- The use of alternative raw material as a fluxing agent in hard porcelain bodies
H. Yildizay*
Kutahya Dumlupinar University, Kutahya Vocational School of Art Science, Department of Tile Arts and Design, Germiyan Campus, Kutahya 43100, Turkey
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this study, pumice partially or fully as a replacement for potassium feldspar to chosen hard porcelain compositions. Pumice was added into the porcelain body in proportions 0, 5, 10, 15, 25 wt.%. The chemical and physical properties, phase-microstructure analysis of samples was evaluated and compared with standard porcelain composition. The crystal phases were found as quartz, mullite and amorphous phase. It was found that the just pumice added porcelain bodies fired at 1380 ℃ had good technical features such as lineer shrinkage, water absorption, bulk density, and flexural strength 50.4 MPa. SEM (scanning electron microscopy) and XRD (X-ray diffraction) analysis results showed the high flexural strength was due to the formation of high mullite crystalline content in the porcelain body and the densification of microstructures. The technological and microstructural features of new hard porcelain bodies showed the suitability of using pumice instead of potassium feldspar as the fluxing agent in hard porcelain bodies
Keywords: Hard porcelain, Pumice, Fluxing agent, Characterization, Materials
In general, porcelains are classified according to their firing temperatures. “Hard porcelains” are usually fired between 1380 ℃ and 1460 ℃. “Soft porcelains” are fired at lower temperatures around 1200 ℃. Hard porcelain structure consists of approximately 30% crystalline and 70% glass. These kinds of porcelains are defined as highly vitreous (glassy) ceramic [1-4]. The formulations of hard porcelain have three main components which are silica as filler for the structure, kaolin for plasticity, and feldspar for fluxing. These components are necessary for the most efficient processing to enhance the final product’s performance. Triaxial porcelain formulations (SiO2–Al2O3–K2O) often include 25 wt.% feldspar, 50 wt.% of plastic component, and 25 wt.% silica for obtaining hard porcelain [5-12].
Feldspars are included in ceramic body’s formulations since they add fluxing ability to the product. Generally, potassium feldspar is considered and employed as the primary fluxing component in the industrial manufacture process of hard porcelain bodies. Many of natural feldspar resources have been exhausted or become uneconomical [13, 14]. For this reason, numerous research began investigating the alternative fluxing components that can be used instead of feldspar while manufacturing ceramic bodies and glazes [15].
Immense sources for pumice are present across the globe and they can be an alternative source for feldspar. According to the records, currently, fifteen countries around the globe produce and manufacture pumice. Having a reserve of nearly 8 billion m3, the world's largest producer of pumice is Turkey, followed by Italy, Chile, Uganda, and Greece [16].
Pumice is mainly composed of SiO2 and Al2O3. It is a volcanic rock which is glassy, amorphous, and porous. During volcanic eruptions, liquid lava spreads into the air as foam and this foam naturally contains gas bubbles; so, the pumice is formed [17].
