- Preparation of decorative slag glass-ceramics and research on the solidification of heavy metals
Yang Tanga, Xiaodong Haoa, Zhenxiang Fanga, Xinyu Baia, Guangyu Wanga, Hongxia Zhanga, Leibo Denga, Hua Chena,b, Ming Zhaob and Yongsheng Dua,b,*
aCollege of Science, Inner Mongolia University of Science and Technology, Baotou 014010, China
bCollaborative Innovation Center of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Glass-ceramics with different CuO additions were prepared with Fe2O3 and Cr2O3 as composite nucleating agents, rare earth-containing blast furnace slag (REBFS) as the main raw material. The existence state and stability of heavy metals Cu, Mn and Cr in glass-ceramics were investigated. The results showed that Cr ions contributed to the generation of spinel phase, while Cu ions and Mn ions can enter the spinel crystal and exist stably, which indicated that there was a synergistic solidification of Cu, Mn and Cr. The depolymerization effect of the copper ions contributed to the transformation of the crystal morphology from dendrites to spherulites. Moreover, the increase in CuO contents promoted glass-ceramics from green to copper red. Based on the leaching experimental data analysis of heavy metals in glass-ceramics, the leaching concentration of heavy metals Cu, Cr and Mn were much lower than the standard leaching toxicity limit of hazardous waste (GB5085.3–2007, China). The results showed that the conversion of REBFS into environmentally friendly glass-ceramics can realize solid waste resource utilization
Keywords: REBFS, Glass-ceramics, Heavy metals, Leaching characteristics, Colourisation
The demand for mineral resources has been increased in the past decade. As one of the most important mineral resources, the co-associated polymetallic mineral resources have been consumed massively annually in China. As a result, the tailings/slag emissions have increased rapidly, which has led to severe environmental pollution and land occupation [1-4]. Especially, the heavy metals (Cu, Pb, Zn, Cr, Mn, etc) in co-associated polymetallic tailings/slag caused serious threat to environment and human health due to their extreme toxicity. For example, it is reported that about 8.0 million tons of rare earth-containing blast furnace slag (REBFS) originated from Bayan Obo rare-earth iron ore deposit in Baotou of Inner Mongolia were generated each year, which is identified as a hazardous waste because of the multiple kinds of heavy metals [5]. Therefore, harmless treatment of above tailings/slag is an important problem to be solved urgently. Its sustainable utilization ways mainly included the preparation of cement, slag brick, glass-ceramics or recycling of valuable metal from tailings/slag, etc [6-8]. However, there are still some problems in the recycling of valuable metals, such as low recovery, high production cost and secondary environment pollution. Besides, the solidification characteristic of heavy metals is a major factor limiting the use of tailings/slag as building materials. At present, the disposal/treatment strategies such as the chemical chelating agent stabilization, high temperature solidification, and cement solidification have been considered to be efficient [9-11]. Among, the preparation of high-performance tailings/slag glass-ceramics not only consumes a large amount of industrial solid wastes, but also realizes the high temperature solidification of multiple heavy metals. According to the report of Li et al., the glass-ceramics were prepared from screen glass, ceramic polishing slag and waste incineration fly ash with high performance of the micro-hardness of 7.2 GPa, bending strength of 86.5 MPa, and water absorption of 0.03% [12]. Cu, Cr , Cd, Pb, Ni and other heavy metals in glass-ceramics were stabilized effectively in the glassy matrix or crystalline phases. The curing effect of Pb and Cu in the prepared glass-ceramics derived from wastes was investigated by Liu et al., and it can be found that the leaching concentrations of Cu and Pb were well below the regulatory standard limits (GB5085.3–2007, China). These results show that it is a reliable way to utilize the Cu- and Pb-rich solid wastes in the form of environment-friendly glass-ceramics [13].
Determining the existence state of heavy metals is a critical factor to improve the curing effect, since the species and existence of heavy metals in glass-ceramics can greatly affect the leaching concentrations. For instance, the glass-ceramics were prepared from stainless steel pickling sludge and fly ash by melting method [14]. The results indicated that Cr and Ni can exist in the spinel crystalline phase stably by solid solution or chemical substitution. Cu, Zn, and Pb, evenly existed in the glass matrix by physical coating solidification. According to the curing effect of heavy metals in bricks made of lead-zinc tailings from Li et al., the relative contents of MgO, Al2O3, SiO2, CaO, and Fe2O3 determined the stability of toxic heavy metals Zn, Pb, Cd, and Cu in the bricks, and too much Al2O3 and SiO2 were not conducive to the formation of stable framework of Pb [15]. Pi et al. found that the leaching efficiency of copper in glass-ceramic was higher than that of zinc under the same condition [16]. However, there are few researches on synergetic effect of the curing mechanism and existing state of heavy metals in the glass-ceramics produced from co-associated polymetallic tailings/slag, which usually contains many kinds of heavy metals.
Besides, heavy metals in glass-ceramics are also important to the development of color rendering properties. The decorative glass-ceramic with different colors can be prepared by adding relevant heavy metals. Tang et al. reported that phosphorus slag was used as the main raw material, Fe2O3 and MnO2 as a coloring agent for preparation of beige glass-ceramics [17]. Yang et al. researched the influence of coloring agents (Fe2O3+Co2O3+Ni2O3) on the colourisation and structure of the LAS glass ceramic [18]. The results of Suzuki et al. showed that the color of Ni-doped glasses was drastically changed from brownish yellow to green [19]. Xue et al. studied the effect of CuO on the mechanical properties and color of glass-ceramics from granite wastes [20]. It was found that the glass-ceramic with 5 wt% CuO dopant was the most stable, which exhibited the color of copper red. The coloring mechanism was that adding CuO can facilitate the formation of Cu2O crystalline phase, which led to the coloring of glass-ceramics. Based on the above research, it has been found that there have been a large number of reports on the effect of agent in the glass-ceramic on its coloring performance and the coloring mechanism of decorative glass-ceramics. However, there is still lack of in-depth research on the stability of the coloring agent in glass-ceramics, which is not favorable for the long-term use of the decorative glass-ceramics in complex environments. In addition, the leaching characteristic of coloring agent in glass-ceramics is an vital parameter for determining the use safety, but related research reports are rarely involved.
In this work, the decorative glass-ceramics are produced from REBFS by melting method and CuO are added as coloring agent. The effects of CuO on the crystallization, colourisation and physicochemical properties of glass-ceramics are studied. Furthermore, the stability and leaching characteristic of coloring agent in glass-ceramics are discussed by the erosion-corrosion experiments and leaching experiment, respectively. Meanwhile, the existing state and synergistic curing characteristics of heavy metals Cr, Mn and Cu in glass-ceramics are studied deeply. The above investigation will give important theoretical support to the preparation of decorative glass-ceramics with low leaching of heavy metals and high color stability. The glass-ceramics in copper red color is expected to be used as decorative materials in building.
Sample preparation
The REBFS used in this study was obtained from Bayan Obo rare-earth iron ore deposit in Baotou of Inner Mongolia. Its chemical composition is displayed in Table 1. According to the composition of the augite crystalline phase of glass-ceramic in the phase diagram, the base glass composition was chosen to be 49.93%SiO2, 23.01%CaO, 9.53%MgO and 7.29%Al2O3 (wt.%), and a homogeneous mixture of 63.19BFS-27.41SiO2-3.69Na2CO3-3.11MgO-1.3Fe2O3-1.3Cr2O3-XCuO (where X=0, 0.64, 1.28, 1.91, 3.14, 4.34 wt.%) was used to prepare the glass-ceramics by melting method, corresponding to samples number Cu0, Cu1, Cu2, Cu3, Cu5 and Cu7, respectively.
First of all, the fully mixed powders were melted at 1500 ℃ for 3 h in a corundum crucible. The residual melt was quenched by water and smashed into powders, and the differential scanning calorimetry (DSC) was conducted to establish the adequate heat treatment. After that, the remaining melt was cast on a preheated stainless steel mold, which was then annealed in another resistance furnace at 600 °C for 5 h. Finally, according to the DSC results, glass-ceramics can be prepared by the above-mentioned two-step heat treatment.
Techniques of characterization
The thermal behavior of glass was measured by DSC (NETZSCH 449) from room temperature to 1200 ºC with a heating rate of 10 ºC/min. To determine the crystal phase of glass-ceramics, powder X-ray diffraction (XRD, PANalytical) was performed at room temperature, applying CuKα radiation with the 2θ range of 10°-80°. The glass-ceramics were polished and sprayed with gold, and the field emission scanning electron micro- scope (FESEM, Supra 55, Zeiss) equipped with the energy dispersive X-ray spectroscopy (EDX) was used to characterize the surface morphology and composition. A qualitative determination of the chemical valence states of copper in glass-ceramics can be made from measurements of X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi). Leaching characteristic of heavy metals in glass-ceramics were determined using the standard of Solid Waste Extraction Procedure for Leaching Toxicity Sulfuric Acid and Nitric Acid Method (HJ/T 299-2007) and Identification Standards for Hazardous Wastes-Identification of Leaching Toxicity (GB/T5085.3-2007). Firstly, the bulk glass-ceramic samples with dimensions of 3 mm × 4 mm × 20 mm was prepared and leached with a mixed solution of nitric acid and sulfuric acid with the pH value of 3.20 ± 0.05 according to the standard HJ/T 299-2007. The contents of heavy metals in leaching solution were distinguished by ICP-MS (Inductively Coupled Plasma-Mass Spectrometry), and the solidification effect of the heavy metals was evaluated according to the standard GB/T5085.3-2007. The erosion-corrosion experiments of polished block glass-ceramics samples were performed with a mixed solution of nitric acid and sulfuric acid, which is stirred with a magnetic stirrer at 30 ± 2 r/min for 20 hours at room temperature. Subsequently, the topography of erosion–corrosion surface was examined by FESEM. Microhardness measurements of glass-ceramics were performed by the Vickers hardness tester (HV-50A) under a load of 4.9 N, and the three-dimensional indent morphology can be obtained using laser confocal microscope (VL2000DX-S, Japan). The bulk density of the glass-ceramic was determined using the Archimedes technique. The glass-ceramic samples with a standard size of 3 × 4 × 40 mm were prepared and the bending strength was tested by the three-point bending technique on an electronic universal testing machine (CSS-88000). According to the standard of JC/T2283-2014 in China, the acid resistance behavior was of glass-ceramics were determined by the quality of pellets of 0.5-1.0 mm in size before and after acid etching in 20 wt% H2SO4 solutions at 100 ºC.
Thermal analysis
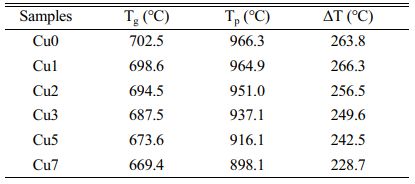
Fig. 1 is the DSC curve of CaO-MgO-Al2O3-SiO2 (CMAS) glass-ceramics with various CuO additions at the heating rate of 20 ℃/min. The obvious crystallization exothermic peak in Fig. 1 indicates that the glass-ceramics have strong crystallization ability, since the nucleating agents of Fe2O3 and Cr2O3 in the experiment can strongly promote the bulk crystallization [21]. It can also be found that the glass transition temperature Tg and crystallization exothermic peak temperature Tp both decrease gradually with the increase of CuO additions [22]. In addition, the full width at half maximum (FWHM) of the exothermic peak show a tendency of decrease and the intensity of the crystallization exothermic peak gradually increase with increasing the amount of CuO addition. This phenomenon indicates that copper ion can effectively decrease the glass viscosity and break the network structure of [Si-O], which can obviously promote crystallization and reduce the integrity of the glass structure. The index of the thermal stability of glass phase can also be estimated by ΔT (where ΔT = Tp-Tg), which is shown in Table 2. It is obvious that ΔT shows a decreasing trend overall with the increase of CuO additions, which indicate that the glass forming ability is gradually weakening [23]. This phenomenon is probably because copper ion has two valence states in glass-ceramics, namely Cu+ and Cu2+, which all can act as the modifier for the glass network. The increase in CuO content can reduce the polymerization level of the glass network and promote crystallization of the glass-ceramics. Furthermore, the nucleation temperature and crystallization temperature of glass-ceramics can also be determined by DSC result. The nucleation temperature is selected as 750 ℃ above the Tg of about 50-100 ℃, and the crystallization temperature is selected as 980 ℃ according to the obvious Tp.
Crystalline phase of glass-ceramics
The influence of various CuO addition on the crystal phases of glass-ceramics is exhibited in Fig. 2. The result shows that the augite phase is the primary crystal phase in all samples, as well as secondary phase of wollastonite (CaSiO3). The samples all show obvious diffraction peaks of augite, which indicate that both Fe2O3 and Cr2O3 could be utilized as the composite nucleation agents to improve the generation of main crystal phase by forming nuclei of spinel. It can also be found that adding CuO into the glass-ceramics has no obvious influence on the main crystal phase. The formation of wollastonite may be due to excessive Ca content in REBFS, and the possible reaction equations are as follows:

