- Influence of ZnFe2O4 substitution on the microstructural and magnetic properties of M-type Sr0.1Ca0.4La0.5Fe12O19 hexagonal ferrites
Xiubin Zhao and Ailin Xia*
School of Materials Science and Engineering, Anhui University of Technology, Maanshan 243002, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The M-type ferrite Sr0.1Ca0.4La0.5Fe12O19 magnetic powders with different ZnFe2O4 substitution amounts (Rm, 0, 1%, 3%, 5%, 7%, and 9%) were obtained using a ceramic process. The structure of specimens were examined by using an X-ray diffractometer. All the specimens exhibited a typical single-phase hexagonal M-type structure, and the particles in specimens were uniformly distributed in size. The VSM study indicated the specimen with Rm=9% had the maximum saturation magnetization of 70.22 emu/g, and the residual magnetization and the coercivity of specimens increased firstly and decreased later with the increase of Rm. The specimen with Rm=3% exhibits the best comprehensive magnetic properties
Keywords: Ferrite, ZnFe2O4 substitution, Ceramic method, Magnetic properties
Hexagonal M-type AFe12O19 (A=Sr, Ba and Pb) ferrite is one of the most widely used hard magnetic materials mainly due to its fine corrosion resistance, low cost and good chemical stability [1-7]. In order to improve the magnetic properties of M-type hexaferrites, adding or doping some elements is widely studied. The substitution of rare earths and transition metals such as La3+, Ce3+, Ni2+, Ti4+, Co2+, Mn2+, Cu2+, Mg2+, and Zn2+ on Fe3+ have been investigated extensively as well as some co-substitutions, such as Co-Ti, La-Co, La-Cu and La-Zn [8-17]. The M-type hexaferrites with high magnetic properties are mainly the La-Co substituted BaFe12O19 (BaM) and SrFe12O19 (SrM) ferrites. In order to reduce the cost of M-type hexaferrites, Zn2+ ions were used to replace Co2+ ions