Pumice is used in construction, textile, chemistry, agriculture as well as other fields such as jewelry, metal and glass industries due to its physical and chemical characteristics. And since it is abundant in nature, it is considerably economic. Pumice is resistant against physical and chemical factors. Pumice has natural color varies from black to grey, brown, and white. Pumice has higher silica content and can be widely used as a building material in the world [18].
The high porosity and soft structure of the pumice promotes easy and economic manufacturing. Plus, it is convenient for using in ceramic production thanks to its chemical structure. Nevertheless, research regarding the pumice usage in ceramic and porcelain manufacturing are not enough. According to our literature review, only a few research have been conducted in terms of pumice usage in ceramics [13, 19-21].
In this study, the use of pumice as an alternative flux additive instead of potassium feldspar in porcelain body composition was investigated. For this purpose, the changes in physical properties and structural composition of porcelain using different amounts of pumice were analyzed.
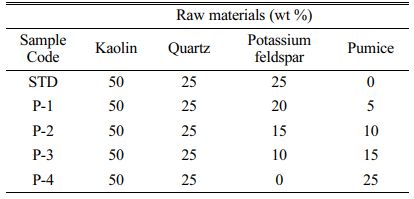
For this study, samples of pumice were obtained the from Denge Bims Pumice Plant mined in Nevsehir, Turkey. The chemical analysis of minerals and porcelain compositions were identified using an Spectro XEPOS 5 Model XRF. The compositions of raw materials are seen in Table 1.
Mineralogical analysis of the pumice and porcelain bodies were determined by XRD with the help of a Rigaku MiniFlex Model diffractometer, CuKα radiation, 2θ = 5 to 70º, scanning speed 1º/min. In XRD, an analysis of pumice the glassy behavior was viewed (Fig. 1).
The standard hard porcelain composition was chosen as the one which includes 25% quartz, 25% potassium-feldspar, and 50% kaolin. In this study, instead of potassium-feldspar, partially or fully pumice was used at selected porcelain compositions. Pumice was added into the standard porcelain body in proportions 0, 5, 10, 15, 25 wt.%. The prepared porcelain combinations were coded as STD, P-1, P-2, P-3, and P-4 in Table 2 can be seen.
Porcelain compositions were milled with an aqueous medium which contains 70 wt.% solids along with 0.01 wt.% dispersant for 2 h with using three different sized alumina balls. Prepared slurries were sieved 63 μm and then poured into the molds with dimensions 3.5 × 30 × 1 cm3. Slip casting method was used for shaping, so which could obtain green body with high strength [22]. The green bodies first dried at room temperature for 24 hours and then dried in oven at 105 ºC for 2 hours. After drying, they were placed into a furnace and sintered at 1380 °C for 6.5 hours. The samples of the fired hard porcelain test bars were given in Fig. 2.
Measuring bulk density (ρb), water absorption (wa), linear shrinkage (Ls) of porcelain samples, the sintering behavior of the samples was assessed.
The linear shrinkage, Ls (%), of fired samples was calculated by the equation below, Eq. (1):
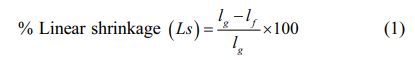
Where lg and lf represents the height (mm) of green and fired product, respectively.
Water absorption (wa) was noted for every sample by setting the dry weight at 105 ºC initially and then, identifying the wet weight after immersion in boiling water for 2 hours and finally, cooling according to the TST 800 EN 997 standards Eq. (2). The value of bulk density (ρw) was calculated with the help of the Archimedes principle depending on the sample’s wet weight, where (wd) is the sample’s dry weight, (ws) is the weight of solid suspended in water, (ρw) is the water density at room temperature Eq. (3) [23].
The results were obtained by the average value of five samples for each measurement.

