- Autotrophic and mixotrophic culture of electro-active microalgae with electron supplied from electrodes for CO2 conversion
Aswathy Udayan#, Seongcheol Kang# and Byoung-In Sang*
Department of Chemical Engineering, Hanyang University, 222 Wangshimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Electrochemical technologies that involve microorganisms are considered to be promising for sustainable applications. Microalgae can be used for carbon capture through photosynthesis which can directly fix carbon dioxide (CO2). The conversion of CO2 into fuel energy and other high value metabolites without pollution can contribute to reduce CO2 emissions with more economic value. Light energy to biomass conversion efficiency is a major challenge in microalgal cultivation. Electrode assisted cultivation techniques for improved photosynthetic and carbon (CO2) fixation metabolism for growth and biomass productivity have rarely been explored for microalgae. Light limitation, which leads to the loss of photosynthetic efficiency that in turn leads to decreased microalgal growth, is a major problem in large scale cultivation systems. Here, we summarise the ability of microalgae to perform extracellular electron uptake from cathode material for efficient biomass production and CO2 conversion. The present review provides insights into the possible development of electroactive microalgae under autotrophic and mixotrophic conditions for efficient CO2 conversion. Using the current knowledge of bioelectrochemistry and learning lessons from electroactive bacteria, we propose a proof of concept for electroactive microalgae and their future applications in CO2 sequestration
Keywords: Electroactive microalgae, CO2 fixation, Extracellular electron uptake, Autotrophy, Mixotrophy
Microalgae are unicellular photosynthetic organisms capable of producing high-value metabolites such as carbohydrates, lipids, proteins, polyunsaturated fatty acids, vitamins, and pigments. Microalgal biomass has garnered tremendous interest for producing nutraceuticals, pharmaceuticals, therapeutics, food supplements, feed, biofuel, bio fertilizers, etc. due to its high content of lipids and other high-value metabolites. Microalgal biomass has the potential to convert solar energy to organic materials and potential metabolites of nutraceutical and industrial value [1]. Microalgae have several advantages in comparison with higher plants. They have a high efficiency of fixing carbon dioxide (CO2) and subsequently convert it into biomass and compounds of potential interest [2]. Ceramic carriers were used to achieve highest carbon reduction of approximately 53% by using photosynthetic bacteria [2]. Moreover, microalgae have a shorter life cycle and higher photosynthetic efficiency than higher plants. However, despite the research efforts made in the past few decades, the cost of microalgal products has remained very high, compared with that of agricultural plant products. Hence, understanding the full potential of microalgae for the sustainable production of biomass and other products are necessary.
Electroactive microorganisms have gained substantial attention recently as they allow the flow and exchange of electrons between intracellular or extracellular redox electroactive donors or acceptors [3]. Electroactive microorganisms are currently applied in microbial fuel cells (MFCs) and microbial electrosynthesis (MES) [4]. Electro-activated microalgae are a proof of concept that the application of renewable electricity as an electron donor in microorganisms.
The application of renewable electricity in micro- organisms like bacteria has already been exploited for the production of metabolites and biomass [5]. Currently, the focus of microbial electrosynthesis is limited to acetogens and methanogens [6]. Different types of mechanisms are involved in the transport of externally produced electrons to the electron transport chain (ETC) of host microorganisms [7]. Two types of electron transfer can be achieved: direct electron transfer (DET) and mediated electron transfer (MET). Cupriavidus necator shows MET using hydrogen as an electron mediator and presents a higher solar energy to biomass conversion rate of 9.7% compared with microalgae that have a solar energy to biomass conversion rate of 3% and 6% at large-scale outdoor cultivation systems and small-scale laboratory conditions, respectively [8, 9]. Due to ineffective light-to-biomass conversion, productivity typically decreases in photo bioreactors (PBRs), resulting in a lower photosynthetic efficiency (9-10%) than the theoretical maximum (corresponding to ~80 g biomass m-2 day-1) [10]. Algal light conversion efficiency is reported to fall between 3% and 5% in PBRs used for large scale cultivation [11]. Therefore, the controlled cultivation of microalgae with efficient photosynthetic activity in a commercially viable manner is necessary.
Based on the physiology of microalgae, different cultivation systems and bioreactors can be developed to enhance light absorption and substrate utilization. Such systems should be able to provide sufficient nutrients and photons (not excess) to prolong the logarithmic growth of microalgae, which is necessary for sufficient product accumulation [12]. Mixotrophic cultivation systems can enhance growth and metabolite production in microalgae. Although these systems provide sufficient organic nutrients and energy for the growth of microalgae, they have a relatively poor photosynthetic efficiency (~2-6%) to fix CO2 and only 5-10% of the allocated carbon is used to produce lipids and other secondary metabolites [13]. Therefore, metabolic and genetic engineering technologies are being considered to enhance microalgal production [14]. Among these, manipulation of the Calvin–Benson–Bassham (CBB) cycle and chloroplast electron transport chain are of potential interest for improving the photosynthetic efficiency of microalgae by increasing the efficiencies of CO2 fixation and light harvesting [1].
