- Synthesis of Co:MgAl2O4 nano-powders for saturable absorber
Wang Chuanyuna,b, Yang Weia,b, Wang Zhiqia,b, Liu Bina,b, Li Shihuaa,b, Lu Taoa,b, Li Xuerena,b, Miao Weipenga,b and Luo Weic,*
aState Key Laboratory of Super Abrasives, Zhengzhou 450001, China
bZhengzhou Research Institute for Abrasives & Grinding Co. Ltd., Zhengzhou 450001, China
cLuoyang Institute of Science and Technology, Luoyang 471023, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Uniform Co:MgAl2O4 nano-powders for saturable absorber were prepared using inverse-drip co-precipitation method. Effects of the precipitant and the metal ion solution on the composition and morphology of Co:MgAl2O4 nano-powders were studied. The results show that pure Co:MgAl2O4 nano-powders can be obtained, using the mixed solution of ammonium carbonate and ammonia as the precipitant. The specific surface area of the particles reached 36 m2/g and the primary particle size was 39 nm according to the SEM images and BET results
Keywords: Co:MgAl2O4 ceramic, Saturable Absorber, Nano-powders
Co:MgAl2O4 is a highly efficient saturable absorber for passive Q-switch of 1.5 µm "eye safe" laser generated by passive Q-switching function, which has the high peak power, strong smoke penetration, small transmission attenuation, low solar spectral irradiance strong photoelectric countermeasure [1-3], and is widely used in space optical communication, fast battlefield ranging, especially the laser-lidar of unmanned equipment and other fields [4-6]. In the Co:MgAl2O4 crystal structure, Co2+ can partially replaces Mg2+ ions, because Co2+ and Mg2+ ions have the same valence and similar radius [7]. Therefore, the physical properties of the Co:MgAl2O4 material are similar to those of magnesium aluminum spinel which has good thermal conductivity, high chemical stability and high laser damage threshold [8-10].
In the late 1990s, Ikesue [11] used hip technology to prepare highly transparent Co:MgAl2O4 for the first time in Japan, and the results showed that this transparent ceramic is promising to be used in the passive Q-switching of saturable absorbers and laser resonators. While, there is no further study on the nonlinear absorption performance until to 2014. Then Wajler [12] et.al used SPS technology to prepare Co:MgAl2O4 transparent ceramics with different concentrations, and studied their spectral properties and nonlinear absorption properties. The main raw material used in their research is the high activity spinel powder prepared by