The optical parameters of the porcelain samples, directly fired at 1380 °C, were identified in BYK Spectro-Guide 45/0 Gloss spectrophotometer operating with D65/10. The colorimeter operates on the CIE Lab method. It is a technique utilized in the ceramic production to set the whiteness and color of by three main parameters (Hunter parameters) L* (brightness) from absolute white L*= 100 to absolute black L*= 0, a* (a* > 0 red; a* < 0 green), b* (b* > 0 yellow; b* < 0 blue) developed from the visible spectra [24-26].
The total color change of the samples was calculated using the following formula.

The ∆E value in this formula shows the numerical difference between the L*a*b* values between the two colors. If the ∆E value is less than 1, the difference between the two colors cannot be detected. If the ∆E value is greater than 1 and less than 3.7, the difference between the two colors can be perceived depending on the visual ability of the individual. If the ∆E value is greater than 3.7, the difference between the two colors is perceptible and unacceptable [24-26].
The phases formed in the fired product were identified by XRD and to perform a quantitative phase analysis used a diffraction/reflectivity analysis program based on the Rietveld method MAUD (Material Analysis Using Diffraction).
Additionally, secondary electron images were taken with SEM (Zeiss Supra 40 VPTM Model-Oxford Instruments TM) at the samples’ fractured surfaces for microstructural examinations.
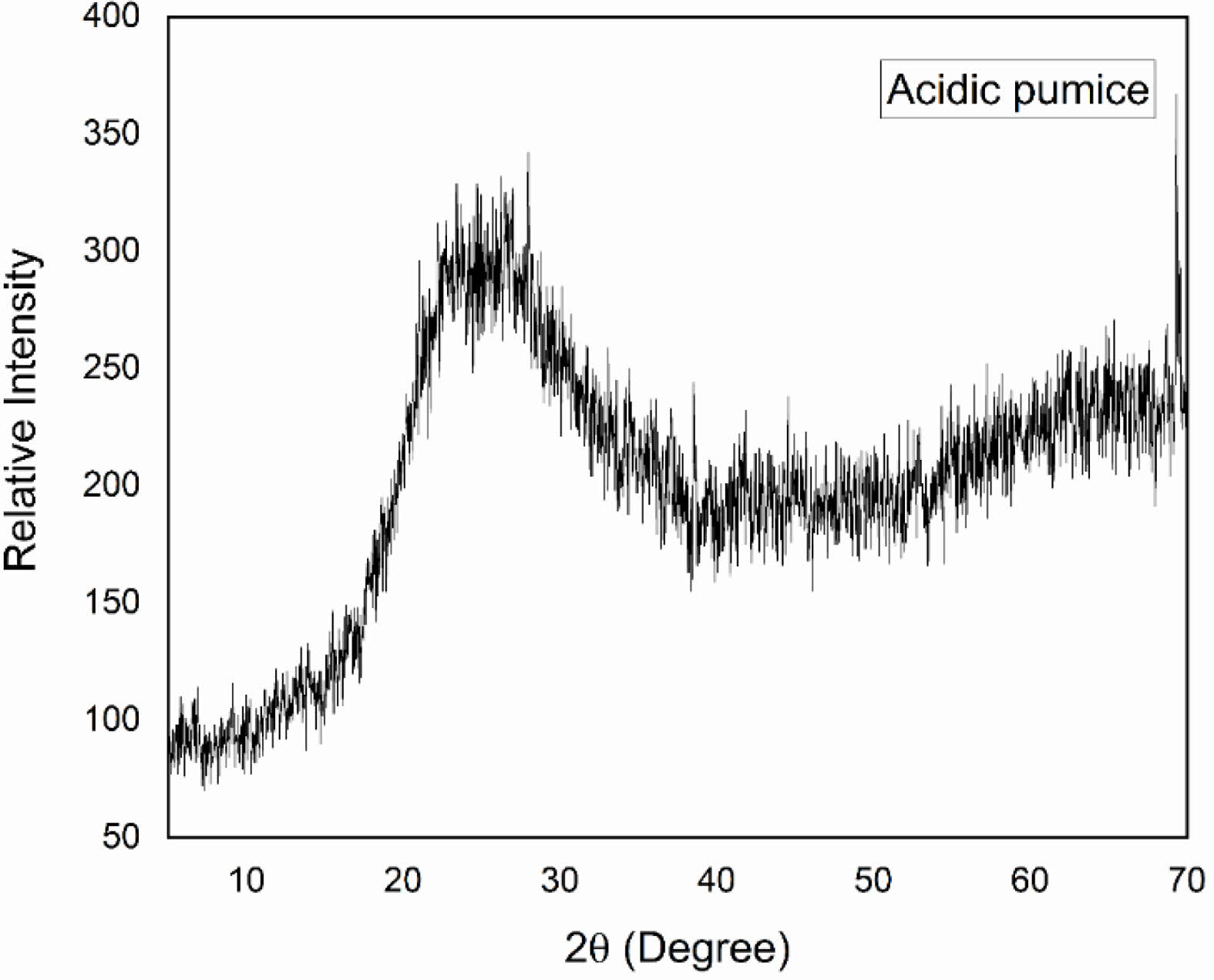
|
Fig. 1 XRD patterns of the pumice. |