Recently, external electron supplementation has gained increasing interest for enhancing the growth and metabolite synthesis in microalgae. Acceleration of carbon and energy flux in microalgae is associated with product biosynthesis. Lipid biosynthetic precursors like acetyl-CoA and NADPH are considered to be important factors for increasing product yield in microalgae [12]. For increasing the capture and delivery of electrons formed by substrate catabolism, extra energy is needed to accelerate the conversion of NADP+ and NADPH in microalgae and this may produce less disturbance on the redox state than the strategy to increase the proportion of NADPH [1]. Therefore, providing electrons through external electrodes has the potential to accelerate energy flux and product yield in microalgae. Mixotrophic cultivation of microalgae using external electrodes can promote the ratio of NADPH/ATP in microalgal cells, which can boost CO2 fixation and improve the yields of biofuels and high-value secondary metabolites from microalgae. Moreover, the external supplementation of electrons can prevent energy loss due to cell shading, which is the major reason for photosynthetic loss in microalgal cultivation systems.
Improving our knowledge of extracellular electron uptake or electro-activated microalgae is critical from both biotechnological and industrial viewpoints [13]. Early research findings have proved extracellular electron uptake by autotrophic bacterial species [14]. Microalgae have been widely used as biophotovoltaics (BPV), wherein the transfer of electrons from microalgal cells to an external electrode takes place [15, 16]. Different types of photo electrodes are developed by researchers for this purpose [17, 18]. However, substantial research has not been conducted on the reverse flow of electrons. Owing to the industrial relevance of efficient microalgal cultivation systems and their emerging biotechnological applications, this review will focus on the possibility of extracellular electron uptake by microalgae in different cultivation modes to achieve maximum CO2 fixation. In this review, we propose a possible mechanism of electro-activated microalgae to overcome the current limitations of CO2 fixation and subsequent microalgal biomass production.
The industrial cultivation of microalgae is often carried out in open ponds or closed PBRs in the presence of sunlight and other chemicals to improve the biomass and metabolite accumulation [19]. When the microalgal cultures attain a high optical density, self-shading occurs, leading to an inhomogeneous distribution of light inside the cultivation system, which will limit the conversion of CO2. As a result, only the cells present at the top layers absorb sufficient incident light, leaving the bottom layers in light-limited conditions; this has major consequences on the photosynthetic metabolism of microalgae [20]. In light-limited conditions, cells do not have sufficient energy for the synthesis of NADPH/ATP, major factors for the Calvin–Benson pathway, and thus, the cells fail to sustain a high biomass growth rate. If the light intensity available is below the compensation point, energy is utilized for cell maintenance rather than CO2 fixation [20]. Culture depth is an important parameter that affects the algal biomass production in large scale cultivation systems. Chisti reported that the culture depth suitable for high biomass production is up to 20 cm [21]. Even if cells are exposed to full sunlight, the chance of excess illumination is low. For example, photosynthesis in Nannochloropsis is reported to be saturated at a light intensity of ~150 µmol photons m-2 s-1, a value below the full sunlight intensity of ~500-2000 µmol photons m-2s-1 (depending on the season) [22]. Microalgal cells can absorb sunlight efficiently even in saturated conditions, which produces 3Chl* (triplet state chlorophyll) that cannot be used for photochemical reactions but generates reactive oxygen species (ROS). Generation of ROS molecules leads to the oxidation of proteins, lipids, and pigments with photosystem II (PSII) D1 subunit as a major target [23]. The damaged proteins and other metabolites are subsequently degraded and resynthesized for regaining the photosynthetic activity [24]. The photosynthetic machinery of microalgae has been evolved to adapt to different light regimes to survive highly variable illumination under natural environments. Microalgae show non-photochemical quenching (NPQ) for the non-radiative de-excitation of excited chlorophyll molecules, which results in the dissipation of excess energy in the form of heat [20]. Antioxidant molecules are also involved in preventing photochemical damage to microalgal cells. Carotenoid molecules act as antioxidants as well as activators of NPQ in microalgal cells, with zeaxanthin being the major carotenoid molecule involved in this process. The cells synthesize zeaxanthin from violaxanthin by the activity of violaxanthin de-epoxidase enzyme under high light conditions; under light-limited conditions, zeaxanthin is converted back to violaxanthin by the activity of zeaxanthin epoxidase enzyme [25]. This photosynthetic regulatory mechanism plays a significant role in photon to biomass conversion efficiency. The repair of damaged PSII needs energy for the synthesis of proteins that can result in the decreased availability of nutrients for microalgal growth [10]. NPQ photo protection mechanisms dissipate energy as heat that decreases the photon to biomass conversion efficiency. For increased biomass productivity and photon to biomass conversion efficiency, an optimal balance should be maintained between photo protection and photon efficiency. In large scale cultivation systems, mixing for gas exchange can also affect light absorption and photosynthetic efficiency. If the mixing is very fast, the zeaxanthin-mediated photo protection mechanism does not occur properly, leading to photo inhibition and decreased productivity [26].