because the cost of ZnO powder is much cheaper than that of CoO powder. L.S. You et al. [18] prepared SrM ferrite substituted by La-Zn via a self-propagating high-temperature synthesis method, and it was found that the La-Zn substitution significantly improved the magnetic properties of M-type strontium ferrite. Vinnik et al. [19] prepared the Zn-substituted BaZnxFe12-xO19 (0 < x < 0.065) single crystals and found that the saturation magnetization (Ms) and coercivity (Hc) depended very sensitively on the amount of Zn-substitution. Asghar et al. [20] synthesized Cr-Zn-doped SrM nanoparticles SrFe12−2x- CrxZnxO19 (x=0.0-0.8) by chemical coprecipitation method and found that both Ms and Hc decreased with the increasing Cr-Zn content, and the dielectric constants, together with the dielectric losses, decreased with the increase of doping content. Xia et al. [2] have synthesized Ba(Zn Ti)xFe12-2xO19 (x = 0.0, 0.2, 0.6, 1.0, 1.4) M-type Ba ferrite powders via a new route by combining a chemical coprecipitation technique and found that small quantities of ZnTi substitutions helps to form single-phase ferrite at a low calcination temperature, the magnetic properties were reduced obviously with the increase of x from 0.2 to 1.4. M.J. Iqbal et al. [21] have prepared the Zr-Zn substituted M type strontium ferrite nanoparticles by a chemical coprecipitation method. The results show that the Ms, magnetic moment and remanent magnetism (Br) increased with the increase of the amount of substitution, but coercivity decreases with the increase of the amount of substitution.
In our previous study, the performance of ferrite can be significantly improved by the exchange coupling between soft and hard magnetic phases. Up to the present, the effects of pure ZnFe2O4 substitution on the microstructural and magnetic properties of the SrCaLa hexaferrites obtained