|
Fig. 2 The samples of the fired hard porcelain test bars. |
|
Table 1 The compositions of raw materials which are employed for hard porcelain bodies (%). |
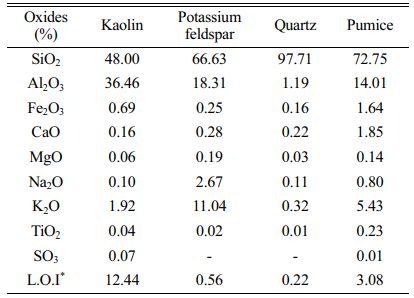
*L.O.I: Loss on ignition. |
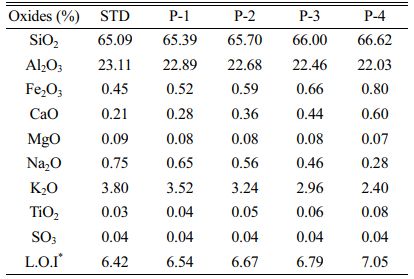
The chemical composition and phase analysis of hard porcelain bodies compositions was specified in Table 3 and Fig. 3 respectively.
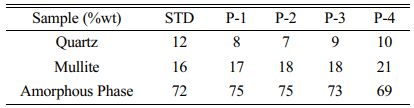
As seen from the XRD results, the porcelain bodies do not contain any crystalline phases other than mullite and residual quartz. The amount of the phases in the fired products were identified by a quantitative phase analysis program based on the Rietveld method MAUD and indicated in Table 4. The combination of pumice and K-feldspar in the porcelain composition led to change amount of the mullite and glass phase.
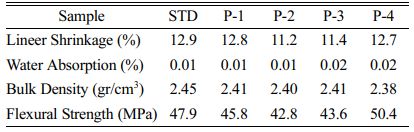
If there is sufficient rate of fluxing oxides, it will help the generation of liquid phase filling the pores, it will promote the gathering closer of grains, and a linear reduction of shrinkage during the firing process and helps to maintain the final product dimensions [27].
Water absorption is a key factor affecting durability of porcelain samples. The less water penetrates a porcelain sample, the more durable is the porcelain samples and the better is its resistance to the natural environment. Ceramics which have water absorption lower than 0.5% are categorized as porcelains [28, 29].
Five test bars and one small porcelain plate were prepared from each porcelain composition to enable measure linear shrinkage, water absorption and bulk density tests. The samples were weighed before and after the tests using an analytical balance. The values of linear shrinkage (Ls) and water absorption (wa) and bulk density (ρb) after firing are presented in Table 5.