American Nano-cerox Company by spray combustion thermal decomposition method. The price of this powders is 10 times higher than that of the current commercial spinel powders, which is not suitable for the needs of large-scale industrial production [13-17]. The Co:MgAl2O4 nano-powders with high purity and good uniformity is necessary to syntheis Co:MgAl2O4 based saturable absorber with good optical quality and spectral performance. However, there is still no commercial Co:MgAl2O4 nano-powders on the market at present due to the cost and price problems [18-20].
In this paper, Co:MgAl2O4 nano-powders with high purity and good uniformity is prepared by the wet process technology, which is simple and cost cheap. The composition, micro morphology and spectral properties of Co:MgAl2O4 nano-powder were studied.
The Co:MgAl2O4 nano-powders is synthesized by reverse drop co-precipitation. Firstly, ammonium carbonate, magnesium nitrate, aluminum nitrate, cobalt nitrate and other raw materials were dissolved in the deionized water, and the obtained solution was filtered to remove the insoluble substances and mechanical impurities which may be introduced in the dissolution process. The true concentration and quantitative ratio of metal ion solution were calibrated. Ammonia, nitric acid and other soluble raw materials are directly used in the experiment.
During the precipitation experiment, a certain amount of metal ion solution shall be weighed according to the designed concentration ratio of Co2+, Mg2+, Al3+ metal ions, and they were stirring-mixed and diluted to the required concentration. The precipitant ammonium carbonate solution is prepared according to the experimental design requirements, and a certain volume of ammonia is added to adjust its pH value. Then, the uniformly mixed metal ion solution is gradually dropped into the continuously-stirring ammonium carbonate solution at a constant rate of 4 ml/min for precipitation reaction.
After the mixed metal ion solution is completely titrated and the precipitation reaction is accomplished, the precipitation mixture should be constantly stirred for aging. After 24 hours of aging, the prepared precipitation precursor was separated from the supernatant by centrifugation, and the precipitation was washed twice with deionized water and absolute ethanol respectively. The washed precursor is placed in a ventilated drying oven and dried to constant weight. The dried precursor was grinded until it can pass through a 200 mesh sieve, and then calcined at a specific temperature to obtain Co:MgAl2O4 nano-powders.
