- Stable temperature dependence of dielectric properties in BaTiO3-Nb2O5-Co3O4 + BaO-V2O5 system
Chang Ho Lee and Jung Rag Yoon*
R&D Center, Samwha Capacitor, Yong-In, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
High reliability components that can operate reliably in harsh and special environments such as aerospace and military fields are required. In this study, the BaTiO3-Nb2O5-Co3O4 composition, which is the basic composition of precious metal electrode multilayer ceramic capacitors with high reliability, was added with the binary BaO-V2O5 composition to confirm the electrical properties and microstructure changes. First, a BaTiO3-Nb2O5-Co3O4 composition having a core-shell structure was prepared, and then a Ba3V4O13 composition was synthesized with a binary BaO-V2O5 composition. Then, 1, 3, and 5 wt% of Ba3V4O13 composition were added to observe changes in electrical properties and microstructure according to firing temperatures. As a result, the maximum dielectric constant 2502, dielectric loss of 0.74%, insulation resistance of 1.04E12 Ω, and X8R temperature characteristics could be satisfied in the firing temperature range of 1150-1210 ℃
Keywords: Aerospace, Precious metal electrode, Multilayer ceramic capacitors
Recently, the remarkable growth of commercial space companies has led to a paradigm shift in the global space industry. As space development is becoming increasingly commercialized and private sector is becoming more involved in space development, with the advent of commercial space, launch vehicles and satellites are becoming more innovative, commercial satellite data is being used more frequently, and industries are merging. All of these factors are lowering the entry barriers to the space industry. Due to these effects, private investment and industrial growth in the aerospace industry are rapidly increasing. Therefore, the demand for parts and materials for aerospace based on advanced technology and reliability is increasing [1, 2]. Semiconductors, sensors, and passive components are used for aerospace electronic components, and products suitable for harsh space environments are required. Multilayer ceramic capacitors (MLCCs), resistors, and inductors are core components in passive components, and they require products that meet the standards for MLCC for space use. Multilayer ceramic capacitors are manufactured by simultaneous sintering of a ceramic dielectric and metal internal electrodes. Furthermore, they are classified into precious metal electrode (PME) and based metal electrode (BME) MLCCs based on the types of internal electrodes used during the sintering process performed at high temperatures. In aerospace applications, PME MLCC was primarily applied with Ag/Pd electrodes. However, since 2017, BME has been newly applied with Ni electrode that meets the MIL-PRF-32535 standard. Although PME MLCC can be sintered in air, occurrence of delamination due to the difference in shrinkage between the ceramic and Ag/Pd electrodes during the sintering process is an issue. In contrast, BME MLCC can make the dielectric very thin and multi-layered to realize high capacitance. When a Ni electrode was used, as sintering should be performed in a reducing atmosphere, positively charged oxygen vacancies were generated in the ceramic. Therefore, deterioration occurred in which the insulation resistance of the dielectric reduced. To solve the issue, rare earth oxides, such as Y2O3, Dy2O3, and Ho2O3, are added to improve the deterioration of insulation resistance [3-6].
Two types of PME MLCCs are available for space: those that use Ag/Pd type primarily for temperature compensation of a paraelectric material with a small change in capacitance depending on temperature, and those that use a ferroelectric as a primary component for high permittivity system with a large capacitance change depending on temperature.
As specified in the EIA (Electronic Industries Association) standard, MLCC is classified into Classes I and II based on dielectric material. Class I dielectric material uses calcium zirconate (CaZrO3)-based and titanium dioxide (rutile, TiO2)-based paraelectric materials, and they exhibit a very small capacitance change due to temperature and low dielectric loss. Class II dielectric material uses a barium titanate (BaTiO3)-based ferroelectric, and the rate of change of the temperature characteristic of the dielectric constant is significant. In addition, although the dielectric constant and dielectric loss change under AC and DC voltages, a high dielectric constant allows for a high capacitance capacitor to be realized [7]. For manufacturing MLCC with Class II characteristics, BaTiO3 was used as a dielectric material and doped with a small quantity of various additives to suppress grain growth, achieve low-temperature sintering, and achieve high reliability. To stabilize the capacitance change rate based on the temperature change (TCC), to reduce the dielectric loss (DF), and to realize high reliability, additives and sintering conditions having a core-shell type grain structure are required. The core is a region composed of a pure BaTiO3 lattice, which is a ferroelectric phase, and the shell region is known as a paraelectric phase, in which an additive concentration gradient exists from the grain boundary to the interior of the grain [8, 9]. For PME MLCC, studies on dielectric ceramics in the BaTiO3-Nb2O5-Co3O4 (BNC) system on the core-shell structure and electrical characteristics based on the Nb/Co ratio, dielectric constant characteristics, and microstructure changes with temperature have been conducted [10-12].
Based on a study by H. Chazono et al. [10, 11], a core-shell structure was formed at a sintering temperature of ≥1250 ℃ regardless of the Nb/Co ratio and added quantities of Nb and Co. Additionally, the temperature coefficient of capacitance (TCC) was increased by X7R (-55 ℃ ~ +125 ℃, capacitance change rate within ±15%), and while having a temperature characteristic, excellent dielectric constant characteristics could be realized. PME MLCC has high reliability in harsh environments by using noble metal. The reliability characteristics of the Ag-Pd internal electrode were excellent. However, the efficiency decreased with increased Pd content. Therefore, a dielectric ceramic is required, that is capable of using a 30 Ag–70 Pd electrode with an Ag content of ≤ 30%, can be sintered at a low temperature, and has high reliability. Generally, low-temperature liquid additives such as CuO, B2O3, Bi2O3, V2O5, Li2CO3, and LiF are added for low-temperature sintering of dielectric ceramics or glass frit is added to lower the sintering temperature. However, due to the instability of the slurry in the MLCC manufacturing process, an issue arises in that sintering or electrical properties are nonuniform or dielectric constant property rapidly deteriorates [13, 14]. Therefore, from BaV2O6, Ba2V2O7, Ba3V2O8, Ba4V2O9, and Ba3V4O13 compositions, which are BaO-V2O5 binary systems known as ULTCC (ultra low temperature co-fired ceramic) capable of sintering at 600 ℃, a Ba3V4O13 (BVO) composition having low-temperature sintering and excellent dielectric constant characteristics was selected. In this study, by adding Ba3V4O13 to the BaTiO3-Nb2O5-Co3O4 ternary composition, a dielectric material for X7R-based PME MLCC with excellent dielectric constant and electrical characteristics, which enabled low-temperature sintering and reliability of aerospace MLCCs, was studied [15, 16].
