- Monitoring and removal technologies of microplastics in the environment
Gopi Kalaiyarasana, Sung Chul Yia,b,* and Byoung-In Sanga,b,*
aDepartment of Chemical Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
bClean-Energy Research Institute, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Microplastic (MP) contamination of the environment is one of the major problems to human and other creatures' health. In recent decades, researchers hardly work to find alternate products for MPs sources, detection, and removal technologies of MPs from various polluted environments. This review discusses the various sources of MPs, ways to minimize the contamination, environmental health effects caused by MPs pollution, and the detection of MPs in various environmental and food samples. Additionally, the sensing mechanism, efficiency, merits, demerits, and challenges in detection techniques for various samples like air, water, and soil samples are described along with suitable examples. Further, the microplastic removal and treatment technologies such as coagulation and flocculation, membrane, biological, filtration, advanced oxidation process, and adsorption technologies are deeply evaluated to gather the necessary knowledge to make a pollution-free environment. In the end, the complications in detection and removal technologies in the current situation and opportunities to overcome the MPs' pollution problem are addressed
Keywords: Microplastics sources, Microplastics detection, Health effects of microplastics, Microplastics Removal, Membranes, Adsorption, Filtration, Coagulation-Flocculation, Environment pollution, Polymers
The global production of plastics increased by over 359 million tons in 2018 [1]. Among them, 13 million tons of plastics are discharged into larger water bodies [2]. That around 80% of plastic waste particles are discharged in microplastics (MPs) which are 0.1 microns to 5000 microns in size [3]. The MPs are synthetic solid particles or polymeric matrices with regular or irregular shapes and are insoluble in water. Based on the sources, MPs are classified into two major categories: primary and secondary microplastics [4]. The microbeads in cosmetics and personal care products, plastic pellets used in manufacturing, and plastic fibers used in artificial fabrics are the best examples of primary microplastics. Deteriorated soda and water bottles, fishing nets, various plastic storage containers, tire wear, and tea bags are the sources of secondary microplastics.
Additionally, MPs have different molecular compositions and structures. Polyester (PEST), fluoropolymers, polypropylene (PP), acrylic, polyethylene (PE), polyamide (PA), etc. have been reported as original materials of microplastics [6]. The health effects of MPs are significantly investigated in marine organisms and humans and documented highly in pieces of literature [7, 8]. Fig. 1 shows the MP's pollution level in the upcoming days in the Eastern Tropical Pacific Ocean. Microplastic pollution will be increased exponentially and is expected to reach 520 MPs per cubic meter at the end of the year 2100 [5]. Many large cities are globally found nearby the coastal area. Hence plastic contamination in the ocean is increasing and spreading over the world. Furthermore, it will be eaten by various fish and other organisms, so seafood safety and quality are affected and threaten human health.
Microplastic pollution could be higher in the soil, around 4-23 times more than in the ocean. The MPs can be spread over miles by the action of wind due to their small size and weight. These MPs have been non-degradable for centuries; hence it has been used as a stratigraphic marker to identify the age of rock deposit or the formation of sand deposit [9]. These MPs show serious health issues to the environment, including humans. It has been noticed in beyond 600 species of organisms [10], in everyday use of table salt [11] and beer, and even in drinking water [12], the atmosphere [8], and marine water [13] anywhere in the continents like Asia, Europe, America, and Antarctica [14].
The risk factors of MPs' pollution of the environment and human health should be investigated clearly. The MPs affect the environment and organisms, including humans, in two ways: physical and chemical effects. The physical effect of MPs depends on their size, shape, and concentration. The chemical effect of MPs is related to hazardous chemicals or materials. MPs can have two varieties of chemicals: (i) additives and polymeric raw materials like monomers or oligomers originating from plastics, and (ii) chemicals attracted from the nearby atmosphere [15]. The chemicals are purposely added as additives during the plastic manufacturing process to provide plastic properties like color and transparency.
Moreover, additives are added to enhance the performance of plastic products, such as resistance to degradation by ozone, temperature, light radiation, mold, bacteria, humidity, and mechanical, thermal, and electrical actions [16]. However, these additives also create health issues directly or indirectly for humans and other organisms when involved with MPs pollution. MPs can cause to create oxidative stress, increased uptake and translocation, DNA damage, inflammatory lesions, neurotoxicity, metabolic disturbances, and increased cancer risk in humans [17]. Microplastic pollution can be detected in various atmospheric samples using a visible light microscope, scanning electron microscope, fluorescence microspore, and stereomicroscope [18]. FTIR and Raman spectroscopies, gas chromatography coupled with mass spectrometry, are also employed in the detection of microplastics in recent years [19].
After identification of the MPs, researchers try to remove them from the environment, such as water, soil, and atmospheric air, to save the health of humans and other organisms. Various technologies, such as coagulation and flocculation, filtration, degradation, distillation, oxidation, adsorption by biological or physiochemical processes, or membrane technologies, are used to remove microplastics. However, every technology has unique merits and demerits in the removal process of microplastics. For example, the filtration technique needs high pressure, and distillation needs elevated temperature. Some other techniques need suitable chemicals or reagents to process the microplastic removal. Therefore, modern technology or development in the existing technology is urgently needed to enhance the performance of microplastic removal in various environments without interfering with neighbors at a low overall cost and high durability.
This review will discuss the sources of microplastic contamination, ways to minimize the contamination, environmental health effects caused by microplastic pollution, and the detection of microplastics in various environmental and food samples. In addition, the microplastic pollution removal and treatment process to create a pollution-free environment, the challenges in the treatment process, and opportunities in the treatment technologies are discussed deeply, along with suitable examples.
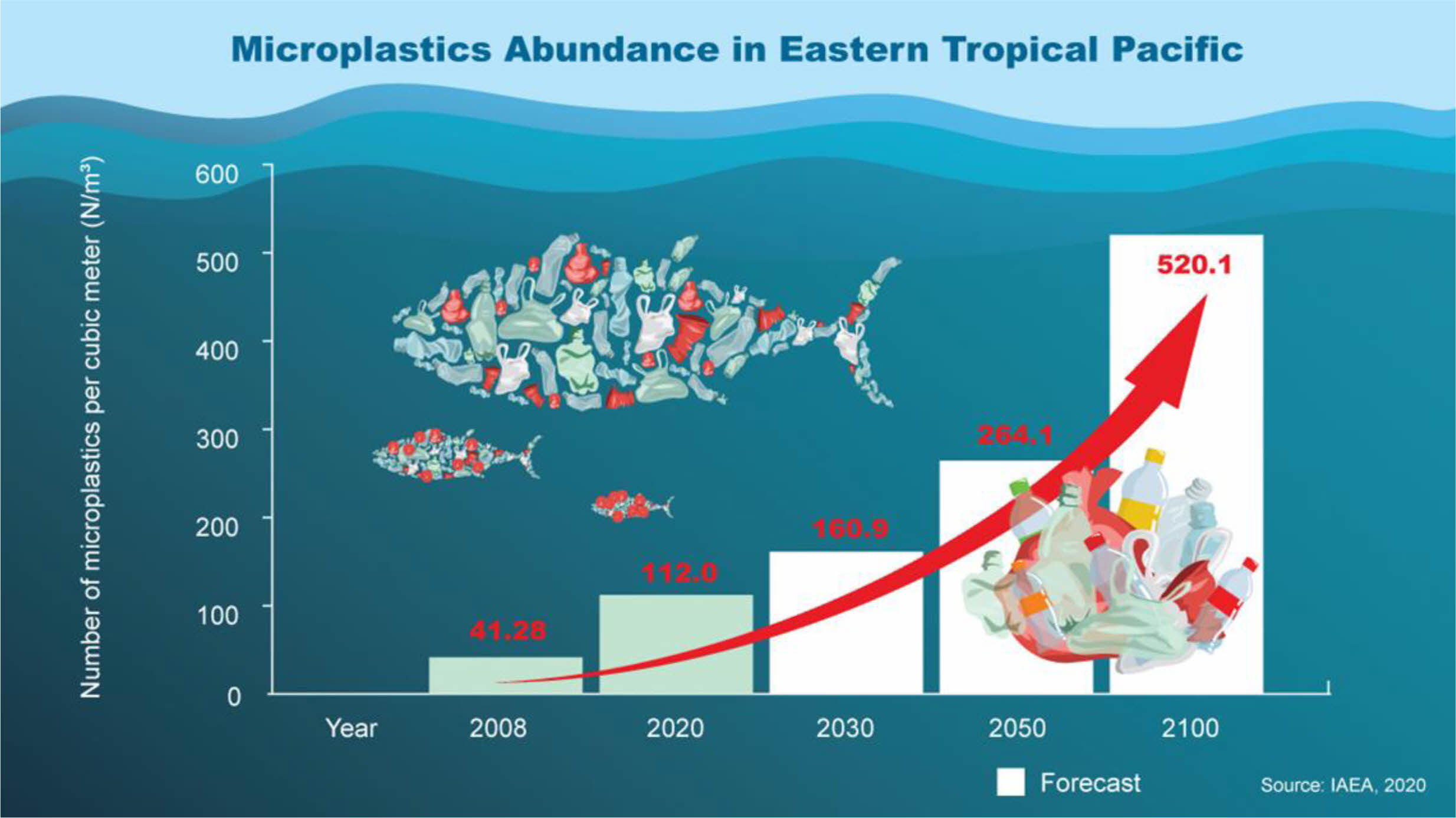
|
Fig. 1 Microplastic pollution level in the upcoming days in the Eastern Tropical Pacific Ocean [5] |