via a ceramic process was still not reported. In this study, influence of ZnFe2O4 additive on the microstructural and magnetic properties of M-type Sr0.1Ca0.4La0.5Fe12O19 hexagonal ferrites was studied, which may help to reduce the use of rare earth elements in high-performance SrM ferrites.
Synthesis of ZnFe2O4 powders
A ceramic process was used to obtain ZnFe2O4 (ZFO) powders. Analytically pure Fe2O3 and ZnO were used as raw materials without further treatment. The mixed powders (Fe2O3 and ZnO) were ball-milled in water for 2 h with an angular velocity of 400 rpm and a ball-to-powder weight ratio of 15:1. The as-milled powders were dried, sifted and then calcined in a muffle at 850 ℃ for 3 h in an air atmosphere. Finally, the calcined samples were pulverized to powders with a size of smaller than 100 μm using a vibration mill.
Preparation of M-type ferrite Sr0.1Ca0.4La0.5Fe12O19 (SrCaLaM)powders with ZFO additive
All samples of Sr0.1Ca0.4La0.5Fe12O19 powders with different amount of ZFO (Rm, 0%, 1%, 3%, 5%, 7% and 9%) were obtained using a ceramic process. The starting materials used were SrCO3 (98% purity), CaCO3 (99% purity), La2O3 (99% purity), and Fe2O3 (98% purity). The mixed powders were milled in water for 2 h with an angular velocity of 400 rpm and a ball-to-powder weight ratio of 15:1, together with ZFO of different Rm. The obtained powders were dried, sifted and then sintered at 1300 ℃ for 2 h in a muffle furnace with an air atmosphere. Finally, the sintered specimens were pulverized to powders with a size of smaller than 100 μm using a vibration mill.
Characterization
The phase composition of the materials was decided by a powder X-ray diffractometer (XRD, Rigaku D/max-2550V/PC) using Cu Kα (λ=1.5406Ǻ) radiation. The 2θ angles were scanned over a range between 10° and 80°. A field emission scanning electron microscope (FESEM, HITACHI S-4800) was utilized to analyze the morphology. The room temperature (RT) magnetic hysteresis loops of samples were measured on a vibrating sample magnetometer (VSM, MicroSense EZ7) with a maximum external field of 1592 kA/m (20000 Oe).