Meanwhile, the cuprite (Cu2O) crystalline phase can appear and the crystallization degree tends to increase gradually with the increase of CuO additions [24]. Generally speaking, Cu+ and Cu2+ are two forms of copper ions in glass-ceramics, while the cuprite can be precipitated in the form of colloid by the aggregation of Cu+, as shown in reaction:

Valence state of copper
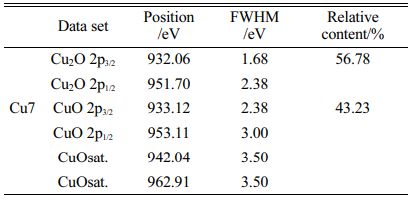
To detect the electronic properties of copper in glass-ceramic, XPS is used for the qualitative analysis of valence states of copper, and the final valence state of copper can be determined by the oxidation state the FWHM/eV, peak position, and multiplet splitting. The XPS fitting spectra of Cu2p are displayed in Fig. 3, and Table 3 shows the valence state distribution of copper in sample Cu7. It can be observed by XPS fitting that the oxidation states of copper in sample Cu7 are Cu2O and CuO, which corresponds to Cu+ and Cu2+ in glass-ceramic, and the relative content of Cu+ and Cu2+ is 56.78% and 43.23%, respectively. The appearance of Cu+ in glass-ceramic is probably because copper oxides undergo the following reaction in the melting process of glass-ceramics:

