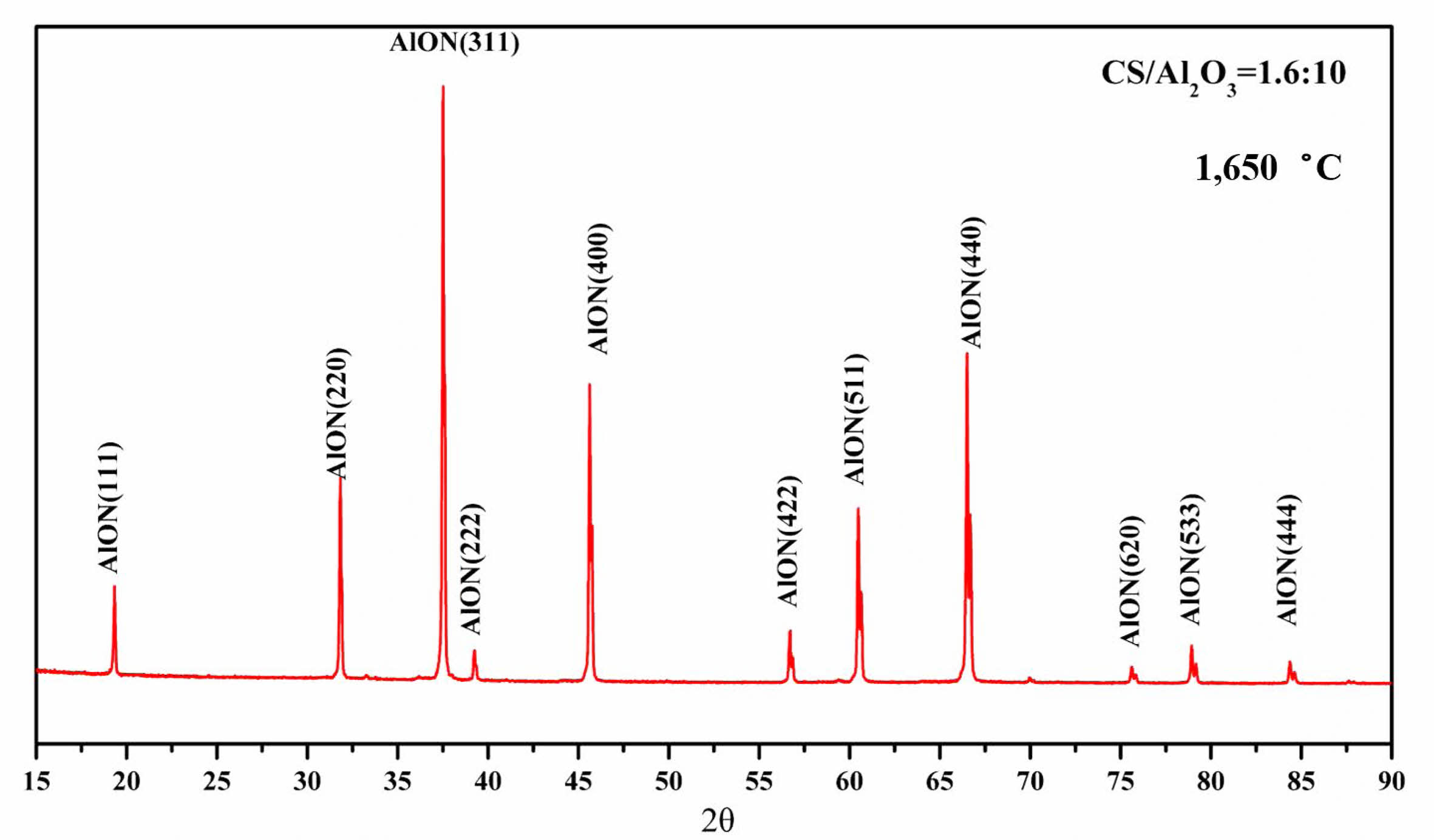
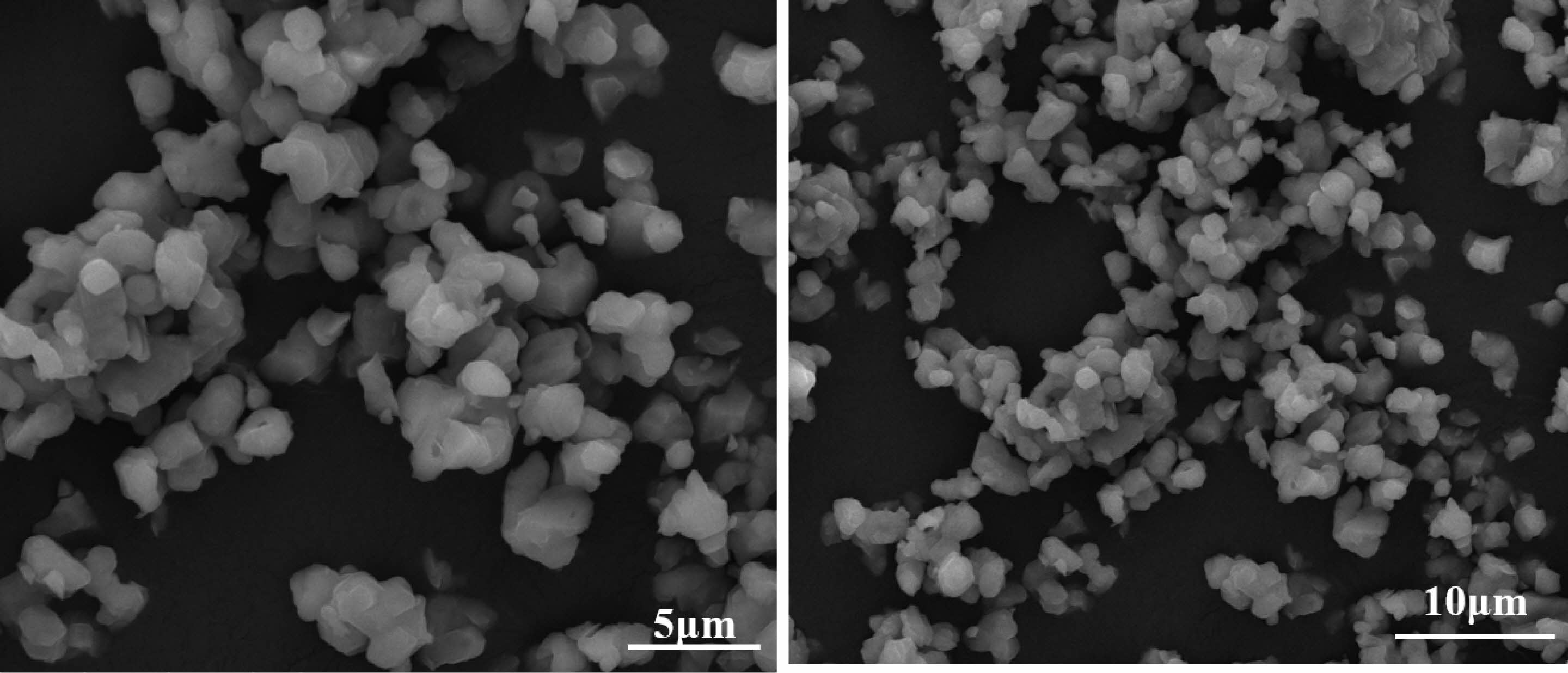
To observe the micro morphology of the powder, pure AlON powder was prepared by SPS calcining at 1,650 °C for 20 min under N2 atmosphere with the best raw material parameters, CS/Al2O3=1.6:10. Figure 9 shows the XRD pattern of AlON powder was prepared by SPS calcined at 1,650 °C for 20 min under N2 atmosphere. The results show that the AlON powder prepared with the best parameters has high purity. The powder prepared with the best parameters was then observed by scanning electron microscopy (SEM). Figure 10 shows the SEM imagery of a sample prepared from the optimal formulation. The high-purity AlON powders are highly uniform, with the size of the nearly spherical particles in the range (1–2) µm.
|
Fig. 3 The infrared absorption spectra of Chitosan and Al2O3/CS precursor. |
|
Fig. 4 The TEM (left), and mass spectrum (right) of Al2O3/C precursor |
|
Fig. 5 The XRD of Al2O3/C precursors with different CS/Al2O3ratio calcined at 1,800 °C by SPS in N2 atmosphere |
|
Fig. 6 he refined patterns of Al2O3/C precursors with different CS/Al2O3 ratio calcined at 1,800 °C by SPS in N2 atmosphere |
|