Preparation of Samples
As a primary composition, barium titanate (BaTiO3, 99%, 300 nm), Nb2O5 (Sigma Aldrich, 99%), and Co3O4 (Sigma Aldrich, 99%) were mixed, as shown in Table 1, and calcined at 1100 ℃ for 2 h. BaTiO3 was synthesized by the solid-state method with an average particle size of 300 nm. As a sintering material, Ba3V4O13 ceramics were prepared by conventional solid ceramic methods by calcining at 600 ℃ for 4 h. High purity BaO (Sigma Aldrich, 99%) and V2O5 (Sigma Aldrich, 99%) were used as starting materials. The powders were weighed based on the composition of BT-Nb-Co (98BaTiO3-1.5Nb2O5-0.5Co3O4)- xBa3V4O13 (x = 0-5 wt%). The mixture was milled with 3 mm diameter ZrO2 balls, and deionized water was used as a medium. After ball milling for 24 h, the slurry was dried at 150 ℃. Afterward, the dry powders were compacted to discs with a diameter and thickness of 10 and 1.0 mm, respectively, using 1 wt% polyvinyl alcohol (PVA) as a binder. Subsequently, it was sintered at a desired temperature for 2 h and then cooled in the furnace. The specimens were printed with silver paste on their upper and bottom surfaces and fired at 600 ℃ for 1 h for electrical measurements.