Around 8.3 billion tons of plastics have been produced since the discovery. Among them, only 9 percent were recycled, and the remaining plastics were mixed into soil or water as macro or microplastics [2]. The naked eye quickly found macro plastics and collected them for recycling. However, microplastics are not visible easily and spread over the globe quickly by wind due to their low weight. As mentioned above, the primary microplastics are small particulates directly released into the environment. These are willingly added to the products such as scrubbing agents in toiletries and cosmetics during manufacturing. In addition, the primary microplastics are also produced by the abrasion of more oversized plastic items in industrial use or maintenance, such as the erosion of tires when driving or the abrasion of synthetic fabrics through the laundry. The secondary microplastics are plastic fragments produced by the action degradation of large plastics, which are mismanaged plastics and accidental loss of fishing nets to the environment. The degradation occurs by applying sunlight or other natural weather and climate conditions. The quantification of microplastics in specific environments is difficult due to a lack of knowledge and technology for estimating uncontrolled, random kinetics followed by the degradation of macro plastics to microplastics. As per the literature, seven significant sources, such as plastic pellets, synthetic textiles, tires, road markings, marine coatings, personal care products, and city clouds of dust, are considered globally primary microplastic pollution. These are unavoidably added to the environment. However, secondary microplastics are produced by mismanaged waste when disposed of various plastics products.
(i) Plastic pellets: These plastic pellets usually exist in the size of 2 to 5 mm or sometimes in powder. These pellets are transformed into plastic products in industries. Unfortunately, these pellets, such as nurdles and mermaid tears, are spilled into the environment during manufacturing, transport, and recycling [20].
(ii) Synthetic textiles: During the washing process of synthetic textiles in an industrial laundry or house, microplastics like micro-fibers are generated via abrasion and shedding fibers. These fibers are discharged and added to sewage water. In the end, the unprocessed sewage water finally ends up in the ocean, creating uncontrollable microplastic pollution. The microfibers of PEST, PE, acrylic, or elastane fibers are released during the washing of textiles. As a result, they are found in the open water and sediments of seawater bodies [21].
(iii) Tires: The tires comprise approximately 60% of styrene-butadiene and other natural rubber or additives [22]. The microplastics were produced from the outer surface of tires when tires get eroded during transport. Releasing of natural rubber as microplastic is not reported enough. However, microplastics formed from synthetic rubbers are reported and have been spread over the environment by wind or rain. Many microplastics that belong to car tires are found in the sea near Norway and Sweden [23].
(iv) Road Markings: The paints, thermoplastics, epoxy, or preformed polymer tapes are used for the road marking. Thermoplastics are widely used in European countries, and paints are globally used by approximately 45% for marking roads [24]. Various weather conditions and vehicle abrasion create microplastics from the road mark. Cause of the wind or rain, they have been spread from one place to another and pollute the environment.
(v) Marine Coatings: Solid coatings, anti-corrosive paints, or anti-fouling paints are applied on the vessel part of marine equipment to protect them. Polyurethane, epoxy, vinyl, and lacquers are plastic materials used for marine coating [25]. Microplastics are created from coatings during construction, maintenance, and usage.
(vi) Personal care products: Plastic microbeads are added to personal care products and cosmetic products as one of the active ingredients to enhance exfoliation or viscosity. It has been directly added to water bodies from hotels, households, hospitals, and beaches. These microbeads as microplastics are detected in various worldwide wastewater [26].
(vii) City dust: This name is given because city dust is not a specific one, and it is a group of various microplastics such as synthetic soles of footwear, synthetic cooking utensils, household dust, city dust, artificial turfs, harbors, and marina, building coating, detergents, etc. The individual impact is minimal, but the impact is heavy when city dust is joined together [24].
These microplastics are spread over the environment through road runoff, wind, rain, and wastewater. More than 3.2 million microplastics are released annually [23]. It will severely affect human and other organisms' health and produce various chronic diseases. Fig. 2
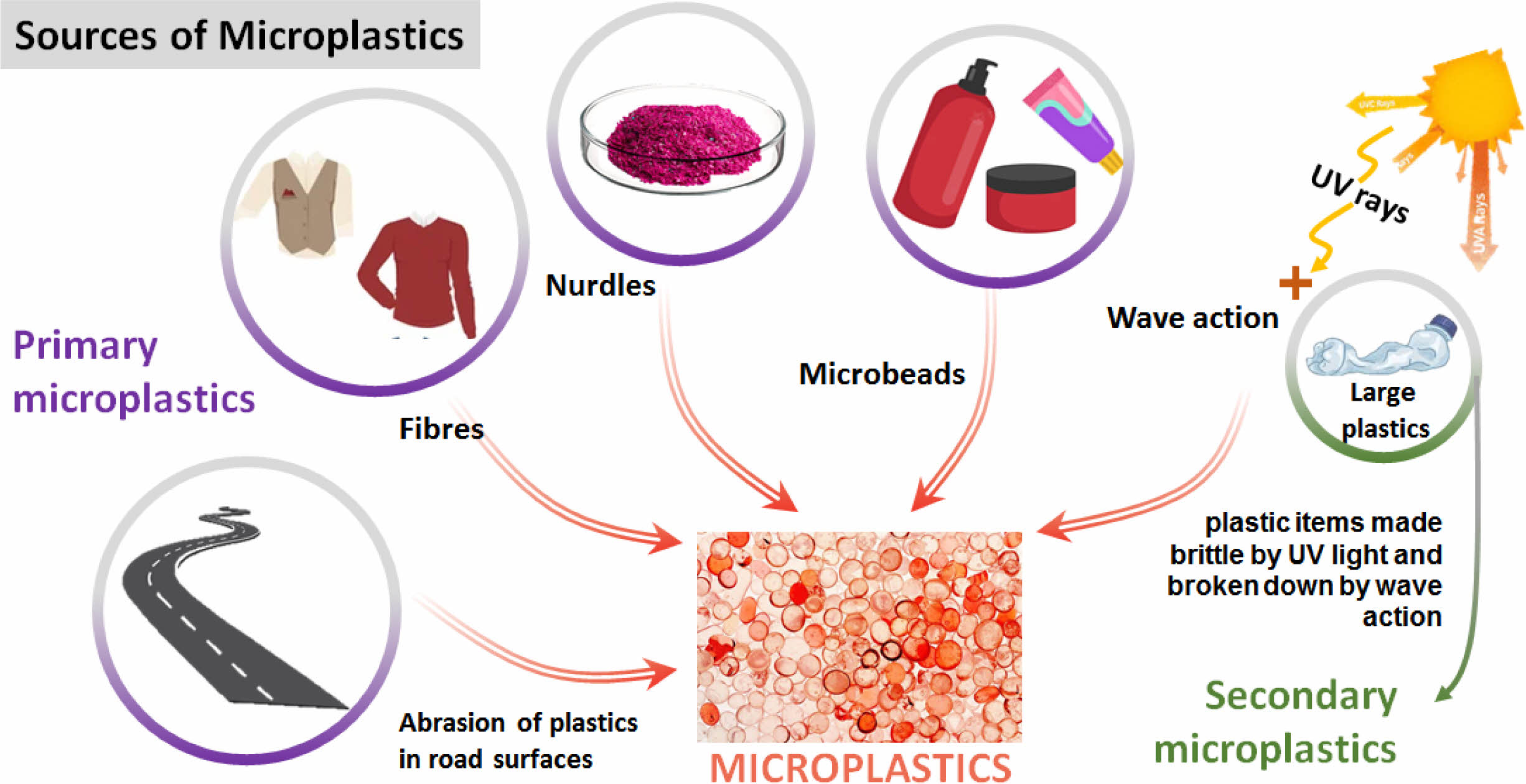
|
Fig. 2 Schematic representation of sources of microplastics in the environment. |
Microplastics have been present in the atmospheric air, water bodies, and various food items. Therefore, microplastics can be taken by inhalation or through diet, which causes serious health issues in humans. Seafood such as fish, shellfish, bivalves, farmed mussels, and other food items like honey and sugar have various microplastics that humans can directly consume when dieting [27]. Microplastics stimulate or boost the human body's immune system when ingested or inhaled by humans. Also, chemical toxicity is created by forming plastic monomers, additives, and other adsorbed pollutants internally in the localized area when microplastics react with human metabolites [27]. However, the toxicity levels for distinct types of microplastics are not investigated elaborately. Hence the lack of knowledge on the health issues related to microplastic consumption forces us to find the lethal dosage of each microplastic.
Microplastics are considered biologically inert, as far as non-toxic. However, continuous administration of microplastics via inhalation or diet will cause them to deposit somewhere and creates a problem [27]. The solubility, size & shape, and surface charge of the microplastics significantly affect the toxicity level of cells or tissues [28]. Through physical action, microplastics' bio persistence can alter the biological reactions with inflammation, oxidative stress, genotoxicity, necrosis, and apoptosis [11]. Microplastics' long-term bio persistence causes tissue damage, carcinogenesis, and fibrosis. Similarly, the chemical components of microplastics, such as a monomer or other fragments, are also involved in health issues like chronic aging and auto-immune diseases [17].