Phase Identification and microstructure
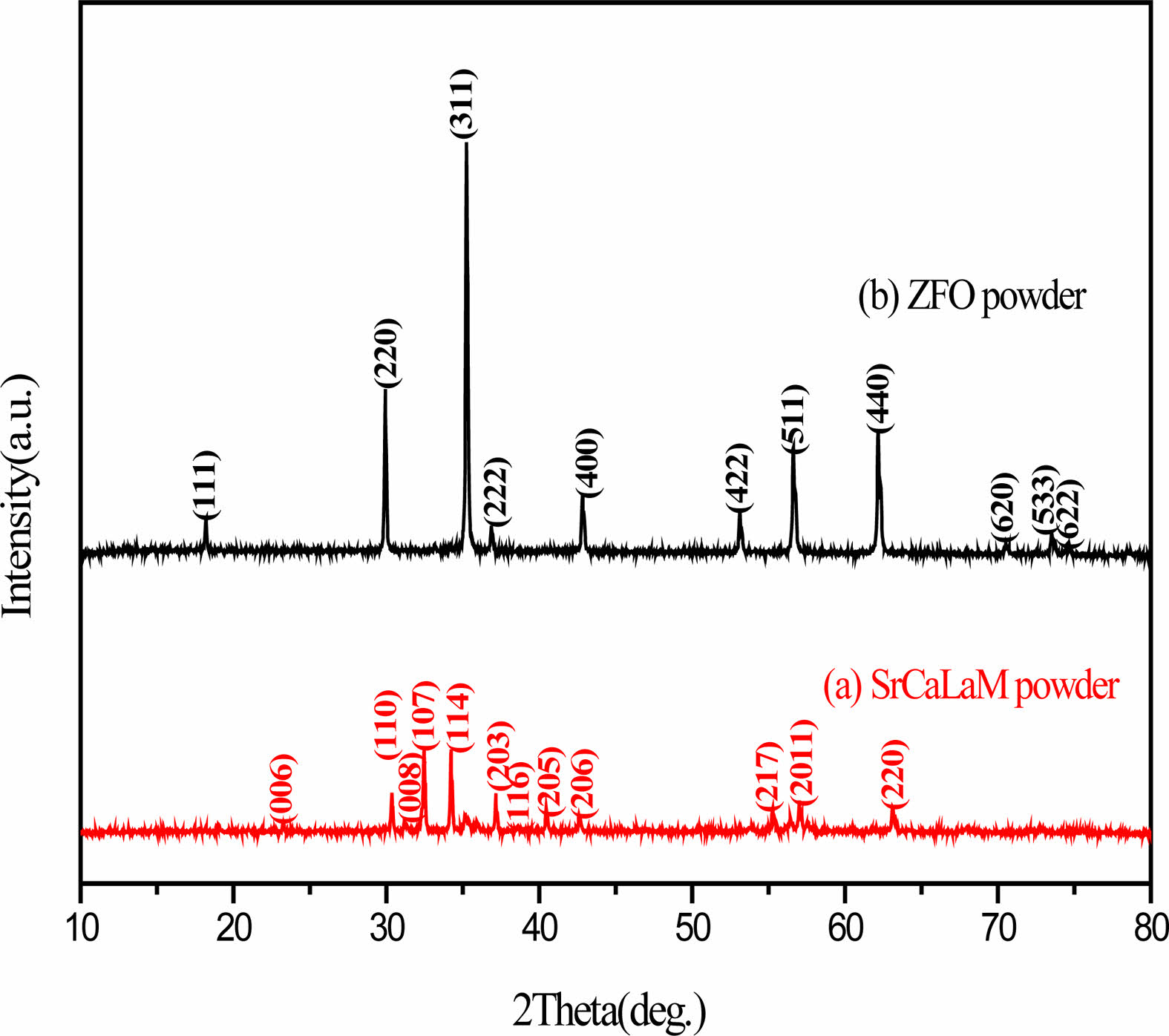
Fig. 1 gives the XRD patterns of the as-synthesized SrCaLaM (Rm=0%) and ZFO powders. The only diffraction peaks from SrCaLaM (Rm=0%) (pdf no 33-1340) and ZFO (pdf no 22-1012) can be found in Fig. 1(a) and (b), respectively, indicating the single-phase structure of specimens.
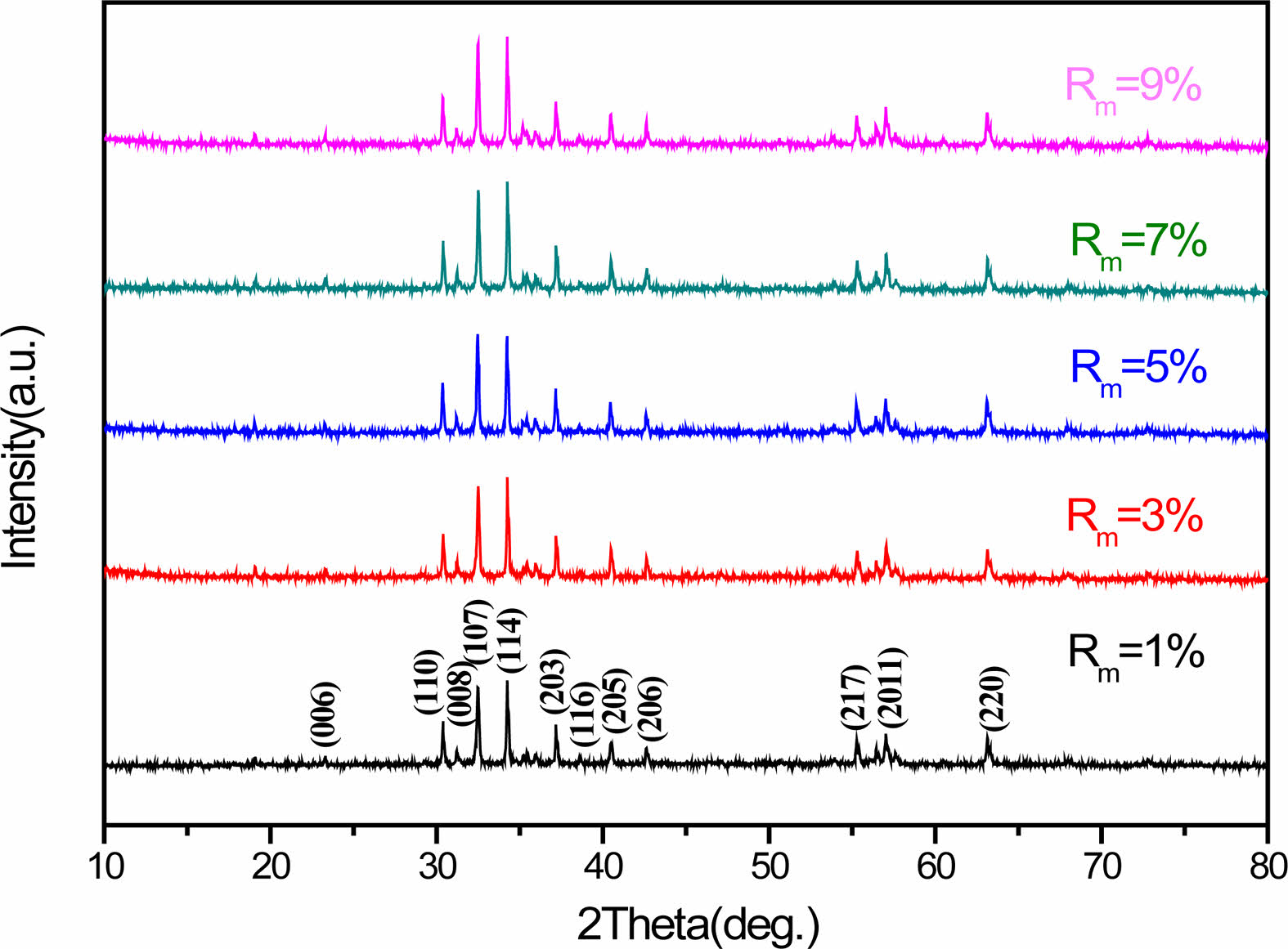
Fig. 2 shows the XRD patterns of the hexaferrite SrCaLaM magnetic powders with different Rm ranging from 1% to 9%. Compared with the standard XRD pattern of strontium ferrite (JCPDS card no. 22-1340), only typical peaks from single-phase magnetoplumbite SrCaLaM was found, which implies that the Zn2+ ions entered into the magnetoplumbite lattice without producing any second phase.
The lattice constants a and c can be calculated using the values of Miller indices (h, k, l) and the inter-planer spacing dhkl corresponding to (107) and (114) peaks according to the following formula (1):
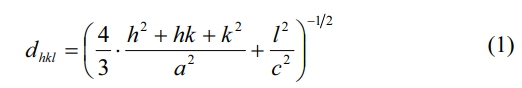
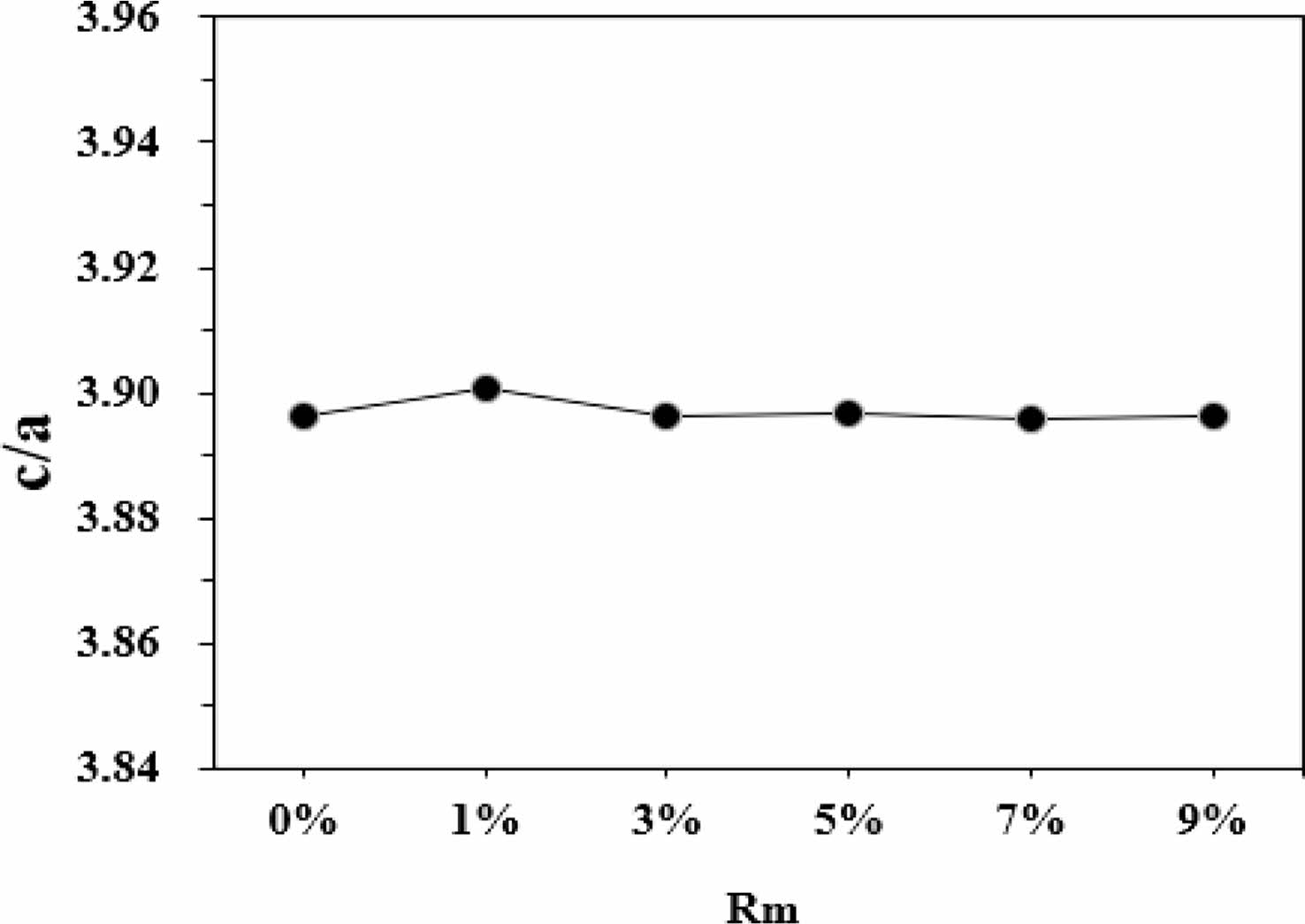
Fig. 3 indicates the change in crystal axis ratio of c/a in different samples. As shown in this figure, the c/a changed slightly with the increase of Rm. According to Verstegen and Stevels [22], the ratio of c/a may present the structure type of Sr ferrites. For the M-type structure, the c/a was assumed to be smaller than 3.98. Herein, the values of c/a range from 3.8960 to 3.9006, which seems to be in well accord with the reported values of M-type structure.
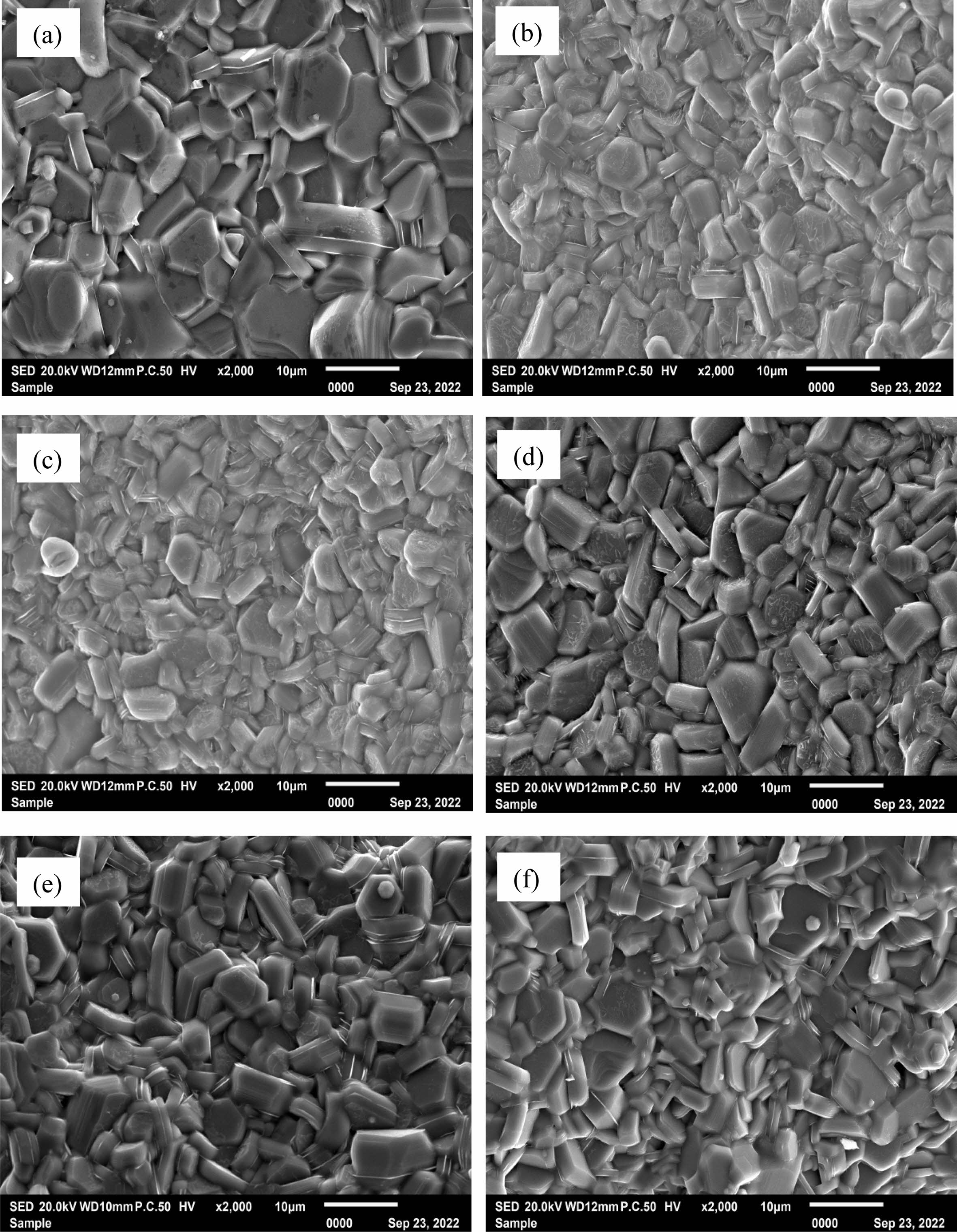