The particle size of Co:MgAl2O4 nano-powders was measured by BET method, and the phase assemblage and microstructure were characterized by XRD and SEM, respectively.
Controlling of composition for Co:MgAl2O4 powder
From the MgAl2O4 phase diagram, it can be seen that there is a solid solution region in the component of spinel. If the spinel is written in the form of oxide: MgO·nAl2O3, where n=Al2O3/MgO, its value can characterize the chemical composition of spinel, usually in the range of 0.9-3.5. For the stoichiometric magnesia alumina spinel with n=1, it has a complete spinel structure. The Co:MgAl2O4 nano-powders prepared in this experiment is a stoichiometric magnesia alumina spinel. It is necessary to adjust the components of Co:MgAl2O4 powder by adjusting the precipitant ammonium carbonate solution and the concentration of metal ions.
The Ksp (solubility product) of Co2+ and Al3+ ions in Co:MgAl2O4 nano-powders is small in alkaline environment, so it is easy to precipitate; However, the Ksp of Mg2+ ion is large and the precipitation rate is low. Increasing the concentration of Mg2+ ion in the metal ion solution can also change the chemical composition of the precipitated powder.
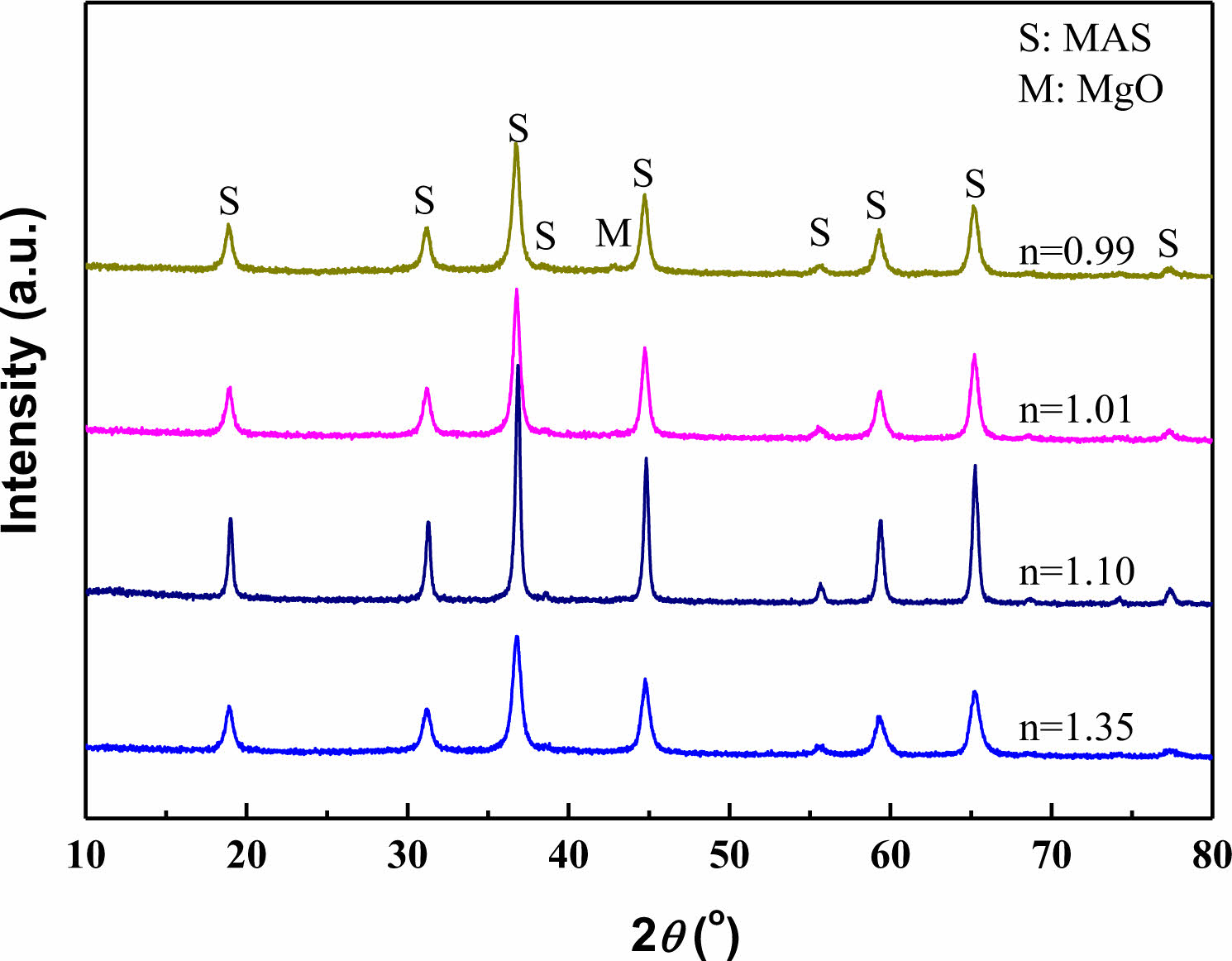
The XRD of Co:MgAl2O4 nano-powders prepared with 1.0 M ammonium carbonate as precipitant and metal ion solution of different proportions is shown in Fig. 1.
It can be seen from the figure that with the increase of Mg2+ concentration in the metal ion solution, the n value of the prepared Co:MgAl2O4 nano-powders can be adjusted to 1, that is, the stoichiometric magnesia alumina spinel phase (MgO·Al2O3), and single-phase spinel can be obtained when n>1. When n<1 is, a small amount of MgO phase begins to appear in the powder due to excessive Mg ions. According to the MgO- Al2O3 binary phase diagram, MgO alumina spinel is a solid solution phase, and the solid solubility of MgO phase in Al2O3 phase is much smaller than that of Al2O3 phase in MgO phase. Therefore, excess Mg2+ ions are easier to precipitate from the Mg-Al spinel phase to form MgO phase. However, there is no other phase peak in the XRD pattern, which also shows that Co2+ has completely entered the Mg-Al spinel lattice and achieved uniform doping.
Controlling of microstructure for Co:MgAl2O4 nano-powders
In the process of preparing ceramics, the morphology of the powder has a very important impact on its properties. The sintering activity of Co:MgAl2O4 nano-powders with uniform morphology will also be improved.