Zheng et al. reported that higher glass melting temperatures yield higher Cu+/Cu2+ ratios [25], thus some amount of Cu2+ can be reduced into Cu+.
Microstructure and elemental analysis of glass-ceramics
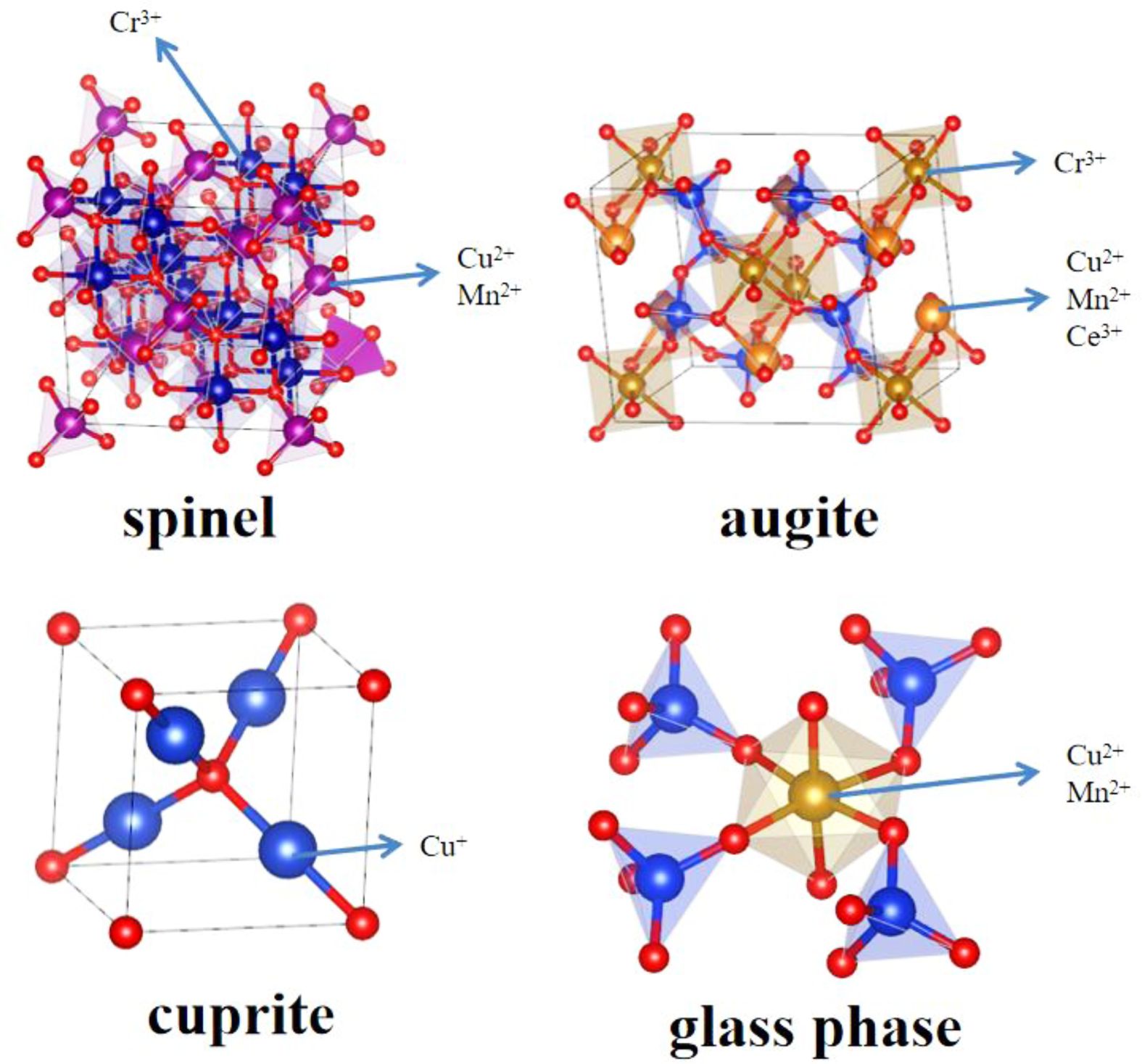
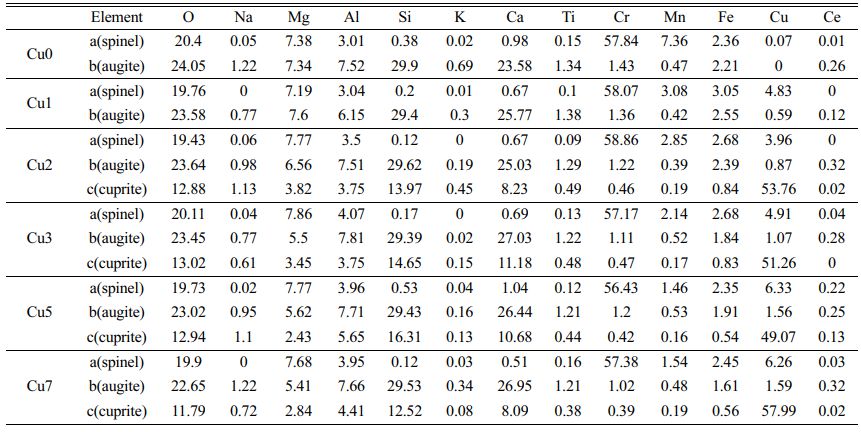
To determine the influence of CuO additions on the microstructure and element distribution of glass-ceramics, SEM and EDS are used to characterize the uncorroded glass-ceramics, and the results are shown in Fig. 4. EDS analysis of bright region (point a and c) and gray region (point b) are also shown in Fig. 4(a), (b) and (c) and Table 4, respectively. It can be found that the glass-ceramics are primarily consisted of spinel phase, augite phase, cuprite crystalline phase and glass phase located at grain boundaries. The results show that the large bright region corresponds to the spinel phase, which are mainly enriched in Cr, Mg, Fe, Mn, Al and O. In the element contents of the gray region, the elements of Ca, Si, Mg, Al and O are higher and these crystals can be regarded as augite phase. It is seen that spinel crystal nucleus could be utilized as heterogeneous nucleation phase to improve the formation of main augite phase, and the normal direction of spinel surface becomes the preferred growth direction of augite in the form of dendrite [26]. Meanwhile, it is also found that rare earth cerium ion intergrowth in REBFS can enter the crystal lattice of augite substitutionally replacing the Ca2+ because of the similar ionic radii, which can improve the mechanical properties of the glass-ceramics since the bond energy of Ce-O is much higher than that of Ca-O [27]. The result of elemental analysis of the small bright region indicates that the crystalline phase is consisted of Cu, Ca, Si and O. Combining the XRD and EDS results, the small plate-like crystal is cuprite phase. As the CuO additions increase, the cuprite phase in glass-ceramics show gradually increasing trend. Meanwhile, EDS analysis also indicate that with the increase of CuO ratios, the relative content of Cu increase and the relative content of Mn (mainly exists as Mn2+) tend to decrease in spinel phase, indicating that a small amount of Cu2+ (ionic radius is 0.73 Å) substitute the Mn2+ (ionic radius is 0.67 Å) due to the similar ion radius. Accordingly, the Mn2+ partly replaced by Cu2+ in spinel phase can exist in the glass phase in the form of manganese oxide octahedron. Furthermore, As the CuO additions increase, the crystalline morphology of augite can be found from dendrite to spherulites like morphology. The reason can be explained by the fact that copper ion can break the [Si-O] net structure and decrease the viscosity, which can increase the degree of bond breaking in glass network and reduce the energy barrier of crystallization of glass. As a result, the increase of nucleation rate leads to the change of crystal