Tipical FESEM images of samples are presented in Fig. 4. It can be observed that the all the samples consist of relatively uniform particles with typical hexagonal structure. It can be found that compared with the sample with Rm=0%, the average particle size of samples with ZFO addictiveis obviously smaller. The addition of ZFO plays the role of refining grain and preventing grain growth. However, with the increase of Rm, the typical morphology and the average particle size of specimens did not change obviously.
Magnetic properties
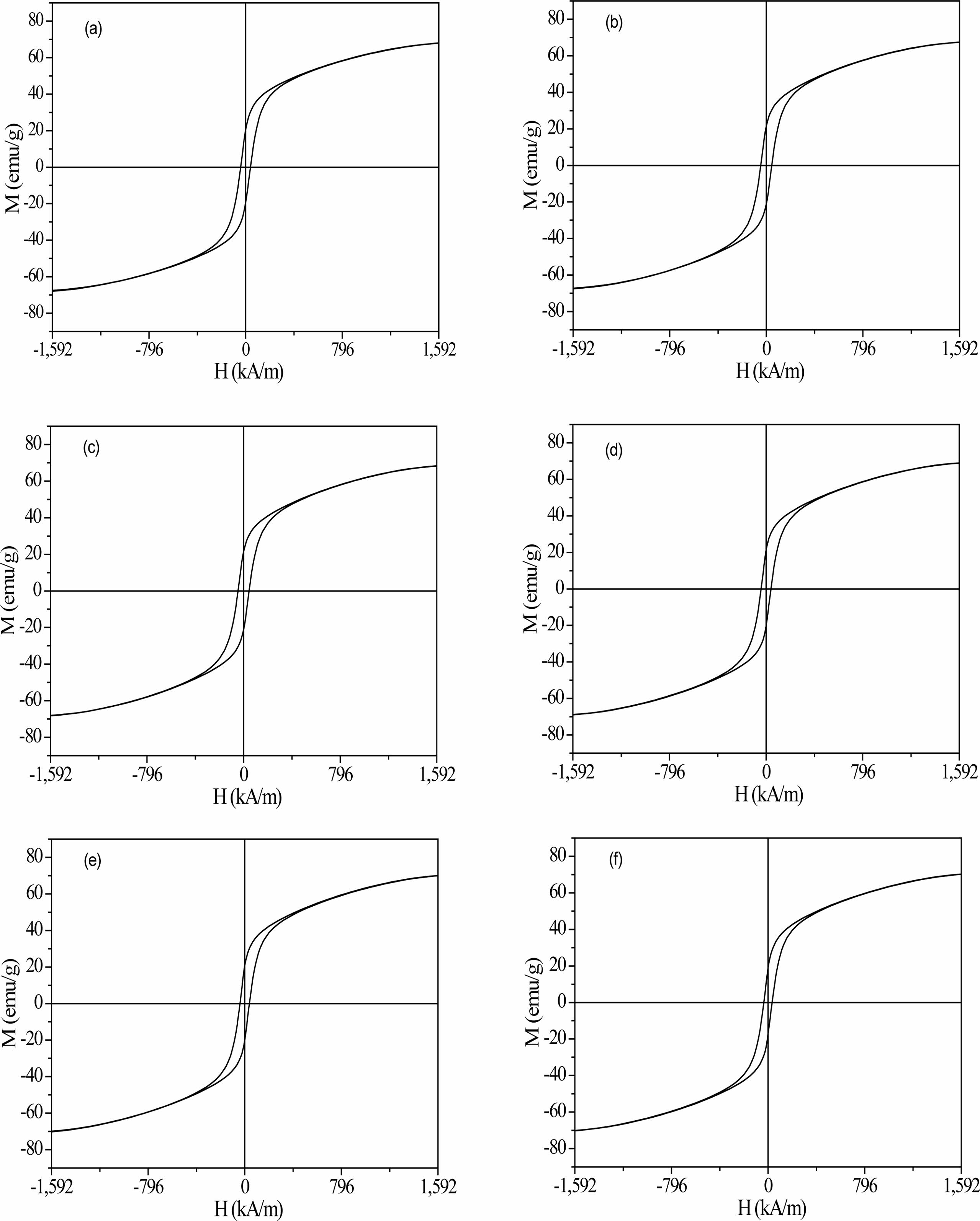
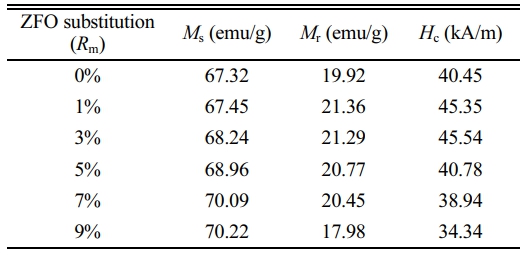
The room-temperature magnetic hysteresis loops of samples with Rm are shown in Fig. 5, and the corresponding Ms, Hc and Mr of samples are listed in Table 1. What should be pointed out is that since the magnetic hysteresis loops were roughly saturated at a field of 1592 kA/m (20000 Oe), the value of magnetization obtained at 1592 kA/m (20000 Oe) is approximately used as Ms.
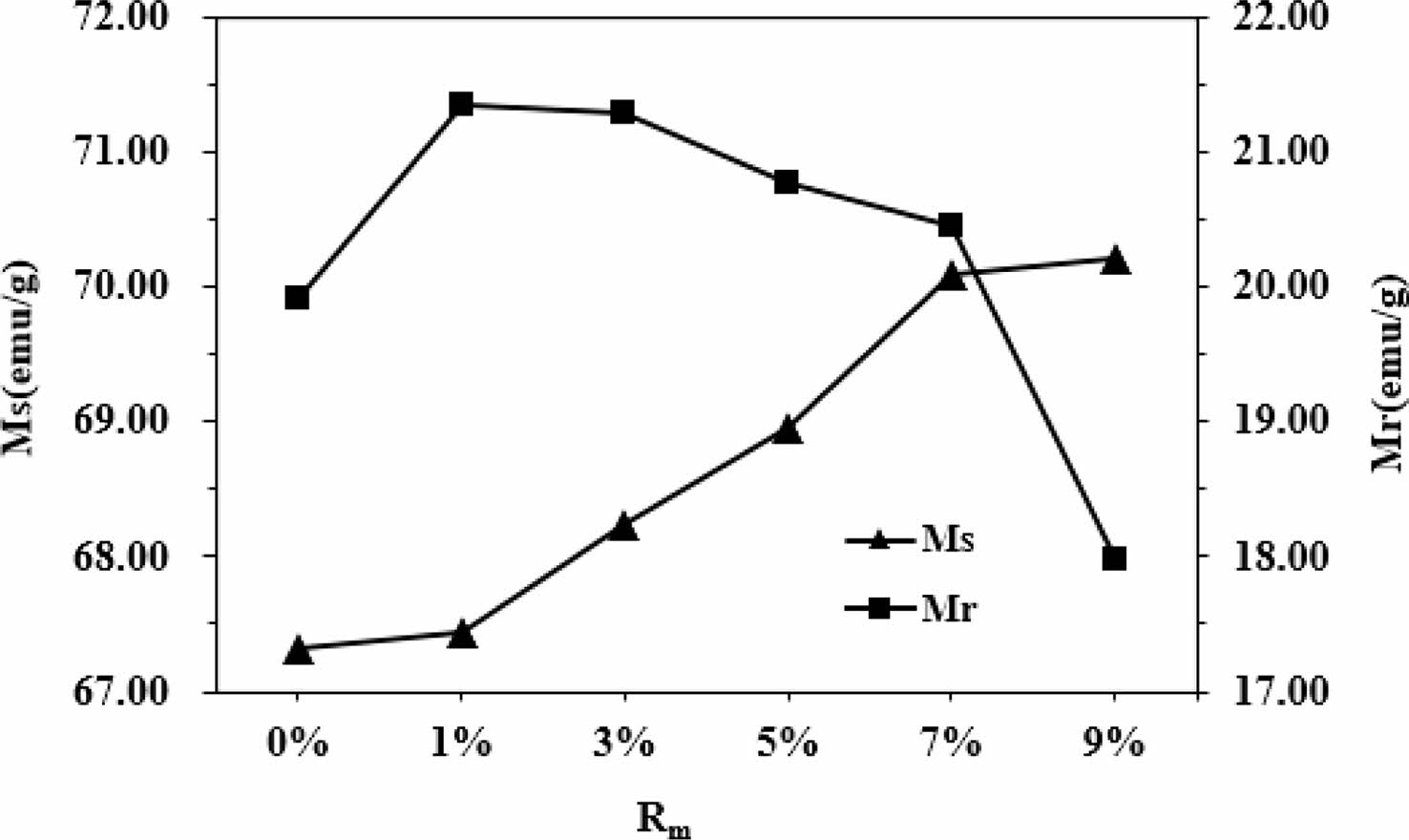
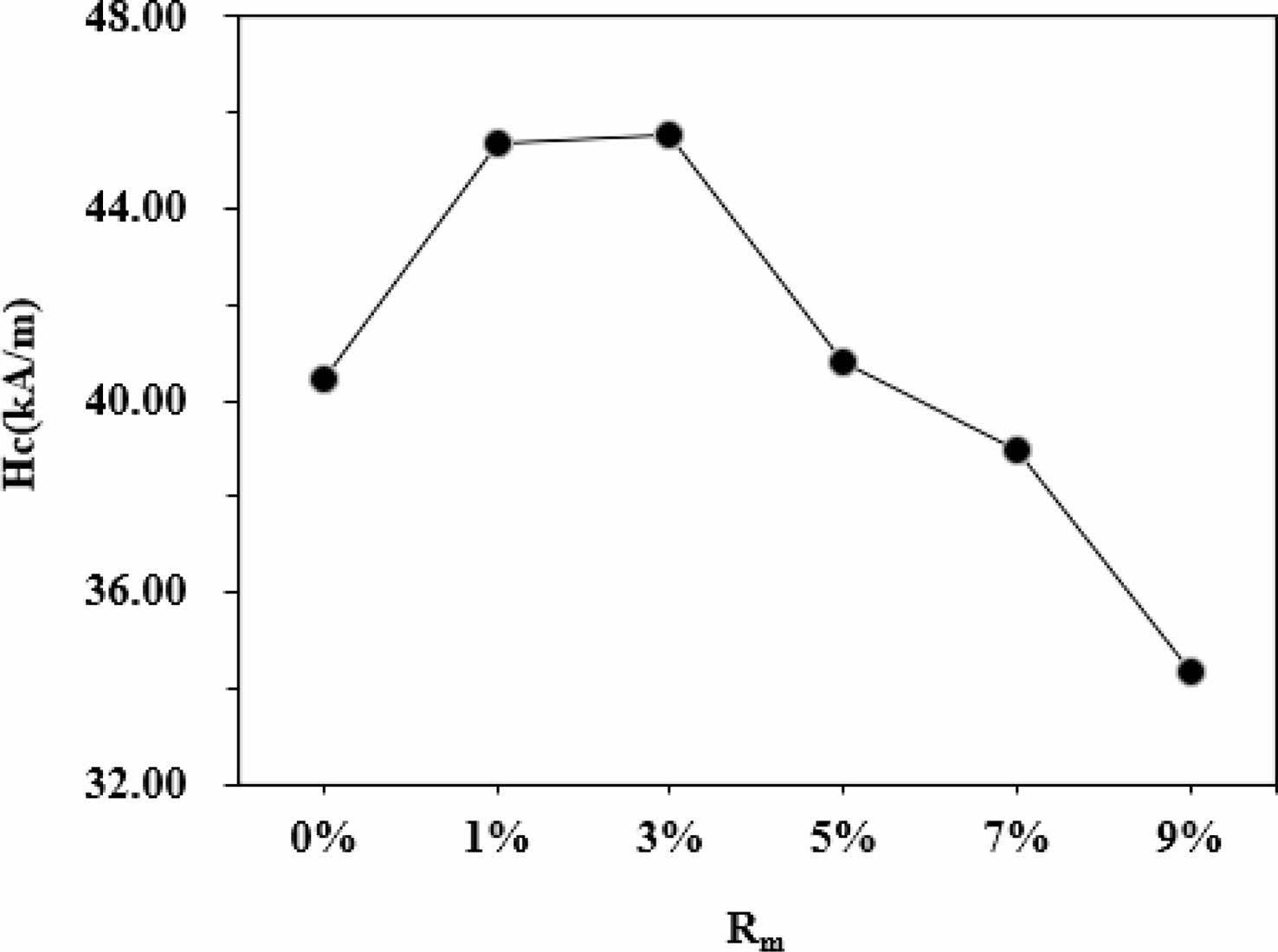
As can be seen from Table 1 and Fig. 6, it appears that the variation of Ms and Mr is obvious for different Rm. The Ms of samples increases with the increase of Rm from 0% to 9%, and it reaches 70.22 emu/g when Rm=9%. The Mr of specimens increases with the increase of Rm from 0% to 1%, while it decreases when Rm increases from 1% to 9%. The maximum Mr is 21.36 emu/g (Rm=1%). The effects of different Rm on the Hc of samples have been presented in Fig. 7 and Table 1. It is apparent that the Hc of samples first increases from 40.45 kA/m (508 Oe) (at Rm=0%) to 45.54 kA/m (572 Oe) (at Rm=3%), which is the maximum value of Hc. However, with a further increase of Rm from 3% to 9%, the Hc decreases continuously to 34.34 kA/m (431 Oe) at Rm=9%. The sample with Rm=3% exhibits the relatively good magnetic properties, among which the Ms is 68.24 emu/g, the Mr is 21.29 emu/g and the Hc is 45.54 kA/m (572 Oe).