Fig. 7 The Al2O3/C with ratio of CS/Al2O3=1.6:10 was calcined at (1,400-1,700) °C by SPS for 20 min under N2 atmosphere. |
|
Fig. 8 The refined patterns of Al2O3/C synthesized with ratio of CS/Al2O3=1.6:10 at (1,600-1,700) °C by SPS for 20 min under N2 atmosphere. |
|
Fig. 9 The XRD pattern of AlON powder was prepared by SPS calcined at 1,650 °C for 20 min under N2 atmosphere with the best raw material parameters. |
|
Fig. 10 The SEM of AlON powder was prepared by SPS calcined at 1,650 °C for 20 min under N2 atmosphere with the best raw material parameters. |
|
Table 2 The lattice parameter and volume of γ-AlON (reference; PDF#04-006-5680) and synthesized AlON with different CS/ Al2O3 ratio at 1,800 °C by SPS in N2 atmosphere. |
|
Table 3 The lattice parameter and volume of synthesized AlON with ratio of CS/Al2O3=1.6:10 at (1,600-1,700) °C by SPS for 20 min under N2 atmosphere. |
The pure AlON powders were prepared by SPS calcining at 1,650 °C for 20 min under N2 atmosphere. The results indicate that the best parameter is CS/Al2O3=1.6:10. The TEM results of Al2O3/C show that the Al2O3 in the precursor is wrapped by a perfect carbon mesh framework, which can prevent the agglomeration of particles in the carbothermal nitridation reaction. The SEM result of AlON powder, which was prepared by SPS calcining at 1,650 °C for 20 min under N2 atmosphere with the best raw material parameters, shows that the high-purity AlON powders are highly uniform, while the size of the nearly spherical particles is in the range (1-2) µm. The pure AlON powder has high sintering activity, which is beneficial to the sintering of high-density and high transparency ceramics. The polymer template strategy uses monomer polymerization to form a continuous carbon network. The carbon skeleton can make the carbothermal reduction reaction take place in the original, thus preventing the excessive agglomeration of grains. The high-purity AlON powder greatly reduces the difficulty of sintering transparent ceramics in the later stage. At the same time, this work provides a new idea for the low-temperature preparation of inorganic powder precursors. We can try to apply polymer polymerization theory to the preparation of inorganic nano powders to obtain powders with special properties.
This work was financially supported by the Guangzhou Postdoctoral Research Fund.
We declare that all authors have no conflict of interest.
- 1. J.W. Mccauley and N.D. Corbin, Progress in Nitrogen Ceramics 65 (1983) 111-118.
-