It is critical to know the physical behavior of a product during sintering in the industry of ceramic. It is seen that the physical analysis results of the prepared porcelain compositions are close to each other and suitable for the working conditions.
As can be understood from the chemical analysis, the amount of CaO in pumice is slightly higher than in other minerals. At higher temperatures, the amount of CaO causes the viscosity of the glassy phase to decrease and it is thought that this helps the mullite phase to develop more easily [30, 31]. It was observed that the flexural strength of the bodies, in which pumice was used instead of K-feldspar, increased. It is thought that this is due to the CaO in the pumice content.
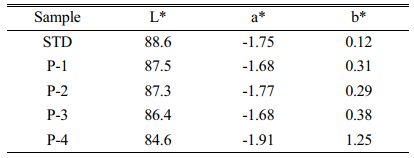
It is known that for the marketing of hard porcelain, whiteness degree has a major role. Thus, we measured the standard body whiteness to get a sense on the whiteness degree and to compare it with the whiteness of pumice added bodies. Table 6 shows the color difference mean values (a*, b*) as well as the L* parameters.
According to the results of our experiments, it was observed that the addition of pumice in the hard porcelain bodies composition had a minor adverse effect regarding the color. This effect is due to the amount of Fe ions present in pumice.
The optical parameters L* (-) black and white (+), a*(-) green and red (+) and b* (-) blue and yellow (+). If we take the standard hard porcelain body whiteness (L*; 88.6) as reference, the closest whiteness value to the standard body (L*; ~87.5) was obtained in P1 and P2 bodies with 5% and 10% pumice additives. So, it is more convenient to be employed in hard porcelain body. Additionally, we found that with the increase of pumice addition into the standard body, the whiteness (L*) decreased while the redness (a*) and yellowness (b*) increased.