In the M-type hexaferrite crystal structure, Fe3+ ions occupy five different sites, including the spin up sites 2a, 2b and 12k as well as the spin down sites 4f1 and 4f2. S.W. Lee et al. [23] and L. Lechevallier et al. [24] reported that Zn2+ ions preferentially replace Fe3+ ions at 4f1 spin-down sites, which will result in an increase of the number of spin-up Fe3+ ions compared with that of spin-down Fe3+ ions. J.M. Bai et al. [25] reported that for a large amount of La-Zn substitution, the substitution of Fe3+ ions by Zn2+ ions can lead to the weakening of the strength of superexchange, which will lead to the transformation of the colinear arrangement of Fe3+ ions to the non-colinear arrangement, accompanied by the spin-inclined structure. Therefore, as the Rm increases from 0% to 1%, the occupation of Zn2+ at 4f1 sites reduces the spin down magnetic moment and enhances the overall magnetic moment, and correspondingly the Mr increases. It is well-known for the M-type hexaferrites that the magnetic moments of Fe3+ ions are arranged collinearly due to existence of superexchange interaction [26]. The decrease of Mr with Rm from 1% to 9% are due to the weakening of Fe3+-O-Fe3+ superexchange interaction strength at the 12k- and 2b-sites. It was found that the Hc of specimens increases first and then decreases with the increase of Rm. It is commonly known that the Hc is mainly dependent on the magnetic crystal anisotropy. Therefore, the increasing Hc for Rm from 0% to 3% can be attributed to the increasing magnetocrystalline anisotropy field after the introduction of ZFO substitution. However, since Zn2+ is a kind of non-magnetic ion, the substitution of magnetic ion Fe3+ will inevitably reduce the total magnetic crystal anisotropy constant and the anisotropy field, thereby