Some airborne fibrous MPs, such as polycyclic aromatic hydrocarbons, take part in the genotoxicity. In addition, the additives like dyes or plasticizers are creating reproductive toxicity, mutagenicity, and carcinogenicity. According to the World Health Organization, particles greater than 5 mm in length and less than 3 mm in diameter, with an aspect ratio of > 3:1, can be considered fibrous MPs [29]. The MPs higher than this size can be inhaled but will undergo mucociliary clearance in the upper airways, leading to gastrointestinal exposure. However, in vitro tests revealed that the fiberoptic MPs of PP, PE, and polycarbonate are exceptionally durable over 180 days in the physiological fluids of the lungs. Polyester fibers are found in the pulmonary tissues that confirm the MPs' existence in the human lungs [30]. These MPs possibly go through into human lungs via inhalation of airborne MPs during a breath. Similarly, polyester, acrylic, or nylon dust are found using the histopathological investigation of lung biopsies in humans who worked in the textile industries [31]. It has been shown that all MPs are not filtered in the human self-protection system like mucociliary clearance. Interstitial lung disease stimulates dyspnoea (breathlessness), coughing, and reduced lung capacity in humans who are working in the para-aramid, nylon, or polyester fibers processing unit [32]. Continues inhalation of airborne fibers up on cell contact, cytotoxicity factors produce inflammation in the lungs. Chronic inflammation continuously produces too many reactive oxygen species which will be leading to create fibrosis and a few cancer types. All plastic products have certain amounts of free radical which is formed during polymerization when manufacturing them. In presence of light or transition metals, the concentration of free radicals will be increased in the plastics. Hence the excess formation of free radicals reacts with the neighboring metabolites in tissues or organs which will later create oxidative stress, cancerous diseases, aging diseases, etc. The less toxic and weekly soluble MPs creates inflammation and lung tumors in rat, but the effect of these MPs in humans is yet to be investigated [33].
The polyethylene-derived MPs with sizes from 0.5 mm to 50 mm stimulate the non-immunological foreign body response. In rabbits, the small-sized PE is more potential than the large-sized PE MPs [34]. The polyethylene terephthalate (PET) MPs with sizes from 0.5 mm to 20 mm is stored in the cytoplasm of histiocytes and the 20 mm to 100 mm sized PET MPs are found in the extracellular tissue. The neighboring tissues are substantially changed by the action of PET MPs and the huge accumulation of PET MPs leads to an affecting on the lymph system [35]. Similarly, polyvinyl chloride (PVC) and polystyrene (PS) MPs are also severely affecting the body. The small-sized PVC MPs are found in the urine, bile, and cerebrospinal fluid of dogs after the ingestion of 200 g of PVC powder into the blood through the venous. The concentration of test solution for ingestion is kept as 10 to 15 PVC particles/mL. The test solution was slowly added to dogs’ blood via venous for about 1-2 hours duration. At the same time, large-sized PVC MPs are found in tissues and organs [36]. Similarly, the PVC MPs seemed in the liver of rats after 10 min of post-esophageal administration. These experiments clearly show that the ingested MPs penetrate through the intestinal wall and be transferred to secondary tissues by the action of lymphatic and portal systems. Cerebral softening, scarring, and micronecroses were examined in the brains of dogs which is investigated for postexposure via femoral artery catheterization into the left ventricular cavity [27].
The household clouds of dust from textile microplastic fibers consist of volatile organic compounds (VOCs) like phthalates and polybrominated diphenyl ethers are associated with health issues like carcinogenicity, reproductive toxicity, and mutagenicity [37, 38]. Apart from VOCs, some other organic pollutants are also adsorbed on the MPs by hydrophobic interaction between the surfaces of MPs and organic pollutants such as organochlorine pesticides, polycyclic aromatic hydrocarbons (PAHs), dichlorodiphenyltrichloroethane (DDTs), and polychlorinated biphenyls (PCBs) leads to potential adverse health issues. These are classified as highly toxic, and involved in endocrine-disrupting, carcinogenesis, mutagenesis, and immunotoxicity. Moreover, a few antioxidants, nonylphenol, and Bisphenol A are released from the matrix of plastic polymers that are severely harmful to human health depending on the concentration.
The lack of evidence for the human health problems by MPs should be perfected such as lethal range, the mechanism between the MPs and humans, sustainability range, and survival limit. Up to now, we have evidence from indirect methods like the health effects of byproducts, adsorbed chemicals, or other pollutants only and subjected to animal models. Hence, the direct health issues caused by the consumption of MPs are yet to be urgently investigated more elaborately. Fig. 3
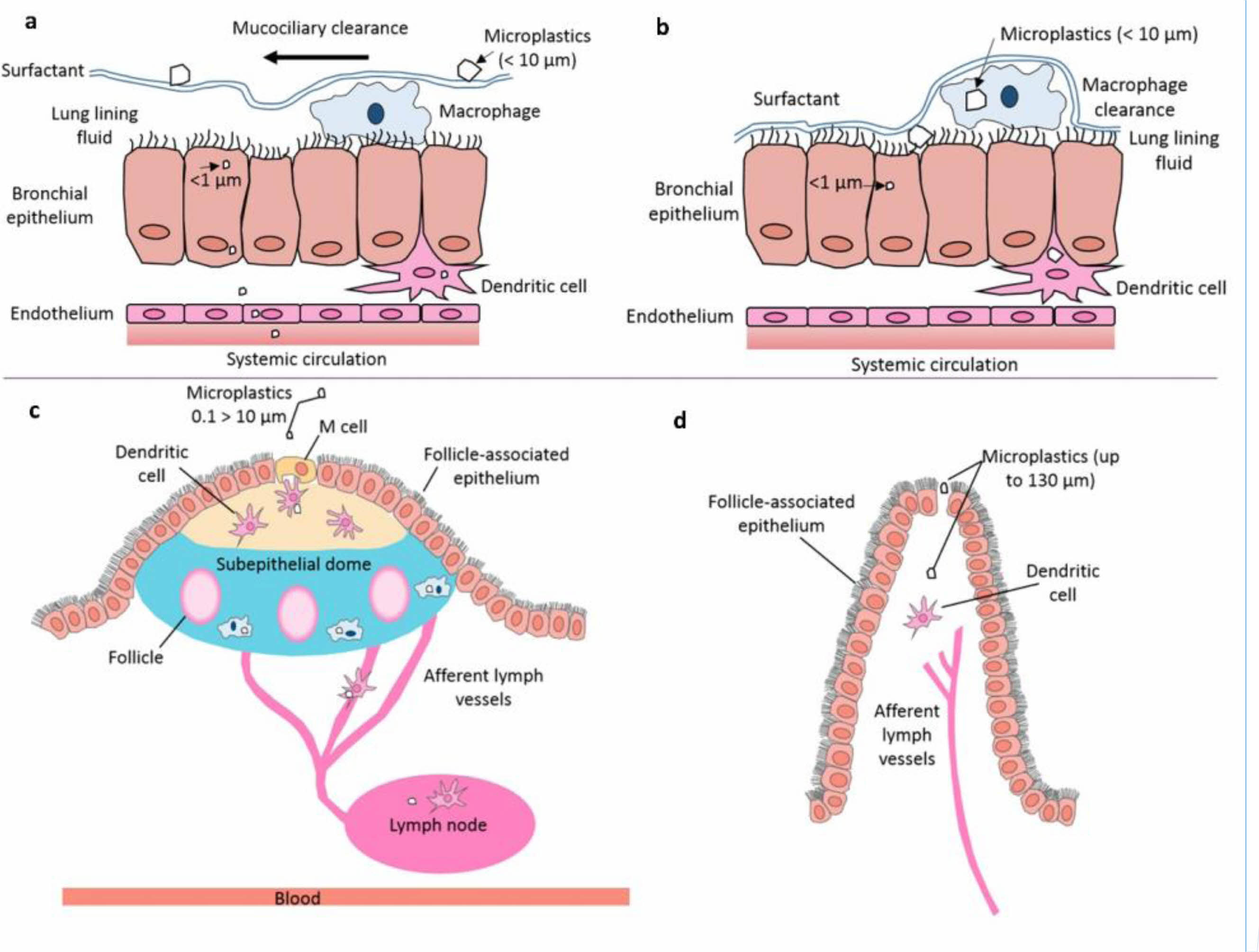
|
Fig. 3 Schematic illustration of MPs (0.1-10 μm) uptake and clearance mechanisms in lungs. (a) Above 1-micron-sized MPs are removed by the action of mucociliary clearance. Hence < 1 μm is possible to enter through the epithelium tissues, (b) if the aerodynamic diameter of a microplastic permits deposition deeper in the lung, it may penetrate the thinner lung lining fluid and contact the epithelium, translocating via diffusion or active cellular uptake. Expected pathways of MPs uptake from the gastrointestinal tract, (c) MPs (0.1 > 10 μm) uptake from the gastrointestinal tract lumen via endocytosis by the M cells of the Peyer’s patches. M cells sample and transport particles from the intestinal lumen to the mucosal lymphoid tissues, (d) microplastic uptake from the gastrointestinal tract lumen via paracellular persorption. “Reprinted (adapted) with permission from [27]. Copyright 2017 American Chemical Society.” |
Detection and quantification of MPs are especially important to control environmental pollution and to develop and check the progress of removal technologies. The experimental procedures for sample preparation and analyses of MPs are different for each type of environmental sample. In most cases, the MPs are identified by the naked eye which is relatively big-sized MPs. however, the quantification of those MPs and analysis of their chemical composition, molecular structure, and shape is difficult when seen by the naked eye. Hence, the development of various detection methods is an urgent requirement.
Detection of MPs in air
To measure the atmospheric MPs, active samplers and Passive atmospheric deposition techniques are used. The atmospheric deposition technique follows the precipitation such as rain, fog, and snow, and particles or aerosols which comes to earth from the atmosphere. In active sampler techniques, the air molecules are continuously pumped to the air sample container where the MPs are analyzed. The passive sampler technique measures the MPs in diffused air only which is diffused due to their kinetic energy. Similarly, spectroscopes and microscopes are also helpful to find the MPs in various environments. For example, the stereomicroscope is used to get information about MPs from three-dimensional viewpoints. The scanning electron microscope, ultraviolet-visible spectroscopy, Fourier transform infrared spectroscopy, pyrolysis gas chromatography coupled with mass spectrometry, Raman spectroscopy, hyperspectral imaging technology-chemometrics, and resonance microwave spectroscopy is also generally used to detect and measure the MPs [39].
Detection of MPs in water