Measurement of samples
Crystal structure and secondary phase formation of the ceramic samples were determined at room temperature by x-ray diffraction (XRD). The surface microstructure of the ceramic samples was observed using scanning electron microscopy (SEM). Transmission electron microscopy (TEM) was used to study the core-shell structures of the sintered disc ceramics. After disc ceramic samples were fired at sintering temperature with silver paste on both sides, the room temperature dielectric constant and dielectric loss were determined by a precision LCR meter (Model E4980A, Aglient) at 1 kHz and 1 Vrms. The temperature-capacitance characteristics of the disc ceramic samples were measured from -55 to 150 ℃ with an LCR Meter (Model E4980A, Agilent) and with an automatic temperature controller at 1 kHz and 1 Vrms. The insulation resistance of the specimens was measured by a digital super megohm meter (Model B2985A, Agilent) at 25 ℃. The bulk density was measured using the Archimedes method.
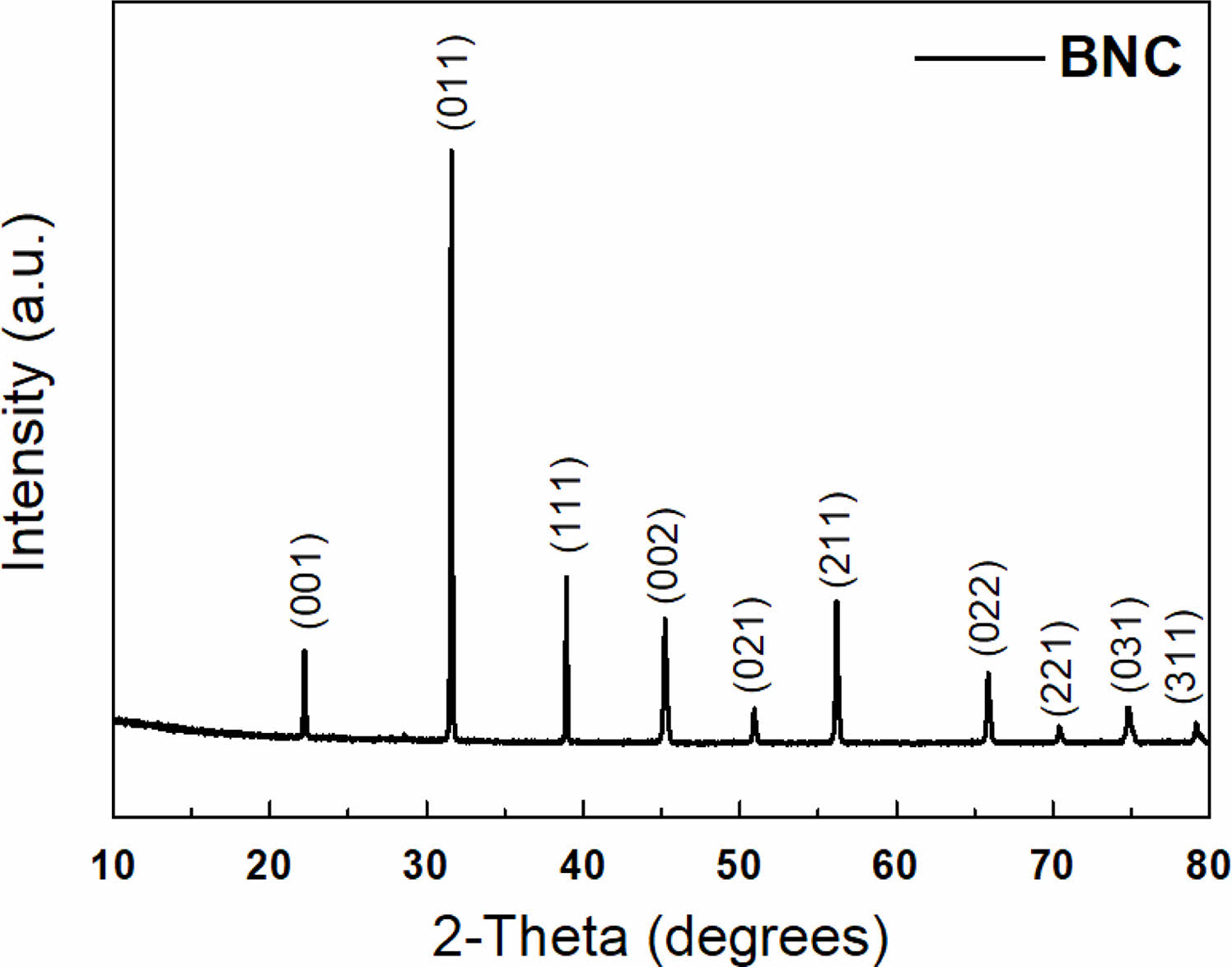
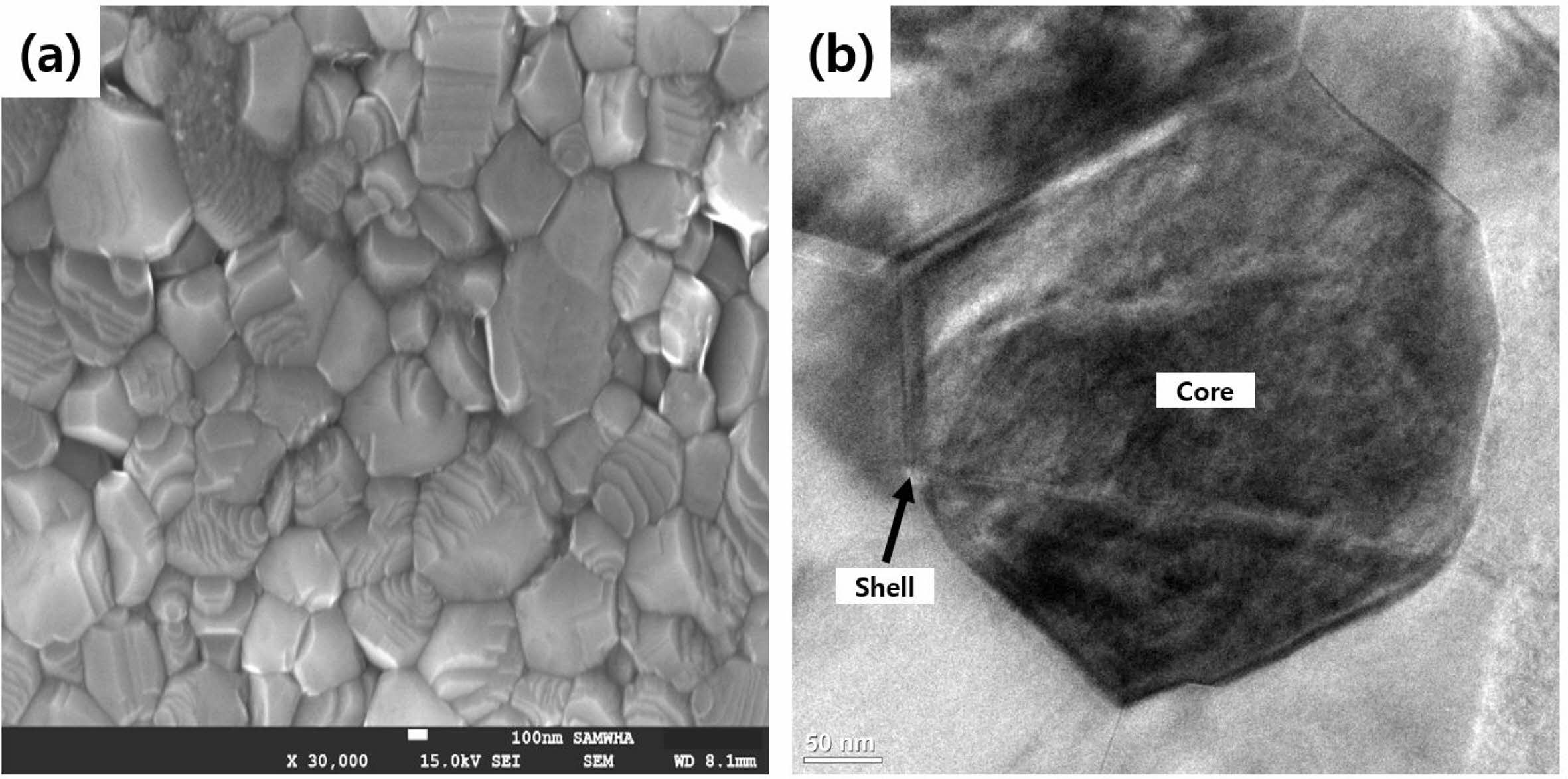
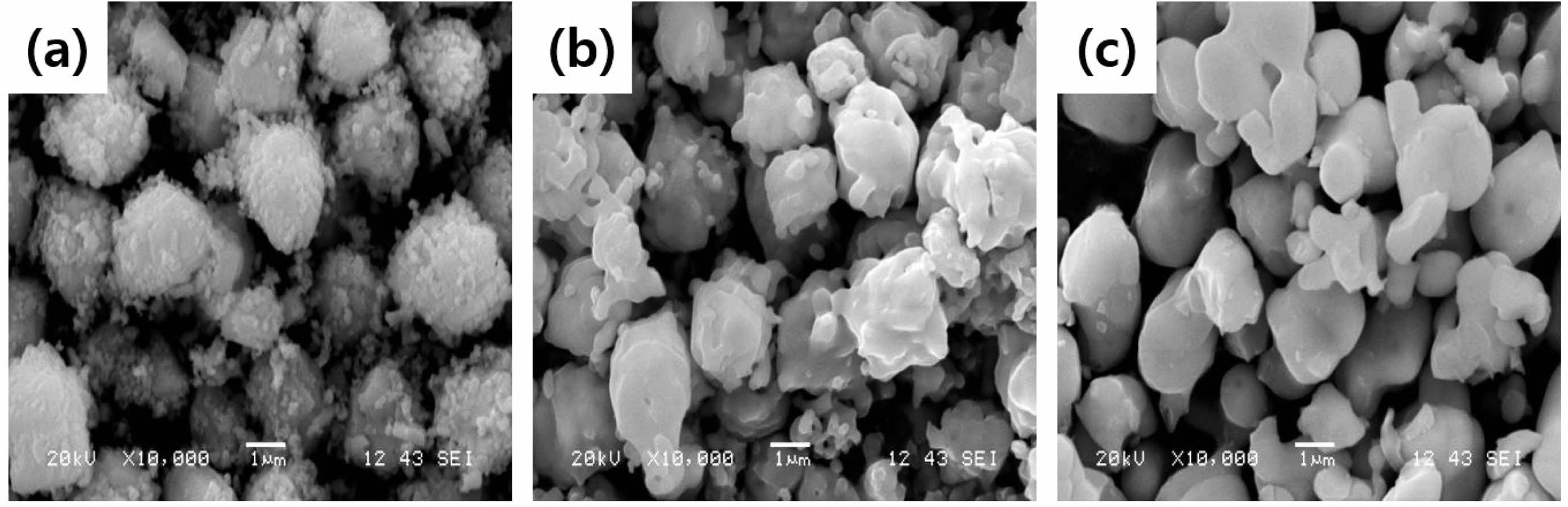