leading to the decrease of Hc. J.C. Corral-Huacuz et al. [27] and X.Q. Shen et al. [28] reported that the decrease of Hc with the increasing Zn2+ ion amount was mainly due to the decrease of magnetic crystal anisotropy constant caused by the substitution of Fe3+ ions with Zn2+ ions.
|
Fig. 1 XRD patterns of as-synthesized SrCaLaM (Rm=0%) and ZFO powders. |
|
Fig. 2 XRD patterns of the hexaferrite SrCaLaM magnetic powders with different Rm. |
|
Fig. 3 The crystal axis ratio of c/a of the hexaferrite SrCaLaM magnetic powders with different Rm. |
|
Fig. 4 Typical SEM images of the hexaferrite SrCaLaM magnetic powders with different Rm.: (a) 0%, (b) 1%, (c) 3%, (d) 5%, (e) 7% and (f) 9%. |
|
Fig. 5 RT magnetic hysteresis loops of specimens with different Rm. Rm: (a) 0%, (b) 1%, (c) 3%, (d) 5%, (e) 7%, and (f) 9%. |
|
Fig. 6 The Ms and Mr of samples with different Rm. |
|
Fig. 7 The Hc of samples with different Rm. |
The M-type ferrite Sr0.1Ca0.4La0.5Fe12O19 ferrite powders with different ZFO substitution amount (Rm, 0%, 1%, 3%, 5%, 7% and 9%) were obtained using a ceramic process. The XRD patterns showed that the single magnetoplumbite phase was obtained in the magnetic powders with the increasing Rm from 0% to 9%. The SEM study manifested that the particles were distributed uniformly in the samples. The VSM study indicated the magnetic properties were affected by the Rm greatly due to the occupation sites of Zn2+. It was found that the sample with Rm=3% exhibits the relatively good magnetic properties of Ms=68.24 emu/g, Mr=21.29 emu/g and Hc=45.54 kA/m (572 Oe). According to this study, the SrM ferrites can be manufactured more economically through adding the low-cost ZFO to reduce the usage of rare earth elements.
- 1. S. Zhang, C.X. Cao, S.B. Su, A.L. Xia, H.Y. Zhang, H.L. Li, Z.Y. Liu, and C.G. Jin, J. Adv. Ceram. 12[4] (2023) 815-821.
-