In the samples in our study, when the change in ∆E value is smaller than 1, the color difference is not perceptible, while ∆E values between 1-2 are detectable. In cases where ∆E values exceeded 2, the color change could be clearly detected.
The result of thermal shock resistance tests, there were no cracks observed in the porcelain bodies. Everyone of the porcelain bodies were in good shape after thermal shocks resistance tests at temperatures 150 °C, 200 °C, and 250 °C, respectively. These also shows that the products will be durable for usage conditions.
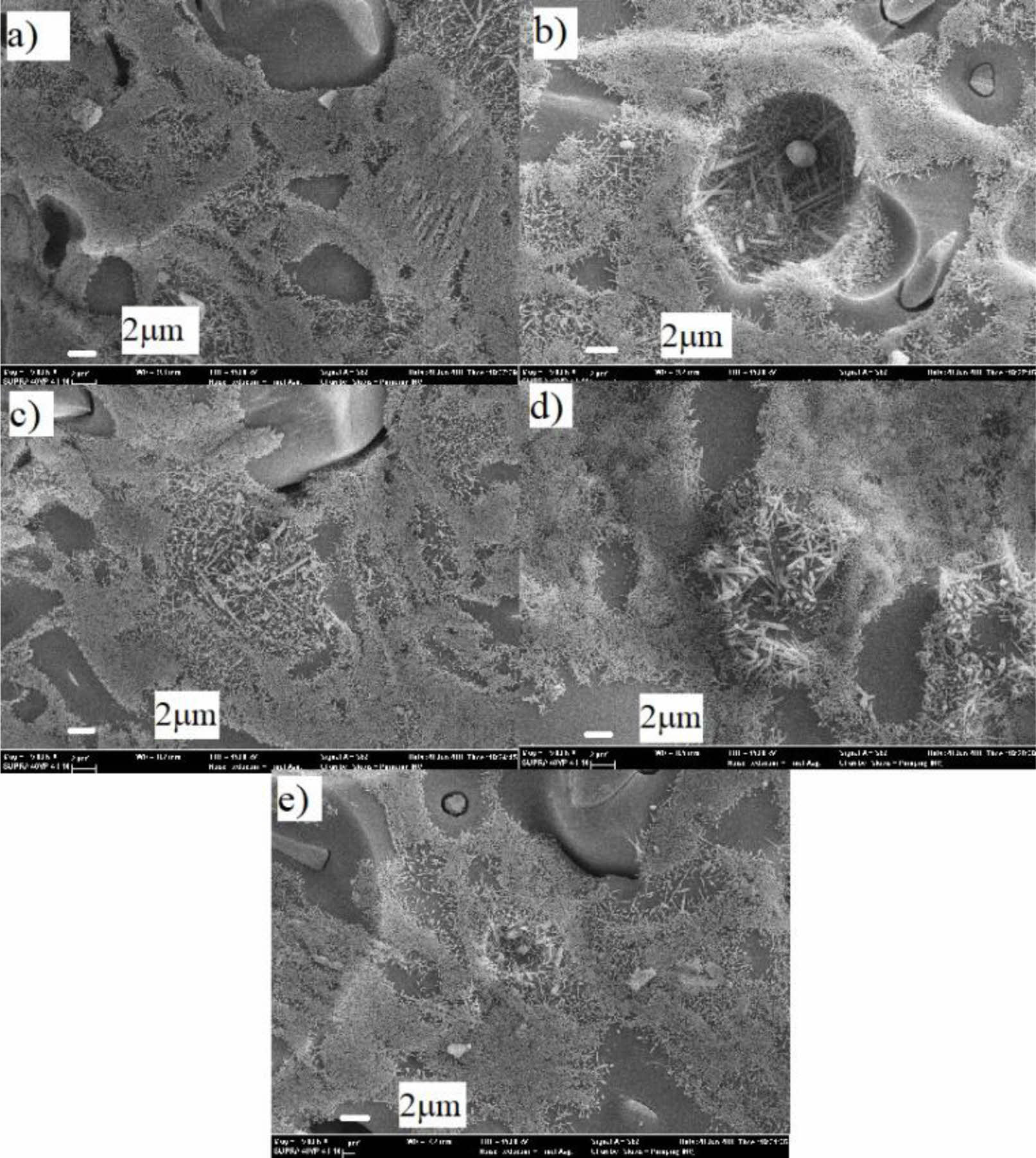
In the microstructural analysis of the crystalline phases detected in all the fired samples were quartz and mullite phases. Quartz is a residual mineral from the original raw materials, and mullite is formed in the process of firing. The SEM micrographs of the samples submitted the typical grain and bond microstructure of porcelain was given in Fig. 4. This microstructure consists of quartz grains bound together by a dense matrix which is composed of primary and secondary mullite crystals. The constantly dispersed glassy phase of interior microstructure led to less porosity and a lower value of water absorption.
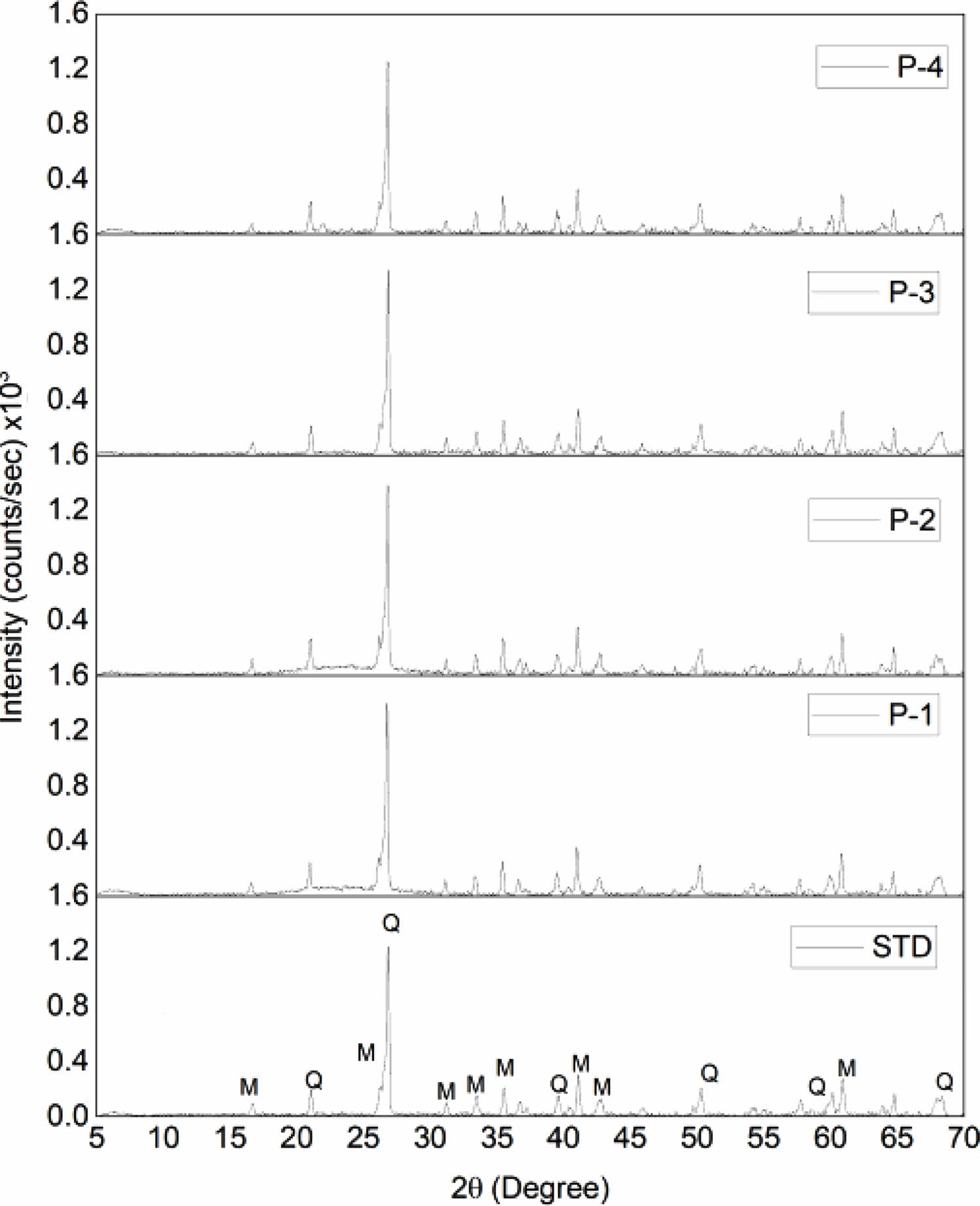
|
Fig. 3 XRD patterns of the STD, P-1, P-2, P-3, P-4 compositions |
|
Fig. 4 SEM micrographs of porcelain body samples (a) STD, (b) P-1, (c) P-2, (d) P-3, (e) P-4. |
|
Table 4 The crystalline and glassy phases amount detected using the MAUD method. |