morphology from dendrite to spherulite. Through the above analysis, the existing forms of heavy metals in glass-ceramics are also summarized in Fig. 5. It is found that heavy metals Cr3+, Cu2+, Ce3+ and Mn2+ can exist stably in the spinel and augite by solid solution. With the increase of CuO content, Cu+ is stably cured in the glass-ceramic as the cuprite phase. Meanwhile, small amount of Cu2+ and Mn2+ can be cured in the glass phase as the manganese oxide octahedron. Therefore, glass-ceramics have a good stabilizing effect on heavy metals overall.
Leaching behaviour of heavy metals
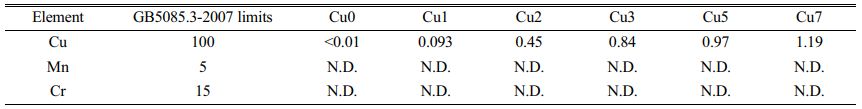
To quantitative determine the curing effect of the glass-ceramics prepared from REBFS on the heavy metals Cr, Cu and Mn, analysis of leach ability of glass-ceramics with various CuO additions are performed in accordance with GB/T5085.3-2007 and HJ/T 299-2007 [28]. Based on the leaching results shown in Table 5, leaching behavior of Cr and Mn is hardly to be detected during the whole period since Cr and Mn can be bound in stable spinel phases to prevent it from leaching [29]. It's worth noting that trace amount of Mn2+ solidified in the glass phase may not be detected in leaching experiment due to the low content. However, as the CuO additions increase, the leaching efficiency of Cu in glass-ceramics increases dramatically. An increase of leaching concentration is mainly caused by the leaching of Cu in cuprite phase and glass phase because of a much lower corrosion resistance in cuprite phase and glass phase. But on the whole, the leaching concentration of heavy metals Cr, Cu and Mn are well below the Chinese standard of GB/T5085.3-2007, which shows that the glass-ceramics has a good stabilizing effect on heavy metals.
Study on the erosion corrosion behaviour
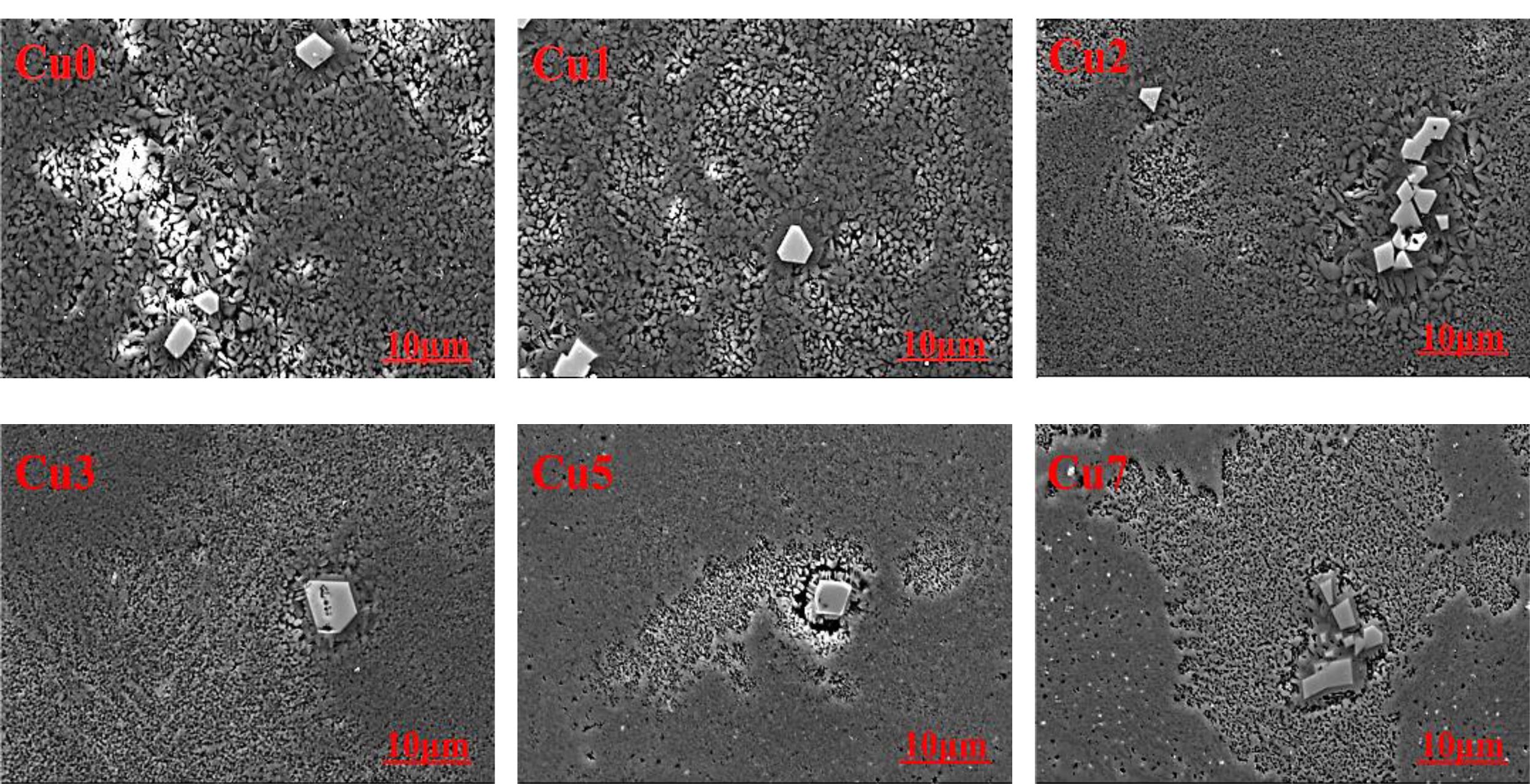
According to HJ/T299-2007 toxicity leaching method, the erosion corrosion experiments of polished block glass-ceramics samples are performed with a mixed solution of nitric acid and sulfuric acid, which is stirred with a magnetic stirrer at 30 ± 2 r/min for 20 hours at room temperature. Then, the corrosion morphology images of glass-ceramics samples are shown in Fig. 6. It is found that the corrosion mainly occurs in areas of glass phase and the white region near the grain boundary corresponds to the glass phase being corroded, which results in the appearance of corrosion pits or holes formed by the dissolving of glass phase in acid solution. As the CuO additions increase, the corrosion regions of glass phase show an increase tendency and corrosion resistance of glass phase is decreased, which can be explained by the fact that copper ions can break the network structure of [Si-O] and decrease the integrity of glass network structure [30]. Meanwhile, the CuO additions have no significant influence on the corrosion resistance of spinel crystal, and spinel