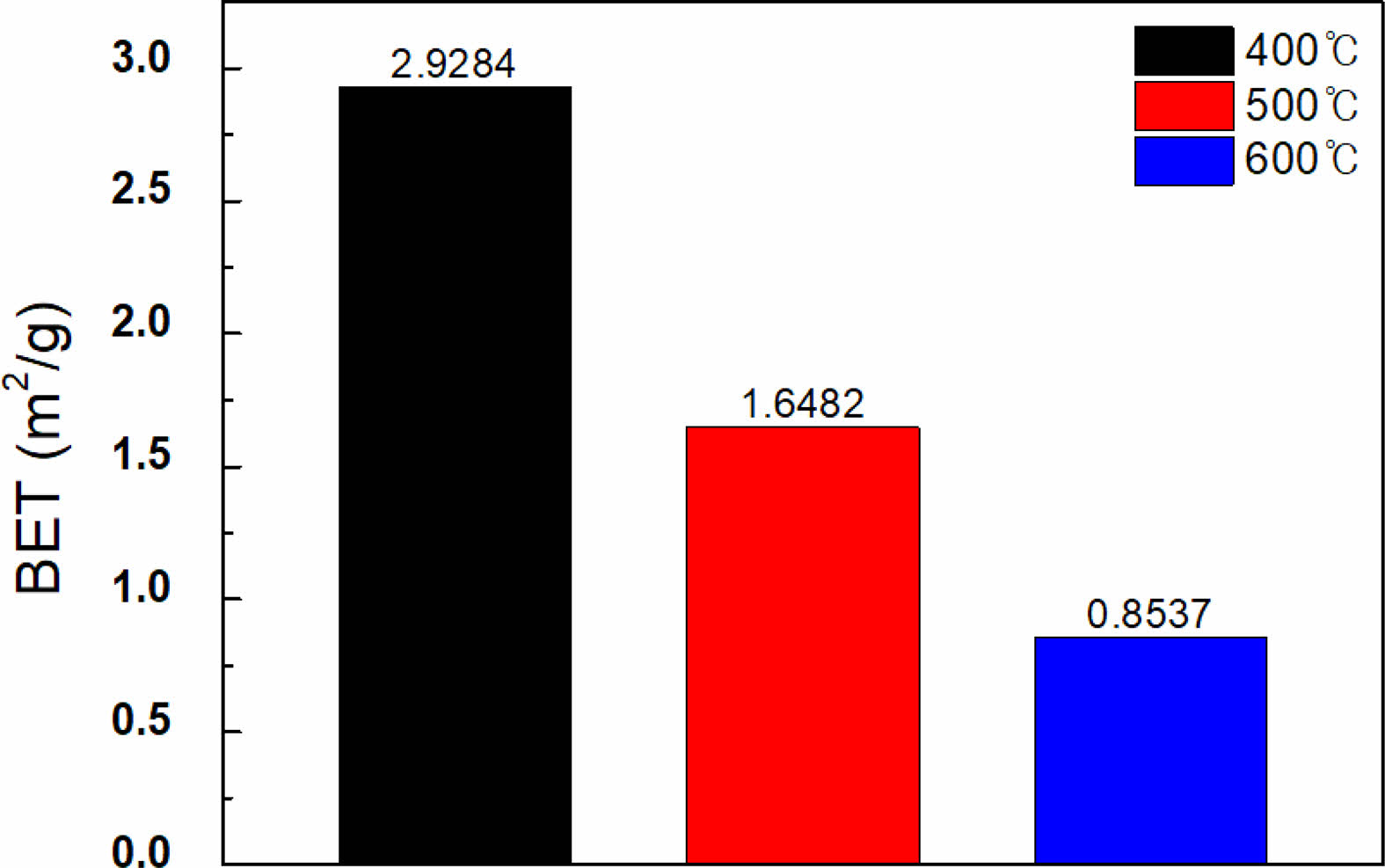
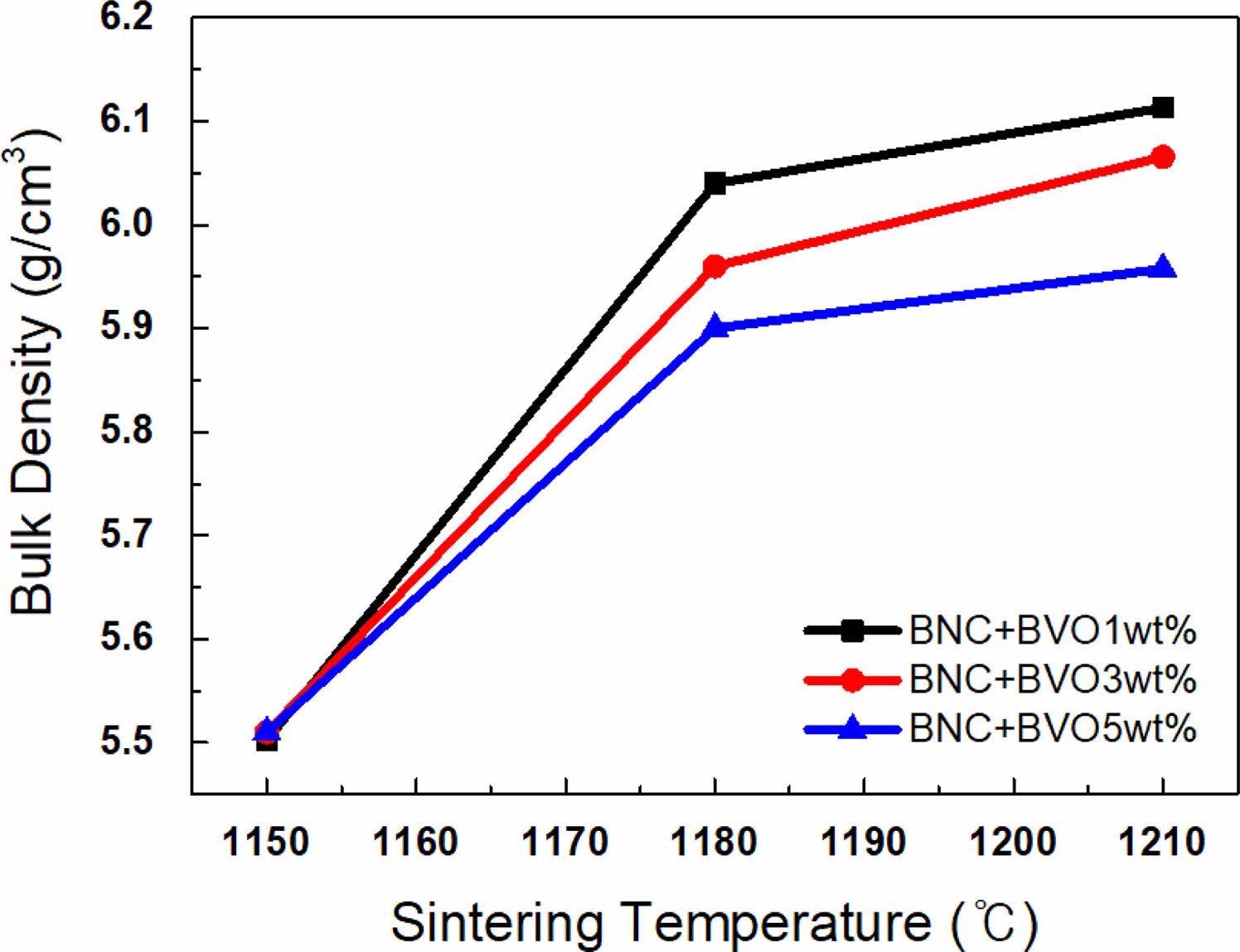
Figure 1 illustrates the XRD result of the synthesized powder sample, as shown in Table 1, with the BaTiO3-Nb2O5-Co3O4 (BNC) ternary composition. The primary phase peaks of BaTiO3 were confirmed, and secondary phase formation based on the addition of Nb2O5 and Co3O4 was not observed. Figure 2(a) shows the SEM analysis result for analyzing the microstructure of the BNC disc sample sintered at a temperature of 1280 ℃, and Fig. 2(b) illustrates the TEM analysis result to confirm the formation of the core-shell structure. From the SEM results presented in Fig. 2(a), a microstructure with uniform grain size can be confirmed without secondary phase of matrix grains. With the TEM analysis result of Fig. 2(b), we can observe the formed core-shell structure. Figure 3 shows the SEM result according to the calcination temperature of Ba3V4O13 (BVO) ceramic, which is a BaO-V2O5 binary composition, acting as a low-temperature sintering additive in the primary BNC composition. The calcination temperatures were 400, 500, and 600 ℃. Furthermore, we observed that the grains agglomerated as the