In this study aimed to assess the potentials for using pumice as a fluxing agent in porcelain manufacturing proportioned by a classical triaxial system: 50% kaolin, 25% feldspar, and 25% quartz. For this reason, 0.5, 10, 15 and 25% pumice by weight was added to the hard porcelain body instead of K-feldspar and sintered 1380 ºC. The results show that the usage of pumice in compositions has similar water absorption, bulk density and shrinkage. The amount of CaO increased with the addition of pumice. It is seen that the viscosity decreases at high temperature and improved the sintering performance of samples. It seems that the decrease in viscosity at high temperature helps to increase the formation of the mullite phase. Maximum flexural strength (50.4 MPa) was obtained with the composition having pumice content 25%. It was determined that the color difference increased depending on the increase in the amount of pumice. Iron impurities in pumice are thought to have a minor adverse effect on porcelain bodies. For this reason, it is thought that the rate of pumice use can be increased by cleaning the iron rate in the pumice with ore enrichment processes such as magnetic method or flotation and reducing it to below 1%.
The inspection result of SEM image of the samples revealed that the sharp corners of the quartz grains were rounded due to the forces of surface tension and the start of their partial solution into the glassy matrix has been observed. Based on these analyses and observations made during this study pumice can be used as substitute or additional source of fluxes in hard porcelain bodies with using enrichment process.
- 1. F. Güngör and B. Altun, Trans. Indian Ceram. Soc. 77 (2018) 192-197.
-