exhibit an excellent corrosion resistance during the whole corrosion process. The corrosion morphology also shows that there is almost no cuprite crystals found on the surface of the samples after the erosion corrosion, and this phenomenon is related to the existing form of cuprite crystals in glass-ceramics. Xue et al. pointed out that cuprite crystals in colloidal states evenly exist throughout the glass-ceramics [20], and the direct-bonding degree between cuprite crystals and glass phase/crystalline phase remains at a relatively low level, which result in the exfoliation of cuprite crystals from glass-ceramics by the erosion corrosion. This phenomenon agrees well with the leaching results.
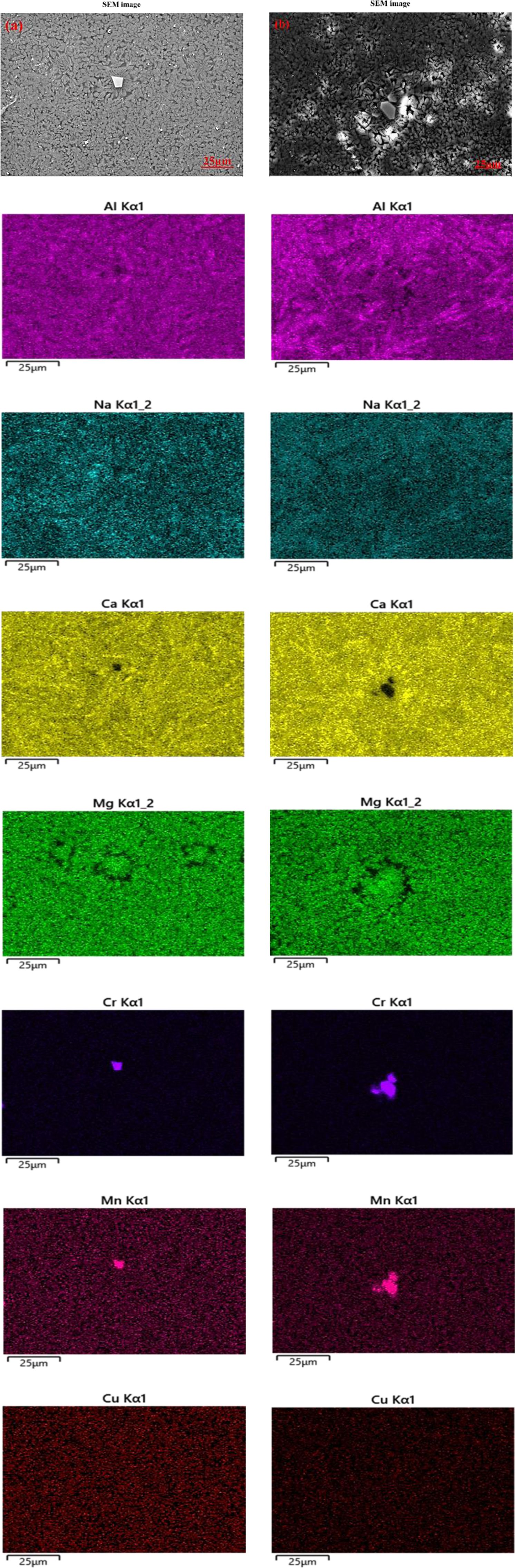
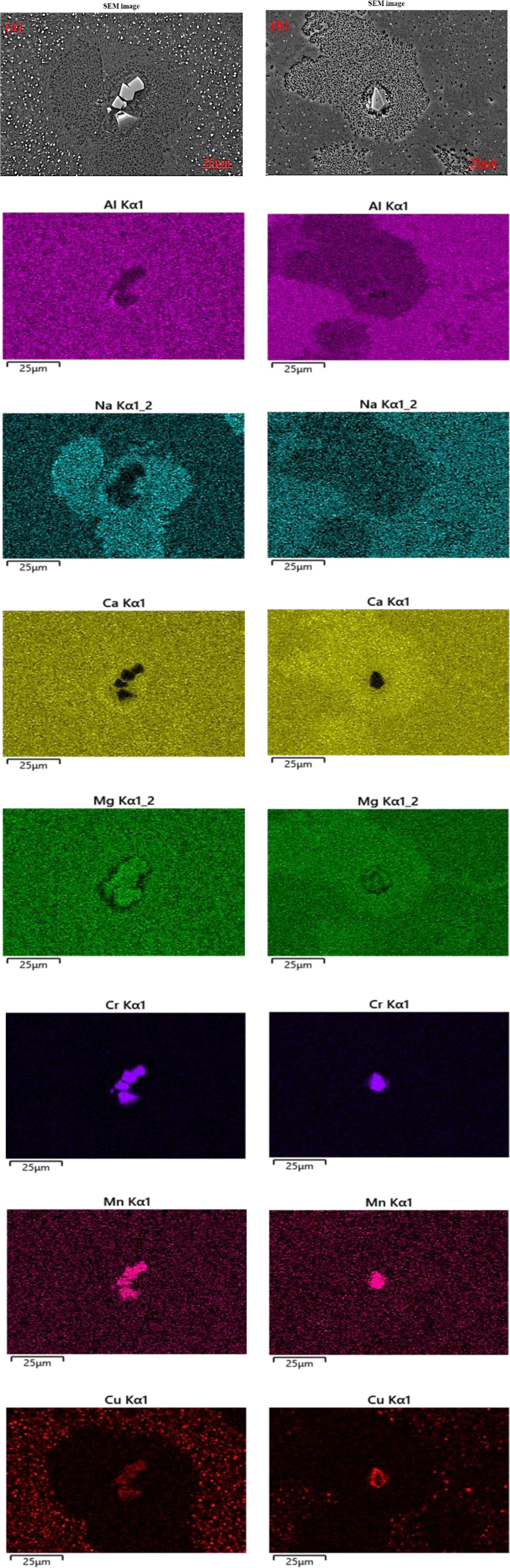
To accurately characterize the elemental distribution in glass-ceramics before and after erosion corrosion, the surface morphologies and elemental mapping of Cu0 and Cu7 are shown in Fig. 7 and Fig. 8, respectively. Compared with the results of sample Cu0 and Cu7 without erosion corrosion, the content of Al and Na is relatively low in serious corrosion regions. It is obvious that this region correspond to glass phase since the basic unit of the glass network structure was regarded to be [AlO4]- tetrahedron, which was charge-balanced by the co-existence of Na+ [31]. In addition, it can be found that the spinel crystal enriched in heavy metals Cr, Cu and Mn is little affected by erosion corrosion. Accordingly, heavy metals Cr, Cu and Mn can be well solidified in spinel structure. On the contrary, the cuprite crystals present in the form of colloidal are corroded more easily, which leads to an increase in leaching concentration of Cu.
Analysis of coloring mechanism
Fig. 9 shows the influence of various CuO additions on the color of glass-ceramics, and the result indicates that the color gradually changes from green to red with the increase of CuO contents. Studies indicate that the existence of iron and chromium ions in glass-ceramics will result in colour of green [32]. Adding CuO is proposed to promote the red coloration in glass-ceramics, and the corresponding coloring mechanism is due to the appearance of cuprite crystals caused by the addition of CuO [33].
Physicochemical properties
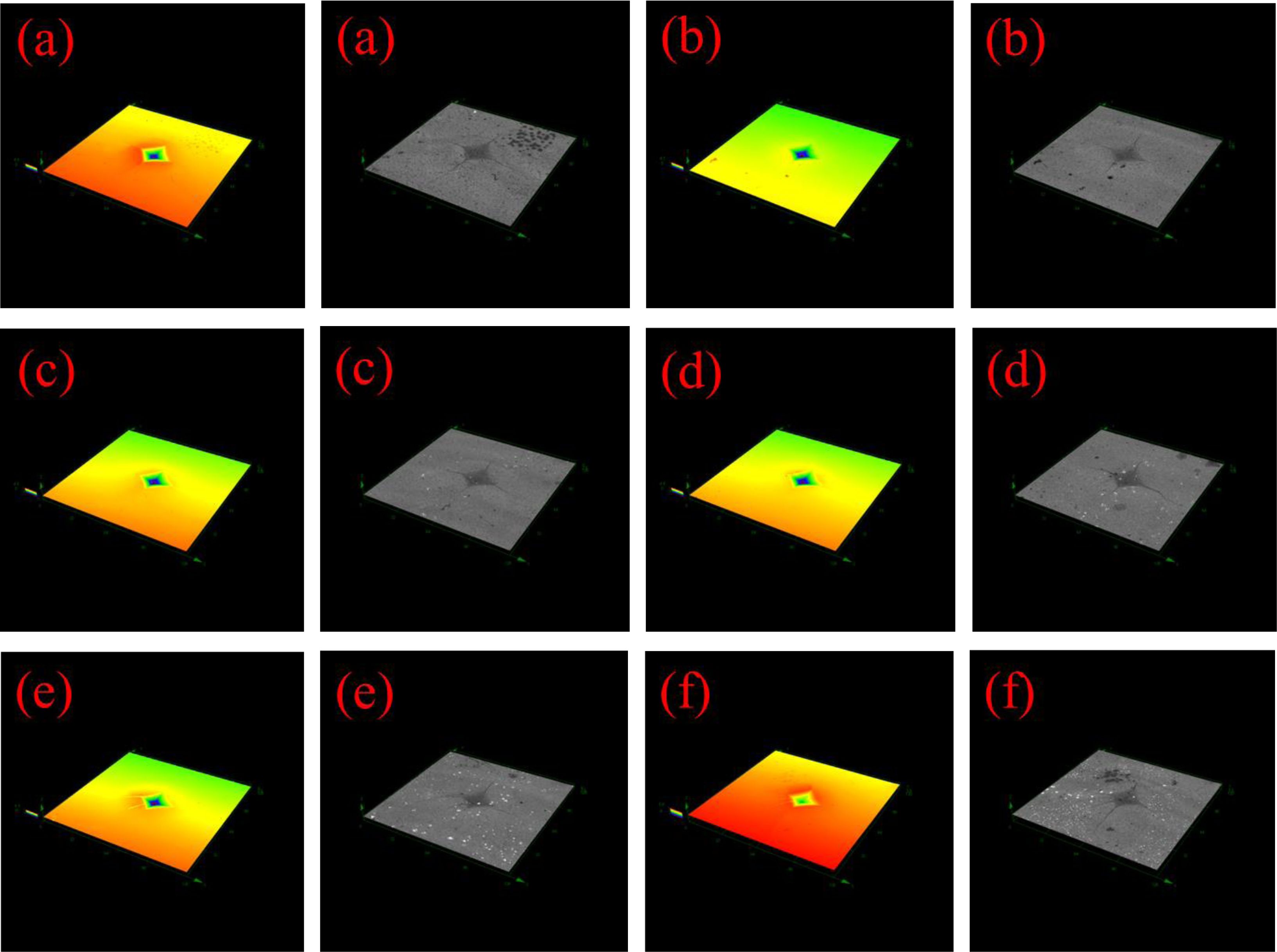
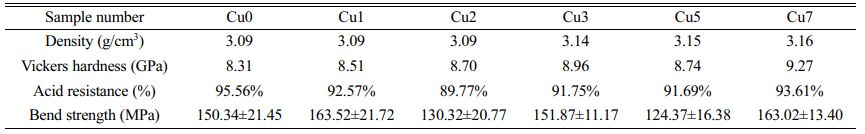
Table 6 shows the mechanical, physical, and chemical properties of glass-ceramics with different CuO additions. As the CuO additions increase, the Vickers hardness and density glass-ceramics demonstrate an increasing trend. It is primarily corresponded to the transformation of crystal morphology of glass-ceramics since the increase of CuO ratios has no obvious effect on phase transformation of main crystalline phases. The crystal morphology of glass-ceramics transforms from dendrites to spherulites with the increase of CuO content, and