calcination temperature increased. The BET results are displayed in Fig. 4 with the calcination temperature of BVO ceramics, and the value of 2.92 m2/g at 400 ℃ decreased by approximately 3.5 times to 0.85 m2/g at 600 ℃. In this study, microstructure and electrical properties were analyzed by adding 1, 3, and 5 wt% of BVO ceramics calcined at 600 ℃ for 4 h to the primary BNC composition. Figure 5 depicts the bulk density values based on the sintering temperature of BNC+BVO (1/3/5 wt%) ceramic disc samples measured by Archimedes method. For sintering temperature of 1150 ℃, the bulk density value of the BNC+BVO ceramic disc samples was approximately 5.5 g/cm3, and no difference was observed due to the change in the added quantity of BVO. For the sintering temperature ≥ 1180 ℃, it has a bulk density ≥ 5.8 g/cm3 similar to the density of pure BaTiO3 ceramic. Additionally, the bulk density decreased as the amount of BVO ceramic increased.
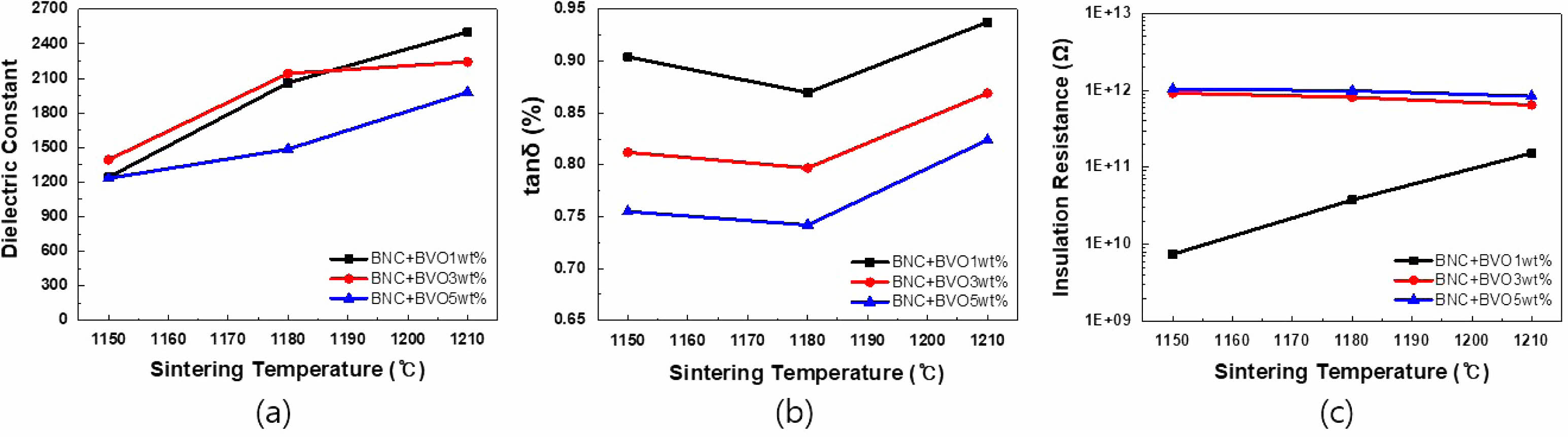
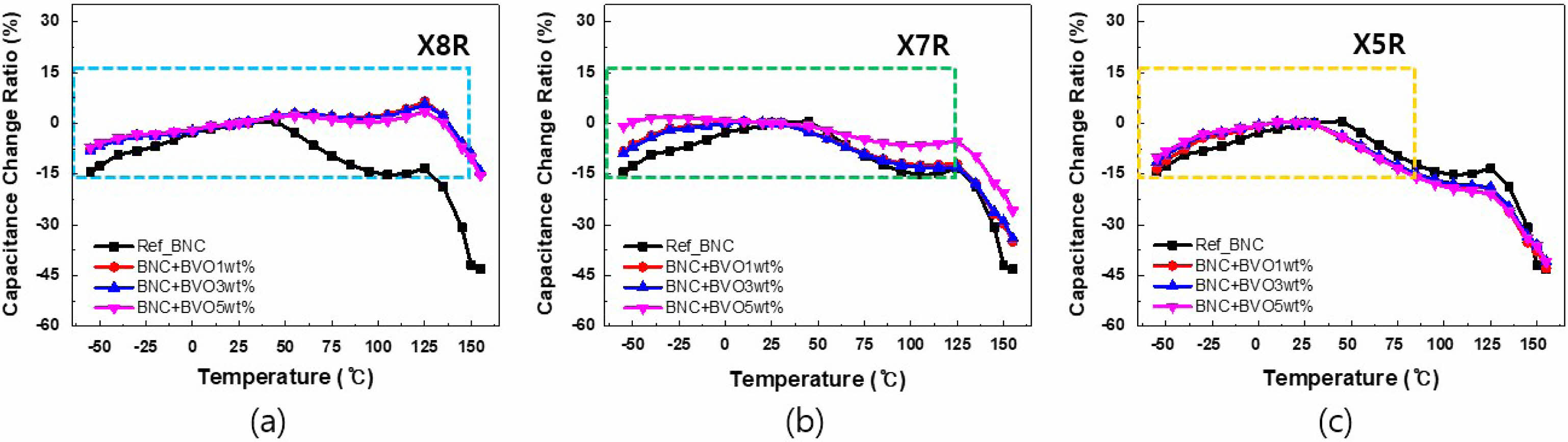
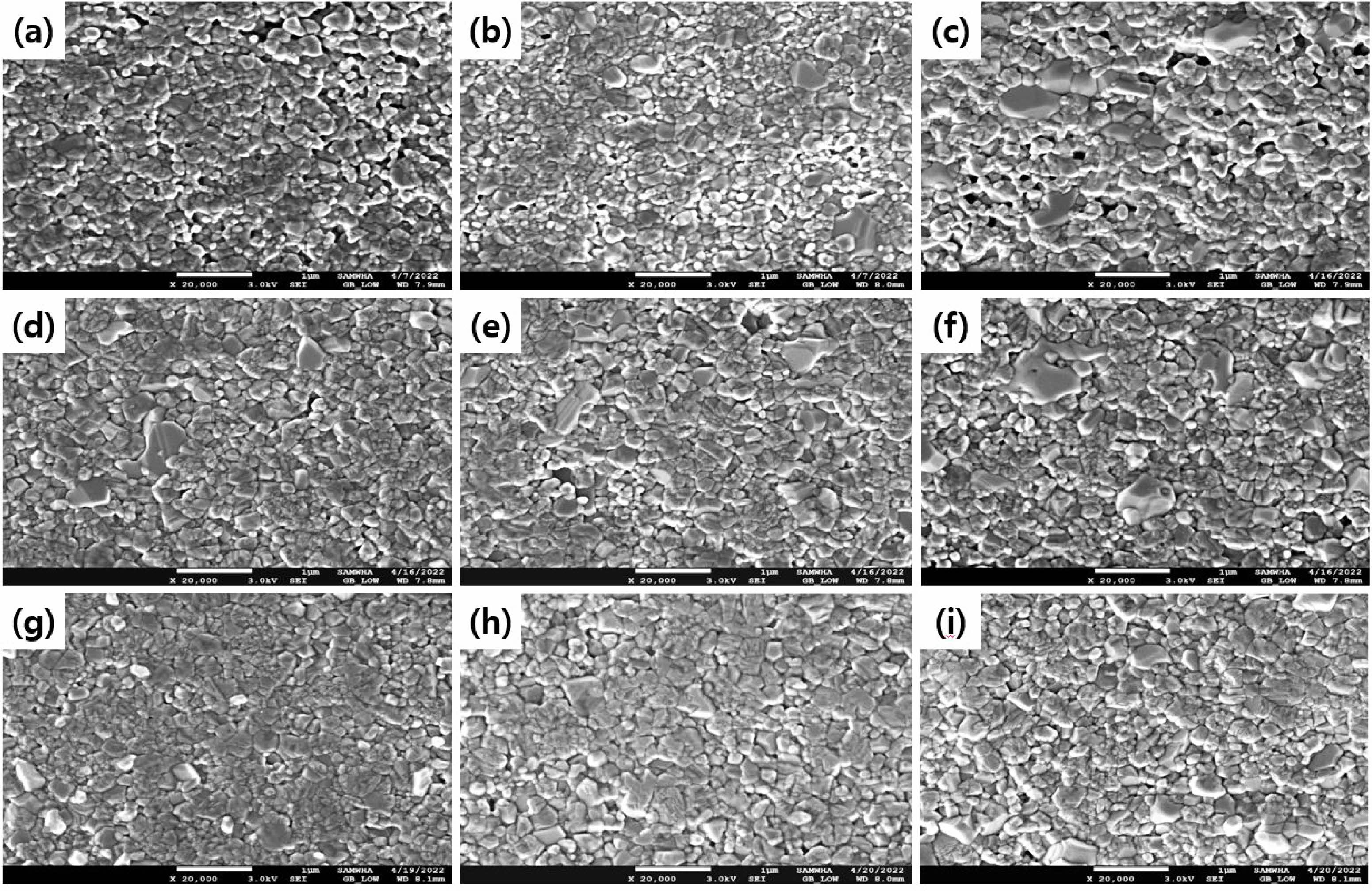
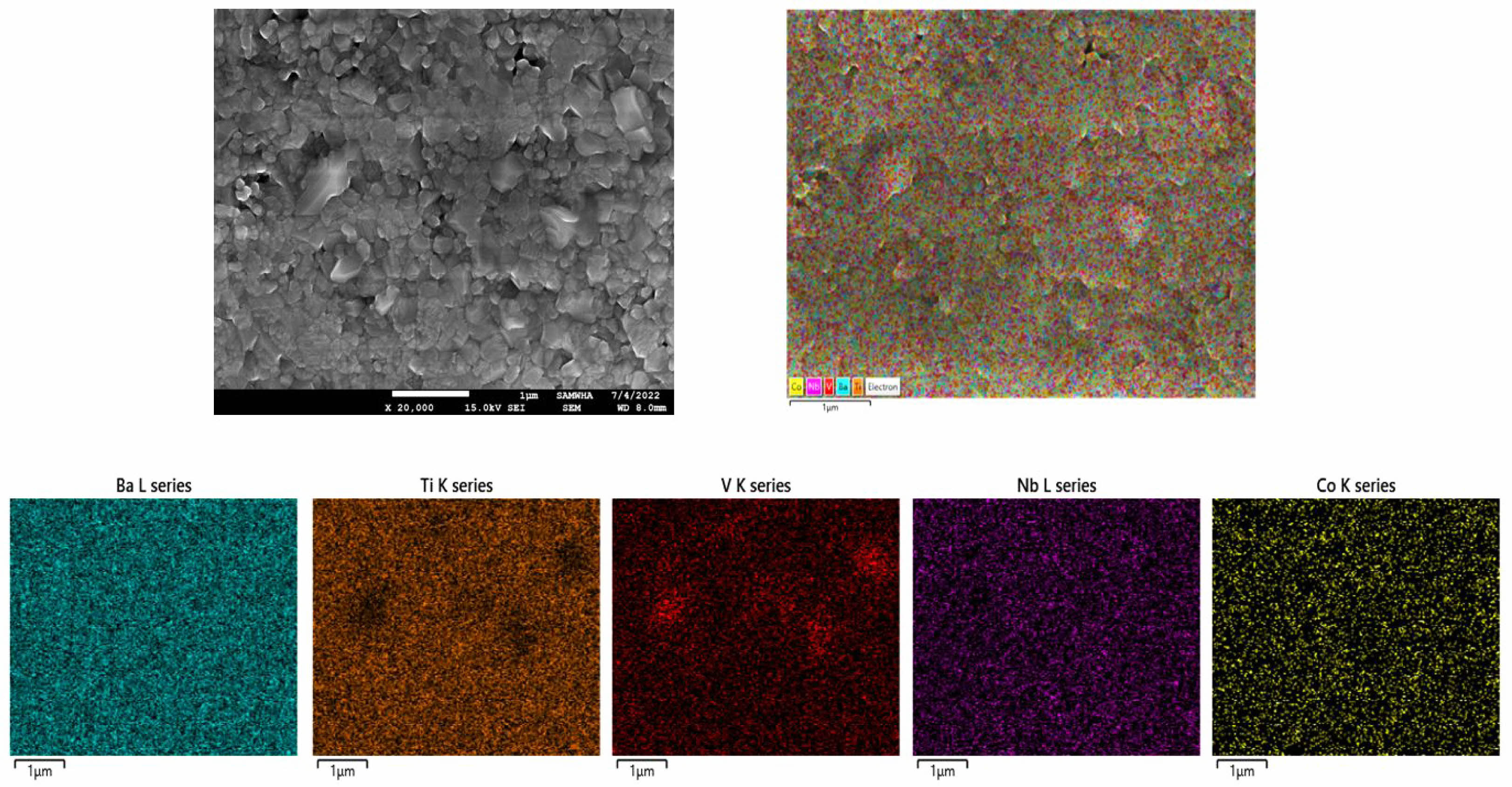