In the sea or oceans, the MPs can be floated or sink. The MPs such as polypropylene (PP) have low density than seawater and it has been floated and dispersed over the entire ocean via gyres caused by oceanic currents. 7040 tons and 28500 tons of MPs are floating on the whole ocean in the size range of 0.33 mm to 1 mm, and 1 mm to 4.75 mm respectively [40]. Greater than 4.75 mm sized plastic particles are weighed as 233.4 kilotons, spread over the ocean. Among them, the north Pacific Ocean is highly contaminated by the MPs followed by the Indian Ocean. Around 4850 trillion pieces of MPs are floated in the global ocean. Some other types of MPs like acrylic MPs have high density than seawater which are sink and accumulate in the deep sea [41].
Recently researchers developed a prototype Raman spectroscope for the detection of MPs in water samples [42]. This device consists of a 5-mW power laser at a wavelength of 405 nm, sample holder, notch filter, diffraction grating, charge-coupled device detectors, microcontroller, and processor. These components are kept in the 3D-printed case. The open-source IoT platform ThingSpeak software is used to communicate the microcontroller and the Wi-Fi-enabled mobile phone. Fig. 4 shows the schematic illustration of the portable device and the photographs of the actual device. The detection range of this device is from 0.015 and 0.035% w/v by MPs in water ratio. This prototype device is significantly important due to its high accuracy, portability, low weight, inexpensive, simplicity, and user friendly. However, it has a few demerits like poor sensitivity due to the use of low power laser light source and the low-sensitive charge-coupled device sensor. When enhancing these two components' properties, then the sensitivity will be increased which may reasonably increase the manufacturing cost, hence the cost for the analyses of MPs in various water samples will be affected. Researchers keep on developing portable, reliable, low-cost sensor devices to detect and check the MP's pollution level in various environmental samples.
Detection of MPs in soil
In the soil samples, the low-density polyethylene (LDPE) and polyvinyl chloride MPs are detected by terahertz spectrometer using the least squares support vector machine algorithm [43]. This technique uses electromagnetic waves from the ranges of 100 gigahertz to 10 terahertz and helps to find the various structural analyses. The authors added the known concentration of MPs externally to the soil samples collected from various places for the analyses and validation of their experimental protocol. The results obtained from the terahertz spectrometer would not affect by the physical or chemical properties of samples and it is extremely sensitive to the specific MPs.
In another report, soil samples are analyzed by transmission electron microscope (TEM) coupled with Energy Dispersive X-Ray Analysis (EDAX) for the detection of various types of MPs [44]. Here, they purified the samples by separation techniques like fractionation before the TEM analyses. Then, the samples are coated on the copper grid which is introduced into the TEM instrument. The size and shape of the MPs are seen by this technique. Further, the EDAX analyses show the elemental composition which is used to calculate the concentration of MPs in soils. In this paper, pyrolysis coupled with gas chromatography and mass spectrometry was also experimented with. The results obtained from both techniques are nearly the same. Hence, the experimental developed protocol for both techniques is useful in the detection of MPs for soil samples.
Mussel and soil samples were collected from the Spree River in Berlin and processed as per the standard operating procedures [45]. The 1 mm size of LDPE pieces was spiked into the samples. Most thermoplastics are melted before the decomposition point and different morphology, structure, and size of LDPE MPs are formed. The authors have taken the above-spiked samples in the aluminum oxide crucible for thermo- gravimetric analyses. The samples were analyzed in the temperature range from 25 to 600 ºC with a 10 ºC per minute heating rate. The thermal extraction twisters i.e., polydimethylsiloxane is used as adsorbers and connected with the TGA outlet. The twisters are subsequently assessed by thermal desorption gas chromatography-mass spectrometry technique. The authors do not find any interfering molecules when detecting LDPE using their proposed method i.e., term extraction and desorption coupled with the gas chromatography-mass spectroscopy technique. Various innovative detection methodologies are evaluated and the merits, demerits, and problems which are addressed and to be solved of available quantification procedures are recently documented [39].
Researchers facing the problem to develop an inexpensive method with highly accurate results for the detection of MPs in various environmental samples like soil, water, or atmosphere samples due to possible contamination of MPs during sample collection or analyses. To validate the developed methods, and to develop quality assurance and quality control procedures, a clean laboratory, standard or blank solution, avoid the plastic materials in a laboratory including textile cloths are needed. Also, the samples should be washed or treated using milli-Q or deionized ultrapure water to develop a successful detection protocol. The samples along with reagents may be needed to filter which should not be plastic made and the samples should be closed with a glass lid or aluminum foil to prevent the contamination of airborne MPs.
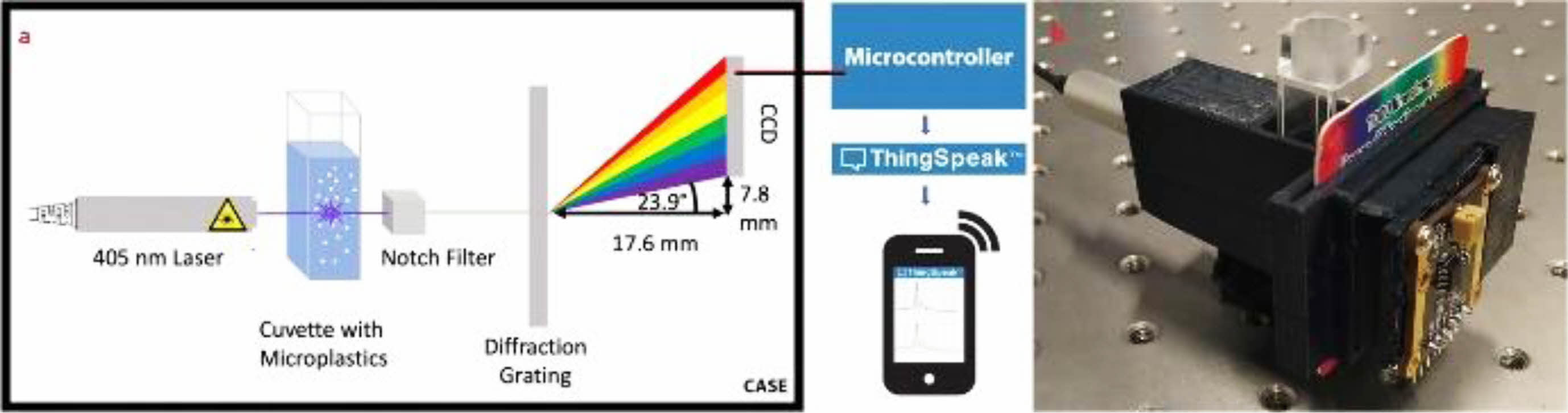
|
Fig. 4 (a) The graphical illustration of the design of the optical detection system, (b) the actual prototype device system in a 3D printed case. Reproduced with permission from Springer Nature [42]. |
As we know, MPs pollution creates various severe environmental and health problems for humans and other living organism. To minimize such growth of diseases and protect our ecosystem from MPs pollution, we need to find alternate microplastic treatment processes or improve the performance of existing processes for the removal of MPs from the soil, water, foods, ocean, and kinds of seafood. Up to now, coagulation and flocculation, filtration using membrane, biological, oxidation, and adsorption techniques are widely used for the removal process concerning the type of MPs. We will look at the working principle, efficiency, merits, and demerits of these techniques with suitable examples along with challenges to overcome in each technique.
Coagulation and Flocculation
The coagulation, flocculation, and sedimentation processes are widely used to separate the MPs or other hazardous impurities. In coagulation, the coagulant which is a highly charged molecule is added to the water to neutralize the charge of the impurities or particles and destabilizes the particle. In the flocculation process, the neutralized particles are aggregated along with each other to form a bigger-sized particle. Then the larger-sized particles or aggregated particles which are called ‘floc’ are getting to settle down. This process is called sedimentation. Several types of coagulants are used in microplastic removal technologies concerning the types of MPs. However, all the coagulant takes a longer time to complete the reaction which is approximately one hour.