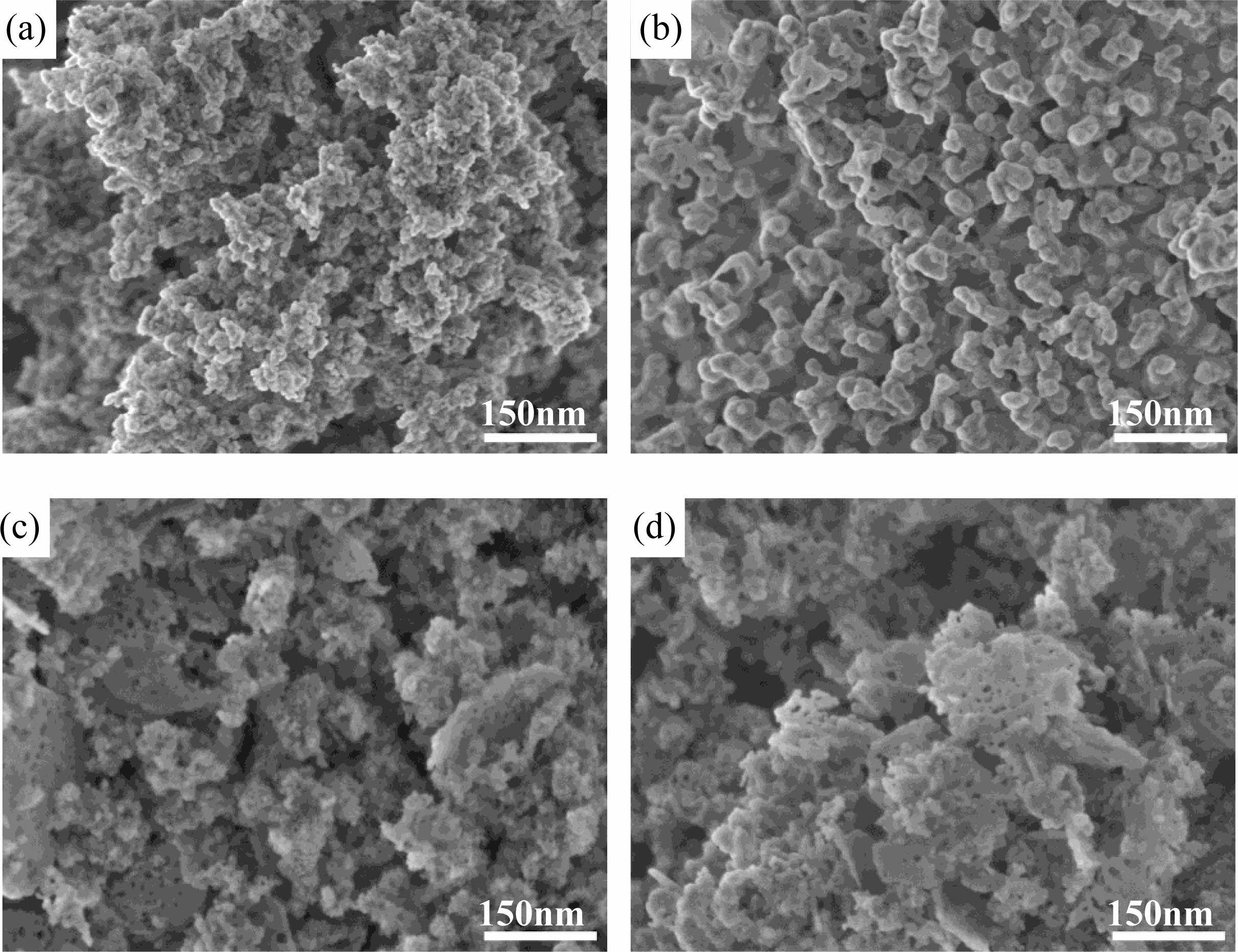
In the coprecipitation process, if the pH value of the precipitant solution system is high, Al3+ ions in the metal ions will easily precipitate in the form of AlOOH or Al(OH)3. AlOOH or Al(OH)3 contains a large amount of hydroxyl, adsorption water and crystallization water, which will cause serious agglomeration of the powder in the drying process, and the sintering performance of the prepared powder is also poor. Therefore, controlling the concentration and pH value of precipitant solution and obtaining hydroxides containing bimetallic ions as much as possible are critical for obtaining uniform powder. Using different precipitant solution systems, the prepared precursor was calcined at 1100 oC for 2 h. The micro morphology of the final Co:MgAl2O4 nano-powders is shown in Fig. 2.
When ammonia water and precipitant (NH4)2CO3 concentrations change, the pH value of the solution also changes, and the morphology of the prepared powder alters significantly. When the concentration of precipitant (NH4)2CO3 was 0.5 M, the microstructure of Co:MgAl2O4 powder was mainly spherical-like particles. After adding ammonia water, the particle size of the powder becomes larger, which may be completely related to the high pH value of the solution, the large precipitation degree of metal ions and the growth of the grains. When the concentration of precipitant (NH4)2CO3 exceeds 1.0 M, the micro morphology of the prepared Co:MgAl2O4 powder shows a large number of porous flake structures. With the increasing of the concentration of (NH4)2CO3, more flake structures are formed. This is mainly because the concentration of (NH4)2CO3 in the reverse drop coprecipitation increases, the pH of the precipitation solution system also increases, and Al3+ ions are easy to precipitate from the solution in the form of AlOOH which has a typical flake structure and also contains a large amount of adsorbed water and crystal water. After high temperature calcination, the flake AlOOH removes the OH group, thus forming a porous flake structure powder. Mg2+ and Co2+ precipitated in the form of bimetallic basic carbonate, dispersed around AlOOH, and reacted with decomposed Al2O3 to form magnesium aluminum spinel during high temperature calcination.
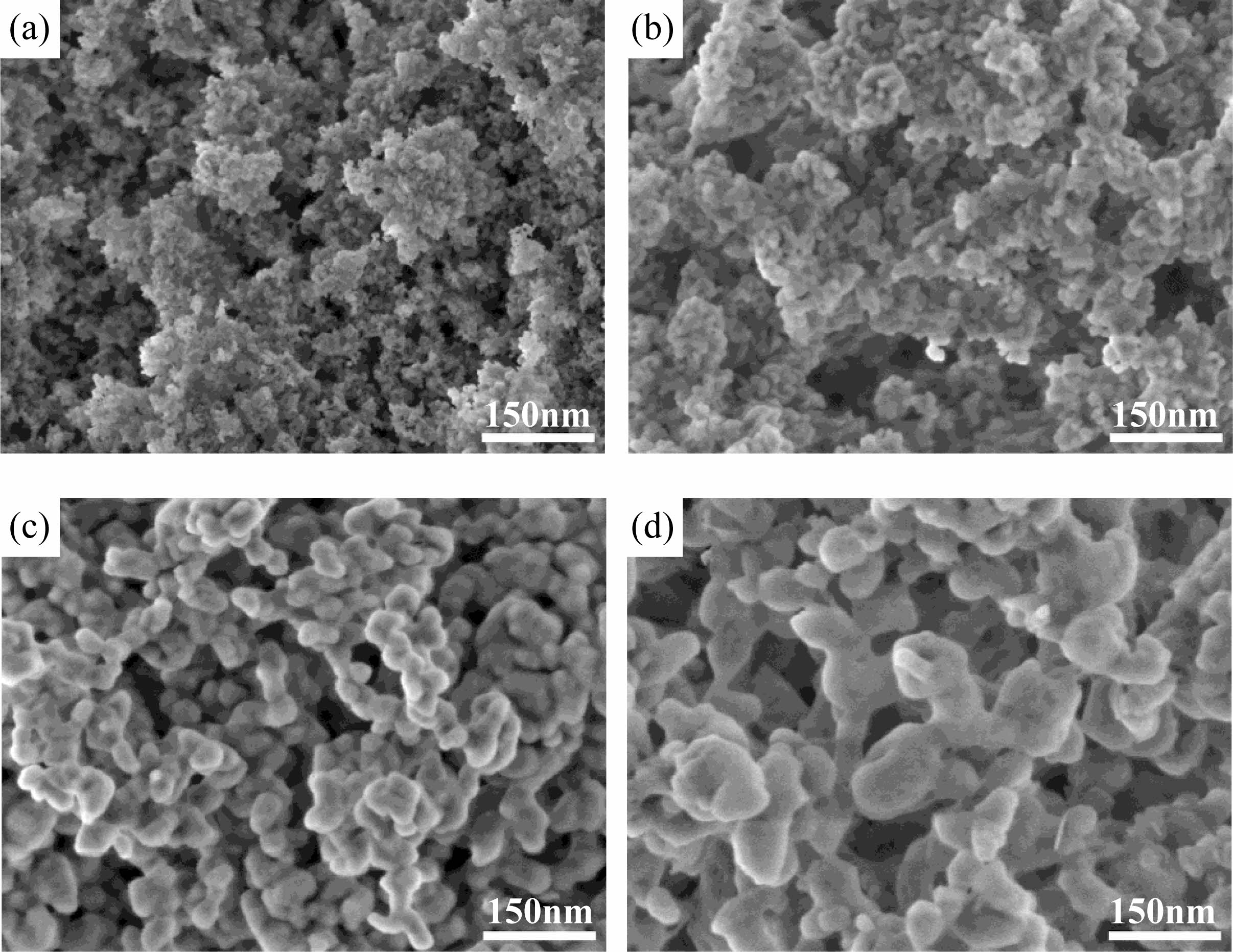