the appearance of spherulites is helpful to enhance the uniformity of crystal distribution and increase the compactness of glass-ceramics [34]. Consequently, the density and hardness of glass-ceramics increase. Fig. 10 shows the Vickers indentation morphology of glass-ceramics with the increase of CuO ratios. It can be found that cracks in the samples of Cu0, Cu1 and Cu2 occur along the diagonal direction of the indentations in square shape. With the continuous additions of CuO, the cracks propagating along the diagonal direction are suppressed and cracks expan producing bypass and deflection, which could increase the Vickers hardness of glass-ceramics. Moreover, as the CuO additions increase, the acid resistance shows a decreasing trend. It may be because that the cuprite crystalline phase with a much lower corrosion resistance shows gradually increasing trend with increasing the CuO additions. The progressive introduction of CuO into the glass-ceramic exerts a complicated influence on its bending strength, which generally tends to first decrease and then increase. Generally speaking, the high aspect ratio of dendrite morphology is helpful to the formation of integrated interlocking crystal structure, which can effectively avoid brittle fracture of glass-ceramics and improve the bending performance. The morphology of glass-ceramics is changed from dendrites to spherulites with the increase of CuO content. As a result, the bending strength of glass-ceramics is decreased gradually. However, the abnormal increasing in bending strength for sample Cu7 may be due to the precipitation of a large number of cuprite phases, which can increase the bending strength of glass-ceramics by the particle pinning effect [35]. In a word, glass-ceramics prepared from REBFS had excellent comprehensive physico- chemical properties, and the crystalline phase and glass phase in glass-ceramics formed a double barrier, which can effectively prevent the leaching of heavy metals [36, 37]. Glass-ceramics can effectively solidify heavy metals and had good economic and environmental benefits [38, 39].
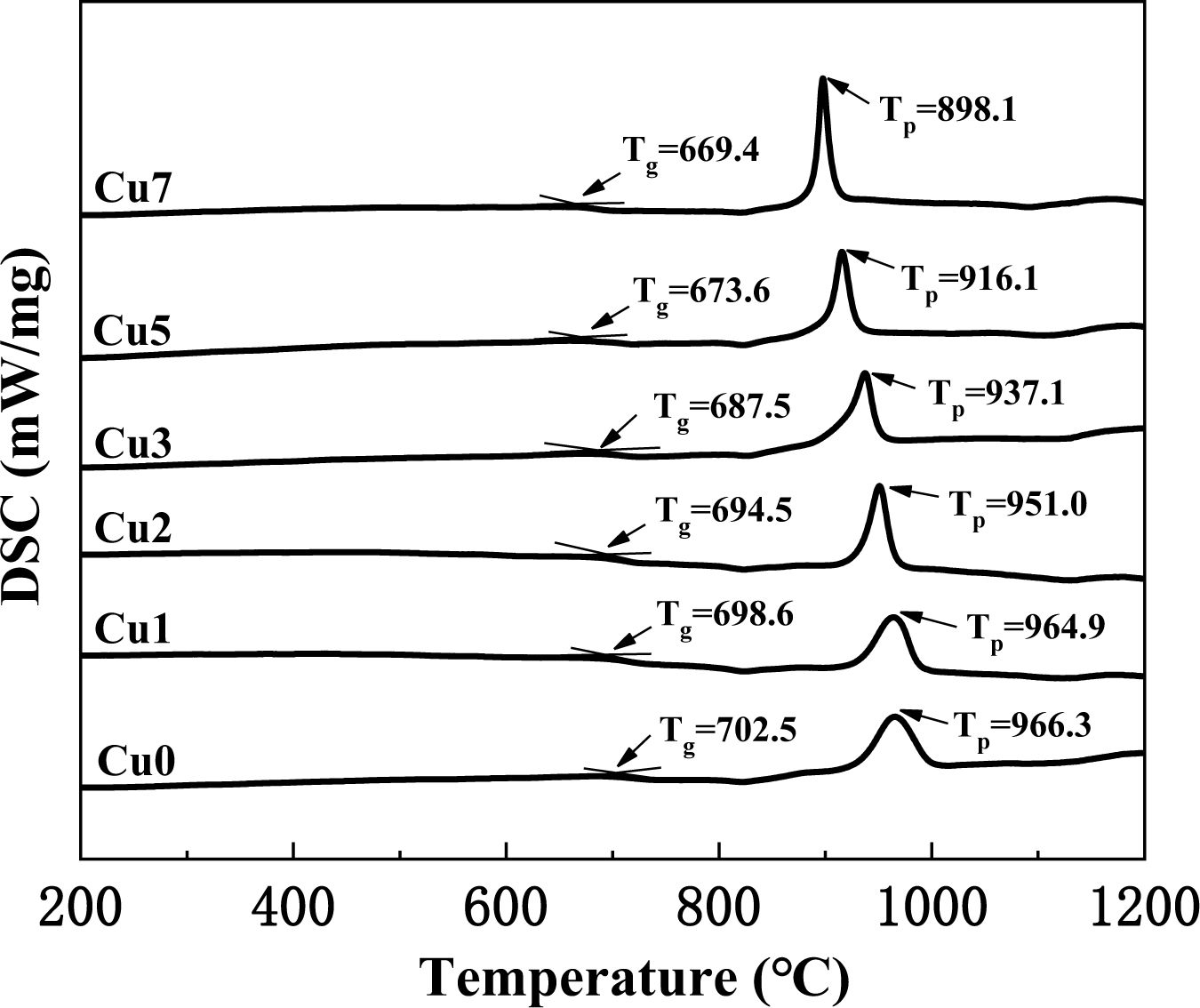
|
Fig. 1 DSC curves of the prepared base glasses with various CuO addition. |
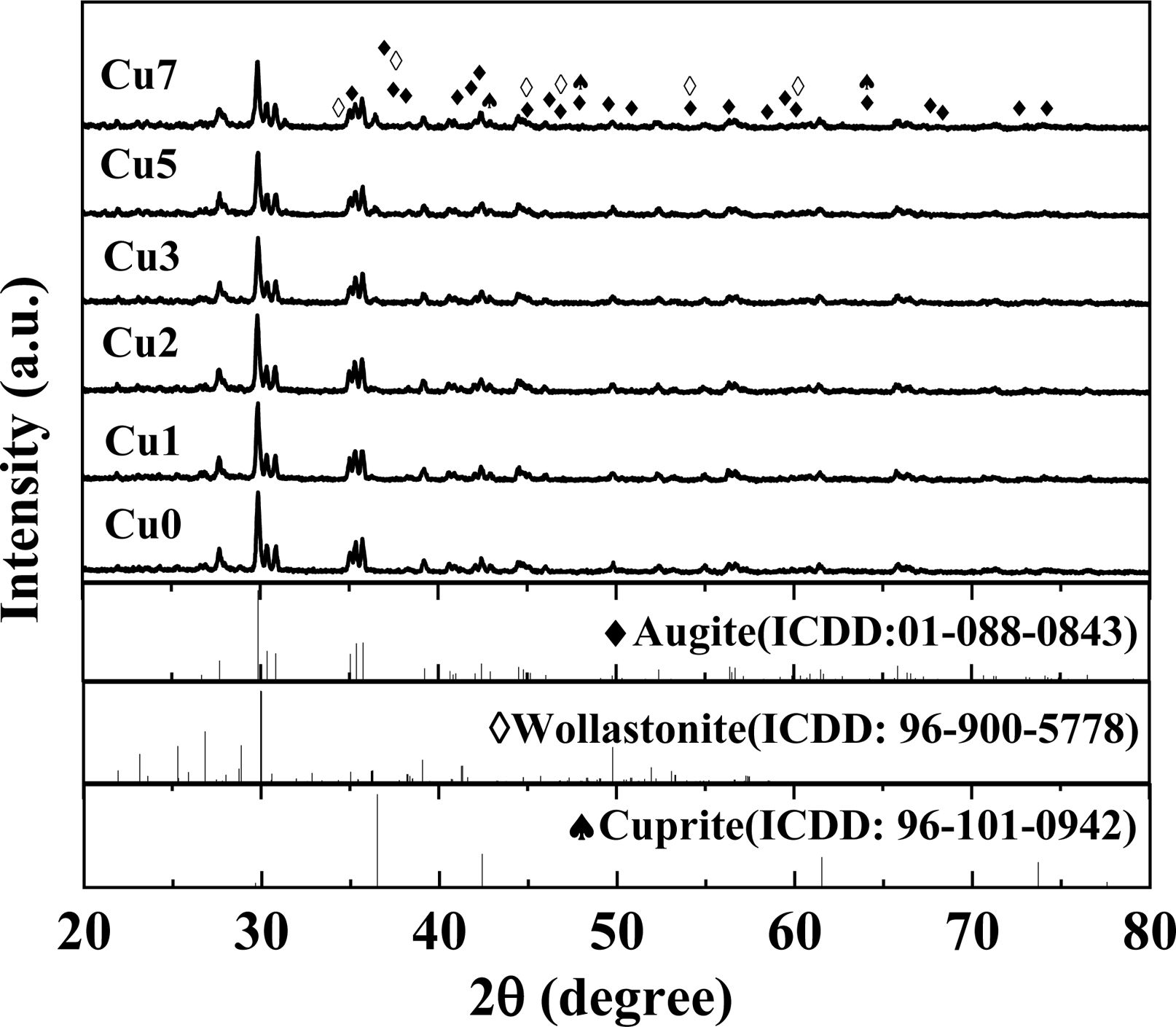
|
Fig. 2 XRD patterns of glass-ceramics with various CuO contents. |
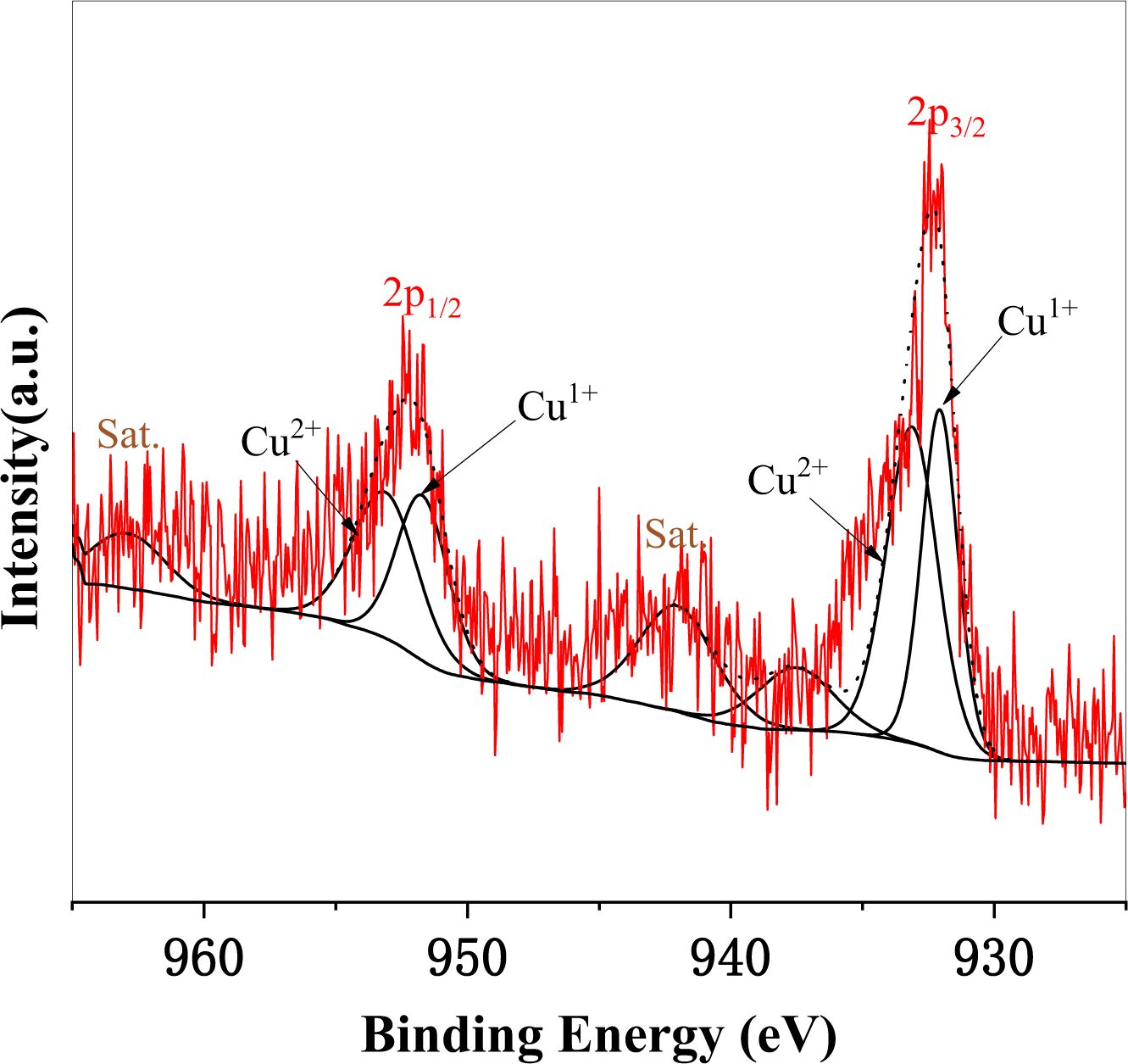
|
Fig. 3 XPS fitting spectra of Cu2p in sample Cu7. |
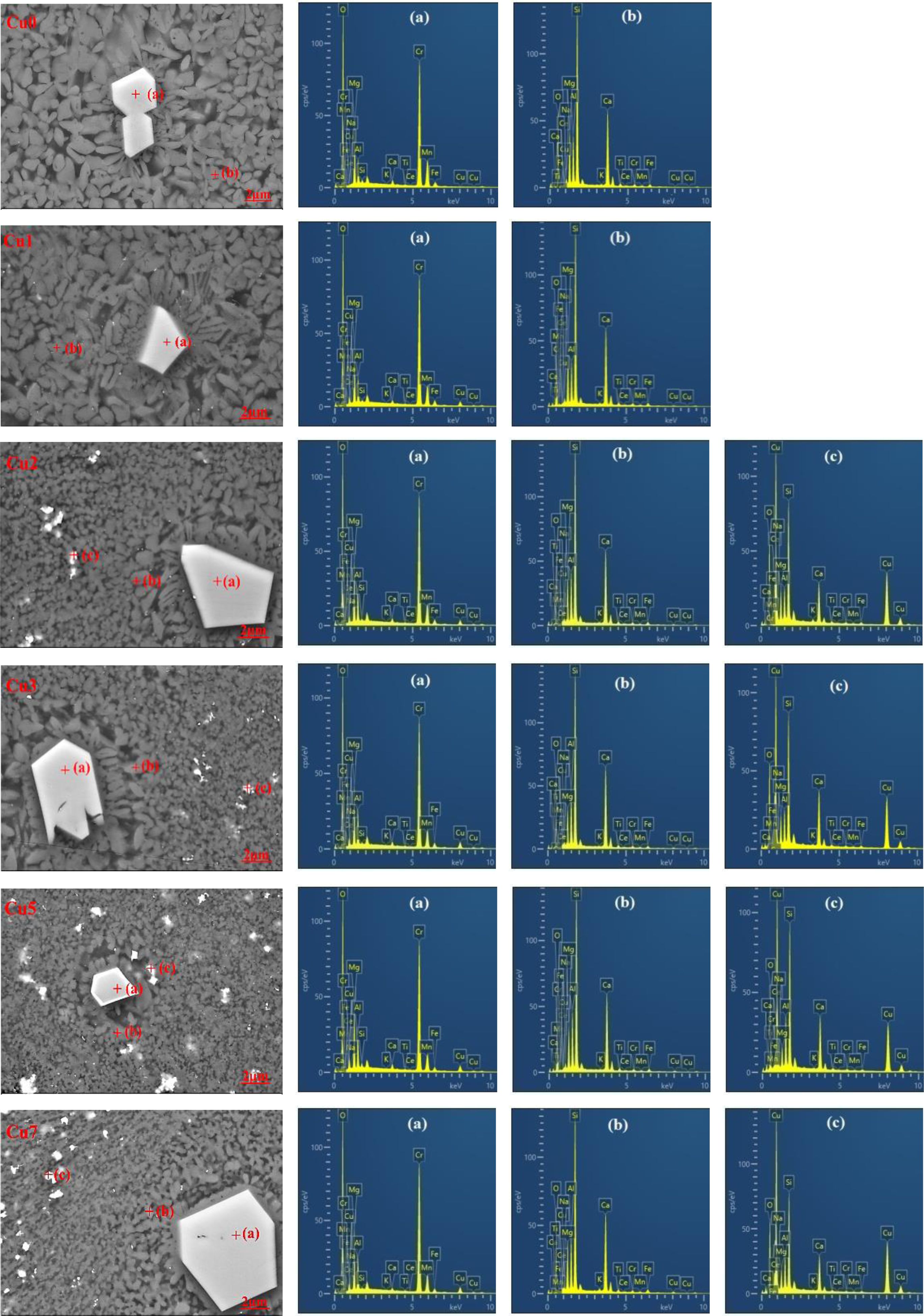
|
Fig. 4 SEM images and EDS data of glass-ceramic samples with different CuO contents. |
|
Fig. 5 Existence state of heavy metals in glass-ceramic. |
|
Fig. 6 SEM image of glass-ceramics with various CuO contents after erosion corrosion. |
|
Fig. 7 SEM image and EDS energy spectrum scan image of sample Cu0: (a) uncorroded (b) corroded. |
|
Fig. 8 SEM image and EDS energy spectrum scan image of sample Cu7: (a) uncorroded (b) corroded. |