For example, the spiked PS microplastics are removed from secondary wastewater treatment plant (WWTP) samples by coagulation and flocculation methods [4]. Polyaluminium chloride, ferric chloride, and polyamine are used as a coagulant to neutralize the charges of particles. The pH was kept between 6.5 to 7.3 to get better results. The efficiency of microplastic removal was measured by the flow cytometry technique. They observed that 99.4% of MPs were removed using the coagulation/flocculation process. The inorganic coagulant such as iron, and aluminum ions show high efficiency than organic coagulants like polyamines in the MPs removal process. The coagulation process was taken 20 minutes and the flocculation and sedimentation process took 30 minutes to complete with better efficiency.
The PE, PS, and PEST-derived pristine and weathered MPs are investigated for a better understanding of the coagulation/flocculation mechanism using alum and aluminum chloralhydrate coagulant [46]. The weather conditions can affect the morphology, roughness factors, and surface charge of MPs which will significantly increase the coagulation and flocculation process. They also discovered that the efficiency of the coagulant and flocculation process is high for aluminum-based coagulants than for organic coagulants like polyacryl- amide. The large-size pristine PE has a low affinity with a coagulant, and the efficiency is extremely low (82%), even at optimized coagulation conditions. They reported that the weathering conditions increases and increasing size decrease the efficiency of MPs removal.
In another report, the removal of the spiked polyethylene MPs is examined using drinking water with aluminum, and iron-based coagulant [47]. Here, aluminum-based coagulants show better efficiency in MPs removal than iron-based coagulants. Authors also suggest that the efficiency of MPs removal in water is depending on the ionic strength and turbidity level. The improved performance in aluminum-based coagulant was achieved, even in neutral pH by the addition of anionic acrylamide to the experimental samples which enhance the rate of formation of aluminum flocs.
The MPs can be removed via an agglomeration mechanism by organosilane such as n-butyltrichlorosilane and isooctyltrichlorosilane as a linker between them [48]. The polluted water composition and reaction temperature are investigated to get better efficiency in MPs removal. The PE, PP, PA, PEST, and PVC are evaluated to calculate the efficiency at different water compositions and temperature ranges from 7.5 ºC to 40 ºC. The level of water contamination and temperature is not significantly affecting the efficiency. However, the polarity and surface chemical functionalities are affecting the efficiency of MPs removal due to kinetics variations in the formation of MPs-organosilane agglomerated particles.
The Styrofoam and rubber MPs were removed from wastewater through electrochemical treatment followed by filtration [14]. They have electrochemically produced the coagulant, Al3+, and Fe3+ from aluminum and iron electrodes by passing current to the wastewater-immersed electrodes. The current density was altered for high efficiency from 10 A/m2 to 20 A/m2 for 0 to 120 minutes. The effect of pH ranges from 4.0 to 10.0 in the sludge was also investigated. The effect of pressure on the membrane was also studied in this article. The 100% efficiency was reached within 10 minutes for the MPs removal at pH 7.0 when allowing a current density of 20 A/m2 to the aluminum-iron electrode combination. This method was confirmed in the WWTPs for one year that shows 100% efficiency when compared to primary, secondary, or tertiary treatment methods.
Membrane, Biological, and Filtration Technologies
Microplastic removal is often a hybrid process and at the last step, the membrane is used. It is like a filtration process, usually, the membrane blocks the MPs which have a higher size than the bore size of the membrane. The membrane should have important properties like chemical and mechanical innerness to enhance durability and should work in a wide pH range irrespective of acidic or alkali nature. Existing membranes can be classified into two major types such as (i) organic-based polymeric membranes which are made up of PAN, high-density PE, Polytetrafluoro- ethylene, polyvinylidene difluoride and (ii) inorganic-based ceramic membranes which are made up of aluminum oxide [49], silicon nitride [50], silicon oxide [49], titanium dioxide [51], titanium boride [52], calcium cobaltite [53] and zirconium carbide, boride [54]. Each membrane has unique merits and demerits, in general, ceramic membranes are inactive to chemicals and have good mechanical strength, but expensive. Polymeric membranes are inexpensive, but they may get damaged when in contact with chemicals or when applying mechanical strength. Hence, we cannot use all membranes for the MPs removal process that push to find the alternate membrane. For example, a membrane was developed from the mixture of reduced graphene oxide (rGO) with polyacrylonitrile to remove the MPs from industrial wastewater [55]. They have perfected the membrane pore size by changing the concentration of rGO from 0.11% to 0.83% w/w with a polyacrylonitrile matrix. The high amount of rGO creates a uniform and large pore size (approx. 150 nm). The developed membrane rejects more than 82% of flocs that are created from ferric chloride coagulant. This membrane has valuable features like anti-fouling properties and easy removal of the cake layers which offers to reuse of the membrane.
The membrane performance was investigated using polyamide and polystyrene microplastics in household filtration systems [56]. The transmembrane pressure (TMP) and flux were elaborately investigated in this paper. The 5 μm of the same pore size cellulose acetate, polycarbonate, and polytetrafluoroethylene membranes are used in this study to filter the PA and PS MPs with the size of 20 μm to 300 μm. All membranes filter the MPs at more than 94% when the concentration of MPs is 100 mg/L. However, the membrane abrasion and fouling phenomenon affect the efficiency of MPs removal in all membrane types. The TMP and flux were depending on the size and shape of MPs, irregularity of MPs, and physiochemical properties of the membrane leading to the effect of the interaction between MPs and membrane. Based on these effects, the PTFE membrane is not suitable, and the cellulose acetate is better than the polycarbonate membrane for household system applications.