From the perspective of sintering kinetics, the calcination temperature of the precursor will directly affect the particle diameter and agglomeration state of the powder. In addition, the thermal analysis of the precursor also shows that the calcination temperature will have an important impact on the chemical composition and grain growth of Co:MgAl2O4 powder. Therefore, the precipitation conditions were optimized: the concentration of precipitant (NH4) 2CO3 was 0.5 m; The volume of ammonia added is 25:800; In the metal ion solution, Mg2+:Al3+=1:1, the titration rate was 4 ml/min, and the aging time was 24 h. The morphology of Co:MgAl2O4 powder is shown in Fig. 3 after the precursor is calcined at 900 °C, 1000 °C, 1100 oC and 1200 oC for 4 hours respectively.
With the increase of calcination temperature, the particles of Co:MgAl2O4 nano-powders continue to sinter and grow. When the calcination temperature is lower than 1000 oC, the particles are small and grow slowly. This is because the low calcination temperature provides weak driving force of nucleation and grain growth, and the particles are not easy to grow. When the calcination temperature exceeds 1000 oC, the particles grow significantly, the size increases, and the particles develop well. In particular, the Co:MgAl2O4 powder calcined at 1100 oC has uniform particle morphology and a small amount of weak agglomeration. When the calcination temperature reaches 1200 oC, the particles continue to grow. However, compared with the powder calcined at 1100 °C, the change of particle size at 1200 oC is not significant at low temperature. At the same time, the degree of agglomeration is increased, and the sintering neck begins to appear between the particles, which is not conducive to the subsequent sintering process of ceramics, indicating that the calcination temperature of the precursor should be lower than 1200 oC.
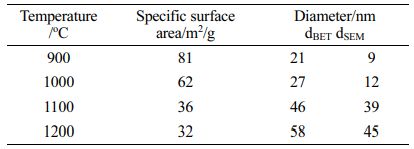
Table 1 shows the specific surface area of Co:MgAl2O4 powder prepared at different calcination temperatures and the particle equivalent diameter calculated according to the specific surface area and SEM spectrum.
Consistent with the SEM observation, Co:MgAl2O4 powder particles grew continuously with the increase of temperature. When the calcination temperature reaches 1100 oC, there is an obvious growth process. The specific surface area of the particles decreases from 62 m2/g to 36 m2/g, and the particle size grows from 12 nm to 39 nm, which is closer to the particle diameter calculated by SEM, indicating that the degree of particle agglomeration is weakened. At the same time, when