Microalgae are photoautotrophic organisms that utilize light as a primary energy source for their metabolism. Light is harvested through phycobilisomes that excites P700 and P680 chlorophyll pigments at the reaction centres of PS I and PS II (Photosystem I and Photo- system II) located in the thylakoid membrane of chloroplasts (Fig. 2). This process aids the extraction of electrons from water and releases oxygen as a by-product [27]. These electrons are then conveyed through PS II, cytochrome b6f, plastocyanin (PC)/Cyt c6, and PSI to ferredoxin (Fd). Ferredoxin acts as the distribution hub for photosynthetic electrons. The photosynthetic electron transport chain (PETC) generates a proton motive force (pmf) across the thylakoid membrane that acts as a driving force for the production of ATP from ADP and Pi by the enzyme ATP synthase (Fig. 1) [28].
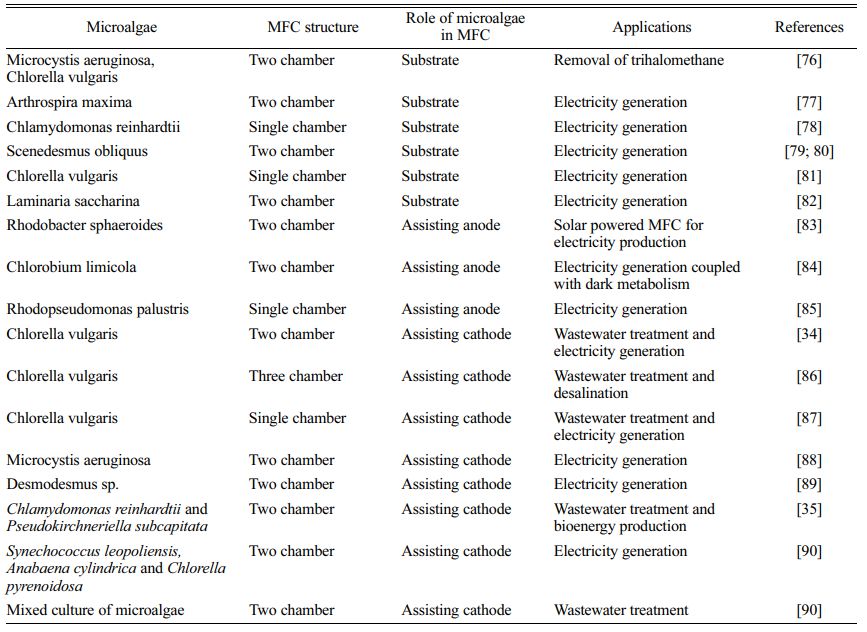
Different types of fuel cells have been developed for wastewater treatment [29]. Electrosynthesis using photosynthetic microorganisms is an emerging field of research. Several studies have been conducted on phototrophic bacteria that have proved the underlying molecular mechanisms and flow of electrons from cathodes to the bacteria for energy transduction and biomass production [2, 6]. Most of the research on microalgae is focused on MFCs for electricity production or wastewater treatment (Table 1). Microalgae that can accept electrons directly from cathode are referred to as electro-activated microalgae.
An MFC is a bio-electrochemical device capable of converting organic or inorganic substrates to energy through microbial metabolism [30]. Organisms capable of doing this are known as electrogens or electro- chemically active microorganisms (EAM) that use the extracellular electron transport (EET) system for exchanging electrons with an insoluble material such as an electrode or metal oxide [31]. MFCs include the incorporation of microalgae in the cathode compartment that depends on the CO2 produced at the anodic compartment as a carbon source. This process facilitates electrogenesis and can be used for wastewater treatment, CO2 sequestration, and the synthesis of commercial products from microalgae (Fig. 2) [32]. Photo-cathodes with microalgae can be considered as a potential technique for the incorporation of photo- synthesis into MFC systems [33] and have benefits such as nutrient removal, CO2 fixation, supply of dissolved oxygen, and algal biomass production [34]. MFCs incorporated with microalgae are used for wastewater treatment for the removal of heavy metals and other nutrients, and the biomass produced by algae can be used to produce biofuels, biofertilizers, etc. [35, 36]. Commault et al. (2014) reported the use of photosynthesis in the cathodic compartment to replace the energy intensive mechanical aeration in bioreactors [37]. As a negative effect, the generation of a high oxygen content in the anodic compartment prevents electrogenesis even with high substrate degradation [38]. High electrogenic activity in the anodic chamber was observed by using photosynthetic bacteria as an anodic catalyst [18]. Oxygen availability at the cathode primarily depends on oxygenic photosynthesis with the help of PSI, PSII, and cytochrome b6f complex to transport electrons from water to NADP+. Small mobile molecules like plastoquinone and plastocyanin carry these electrons, thereby contributing to photo- synthetic energy conversion [39].
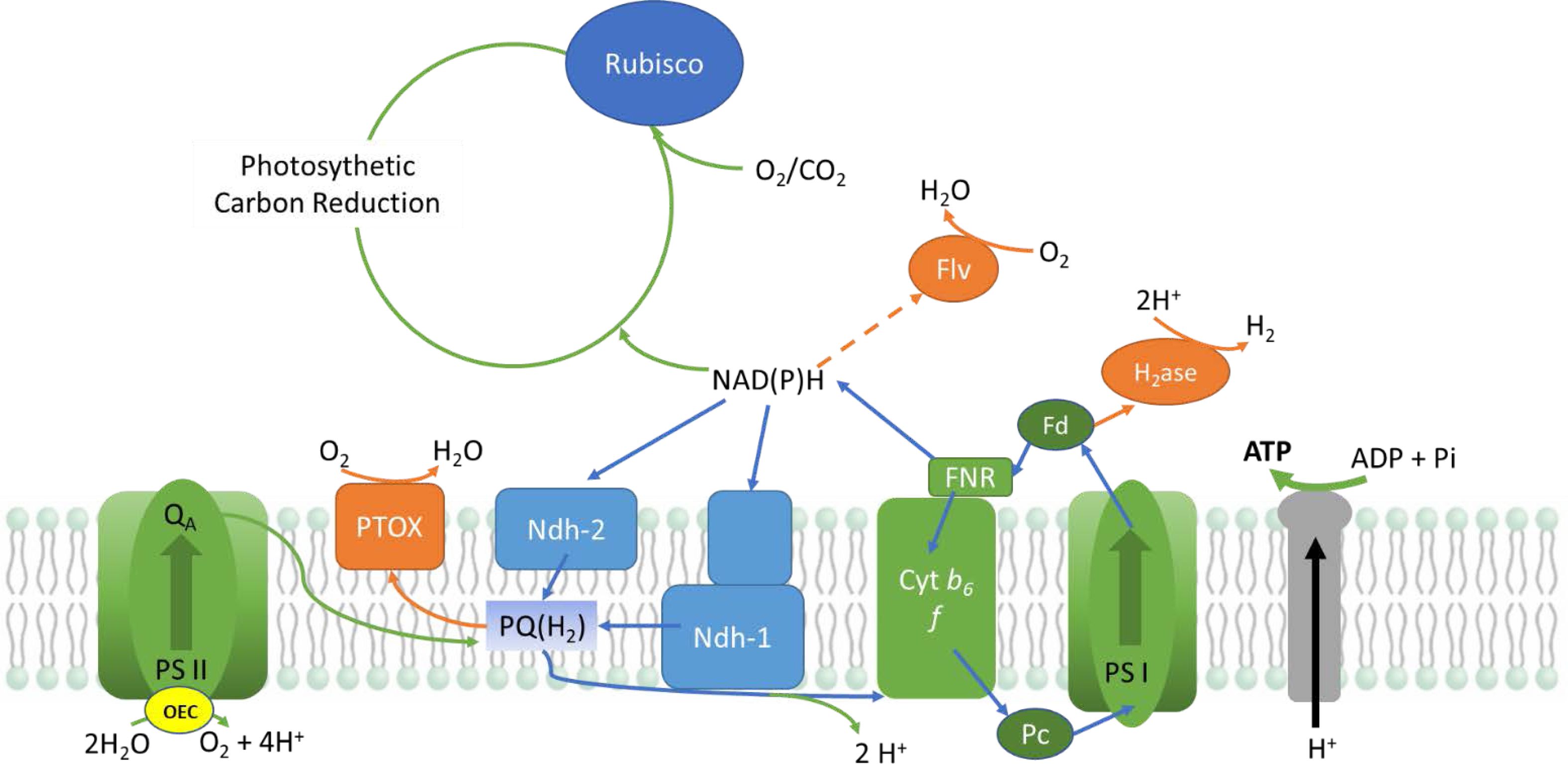
|
Fig. 1 Electron transport during light condition in microalgae (OEC; oxygen evolving complex, PSI & II; photosystem I & II, PTOX; plastoquinol terminal oxidase, PQ; plastoquinone, Ndh 1&2; NADH dehydrogenase like 1&2, Cytb6f; cytochromeb6f, PC; plastocyanin, FNR; ferredoxin-NADP reductase, Fd; ferredoxin, H2ase; hydrogenase, Flv; flavin). |
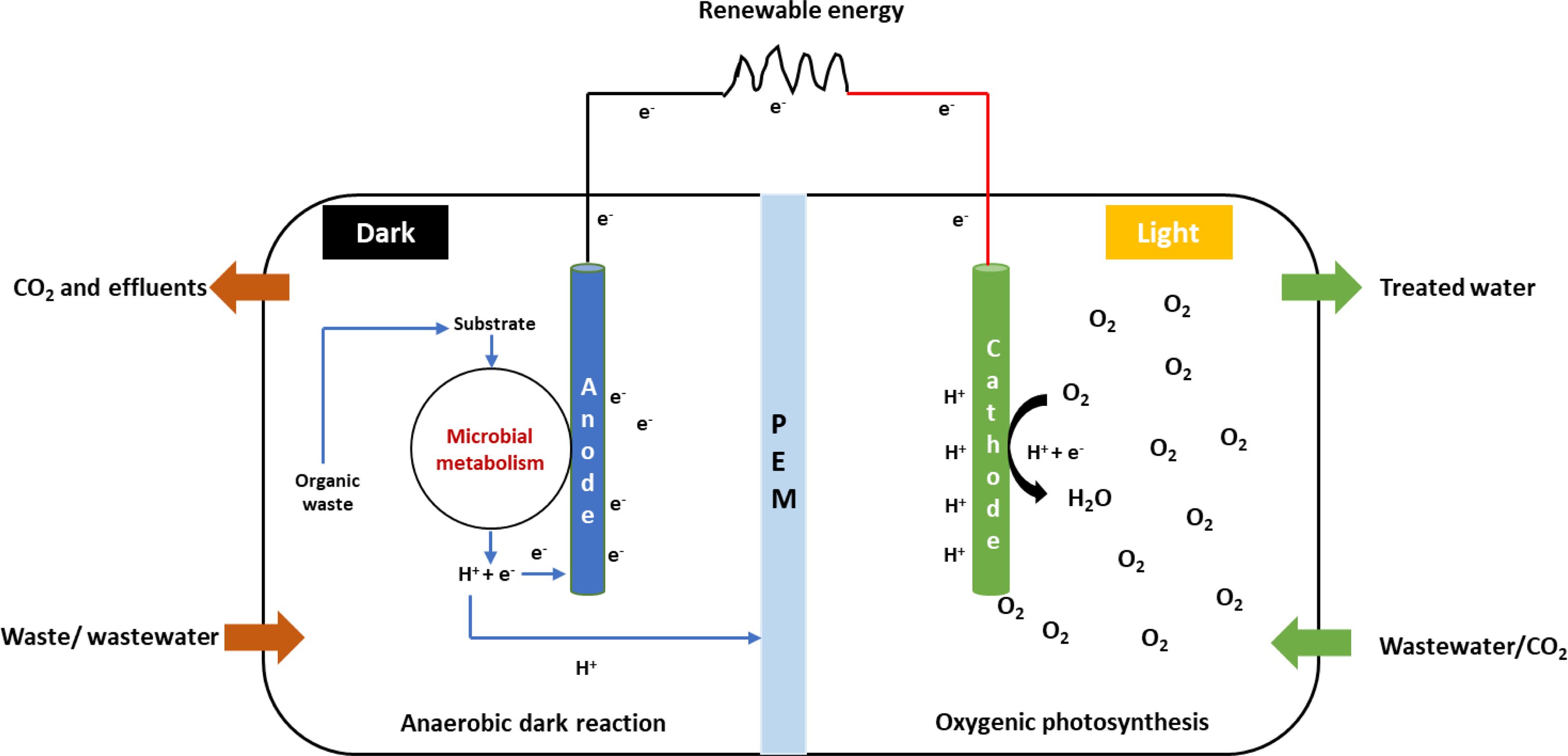
|
Fig. 2 Electron transport process in microbial fuel cell (MFC). |
The carbon-derived chemicals produced from micro- algae have been used in biofuel, nutraceutical, and pharmaceutical industries. However, the major energy source to produce these value-added commodities is photosynthesis, which provides the reducing power and energy. The addition of external electrons to photo- synthetic electron transfer can effectively optimize biosynthetic pathways like the CBB cycle, thereby increasing the biomass and secondary metabolite production in microalgae. However, multiple factors should be taken into consideration for an effective mechanism of electro-activated microalgae.
Autotrophy, heterotrophy, mixotrophy, and photo- heterotrophy are the four major microalgal cultivation modes (Fig. 3). In the photoautotrophic mode of cultivation, microalgae utilize CO2 as the carbon source and sunlight as the energy source for generating organic matter [40]. However, autotrophy is considered to be the most primitive way of microalgal cultivation and has been used since the 1950s in open pond system and closed PBRs [41].