The dynamic membrane technology helps to reduce the cost and time of MPs removal in WWTPs due to reduced energy consumption. The dynamic membrane was formed on the supports or existing membrane during the filtration process. It will increase the TMP and filtration resistance. For example, the dynamic membrane was formed on the 90 μm of supporting mesh during the synthetic wastewater filtration [57]. In this paper, the authors investigate the impact of influent MPs concentration and influent flux with dynamic membranes. The TMP was increased from 80 mm to 180 mm at the water head and total filtration resistance was increased from 2.89 × 10−9 m−1 to 6.52 × 10−9 m−1 during filtration. The increasing influent fluxes from 9 L/h to 21 L/h increase the TMP and filtration resistance. At the same time, the increasing flux decreases the turbidity level in effluent within 10 minutes due to the rapid formation of the dynamic membrane. Similarly, the increasing size of MPs increases the TMP and filtration resistance at all influent fluxes. However, the effluent turbidity was reduced from 200 NTU to less than 1 NTU within 20 minutes of filtration time. This is a good achievement in using dynamic membranes compared to conventional membrane filter techniques.
In recent days, an advanced membrane technique, membrane bioreactor (MBR) also been used in the MPs removal from wastewater. This technique consists of two main parts such as bioreactor and microfiltration or ultra-filtration. In a bioreactor, the MPs are biologically degraded by the action of enzymes or bacteria or other microbes. Then the biologically degraded effluent was filtered by membranes. The MBR setup is usually configured in two ways such as (i) internal/ submerged/ immersed membrane bioreactor and (ii) external/ side stream membrane bioreactor. In the submerged type, the membrane is placed within the biological reactor and immersed in seawater. In the second type, the membrane is placed outside of the bioreactor. The MPs are biologically treated in the reactor, then filtered by a membrane. The type of internal MBR has major advantages like lower cleaning frequencies, and lower energy consumption, similarly side stream MBR can be used in filtration even in a higher concentration of sludge level. However, both types of MBR have a common issue i.e., fouling caused by the deposition of MPs. The fouling effect may reduce the pore size or block the pores of membranes is caused by chemical or physical interaction between MPs with membranes. To overcome the fouling effect, researchers developed anaerobic dynamic membranes along with a bioreactor in both submerged and external methods [58]. The authors achieved over 99% of COD removal efficiency. Hence this is a promising approach for the removal of several types of MPs.
The microplastic particles and fibers in WWTPs and different types of sludges are filtered by advanced MBR technology [59]. The samples are collected from WWTPs for three months at two weeks once. After the conventional activated sludge (CAS) process, the effluent was introduced into MBR. The MBR allows only 0.4 MPs per liter when injecting the 1 MP per litter which is a filtrate of CAS. The developed MBR process has a high retention rate relatively when compared to the CAS process. In another report, 99.9% of MPs removal was achieved using MBR technology [60]. Thirteen diverse types of MPs including PS and PE with sizes ranging from 20 μm to 100 μm are used for the investigation and efficiency of MPs removal by MBR. All samples were collected from four different WWTPs. In this paper, the authors investigated the filtration efficiency of MBR and compared the efficiency with that of disc filter, rapid sand filtration, and dissolved air filtration. The MBR removes MPs around 99.9% (from 6.9 to 0.005 MPs/L) while the rapid sand filter removes 97% (from 0.7 to 0.02 MPs/L), dissolved air flotation removes 95% (from 2.0 to 0.1 MPs/L) and disc filter removes 40% to 98.5% (from 0.5-2.0 to 0.03-0.3 MPs/L). Hence, we can strongly recommend the MBR technology for better MPs removal from diverse types of water and sludges.
In recent days, the reverse osmosis process used to remove the MPs with their tiny pores. The size of pores in the RO membrane is approximately 0.1 nm. Through these pores, almost all contaminants are filtered. However, the bigger problem is the fouling effect in the membrane and high pressure is needed. To minimize the fouling effect, the effluent was pretreated by carbon filters which remove rust, sediment, chlorine, and other contaminants. Hence, the efficiency of MPs removal is higher compared to microfiltration or nanofiltration. Due to the function of higher pressure while the filtration, the RO process could be called a ‘pressure-driven membrane system.’ Heavy metals like lead, arsenic, volatile organic compounds, radioactive particles, fluoride, and chloride also can be removed along with MPs by the RO process. The RO filters have been attached sometimes with MBR to get improved performance in the MP removal.
Distillation filters are also used for the removal of MPs from distinct types of pollutant water. In this process, the effluent flows through the heating coils which are placed in the effluent reservoir of the WWTPs. When the water molecules reach the evaporation temperature, the water gets evaporated. The chemicals which are a lower evaporation point also get to evaporate. The mixture of water and pollutant vapors are passing through the coolant followed by a filtration setup. The water and other chemicals' vapors are condensed into a liquid state at the required temperature and the liquids are filtered to get purified water. Usually, carbon-based filters are used to filter the distilled water to remove the volatile organic compounds and other chemicals which has a low boiling temperature. This is an effective method to remove the MPs from sludge due to certain pollutants removed at the reservoir and a few contaminants removed at filters. However, it required high energy to boil the water leads to making them unsuitable for large-scale treatment processes.
The MPs can be removed from the secondary effluent of WWTPs using biofilter technology in Denmark [61]. The biofilter has four different zones with various dimensions that consist of stone wool. Initially, the effluent has 917 MPs/m3 volume with a weight of 24.8 μg/m3. After using a biofilter, the concentration of MPs is reduced to 197 MPs/m3 with a mass concentration of 2.8 μg/m3. The developed biofilter holds the MPs with generous size and mass significantly. The MPs and other pollutants with a size of >100 μm were filtered effectively. The removal efficiency is about 79%, however, this is not sufficient for use on a large scale.
Advanced oxidation processes
The MPs can be removed from the environment by advanced oxidation processes (AOP) like oxidation, degradation, and photochemical methods. The AOP process converts hazardous MPs and other chemical pollutants into non-toxic products. The AOP can be used independently or followed by filtration, based on the method. The MPs surface gets oxidized by the action of oxidants, most commonly reactive oxygen species. The MPs are decomposed into several types and sizes by the action of homogeneous and heterogeneous AOPs such as UV photolysis, UV/H2O2, O3, light-induced photocatalysis, UV/visible, heat-activated persulphate and peroxymonosulphate, and plasma. The MPs are not fully decomposed in most cases and only surfaces get oxidized. Moreover, the AOP-treated MPs are acts as pollutant carriers to animals and humans due to the adsorption of pollutants on the treated MPs [62].