- 2. K.P. Zheng, H. Wang, P.Y. Xu, H.G. Gu, B.T. Tu, W.M. Wang, S.Y. Liu, and Z.Y. Fu, J. Eur. Ceram. Soc. 41[7] (2021) 4319-4326.
-

- 3. M.M. Ding, T. Wang, B. Maerz, S. Robertson, Z.H. Sun, L.C. Fan, Y. Shi, and H.Z. Wu, Ceram. Int. 45[17] (2019) 21127-21135.
-

- 4. N.N. Belov, N.T. Yugov, A.Y. Sammel, and E.Y. Stepanov, Vestn. Tomsk. Gos. Univ. Mat. Mekhanika. 67 (2020) 69-77.
-

- 5. L.L. Lundin, Adv. Mater. Processes. 164[11] (2006) 45-47.
- 6. Y.Z. Wang, Z. Feng, T. Meng, and R.S. Zhang, Rare Met. Mater. Eng. 44[1] (2015) 101-104.
- 7. A. Fletcher, D. Turner, S. Fairchild, C. Rice, and G. Pitz, Phys. Status Solidi A. 215[2] (2018) 5.
-

- 8. F. Zhang, S.W. Wang, Z. Zhang, and X.Y. Yuan, Rare Met. Mater. Eng. 38 (2009) 403-406.
- 9. Z. Feng, J.Q. Qi, Q.Y. Chen, Y.Z. Wang, X.X. Cao, Y. Yu, C.M. Meng, and T.C. Lu, Ceram. Int. 45[17] (2019) 23022-23028.
-

- 10. Z. Feng, J.Q. Qi, X. Huang, X.F. Guo, Y. Yu, X.X. Cao, Y.Z. Wang, D. Wu, C.M. Meng, and T.C. Lu, J. Am. Ceram. Soc. 102[5] (2019) 2377-2389.
-

- 11. Q. Liu, N. Jiang, J. Li, K. Sun, Y.B. Pan, and J.K. Guo, Ceram. Int. 42[7] (2016) 8290-8295.
-

- 12. J. Lei, F. Ma, Y. Shi, J. Xie, W. Hu, and Z. Shi, Sci. China-Technological Sci. 55[12] (2012) 3405-3410.
-

- 13. F.Z. Ma, J. X Lei, Y. Shi, J.J. Xie, and L. Fei, Adv. Mater. Res. [624] (2012) 42-46.
-

- 14. R.S. Kumar, S. Erkulla, A.K. Khanra, and R. Johnson. J. Sol-Gel Sci. Technol. 93 (2020) 100-110.
-

- 15. B. Naderi-Beni and A. Alizadeh. Ceram. Inter. 46[1] (2020) 913-920.
-

- 16. S. Kamali, M.M. Mohebi, M.H. Taherian, and V. Sabaghi, Appl. Ceram. Technol. 18[3] (2021) 724-738.
-

- 17. H.M. Oh, Y.J. Park, H.N. Kim, J.W. Ko, and H.K. Lee, J. Eur. Ceram. Soc. 41[1] (2021) 775-780.
-

- 18. V. Sabaghi, F. Davar, and M.H. Taherian, Ceram. Inter. 45[3] (2019) 3350-3358.
-

- 19. Z. Ding, D. Qeng, D. Shi, X. Zhou, Y. Li, S. Ran, and Q. Huang, J. Am. Ceram. Soc. 97[10] (2014) 3037-3040.
-

- 20. K.R. Murali and P. Thirumoorthy, J. Alloys Compd. 500[1] (2010) 93-95.
-

- 21. E.S. Zemlyakova, A.V. Tcibulnikova, V.A. Slezhkin, A.Y. Zubin, I.G. Samusev, and B.V. Vryukhanov, J. Polym. Eng. 39[5] (2019) 415-421.
-

- 22. J. Rodriguezcarvajal, Phys. B. 192[1-2] (1993) 55-69.
-

 This Article
This Article
-
2023; 24(4): 675-682
Published on Aug 31, 2023
- 10.36410/jcpr.2023.24.4.675
- Received on Apr 17, 2023
- Revised on Jun 6, 2023
- Accepted on Jul 6, 2023
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Young-Hwan Hani, and Jooseong Kimh,j
-
hSchool of Materials Science and Engineering, Yeungnam University, 280 Daehak-ro, Gyeongsan, Gyeongbuk 38541, Republic of Korea
iWuhan University of Technology, International School of Materials Science and Engineering, 122 Luoshi, Hongshan, Wuhan 430070, People’s Republic of China
jHudensBio Co., Ltd, Gwangju, Republic of Korea
Tel : +82-62-609-7545 Fax: +82-62-574-2805 - E-mail: joorpediem@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.