In mixotrophy, the advantages of autotrophy and heterotrophy are combined to overcome the challenges of microalgal cultivation. In this mode of cultivation, microalgae can utilize both CO2 and exogenous organic compounds such as glucose, fructose, acetate, and glycerol as the carbon source [42]. In mixotrophic cultivation, microalgae can grow heterotrophically using organic carbon for increased biomass and lipid accumulation and can also use inorganic carbon to produce oxygen through photosynthesis, thereby lowering the overall CO2 emissions [43]. Unlike heterotrophy, photosynthetic pigments are illuminated under the mixotrophic mode of cultivation [44]. Mixotrophic cultivation can be used to increase the growth and biomass production of microalgae with minimum contamination and a low cost [45]. The use of PBRs can reduce the chances of contamination even at an increased cost efficiency and a biomass production of ~5-15 g/L can be achieved, which is 3-30 times higher than that achieved under autotrophic cultivation conditions [46]. The mixotrophic cultivation of Chlorella vulgaris exhibited a biomass production of 2 g/L with glucose (1% w/w) as the carbon source [47]. Mixotrophic microalgal cultivation using glucose increased the growth rate of microalgae in comparison with photoautotrophic and heterotrophic cultivation methods [48]. Mixotrophic cultivation can be used to enhance lipid, carbohydrate, and protein accumulation in microalgae. Cultivation of Asterarcys quadricellulareshowed a carbohydrate accumulation of 36.6% in media supplemented with 0.1 g/L [49]. Similarly, C. vulgaris produced a carbohydrate content of 8.74% using a mixture of glucose and glycerol as the carbon source [50]. The extracellular uptake of electrons by autotrophically and mixotrophically cultivated microalgae depends on various conditions like media composition, nature of electron mediators, and electrode material.
However, the phototrophic and mixotrophic cultivation of microalgae has many disadvantages in large-scale cultivation systems. The incorporation of electrons as an energy source in microalgal cultures can be considered to be a solution to this problem. In microbes, during extracellular electron transfer, cells try to make contact with each other through electrically conductive proteins, such as c-type cytochromes situated in outer membranes and Fe-S proteins, conductive pili, or periplasmic extensions, which aid the transfer of electrons across cell membranes [51]. The major technical difficulty in the use of electroactive microalgae is their thick cell wall and the membrane bound organelle chloroplasts, where photosynthesis occurs. This makes the eukaryotic algae different from cyanobacteria and other micro- organisms owing to the difference in the relationship between primary electrogenic membranes and external electrodes [52].
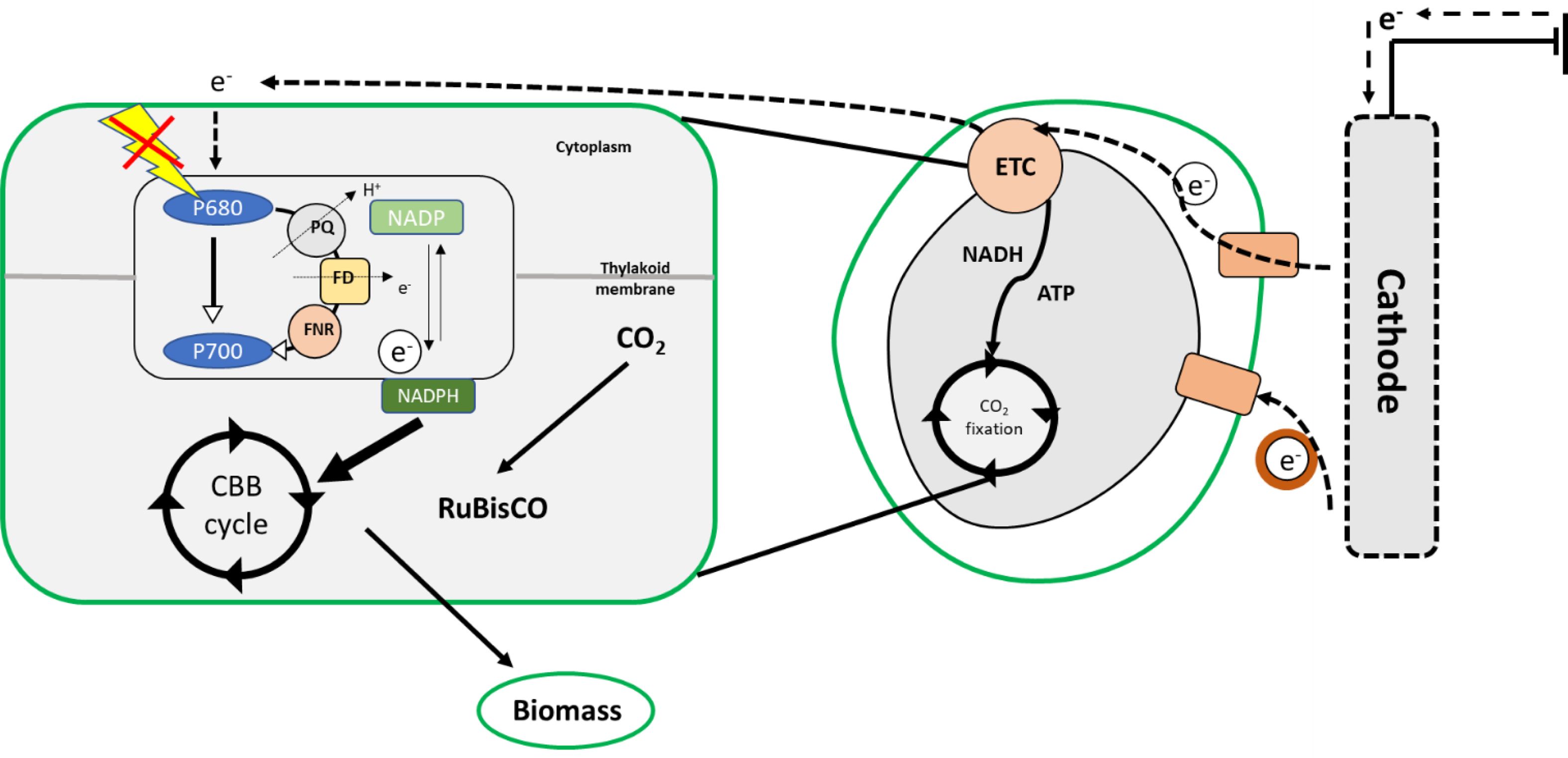
|
Fig. 3 Electron transport during electro-activation in microalgae. |
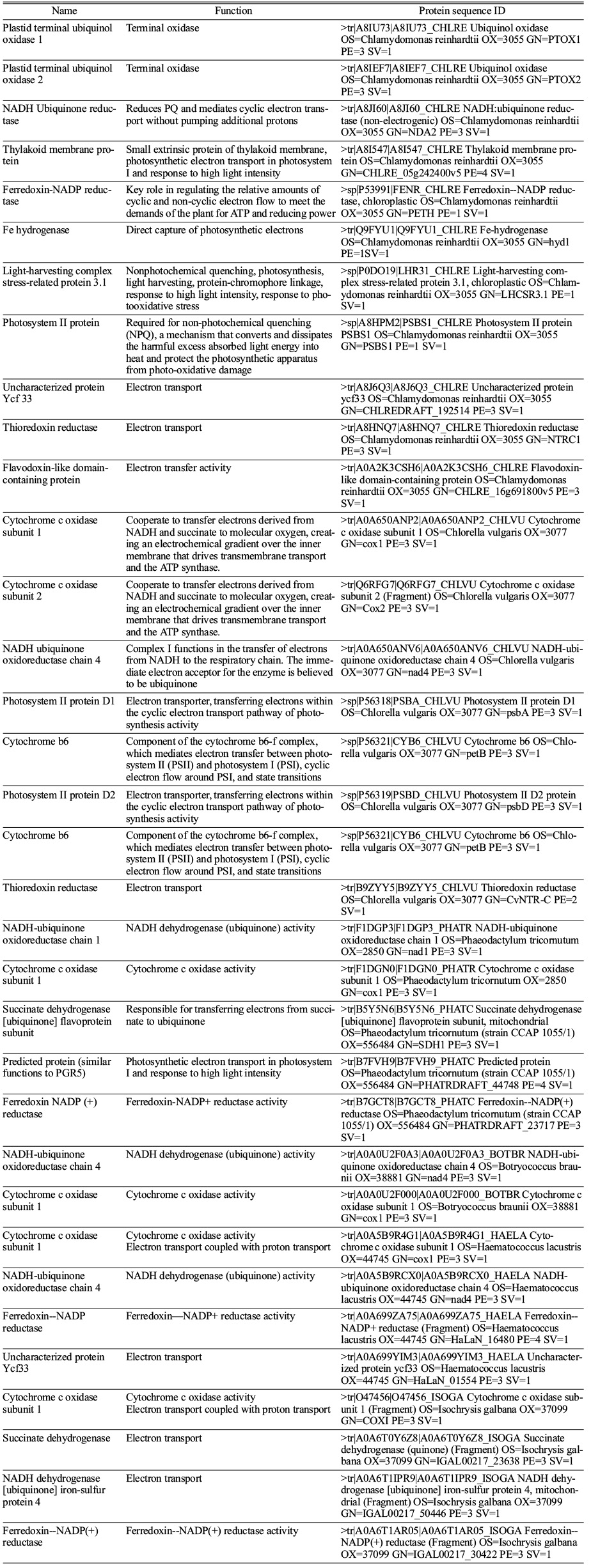
The mechanism of PETC has remained stable during the course of evolution in cyanobacteria and algae except for the light harvesting complex proteins [27]. However, the extracellular electron transport proteins in cyanobacteria have been well studied by Nikkanen et al., 2021 [27]. They proposed the possible electron transport proteins in microalgae that are hypothetical proteins (Table 2). In cyanobacteria, NDH-1 acts as the major electron supplier to ETC followed by SDH, cytochrome oxidase, PTOX, ARTO, FNR, etc. [53-55]. However, the presence of these proteins is predicted in the thylakoid membrane of different microalgal species that raises the possibility of extracellular electron uptake by eukaryotic microalgae under different cultivation conditions (Table 2).
Electron mediators
In photosynthetic microalgae, there is no evidence for direct extracellular electron transfer (DEET). However, bacteria like Shewanella show DEET ability with the help of nanowires situated in protrusions of the outer membrane [56]. In cyanobacteria, SynechocystisDEET has been reported with the help of conductive pili but the mechanism of DEET using pili is still unclear [57]. MET is widely employed in electroactive bacteria like methanogens and acetogens, and exploited using MFCs and bio-photovoltaic systems. Electron mediators have the ability to cross the semipermeable membranes of microalgae and interact with the inorganic or metallic electrode and the photosynthetic biological components [58]. The selection of a suitable electron mediator is necessary for the successful transfer of electrons from cathode to microalgae. The selected electron mediator should have the ability to pass the cell membranes through porin channels so that it can effectively transfer electrons within the cells [59]. Here, we propose a model wherein the anodic and cathodic compartments have the same electron mediator for transferring the electrons to the microalgae. For effective oxidation in the anodic compartment, the electron mediator should meet certain requirements. The mediator should not be toxic to the microalgae, and it should be effectively active for the electro- catalytic oxidation of various metabolites. Moreover, the electron mediator should be stable in the presence of different secondary metabolites and at different temperatures (10-40 C) and different pH ranges (5-9) [60]. There are several reports on the use of ferricyanide, quinone derivatives, neutral red, methyl viologen, etc. as electron mediators in MFCs to capture electrons from microalgae to generate bioelectricity [61]. However, in the case of electro-activated microalgae, the electron mediator should not be toxic to the algal cells. The currently used quinone derivatives are reported as algicides that are toxic to the microalgae at certain concentrations [62, 63]. Conversely, electron mediators such as riboflavin and Fe-EDTA are reported to promote the growth and electron transfer in biocathodes [64, 65]. The redox potential of an electron mediator is a significant factor that contributes to its activity. The redox potential of the artificial electron mediator should be positive enough to carry out fast reduction but negative enough to prevent energy loss. In addition, the kinetics of the microbial reduction and electrochemical oxidation should be as fast as possible [60].
|
Table 2 Predicted extracellular electron transport proteins in different microalgal species (Data obtained from UniProt, https://www.uniprot.org/). |