- 2. S. Wanga, X. Qi, J. Hu, and X. Tian, J. Ceram. Process. Res. 16[3] (2015) 361-365.
-

- 3. A. Capoglu, J. Eur. Ceram. Soc. 31 (2011) 321-329.
-

- 4. D.U. Tulyaganov, S. Agathopoulos, H.R. Fernandes, and J.M.F. Ferreira, J. Eur. Ceram. Soc. 27 (2007) 1665-1670.
-

- 5. Ç. Koca, N. Karakus, N. Toplan, and H.Ö. Toplan, J. Ceram. Process. Res. 13[6] (2012) 693-698.
-

- 6. M.M. Salman and H.T. Nhabih, J. Ceram. Process. Res. 21[3] (2020) 371-377.
-

- 7. S. Wattanasiriwech and D. Wattanasiriwech, J. Ceram. Process. Res. 20[6] (2019) 643-648.
-

- 8. C. Leonelli, F. Bondioli, P. Veronesi, M. Romagnoli, T. Manfredini, G.C. Pellacani, and V. Cannillo, J. Eur. Ceram. Soc. 21 (2001) 785-793.
-

- 9. D. Njoya, M. Hajjaji, A. Baçaoui, and D. Njopwouo, Mater. Charact. 61 (2010) 289-295.
-

- 10. Y. Iqbal and W.E. Lee, J. Am. Ceram. Soc. 83 (2000) 3121-3127.
-

- 11. F.A. Firat, E. Ercenk, and S. Yilmaz, J. Ceram. Process. Res. 13[6] (2012) 756-761.
-

- 12. T. Aydin and A. Kara, J. Ceram. Process. Res. 15[6] (2014) 486-491
-

- 13. H.B. Poyraz, N. Erginel, and N. Ay, J. Eur. Ceram. Soc. 26 (2006) 741-746.
-

- 14. B. Karasu, M. Çak, and Y.G. Yeşilbaş, J. Eur. Ceram. Soc. 21 (2001) 1131-1138.
-

- 15. Z.B. Ozturk and B. Yildiz, Acta Phys. Pol., A. 127 (2015) 1183-1185.
-

- 16. USGS, (https://minerals.usgs.gov/minerals/pubs/commodity/pumice/mcs-2022-pumic.pdf, 2022)
- 17. P. Lura, Proceedings of International RILEM Symposium on Concrete Science and Engineering (2004) 137-151.
- 18. F.N. Degirmenci and A. Yilmaz, Indian J. Eng. Mater. Sci. 18 (2011) 61-68.
- 19. I. Tore, J. Sci. Mod. Eng. 1 (2013) 16-19.
- 20. O.E. Kuzugudenli, Key Eng. Mater. 264-268 (2004) 1427-1430.
-

- 21. Z. Bayer Ozturk and E. Eren Gültekin, Adv. Ceram. Sci. Eng. 3 (2014) 1.
-

- 22. W.P. Tai, K. Kimura, and K. Jinnai, J. Mater. Sci. 37 (2002) 1273-1279.
-

- 23. N. Pandey, S.C. Ram, I. Chakrabarty, and M.R. Majhi, Trans. Indian Ceram. Soc. 79 (2020) 188-195.
-

- 24. A. Salema, S.H. Jazayerib, E. Rastellic, and G. Timellinic, J. Ceram. Process. Res. 10[5] (2009) 621-627.
-

- 25. W.M. Johnston and E.C. Kao, J. Dent. Res. 68 (1989) 819-822.
-

- 26. I.E. Ruyter, K. Nilner, and B. Möller, Dent. Mater. 3 (1987) 246-251.
-

- 27. J.-Y. Hwang, X. Huang, A. Garkida, and A. Hein, J. Miner. Mater. Charact. Eng. 05 (2006) 119-129.
-

- 28. D. Njoya and M. Hajjaji, Trans. Indian Ceram. Soc. 72 (2013) 252-256.
-

- 29. D.Y. Tunçel and E. Özel, Ceram. Int. 38 (2012) 1399-1407.
-

- 30. Z. Bayer Ozturk, J. Ceram. Process. Res. 17[6] (2016) 555-559.
-

- 31. H. Aydin and G. Tokatas, SN Appl. Sci. 1 (2019) 56.
-

 This Article
This Article
-
2023; 24(2): 237-241
Published on Apr 30, 2023
- 10.36410/jcpr.2023.24.2.237
- Received on Jul 25, 2022
- Revised on Oct 24, 2022
- Accepted on Nov 3, 2022
 Services
Services
Shared
 Correspondence to
Correspondence to
- H. Yildizay
-
Kutahya Dumlupinar University, Kutahya Vocational School of Art Science, Department of Tile Arts and Design, Germiyan Campus, Kutahya 43100, Turkey
Tel : 05055276767 Fax: 90-274-4431204 - E-mail: hale.yildizay@dpu.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.