- 2. A.L. Xia, D.X. Du, P.P. Li, and Y.X. Sun, J. Mater. Sci: Mater Electron 22 (2011) 223-227.
-

- 3. M.M. Hessien, M.M. Rashad, M.S. Hassan, and K. El-Barawy, J. Alloy. Compd. 476 (2009) 373-378.
-

- 4. A.L. Xia, C.H. Zuo, L. Chen, C.G. Jin, and Y.H. Lv, J. Magn. Magn. Mater. 332 (2013) 186-191.
-

- 5. L. Qiao, B. Xu, Q. Xi, J. Zheng, and L. Jiang, Ceram. Int. 36 (2010) 1423-1427.
-

- 6. D.T.M. Hue, P. Lampen, T.V. Manh, and V.D. Viet, J. Appl. Phys. 114 (2013) 1191.
-

- 7. F.Y. Wang, J.J. Ji, C.X. Cao, C.Z. Gong, A.L. Xia, H.Y. Zhang, H.L. Li, Z.Y. Liu, and C.G. Jin, J. Phys. Chem. Sol. 163 (2022) 110565.
-

- 8. X. Liu, W. Zhong, S. Yang, Z. Yu, B. Gu, and Y. Du, J. Magn. Magn. Mater. 238 (2002) 207-214.
-

- 9. X.B. Zhao, S. Zhang, J.S. Li, A.L. Xia, and Y.J. Yang, J. Ceram. Process. Res. 24 (2023) 98-102.
-

- 10. Y. Yang, X. Liu, D. Jin, and Y. Ma, Mater. Res. Bull. 59 (2014) 37-41.
-

- 11. Y.J. Yang, F.H. Wang, X.S. Liu, J.X. Shao, and D.H. Huang, J. Magn. Magn. Mater. 421 (2017) 349-354.
-

- 12. C.J. Li, B. Wang, and J.N. Wang, J. Magn. Magn. Mater. 324 (2012) 1305-1311.
-

- 13. B.K. Rai, S.R. Mishra, and V.V. Nguyen, J. Alloys Compd. 550 (2013) 198-203.
-

- 14. Y.J. Yang, F.H. Wang, J.X. Shao, M.L. Li, and D.H. Huang, J. Ceram. Process. Res. 18 (2017) 151-155.
-

- 15. P.A.M. Castellanos, A.C.M. Borges, G.O. Melgar, J.A. Garcia, and E.G. Alcaide, Physica B 406 (2011) 3130-3136.
-

- 16. W. Zhang, B. Yang, H. Xi, L. Wang, X. Lu, and L. Qiao, J. Alloys Compd. 546 (2013) 234-238.
-

- 17. Y.J. Yang, F.H. Wang, J.X. Shao, and Q.L. Cao, J. Ceram. Process. Res. 17 (2016) 615-619.
-

- 18. L. You, Q. Liang, J. Zheng, M. Jiang, L. Jiang, and J. Sheng, J. Rare Earth. 26 (2008) 81-84.
-

- 19. D.A. Vinnik, A.S. Semisalova, L.S. Mashkovtseva, A.K. Yakushechkina, S. Nemrava, S.A. Gudkova, D.A Zherebtsov, N.S. Perov, L.I. Isaenko, and R. Niewa, Mater. Chem. Phys. 163 (2015) 416-420.
-

- 20. P.F.S. Pereira, A.P.D. Moura, I.C. Nogueira, M.V.S. Lima, E. Longo, P.C.D.S. Filho, O.A. Serra, E.J. Nassar, and I.L.V. Rosa, J. Alloys Compd. 526 (2012) 11-21.
-

- 21. M.J. Iqbal, M.N. Ashiq, and P.H. Gomez, J. Alloys Compd. 478 (2009) 736-740.
-

- 22. G.B. Teh, Y.C. Wong, and R.D. Tilley, J. Magn. Magn. Mater. 323 (2011) 2318-2322.
-

- 23. S.W. Lee, S.Y. An, I.B. Shim, and C.S. Kim, J. Magn. Magn. Mater. 231 (2005) 290-291.
-

- 24. L. Lechevallier, J.M. Le. Breton, J.F. Wang, and I.R. Harris, J. Phys: Condens. Matter. 16 (2004) 5359-5376.
-

- 25. J.M. Bai, X.X. Liu, T. Xie, F. Wei, and Z. Yang, Mater. Sci. Eng. B 68 (2000) 182-185.
-

- 26. Y.J. Yang, F.H. Wang, J.X. Shao, X.S. Liu, S.J. Feng, and J.S. Yang, J. Magn. Magn. Mater. 401 (2016) 1039-1045.
-

- 27. J.C. Corral-Huacuz, and G. Mendoza-Suarez, J. Magn. Magn. Mater. 245 (2002) 430-433.
-

- 28. X.Q. Shen, M.Q. Liu, F.S. Song, and Y.W. Zhu, Appl. Phys. A 104 (2011) 109-116.
-

 This Article
This Article
-
2023; 24(4): 634-639
Published on Aug 31, 2023
- 10.36410/jcpr.2023.24.4.634
- Received on Mar 18, 2023
- Revised on Jun 17, 2023
- Accepted on Jul 6, 2023
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ailin Xia
-
School of Materials Science and Engineering, Anhui University of Technology, Maanshan 243002, China
Tel : +86 13665557919 Fax: +86 05552311570 - E-mail: alxia@126.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.