The cultivation of microalgae under different cultivation modes has various benefits and disadvantages. In phototrophic cultivation, light is the sole source of energy and when it comes to large scale cultivation systems, microalgae are not able to use light effectively. To solve this problem, mixotrophic cultivation systems have been introduced. However, in mixotrophy, the presence of expensive carbon sources and contamination with bacteria have been reported as major drawbacks. Thus, here we propose a mechanism to introduce external electrons as an additional source of energy in the normal growth medium under phototrophic or mixotrophic cultivation. The conductivity of the medium is a crucial factor for the cultivation of electro-activated microalgae. Moreover, the electron mediator and carbon source should work together for the efficient supply of electrons to the microalgal species selected.
Temperature, pH, applied current, and continuous nutrient supply are also crucial factors in microbial electrosynthesis [66]. The supply of gases such as CO2 and light intensity also affect the MES in different bacterial and cyanobacterial species. However, reports on how these parameters affect microalgal electrosynthesis are still lacking. Changing the intrinsic properties of the reactor, such as temperature, salinity, and pressure, can clearly affect the viability of electroactive microalgae. Some researchers demonstrated the enhanced activity of MFCs under thermophilic temperatures above 45 C that showed efficient substrate solubility, high microbial activity, efficient mass transfer, and low risk of contamination [67, 68]. However, most microalgae prefer mesophilic cultivation conditions and high temperatures may affect the viability of microalgae. The electrolyte nature and concentration can also affect electrosynthesis. The concentration of electrolyte significantly affects the conductivity of the cultivation medium and decreases the ohmic drop in the reactor [69]. Halophilic organisms have reported to have efficient MES activity [70]. This can be an advantage for marine microalgae that grow normally under halophilic conditions. The identification of marine microalgae with the ability of extracellular electron transfer and the modification of cultivation conditions could be effectively to develop electroactive organisms.
In addition, the electrolyte pressure can be used to enhance CO2 availability in the microalgal culture. An optimal soluble CO2 concentration is necessary for substrate specific consumption and maximum growth. The use of thicker electrode materials and different current densities can affect the available CO2 in the bioreactor. Moreover, a high temperature and salinity have a negative impact on CO2 availability. More research is needed to understand the factors that affect the electroactivity of microalgae under phototrophic and mixotrophic conditions. In addition, the optimization of cultivation conditions and design of bioreactors are necessary.
As a result of photosynthesis, microalgae can accumulate biomass and high-value metabolites. The application of an electric field has been shown to increase the growth and productivity of bacteria, fungi, and algae. The cellular machinery shows increased fermentation kinetics, increased synthesis of enzymes and RNAs, increased cell division, and increased mass transfer across the cellular membrane. The application of a low intensity electric field can significantly reduce the lag phase of Saccharomyces cerevisiae [71]. The polarization of Volvox sp. has been observed under the influence of electric stimuli [72]. The application of a static electric field of 2.7 kV/cm led to a 51% increase in the growth of C. vulgaris [73]. However, the application of electrostimulation on microalgal cultures for enhanced product and metabolite accumulation is still limited. The limited studies and finding an ideal candidate for electroactive microalgae are the current challenges in the development of a new production process.
Electro-technologies can be scaled up linearly in a cost-effective manner because they do not involve mechanical stress or diffusional processes. However, their industrialization faces several practical challenges like the requirement of a large electrode surface area, large electrode gap, large treatment chamber, and high flow rate. Moreover, effective electrode materials should be developed for successful microalgal cultivation in cathode compartment [74]. The need for generators increases at larger scales, consequently increasing the output voltage, high frequency pulses, and high energy capacity [75]. The major challenge in practical applications is finding power sources capable of producing high voltage and current outputs with a specific pulse and frequency. However, the development of generators and control systems for increased production in a cost-effective manner can solve the problem. More research is needed for the development of electro-activated microalgae to make them practically effective at a large scale.
The development of microalgae as a candidate for green chemical synthesis is currently challenging due to ineffective light to biomass conversion efficiency. The electroactive microorganisms have been gaining attention because of their high biomass and metabolite production rates. Electroactive microalgae are still in a proof-of-concept stage, and more research is needed to understand the external electroactive mechanism and production process. Advances in tools for screening electroactive organisms can provide a path for the development of electroactive microalgae. It is also important to understand the rate-limiting factors in electroactive microalgae to scale up the production process. Future research in this field can make microalgal biorefinery more economically viable for chemical synthesis.
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 2019281010007B), and by the “Graduate School of Post Plastic Specialization” of Korea Environmental Industry & Technology Institute grant funded by the Ministry of Environment, Republic of Korea.
- 1. H. Sun, W. Zhao, X. Mao, Y. Li, T. Wu, and F. Chen, Biotechnol. Biofuels 11[1] (2018) 1-23.
-