the temperature exceeds 1100 oC, the growth of particles tends to be gentle, and the degree of agglomeration between particles increases. The results also show that 1100 oC is the most reasonable calcination temperature for the precursor prepared with optimized process parameters. After calcination at 1100 oC, Co:MgAl2O4 nano-powders has good particle uniformity, weak agglomeration degree and small size.
|
Fig. 1 XRD patterns of Co:MgAl2O4 nano-powders by different ratios of metal ion. |
|
Fig. 2 SEM microscopic morphology of Co:MgAl2O4 nanopowder prepared by different precipitant solutions: (a) 0.5 M (NH4)2CO3, (b) 0.5 M (NH4)2CO3+NH3·H2O, (c) 1.0 M (NH4)2CO3+NH3·H2O, (d) 1.5 M (NH4)2CO3+NH3·H2O. |
|
Fig. 3 SEM spectrum of Co:MgAl2O4 powder at different calcination temperatures: (a) 900 ℃, (b) 1000 ℃, (c) 1100 ℃, (d) 1200 ℃ |
|
Table 1 the specific surface area and diameter of Co:MgAl2O4 nano-powders calcined at different temperatures. |
In this study, Co:MgAl2O4 nano-powder was prepared by reverse drop co-precipitation, and the chemical composition and morphology of the prepared powder were characterized. The results show that:
(1) The chemical composition of Co:MgAl2O4 nano-powder can be controlled by adjusting the concentration of precipitant solution and metal ions. Although the prepared powders with different chemical components have a single magnesium aluminum spinel structure, the chemical composition is very different, which is mainly related to the fact that MgAl2O4 is a kind of solid solution, and the solid solution range of MgO and Al2O3 is large. Stoichiometric Co:MgAl2O4 nano-powders can be prepared by reasonably controlling the proportion of ion concentration in precipitant solution and metal solution.
(2) With the increase of the concentration of precipitant (NH4)2CO3 and the addition of ammonia, the pH of the precipitation solution system also increases, and the AlOOH with flaky structure is easy to precipitate from the solution. Even after high-temperature calcination, the prepared Co:MgAl2O4 powder still has the flaky structure. However, the powder is easy to agglomerate when the calcination temperature increases to higher than 1100 oC owing to the augmented driving force. Therefore, to obtain Co:MgAl2O4 powder with uniform morphology, the concentration of (NH4)2CO3 should be controlled to 0.5 M, and the calcination temperature of precursor to 1100 oC.
- 1. Y.J. Chen, Y.F. Lin, Y.Q. Zou, Z. Luo, and Y. Huang, Opt. Express 20[9] (2012) 9940-9947.
-