Figure 6 is a graph of the dielectric constant, tanδ, and insulation resistance based on the firing temperature. Because of the dielectric constant shown in Fig. 6(a), the dielectric constant increased with the firing temperature regardless of the added quantity of BVO. Figure 6(b) shows the tanδ result, and practically, no change was observed in loss until the firing temperature of 1180 ℃. However, tanδ increased rapidly at a temperature of >1180 ℃. In addition, in BVO 1 and 3 wt%, no difference in insulation resistance was observed due to the change in the firing temperature. However, when BVO 5 wt% was added, the initial insulation resistance greatly decreased and increased with the firing temperature, as shown in Fig. 6(c). Capacitance is a crucial characteristic for MLCC rate, and the changes in capacitance with temperature are shown in Figs. 7(a)-(c). Figure 7(a) illustrates the firing results at 1150 ℃, and all compositions of BNC+BVO showed a dramatic improvement in temperature characteristics compared to the reference BNC compositions, satisfying even the X8R (-55 to +150℃) characteristics. Figure 7(b) shows the results at a firing temperature of 1180 ℃. Additionally, the X7R (-55 to +125 ℃) characteristics were rarely satisfied when 1 and 3 wt% of BVO were added, whereas the addition of 5 wt% BVO showed more stable temperature characteristics. However, when the firing temperature was 1210 ℃, the temperature characteristics deteriorated compared to the reference BNC composition, and all compositions satisfied the X5R (-55 to +85 ℃) characteristics only, regardless of the added quantity of BVO. Figures 8 (a)-(i) depict the results of SEM analysis to confirm the microstructure change due to the firing temperature and the added quantity of BVO. In all the results, traces of liquid phase sintering can be observed, and the traces of liquid phase sintering increased with the added quantity of BVO and firing temperature. Among the various firing and added BVO quantity variables, EDS analysis of the composition fired at 1180 ℃ and added with 3 wt% BVO is performed, as shown in Fig. 9. The uniformly distributed BNC composition could be confirmed through the Nb (niobium) and Co (cobalt) peaks, and the traces of liquid phase sintering by the addition of BVO could be confirmed through Ti (titanium) peak in empty areas and concentrated V (vanadium) peaks.