Photolytic decomposition is caused by light, especially UV or visible. The hydroxyl radical (OH●), singlet oxygen (1O2), organoperoxy radicals (ROO●), carbonate radicals (CO3●-), triplet organic matter (3OM●), hydrated electrons (e-aq) are generated during the irradiation of light, especially UV. The physicochemical properties of PS, PET, PE, and PVC are changed when treated with UV irradiation [63]. The degradation pathways are overly complicated to understand. The efficiency of degradation is depending on the irradiation time, and type of UV such as 200-280 nm (UV-C), 280-315 nm (UV-B), and 315-400 nm (UV-A). Effective deformations like cracks, wrinkles, and protrusions are formed on the plastic materials by irradiation of shorter wavelength (UV-C) i.e., 254 nm compared to UV-B or UV-A [62]. Moreover, the efficiency of photolytic degradation was increased when creating a vacuum at the reactor. The hydrophobicity of MPs was also decreased after the photolytic reaction, which leads to the weakened adsorption of other organic pollutants.
UV photolysis is remarkably effective in presence of H2O2 molecules. The H2O2 will produce OH●, and O● at once and in massive quantities when exposed to UV rays. These radicals are highly reactive towards plastics, hence the degradation taking place. The changes in embrittlement, surface morphology, carbonyl index (CI), glass transition temperatures, crystallinity, hydrophobicity, and average molecular weight of PS were examined throughout the treatment process using UV/H2O2 protocol [64]. The surface roughness, crystallinity and embrittlement, and CI were increased in PS after the treatment. However, the hydrophobicity and average molecular weight was decreased due to the chain scission reactions and formation of various functional groups on the surfaces due to oxidation by radicals.
Similarly, ozone is a strong oxidant and is highly investigated for the degradation of MPs. The surface oxidation of MPs by ozone leads to the removal and decomposition of MPs in various environmental samples. The degradation via the aqueous phase ozonation process was examined using PE MPs [65]. They applied 4 mg/min to 7 mg/min of ozone to the PE MPs for 60, 120, and 180 minutes and the ozone uptake was measured by iodometry procedure. The higher concentration of ozone and longer reaction time would enhance the uptake of ozone by up to 44%. The carbonyl and hydroxyl groups are increased on the PE MPs after the ozone treatment process that was measured by FTIR and XPS analyses. The authors finally concluded that the reaction time, 120 min greatly enhanced the degradation reaction than the concentration of oxidant in the ozone treatment process. Various types of MPs from WWTPs are removed up to 99% using an ozone treatment process when performing it with coagulation procedures [66].
Some heterogeneous photocatalyst was also used in the removal of MPs. For instance, TiO2 nanoparticles film was developed for the degradation of PS and PE MPs under UV illumination [67]. The film was developed from a mixture of TiO2 nanoparticles and Triton X-100. The as-prepared film shows 98.4% of mineralization in 400 nm-sized PS microspheres within 12 hours. For PE photodegradation, high efficiency occurred after 36 hours of treatment. In both MPs, the carbon dioxide was released as end products, and the formation of carbonyl, hydroxyl, and carbon-hydrogen functional groups are also found after the photo- degradation process. Authors believe that this method is low-cost, green, and highly effective towards MPs removal from the environment.
Compared to UV-induced degradation, visible-driven degradation is a greener approach. The ZnO nanorods are synthesized to remove the PP MPs in a continuous flow system [68]. More than 65% of MPs volume was decreased when treating visible light for two weeks to polluted water by ZnO nanorods. The decomposition products such as formaldehyde, acetone, acetaldehyde, butyraldehyde, acetyl acetate, and hydroxy pentyl were detected by GC-MS and most of them are non-toxic or less toxic. The visible light absorption of ZnO nanorods can be increased by the deposition of platinum on the surfaces of nanorods. The ZnO-Pt nanocomposite was used to decompose the 50 μm sized LDPE MPs [69]. The efficiency of degradation was enhanced up to 78% as expected under visible light irradiation.
Adsorption technologies
The high surface area of MPs leads to adsorbing many toxic chemicals and releases them in a different environment. The MPs are acting as a carrier for the toxic chemical from one place to another place. The adsorption is occurred by surface functional groups via physiochemical interactions like Van der Waals force or ion exchange. The same phenomena can be used to remove MPs from the environment. For example, the marine algae of Fucus vesiculosus adsorb various MPs on its surface due to the presence of alginic acid in its cell wall [70]. The adsorption is directly proportional to the amount of carboxylic acid present on the surface of algae. 20 μm sized PS MPs were used for the investigation. 94.5% of MPs are adsorbed on the algae surface of seaweed. The positively charged PS MPs are more highly adsorbed than negatively charged PS MPs due to the electrostatic interaction between algae and MPs [71]. It is confirmed by the green algae Pseudokirchneriella subcapitata whichwas used to remove PS MPs with a size of 20 nm to 500 nm at various surface charges through the adsorption mechanism [72]. The positively charged MPs were highly adsorbed on the unicellular green algae.
Challenges and opportunities
In this review, we have briefly discussed one of the major environmental pollution problems which is microplastics. The possible sources of MPs in various environments like soil, water, and air were discussed. The detection and determination of MPs using the device with high accuracy, low cost, and high durability and adapting to several types of samples without any complicated analysis procedures are not established sufficiently and are under the development condition up to now. Similarly, the health effects on humans and other living organisms are weakly investigated and evidenced. Up to now, we have extremely limited reports in clinical toxicology for direct or indirect consumption of MPs. More fundamental and advanced studies are needed to understand the MPs' toxicology, lethal range, a working mechanism in organs, and immunity function of humans & other animals. This will help and be important to the development of medicine and therapeutic methods. We have huge reports on the removal or treatment process for the MPs. However, those are mostly expensive, have high-energy consumption, complicated procedures, low individual efficiency, and produce secondary toxic small molecules. A lot of challenges must be addressed in the removal process. The reuse of coagulants, fouling in the membrane process, high pressure in the filtration, high energy consumption, low efficiency, or longer time in the photolytic degradation are big challenges in the MPs treatment process that to be addressed clearly to make a pollution-free environment.
This work was supported by Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (No. 2021010000001B, Development of new conversion process for biodiesel production from low-grade feedstock).
- 1. M. Shen, G. Zeng, Y. Zhang, X. Wen, B. Song, W. Tang, Sci. Total Environ. 697 (2019) 134200.
-