- 2. Y. Kim and S. Lee, J. Ceram. Process. Res. 18[4] (2017) 285-290.
-

- 3. C. Koch and F. Harnisch, Front. Microbiol. 7 (2016) 1890.
-

- 4. O. Choi and B.I. Sang, Biotechnol. Biofuels 9[1] (2016) 1-14.
-

- 5. B.S. Koh and S.C. Yi, J. Ceram. Process. Res. 18[11] (2017) 810-814.
-

- 6. J. Lee, J. Chun, O. Choi, and B.I. Sang, J. Ceram. Process. Res. 21[5] (2020) 602-608.
-

- 7. L. Shi, H. Dong, G. Reguera, H. Beyenal, A. Lu, J. Liu, H.Q. Yu, and J.K. Fredrickson, Nat. Rev. Microbiol. 14[10] (2016) 651-662.
-

- 8. M. Cuaresma, M. Janssen, C. Vílchez, and R.H. Wijffels, Bioresour. Technol. 102[8] (2011) 5129-5137.
-

- 9. C. Liu, B.C. Colón, M. Ziesack, P.A. Silver, and D.G. Nocera, Science 352[6290] (2016) 1210-1213.
-

- 10. A. Melis, L. Zhang, M. Forestier, M.L. Ghirardi, and M. Seibert, Plant Physiol. 122[1] (2000) 127-136.
-

- 11. S. Subramanian, A.N. Barry, S. Pieris, and R.T. Sayre, Biotechnol. Biofuels 6[1] (2013) 1-12.
-

- 12. T. Nakajima, H. Hanawa, and T. Tsuchiya, J. Ceram. Process. Res. 17[5] (2016) 485-488.
-

- 13. K.S. Vuoristo, A.E. Mars, J.P. Sanders, G. Eggink, and R.A. Weusthuis, Trends Biotechnol. 34[3] (2016) 191-197.
-