|
Fig. 9 The effect of different CuO additions on the color of glass-ceramics. |
|
Fig. 10 Vickers indentation morphology of glass-ceramics with various CuO additions: (a) Cu0, (b) Cu1, (c) Cu2, (d) Cu3, (e) Cu5 and (f) Cu7. |
|
Table 2 Phase transition temperatures of glass-ceramics with various CuO addition. |
|
Table 4 Elemental analysis of a (spinel phase), b (augite phase) and c (cuprite)in Fig. 4 (wt%) |
|
Table 5 Leaching test results of glass-ceramics with different CuO contents (mg/L) |
REBFS was successfully used as the main raw material to prepare glass-ceramics, and the effect of CuO on the crystallization, chemical stability, coloring mechanism and properties of glass-ceramics were studied. The main conclusions of this work are as follows:
1. Copper ions had two valence states in glass-ceramics, namely Cu+ and Cu2+, and Cu+ content was higher than Cu2+ content. Cu+ ions were found to mainly exist in the cuprite crystals and Cu2+ ions mainly exist in spinel crystals, augite and glass phase. Meanwhile, copper ion in glass phase can break the network structure of [Si-O] and decrease the viscosity, thus causing the crystal morphology of augite changed from dendrite to spherulites.
2. Glass-ceramics prepared from REBFS had excellent comprehensive physicochemical properties. The increase of CuO contributed to the increase of the density and Vickers hardness of glass-ceramics, while the acid resistance and bending strength slightly decreased.
3. The leaching tests showed that the leaching concentration of Cr, Cu and Mn in glass-ceramics were far below the national standard of GB/T5085.3-2007 in China. Glass-ceramics had a good stabilizing effect on heavy metals overall.
4. The colour of glass-ceramics gradually changed from green to red with the increase of CuO contents. The glass-ceramics in copper red color is expected to be used as decorative materials in building, which not only realized the harmless utilization of REBFS but also friendly to the environment.
This work was supported by National Natural Science Foundation of China (grant number 52060021), Fundamental Research Funds for Inner Mongolia University of Science & Technology, Natural Science Foundation of Inner Mongolia Autonomous Region (grant number 2022LHMS05002 and 2022LHMS05005) and Inner Mongolia University of Science and Technology Plan (0903058002).
- 1. G.H. Ai, Y.F. Liu, Y. Wang, and Y.L. Chen, J. Residuals Sci. Tech. 12[1] (2015) 17-23.
-