|
Fig. 1 XRD patterns of BNC powder sintered at 1280 ℃ for 1 hr. |
|
Fig. 2 (a) SEM image and (b) TEM image of BNC ceramic sintered at 1280 ℃ for 1 hr. |
|
Fig. 3 SEM images of Ba3V4O13 ceramics by calcination tempeature, (a) 400 ℃, (b) 500 ℃, and (c) 600 ℃ for 4 hr. |
|
Fig. 4 BET result of Ba3V4O13 ceramics by calcination temperature |
|
Fig. 5 Bulk density versus sintering temperature in BNC+ Ba3V4O13 ceramics. |
|
Fig. 6 Electrical Properties of BNC+Ba3V4O13 ceramics according to the sintering temperature. (a) dielectric constant, (b) tanδ, and (c) insulation resistance. |
|
Fig. 7 Temperature coefficient of capacitance (TCC=∆C/C25℃) curves. (a) sintered at 1150 ℃, (b) sintered at 1180 ℃, and (c) sintered at 1210 ℃. |
|
Fig. 8 SEM images of BNC+Ba3V4O13 ceramics according to the sintering temperatur and BVO content. (a) 1 wt% at 1150 ℃, (b) 3 wt% at 1150 ℃, (c) 5 wt% at 1150 ℃, (d) 1 wt% at 1180 ℃, (e) 3 wt% at 1180 ℃, (f) 5 wt% at 1180 ℃, (g) 1 wt% at 1210 ℃, (h) 3 wt% at 1210 ℃, and (i) 5 wt% at 1210 ℃. |
|
Fig. 9 EDS images of BNC+Ba3V4O13 (3 wt%) ceramics sintered at 1180 ℃ for 1 hr |
In this study, to develop a dielectric composition with stable temperature characteristics, a BaTiO3-Nb2O5-Co3O4 (BNC) composition was added to a BaO-V2O5 (BVO) binary composition as an additive and its electrical properties and microstructures were analyzed. First, a core-shell structured BNC composition having no secondary phase and uniform particle size was analyzed by XRD, SEM, and TEM. To secure temperature characteristics based on the BNC composition, the Ba3V4O13 composition was synthesized by mixing a BaO+V2O5 binary composition in a 3:2 molar ratio and calcining at 600 ℃ for 4 h. Afterward, 1, 3, and 5 wt% of the BVO composition were added to the BNC composition, and electrical properties such as bulk density, dielectric constant, tanδ, insulation resistance, and temperature characteristics were measured. In addition, microstructural changes were observed through SEM and EDS analysis. Therefore, a composition with a maximum dielectric constant of 2502, tanδ of 0.74%, high insulation resistance of 1.04E12 Ω, and even X8R characteristics was developed in the firing temperature range of 1150-1210 ℃. Additionally, we demonstrated the role of the BVO composition as an additive in the BNC composition, acting as a liquid phase sintering agent and lowering the sintering temperature without generating a secondary phase through SEM and EDS analysis. The developed BNC+BVO composition is considered to require continuous research and development for application to aerospace and military applications requiring high reliability characteristics with high dielectric constant and stable temperature characteristics.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021M1A3B2A01076907)
- 1. C.Y. Hwang, Space Policy Research. 14 (2021)
- 2. A. Teverovsky, “Leakage Currents in Low-Voltage PME and BME Ceramic Capacitors,” presented at the 7th International Conference on Electroceramics (ICE2015), Penn State Conference Center, State College PA, USA, (2015).
- 3. K. Hong, T.H. Lee, J.M. Suh, S.H. Yoon, and H.W. Jang, J. Mater. Chem. 7 (2019) 9782-9802.
-

- 4. T. Kirianov, H. Hagiwara, Kishi, and H. Ohsato, Appl. Phys. Part 1 41[11] (2002) 6934.
- 5. C.H. Lee and J.R. Yoon, Trans. Electr. Electron. Mater. 22[4] (2021) 424-431.
-

- 6. L. Chen, H. Wang, P. Zhao, C. Zhu, Z. Cai, Z. Cen, L. Li, and X. Wang, J. Am. Ceram. Soc. 102[7] (2019) 4178-4187.
-

- 7. Y. Mizuno1, H. Chazono, and H. Kishi, Jpn. J. Appl. Phys. 42[1] (2003) 1-15.
- 8. B.S. Rawal, M. Kahn, and W.R. Buessem, Adv. in Ceram. 1 (1981) 172-188.
- 9. S.K. Chiang, N.E. Lee, and D.W. Ready, Ceram. Bull. 66 (1987) 1230.
- 10. H. Chazono and H. Kishi, J. Am. Ceram. Soc. 82[10] (1999) 2689-2697.
-

- 11. F.K. Hennings and B.S. Schreinemacher, J. Eur. Ceram. Soc. 14 (1994) 463-467.
-

- 12. H. Chazono and H. Kishi, J. Am. Ceram. Soc. 83[1] (2000) 101-106.
-

- 13. A. Randall, S.F. Wang, D. Laubsher, J.P. Dougherty, and W. Huebner J. Mater. Res. 8[4] (1993) 871-879.
-

- 14. I. Hsing, H.C. Shiung, H.C. Chun, and L.F. Shen, Mat. Chem. and Phy. 113 (2009) 658-663.
-

- 15. U.A. Neelakantan, S.E. Kalathil, R. Ratheesh, J. Inorg. Chem. 2015[2] (2015) 305-310.
-

- 16. B. Masin, K. Ashok, S. Vishnu, H. Sreemoolanadhan, and K. Prabhakaran, Ceram. Int. 48[15] (2022) 22520-22526.
-

 This Article
This Article
-
2023; 24(3): 588-593
Published on Jun 30, 2023
- 10.36410/jcpr.2023.24.3.588
- Received on Jan 26, 2023
- Revised on Mar 22, 2023
- Accepted on May 25, 2023
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jung Rag Yoon
-
R&D Center, Samwha Capacitor, Yong-In, Korea
Tel : +82-31-330-5765 Fax: +82-31-332-7661 - E-mail: yoonjungrag@samwha.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.