- 14. K. Qiao, T.M. Wasylenko, K. Zhou, P. Xu, and G. Stephanopoulos, Nat. Biotechnol. 35[2] (2017) 173-177.
-

- 15. P.M. Shrestha and A.E. Rotaru, Front. Microbiol. 5 (2014) 237-237.
-

- 16. D. Gupta, M.C. Sutherland, K. Rengasamy, J.M. Meacham, R.G. Kranz, and A. Bose, mBio 10[6] (2019) e02668-02619.
-

- 17. W. Wunderlich, T. Oekermann, L. Miao, N.T. Hue, S. Tanemura, and M. Tanemura, J. Ceram. Process. Res. 5[4] (2004) 343-354.
- 18. S. Chinnasamy and S. Ramanathan, J. Cerm. Process. Res. 21[1] (2020) 123-130.
-

- 19. J.H. De Vree, R. Bosma, M. Janssen, M.J. Barbosa, and R.H. Wijffels, Biotechnol. Biofuels 8[1] (2015) 1-12.
-

- 20. G. Perin, A. Bellan, A. Bernardi, F. Bezzo, and T. Morosinotto, Physiol. Plant. 166[1] (2019) 380-391.
-

- 21. Y. Chisti, in “Algae Biotechnol.” (Springer, 2016) 21-40.
-

- 22. E. Sforza, D. Simionato, G.M. Giacometti, A. Bertucco, and T. Morosinotto, PLoS ONE 7[6] (2012) e38975.
-

- 23. N. Murata, S. Takahashi, Y. Nishiyama, and S.I. Allakhverdiev, Biochimica et Biophysica Acta (BBA)-Bioenergetics 1767[6] (2007) 414-421.
-

- 24. Y. Kato, X. Sun, L. Zhang, and W. Sakamoto, Plant Physiol. 159[4] (2012) 1428-1439.
-

- 25. P. Kuczynska, M. Jemiola Rzeminska, and K. Strzalka, Mar. Drugs. 13[9] (2015) 5847-5881.
-

- 26. R. Goss and T. Jakob, Photosynth. Res. 106[1] (2010) 103-122.
-

- 27. L. Nikkanen, D. Solymosi, M. Jokel, and Y. Allahverdiyeva, Physiol. Plant. (2021).
-

- 28. C.W. Mullineaux, Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837[4] (2014) 503-511.
-

- 29. M. Marappan, M.K. Vijaykrishananb, K. Palaniswamy, K. Manoharan, and J. Arumughan, J. Ceram. Process. Res. 22[2] (2021) 131-142.
-

- 30. M.S. Guzman, K. Rengasamy, M.M. Binkley, C. Jones, T.O. Ranaivoarisoa, R. Singh, D.A. Fike, J.M. Meacham, and A. Bose, Nature Commun. 10[1] (2019) 1-13.
-

- 31. L.E. Doyle and E. Marsili, Bioresour. Technol. 195 (2015) 273-282.
-

- 32. S.V. Mohan, S. Srikanth, P. Chiranjeevi, S. Arora, and R. Chandra, Bioresour. Technol. 166 (2014) 566-574.
-

- 33. L. Xiao and Z. He, Renew. Sust. Energ Rev. 37 (2014) 550-559.
-

- 34. L. Xiao, E.B. Young, J.A. Berges, and Z. He, Environ. Sci. Technol. 46[20] (2012) 11459-11466.
-

- 35. L. Christenson and R. Sims, Biotechnol. Adv. 29[6] (2011) 686-702.
-

- 36. M. Karthikeyan, M. Muthukumar, P.Karthikeyan, and C. Mathan. J. Ceram. Process. Res. 20[5] (2019) 490-498.
-

- 37. A. Commault, G. Lear, P. Novis, and R. Weld, N.Z.J. Bot. 52[1] (2014) 48-59.
-

- 38. G.V. Subhash, R. Chandra, and S.V. Mohan, Bioresour. Technol. 136 (2013) 644-653.
-

- 39. L. Taiz, E. Zeiger, I.M. Moller, and A. Murphy, in “Plant physiology and development” (Sinauer Associates Incorporated, 2015) p.165-196.
- 40. S. Smetana, M. Sandmann, S. Rohn, D. Pleissner, and V. Heinz, Bioresour. Technol. 245 (2017) 162-170.
-

- 41. A. Lodi, L. Binaghi, D. De Faveri, J.C.M. Carvalho, and A. Converti, Ann. Microbiol. 55[3] (2005) 181-185.
- 42. G. Zuccaro, A. Yousuf, A. Pollio, and J.P. Steyer, in “Microalgae cultivation for biofuels production” (Academic Press, 2020) p.11-29.
-

- 43. C. Yang, Q. Hua, and K. Shimizu, Biochem. Eng. J. 6[2] (2000) 87-102.
-

- 44. Y. Alkhamis and J.G. Qin, J. Appl. Phycol. 28[1] (2016) 35-42.
-

- 45. J. Zhan, J. Rong, and Q. Wang, Int. J. Hydrog. Energy 42[12] (2017) 8505-8517.
-

- 46. M.I. Pereira, B.M. Chagas, R. Sassi, G.F. Medeiros, E.M. Aguiar, L.H. Borba, E.P. Silva, J.C.A. Neto, and A.H. Rangel, PLoS ONE 14[10] (2019) e0224294.
-

- 47. Y. Liang, N. Sarkany, and Y. Cui, Biotechnol. Lett. 31[7] (2009) 1043-1049.
-

- 48. Perez-Garcia and Y. Bashan, in “Algal biorefineries” (Springer, 2015) p.61-131.
-

- 49. Oliveira, S. Gianesella, V. Silva, T. Mata, and N. Caetano, Energy Procedia 136 (2017) 468-473.
-

- 50. W.B. Kong, H. Yang, Y.T. Cao, H. Song, S.F. Hua, and C.G. Xia, Food Technol. Biotechnol. 51[1] (2013) 62.
- 51. R. Karthikeyan, R. Singh, and A. Bose, J. Ind. Microbiol. Biotechnol. 46[9-10] (2019) 1419-1426.
-

- 52. L.T. Wey, P. Bombelli, X. Chen, J.M. Lawrence, C.M. Rabideau, S.J. Rowden, J.Z. Zhang, and C.J. Howe, Chem. Electro. Chem. 6[21] (2019) 5375.
-

- 53. T. Ogawa and H. Mi, Photosynth. Res. 93[1-3] (2007) 69-77.
-

- 54. D.J. Lea Smith, N. Ross, M. Zori, D.S. Bendall, J.S. Dennis, S.A. Scott, A.G. Smith, and C.J. Howe, Plant Physiol. 162[1] (2013) 484-495.
-

- 55. A. Udayan, A.K. Pandey, P. Sharma, N. Sreekumar, and S. Kumar, Systems Microbiology and Biomanufacturing 1[4] (2021) 411-431.
-

- 56. F. Wang, Y. Gu, J.P. O'Brien, S.M. Yi, S.E. Yalcin, V. Srikanth, C. Shen, D. Vu, N.L. Ing, A.I. Hochbaum, E.H. Egelman, and N.S. Malvankar, Cell 177[2] (2019) 361-369.
-