- 2. B. Wang, X.Y. Yuan, L. Han, X.Y. Wang, and L.J. Zhang, Environ. Earth Sci. 74[6] (2015) 5087-5096.
-

- 3. X.H. Chen, H.X. Guo, and X.H. Cheng, Environ. Geotech. 5[2] (2018) 107-113.
- 4. T. Chen, C. Lei, B. Yan, L.L. Li, D.M. Xu, and G.G. Ying, Environ. Sci. Pollut. R. 25[36] (2018) 36702-36711.
-

- 5. B.W. Li, L.B. Deng, X.F. Zhang, and X.L. Jia, J. Non-Cryst. Solids 380 (2013) 103-108.
-

- 6. Y.L. Zhao, J.P. Qiu, M.A. Zhengyu, Z.B. Guo, and H. Liu, Powder Technol. 375 (2020) 539-548.
-

- 7. H. Park, Y. Jeong, Y.B. Jun and J.E. Oh, Constr Build Mater. 122 (2016) 343-353.
-

- 8. G.B. Wang, Q.Q. Zhang, W.C. Du, R.Z. Lin, J.H. Li, F.X. Ai, Y. Yin, R. Ji, X.R. Wang, and H.Y. Guo, Ecotoxicol Environ Saf. 207 (2021) 111275.
-

- 9. W.C. Ma, D.M. Chen, M.H. Pan, T.B. Gu, L. Zhong, G.Y. Chen, B.B. Yan, and Z.J. Cheng, J. Environ. Manage. 247 (2019) 169-177.
-

- 10. J.G. Jiang, J. Wang, X. Xu, W. Wang, Z. Deng, and Y. Zhang, J. Hazard. Mater. 113[1-3] (2004) 141-146.
- 11. Y.L. Pi, W.H. Zhang, Y.S. Zhang, W.T. Zou, Z.X. Zhang, and F. Wu, Ceram. Int. 47[17] (2021) 24663-24674.
-

- 12. B.Q. Li, Y.P. Guo, and J.Z. Fang, J. Alloy. Compd. 881 (2021) 159821.
-

- 13. L. Liu, Y.L. Li, and Z.K. Zhang, J. Clean. Prod. 269 (2020) 122417.
-

- 14. S.Z. Zhao, Q. Wen, X.Y. Zhang, B. Liu, and S.G. Zhang, Ceram. Int. 47[15] (2021) 21599-21609.
-

- 15. C. Li, Q. Wen, M. Hong, Z. Liang, Z. Zhuang, and Y. Yu, Constr. Build. Mater. 134 (2017) 443-451.
-

- 16. Y.L. Pi, W.H. Zhang, Y.S. Zhang, W.T. Zou, Z.X. Zhang, and F. Wu, Ceram. Int. 47[17] (2021) 24663-24674.
-

- 17. L.Y. Tang, R.H. Han, and J. Quan, J. Wuhan Univ. Technol. 34[6] (2012) 6-9.
- 18. H. Yang and X.Z. Guo, Int. J. Mater. Prod. Tec. 37[3-4] (2010) 280-286.
-

- 19. T. Suzuki, K. Horibuchi, and Y. Ohishi, J. Non-Cryst. Solids 351[27-29] (2005) 2304-2309.
-

- 20. J.W. Xue, J.W. Zhong, Y.R. Mao, C.H. Xu, W. Liu, and Y.Q. Huang, Ceram. Int. 46 (2020) 23186-23193.
-

- 21. G.Y. Wang, Y.S. Du, J. Ma, H.X. Zhang, S.L. Ouyang, L.B. Deng, H. Chen, and M. Zhao, J. Ceram. Process. Res. 22[6] (2021) 665-674.
-

- 22. S.A.M. Abdel-Hameed, M.A. Marzouk, and A.E. Abdel-Ghany, J. Non-Cryst. Solids 357[24] (2011) 3888-3896.
-

- 23. H.A. Elbatal, Z.S. Mandouh, H.A. Zayed, S.Y. Marzouk, G.M. Elkomy, and A. Hosny, J. Non-Cryst. Solids 358 (2012) 1806-1813.
-

- 24. E.M.A. Hamzawy and C. Leonelli, Glass Technol. 46[3] (2005) 281-286.
- 25. H.X. Zheng, M.W. Colby, and J.D. Mackenzie, J. Non-Cryst. Solids, 127[2] (1991) 143-150.
-

- 26. H.R. Li, S.Y. Liu, W.C. Xu, Y.X. Zhang, Y. Shi, J. Ma, S.L. Ouyang, and Y.S. Du, J. Eur. Ceram. Soc. 41[2] (2021) 1603-1612.
-

- 27. H. Chen, B. W.Li, M. Zhao, X.F. Zhang, Y.S. Du, Y. Shi, and J.S. McCloy, J. Non-Cryst. Solids 524 (2019) 119638.
-

- 28. S.L. Ouyang, Y.X. Zhang, Y.X. Chen, Z.W. Zhao, M. Wen, B.W. Li, Y. Shi, M.Z. Zhang, and S.L. Liu, Sci. Rep. 9 (2019) 1964.
-

- 29. J. Ma, Y. Shi, H.X. Zhang, S.L. Ouyang, L.B. Deng, H. Chen, M. Zhao, and Y.S. Du, Mater. Chem. Phys. 261 (2021) 124213.
-

- 30. M.V.S. Rao, S. Suresh, T. Narendrudu, A.S. Kumar, G.C. Ram, and D.K. Rao, J. Mol. Struct. 1125 (2016) 624-632.
-

- 31. H.X. Zhang, Y.S. Du, X.W. Yang, X.F. Zhang, M. Zhao, H. Chen, S.L. Ouyang, and B.W. Li, J. Non-Cryst. Solids, 482 (2018) 105-115.
-

- 32. G.X. Gayo and A.E. Lavat, Ceram. Int. 44[18] (2018) 22181-22188.
-

- 33. T.M. Gross, J. Lahiri, A. Golas, J. Luo, F. Verrier, J.L. Kurzejewski, D.E. Baker, J. Wang, P.F. Novak, and M.J. Snyder, Nat. Commun. 10 (2019) 1979.
-

- 34. X. Chen, T.C. Chadwick, R.M. Wilson, R. Hill, and M.J. Cattell, J. Dent. Res. 89[12] (2010) 1510-1516.
-

- 35. D.D. Feng, Y.M. Zhu, F.F. Li, and Z.H. Li, J. Eur. Ceram. Soc. 36[10] (2016) 2579-2585.
-

- 36. W. Dang and H.Y. He, J. Ceram. Process. Res. 21[1] (2020) 69-74.
-

- 37. W.T. Zou, W.H. Zhang, Y.L. Pi, Y.S. Zhang, L. Zhang, and Y. Chen, Ceram Int. 48 (2022) 5040-5053.
-

- 38. C.H. Li, W. Zhao, J.L. Zhang, W. Lu, P. Li, B.J. Yan, and H.W. Guo, J. Ceram. Process. Res. 22[4] (2021) 409-420.
-

- 39. N. Gao, Y.X. Chen, C.G. Wang, Y.L. Guo, and J.Y. Zhang, J. Ceram. Process. Res. 22[6] (2021) 714-721.
-

 This Article
This Article
-
2023; 24(1): 58-68
Published on Feb 28, 2023
- 10.36410/jcpr.2023.24.1.58
- Received on Jun 14, 2022
- Revised on Aug 17, 2022
- Accepted on Sep 3, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yongsheng Du
-
aCollege of Science, Inner Mongolia University of Science and Technology, Baotou 014010, China
bCollaborative Innovation Center of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, China
Tel : +86-472-5954358 Fax: +86-472-5954358 - E-mail: dys1111@imust.edu.cn







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.