- 2. Y. Liu, J. Liu, C.C. Liu, R.L. Niu, L.H. Zheng, L.B. Su, and J. Xu, Laser Phy. 21[3] (2011) 472-476.
-

- 3. P. Xu, C.T. Xia, J.Q. Di, X. Xu, Q. Sai, and L. Wang, J. Cryst. Growth 361 (2012) 11-15.
-

- 4. Y.V. Terekhov, D.V. Martyshkin, V.V. Fedorov, I.S. Moskalev, and S.B. Mirov, Laser Phy. 24[2] (2014) 025003.
-

- 5. G. Boulon, G. Alombert-goget, Y. Guyot, M. Guzik, T. Epicier, N.P. Blanchard, L. Chen, L. Hu, and W. Chen, J. Mater. Chem. C 44[2] (2014) 9385-9397.
-

- 6. N.V. Kuleshov, V.P. Mikhailov, V.G. Scherbitsky, P.V. Prokoshin, and K.V. Yumashev, J. Lumines. 55[5-6] (1993) 265-269.
-

- 7. K.V. Yumashev, Applied Optics 38[30] (1999) 6343-6346.
-

- 8. K.V. Yumashev, I.A. Denisov, N.N. Posnov, P.V. Prokoshin, and V.P. Mikhailov, Appl. Phy. B-Lasers Opt. 70[2] (2000) 179-184.
- 9. K.V. Yumashev, I.A. Denisov, N.N. Posnov, N.V. Kuleshov, and R. Moncorge, J. Alloy. Comp. 341[1-2] (2002) 366-370.
-

- 10. N.V. Kuleshov, V.G. Scherbitsky, V.P. Mikhailov, S. Kück, J. Koetke, K. Petermann, and G. Huber, J. Lumines. 71[4] (1997) 265-268.
-

- 11. A. Ikesue and L.A. Yan, J. American Ceram. Soc. 89[6] (2010) 1936-1944.
-

- 12. A. Wajler, A. Kozlowskam M. Nakielska, K. Lesniewska-Matys, A. Sidorowicz, D. Podniesinski, and P. Putyra, J. American Ceram. Soc. 97[6] (2014) 1692-1695.
-

- 13. J.G. Li, T. Ikegami, J.H. Lee, T. Mori, and Y. Yajima, Ceram. Int. 27[4] (2001) 481-489.
-

- 14. J.G. Li, T. Ikegami, J.H. Lee, and T. Mori, J. American Ceram. Soc. 83[11] (2000) 2866-2868.
-

- 15. B.S. Choi, O.G. Jeong, J.C. Park, J.W. Kim, S.J. Lee, J.H. Ryu, J.I. Lee, and H. Cho, J. Ceram. Process. Res. 17[7] (2016) 778-781.
-

- 16. R.-T. Wang, X.-P. Liang, Y. Peng, Xiao-wei Fan, and Jian-xin Li, J. Ceram. Process. Res. 11[2] (2010) 173-175.
-

- 17. J. Chandradass and K.H. Kim, J. Ceram. Process. Res. 11[1] (2010) 96-99.
-

- 18. V. Vitkin, P. Loiko, L. Basyrova, A. Polishchuk, D. Zavirukha, J. Klimke, and A. Goldstein, IEEE (2022) pp.1-1.
-

- 19. Y. Jing, Q. Liu, S. Su, X. Li, Z. Liu, J. Wang, and J. Li, J. Inorgan. Mater. 36[8] (2021) 877-813.
-

- 20. S. Su, Q. Liu, Z. Hu, X. Chen, H. Pan, X. Liu, L. Wu, and J. Li, J. Alloy. Comp. 797 (2019) 1288-1294.
-

 This Article
This Article
-
2023; 24(2): 374-378
Published on Apr 30, 2023
- 10.36410/jcpr.2023.24.2.374
- Received on Aug 30, 2022
- Revised on Mar 7, 2023
- Accepted on Mar 9, 2023
 Services
Services
Shared
 Correspondence to
Correspondence to
- Luo Wei
-
Luoyang Institute of Science and Technology, Luoyang 471023, China
Tel : 13693793872 - E-mail: luowei@lit.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.