- 57. K. Senturk and M. Yilmaz, MedFAR 3[1] (2021) 1-9.
- 58. V. Hartmann, D. Harris, T. Bobrowski, A. Ruff, A. Frank, T.G. Pomorski, M. Rogner, W. Schuhmann, N. Adir, and M.M. Nowaczyk, J. Mater. Chem. 8[29] (2020) 14463-14471.
-

- 59. H. Kowata, S. Tochigi, H. Takahashi, and S. Kojima, J. Bacteriol. 199[19] (2017).
-

- 60. B.E. Logan, in “Microbial fuel cells” (John Wiley & Sons, 2008) p.61-84.
- 61. G. Longatte, A. Sayegh, J. Delacotte, F. Rappaport, F.A. Wollman, M. Guille Collignon, and F. Lemaitre, Chem. Sci. 9[43] (2018) 8271-8281.
-

- 62. R.L. Heydorn, C. Engel, R. Krull, and K. Dohnt, Chem. Bio. Eng. Rev. 7[1] (2020) 4-17.
-

- 63. Y. Zhang, Z. Zhang, W. Liu, and Y. Chen, Sci. Total Environ. 744 (2020) 140652.
-

- 64. W. Huang, J. Chen, Y. Hu, J. Chen, J. Sun, and L. Zhang, Int. J. Hydrog. Energy 42[4] (2017) 2349-2359.
-

- 65. Q. Liu, K. Yu, P. Yi, W. Cao, X. Chen, and X. Zhang, Environ. Sci. Pollut. Res. Int. 26[19] (2019) 19540-19548.
-

- 66. P. Dessi, P. Chatterjee, S. Mills, M. Kokko, A.M. Lakaniemi, G. Collins, and P.N.L. Lens, Bioresour. Technol. 294 (2019) 122115.
-

- 67. B.E. Logan, R. Rossi, A.a. Ragab, and P.E. Saikaly, Nat. Rev. Microbiol. 17[5] (2019) 307-319.
-

- 68. A. Prevoteau, J.M. Carvajal Arroyo, R. Ganigue, and K. Rabaey, Curr. Opin. Biotechnol. 62 (2020) 48-57.
-

- 69. M. Dopson, G. Ni, and T.H. Sleutels, FEMS Microbiol. Rev. 40[2] (2015) 164-181.
-

- 70. J.R. Mattar, M.F. Turk, M. Nonus, N.I. Lebovka, H. El Zakhem, and E. Vorobiev, Bioelectrochemistry 103 (2015) 92-97.
-

- 71. Y. Hayashi and K. Sugawara, Phys. Rev. E Stat. Nonlin. Soft. Matter. Phys. 89[4] (2014) 042714.
-

- 72. H. Nezammahalleh, F. Ghanati, T.A. Adams, M. Nosrati, and S.A. Shojaosadati, Bioresour. Technol. 218 (2016) 700-711.
-

- 73. T. Kotnik, W. Frey, M. Sack, S.H. Meglic, M. Peterka, and D. Miklavcic, Trends Biotechnol. 33[8] (2015) 480-488.
-

- 74. Y.H. Yun, I.R. Jo, Y.H. Lee, V.H.V. Quy, and K.S. Ahn, J. Ceram. Process. Res. 21 (2020) 33-40.
-

- 75. A. Golberg, M. Sack, J. Teissie, G. Pataro, U. Pliquett, G. Saulis, T. Stefan, D. Miklavcic, E. Vorobiev, and W. Frey, Biotechnol. Biofuels 9[1] (2016) 1-22.
-

- 76. A. Golberg, M. Sack, J. Teissie, G. Pataro, U. Pliquett, G. Saulis, T. Stefan, D. Miklavcic, E. Vorobiev, and W. Frey, Biotechnol. Biofuels 9[1] (2016) 1-22.
-

- 77. H. Wang, D. Liu, L. Lu, Z. Zhao, Y. Xu, and F. Cui, Bioresour. Technol. 116 (2012) 80-85.
-

- 78. A.E. Inglesby, D.A. Beatty, and A.C. Fisher, Rsc Advances 2[11] (2012) 4829-4838.
-

- 79. K. Nishio, K. Hashimoto, and K. Watanabe, Biosci. Biotechnol. Biochem. (2013) 120833.
-

- 80. N. Rashid, Y.F. Cui, M.S.U. Rehman, and J.I. Han, Sci. Total Environ. 456 (2013) 91-94.
-

- 81. Y. Cui, N. Rashid, N. Hu, M.S.U. Rehman, and J.I. Han, Energy convers. Manage. 79 (2014) 674-680.
-

- 82. S.B. Velasquez‐Orta, T.P. Curtis, and B.E. Logan, Biotechnol. Bioeng. 103[6] (2009) 1068-1076.
-

- 83. V. Gadhamshetty, D. Belanger, C.J. Gardiner, A. Cummings, and A. Hynes, Bioresour. Technol. 127 (2013) 378-385.
-

- 84. Y. Cho, T. Donohue, I. Tejedor, M. Anderson, K. McMahon, and D. Noguera, J. Appl. Microbiol. 104[3] (2008) 640-650.
-

- 85. J.P. Badalamenti, C.I. Torres, and R. Krajmalnik‐Brown, Biotechnol. Bioeng. 111[2] (2014) 223-231.
-

- 86. D. Xing, Y. Zuo, S. Cheng, J.M. Regan, and B.E. Logan, Environ. Sci. Technol. 42[11] (2008) 4146-4151.
-

- 87. A. Kokabian and V.G. Gude, Environ. Sci: Process. Impacts 15[12] (2013) 2178-2185.
-

- 88. Y. Zhang, J.S. Noori, and I. Angelidaki, Energy Environ. Sci. 4[10] (2011) 4340-4346.
-

- 89. P.J. Cai, X. Xiao, Y.R. He, W.W. Li, G.L. Zang, G.P. Sheng, M.H.W. Lam, L. Yu, and H.Q. Yu, Biosens. Bioelectron. 39[1] (2013) 306-310.
-

- 90. Y. Wu, Z.J. Wang, Y. Zheng, Y. Xiao, Z.H. Yang, and F. Zhao, Appl. Energy 116 (2014) 86-90.
-

- 91. X.A. Walter, J. Greenman, and I.A. Ieropoulos, Algal Res. 2[3] (2013) 183-187.
-

 This Article
This Article
-
2023; 24(1): 29-39
Published on Feb 28, 2023
- 10.36410/jcpr.2023.24.1.29
- Received on May 25, 2022
- Revised on Jul 12, 2022
- Accepted on Jul 21, 2022
 Services
Services
- Abstract
introduction
challenges in microalgae cultivation
electron transport mechanism in microalgae
proposed mechanisms of electro-activation in microalgae
predicted extracellular electron transport proteins
cultivation conditions and reactor design
challenges and future perspectives
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Byoung-In Sang
-
Department of Chemical Engineering, Hanyang University, 222 Wangshimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
Tel : +82-2-2220-2328 Fax: +82-2-2220-4716 - E-mail: biosang@hanyang.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.