- 2. J.R. Jambeck, R. Geyer, C. Wilcox, T.R. Siegler, M. Perryman, A. Andrady, R. Narayan, K.L. Law, Science 347 (2015) 768-771.
-

- 3. Z.D. Taha, R. Md Amin, S.T. Anuar, A.A.A. Nasser, E.S. Sohaimi, Sci. Total Environ. 786 (2021) 147466.
-

- 4. K. Rajala, O. Grönfors, M. Hesampour, A. Mikola, Water Res. 183 (2020) 116045.
-

- 5. J. Orayeva, World Oceans Day 2020 : New IAEA Research Records Dramatic Increase in Microplastic Pollution in Eastern Tropical Pacific Ocean, 15 (2020) 1-5.
- 6. W. Courtene-Jones, B. Quinn, S.F. Gary, A.O.M. Mogg, B.E. Narayanaswamy, Environ. Pollut. 231 (2017) 271-280.
-

- 7. L. Svetlichny, M. Isinibilir, T. Mykitchak, K.M. Eryalçın, E.E. Türkeri, E. Yuksel, A.E. Kideys, Reg. Stud. Mar. Sci. 43 (2021) 101650.
-

- 8. J.C. Prata, Environ. Pollut. 234 (2018) 115-126.
-

- 9. J.A. Brandon, W. Jones, M.D. Ohman, Sci. Adv. 5 (2019).
-

- 10. B. Toussaint, B. Raffael, A. Angers-Loustau, D. Gilliland, V. Kestens, M. Petrillo, I.M. Rio-Echevarria, G. Van den Eede, Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 36 (2019) 639-673.
-

- 11. D. Peixoto, C. Pinheiro, J. Amorim, L. Oliva-Teles, L. Guilhermino, M.N. Vieira, Estuar. Coast. Shelf Sci. 219 (2019) 161-168.
-

- 12. S.A. Mason, V.G. Welch, J. Neratko, Front. Chem. 6 (2018) 407.
-

- 13. C. Campanale, F. Stock, C. Massarelli, C. Kochleus, G. Bagnuolo, G. Reifferscheid, V.F. Uricchio, Environ. Pollut. 258 (2020) 113284.
-

- 14. C. Akarsu, H. Kumbur, A.E. Kideys, Water Sci. Technol. 84 (2021) 1648-1662.
-

- 15. Campanale, Massarelli, Savino, Locaputo, Uricchio, Int. J. Environ. Res. Public Health. 17 (2020) 1212.
-

- 16. J.N. Hahladakis, C.A. Velis, R. Weber, E. Iacovidou, P. Purnell, J. Hazard. Mater. 344 (2018) 179-199.
-

- 17. A. Rahman, A. Sarkar, O.P. Yadav, G. Achari, J. Slobodnik, Sci. Total Environ. 757 (2021) 143872.
-

- 18. G. Chen, Z. Fu, H. Yang, J. Wang, Trends Anal. Chem. 130 (2020) 115981.
-

- 19. A. Baruah, A. Sharma, S. Sharma, R. Nagraik, Int. J. Environ. Sci. Technol. 19 (2022) 5721-5730.
-

- 20. R. Essel, L. Engel, M. Carus, Umweltbundesamt. 64 (2015) 48. (accessed September 27, 2022).
- 21. M.A. Browne, P. Crump, S.J. Niven, E. Teuten, A. Tonkin, T. Galloway, R. Thompson, Environ. Sci. Technol. 45 (2011) 9175-9179.
-

- 22. P. Sundt, P. Schulze, F. Syversen, Sources of microplastic-pollution to the marine environment, Norwegian Environment Agency, Plast. Cosmet. (A Fact Sheet. UNEP); UNEP Norw. North Sea, Norw. (2015).
- 23. D. Friot, J. Boucher, Primary microplastics in the oceans | IUCN Library System, 2017. (accessed September 28, 2022).
- 24. C. Lassen, S.F. Hansen, K. Magnusson, F. Noren, N.I.B. Hartmann, P.R. Jensen, T.G. Nielsen, A. Brinch, Microplastics - Occurrence, effects and sources of releases to the environment in Denmark, 2015. (accessed September 27, 2022).
- 25. OECD, Emission Scenario Document on Coating Industry (Paints, Lacquers and Varnishes), Ser. Emiss. Scenar. Doc. (2009) 1-201. (accessed September 27, 2022).
- 26. A.G.J. Driedger, H.H. Dürr, K. Mitchell, P. Van Cappellen, J. Great Lakes Res. 41 (2015) 9-19.
-

- 27. S.L. Wright, F.J. Kelly, Environ. Sci. Technol. 51 (2017) 6634-6647.
-

- 28. K. Kannan, K. Vimalkumar, Front. Endocrinol. (Lausanne). 12 (2021) 1-19.
-

- 29. World Health Organization. Determination of airborne fibre number concentrations: a recommended method, by phase-contrast optical microscopy (membrane filter method). (1997) 1-53.
- 30. J.L. Pauly, S.J. Stegmeier, H.A. Allaart, R.T. Cheney, P.J. Zhang, A.G. Mayer, R.J. Streck, Cancer Epidemiol. Biomarkers Prev. 7 (1998) 419-428. (accessed September 30, 2022).
- 31. J. Cortez Pimentel, R. Avila, A. Galvao Lourenco, Thorax. 30 (1975) 204-219.
-

- 32. W.L. Eschenbacher, K. Kreiss, M.D. Lougheed, G.S. Pransky, B. Day, R.M. Castellan, Am. J. Respir. Crit. Care Med. 159 (1999) 2003-2008.
-

- 33. P.J.A. Borm, D. Höhr, Y. Steinfartz, I. Zeitträger, C. Albrecht, Inhal. Toxicol. 12 (2000) 225-231.
-

- 34. T. Kubo, K. Sawada, K. Hirakawa, C. Shimizu, T. Takamatsu, Y. Hirasawa, J. Biomed. Mater. Res. 45 (1999) 363-369.
-

- 35. H.G. Willert, M. Semlitsch, L.F. Peltier, Clin. Orthop. Relat. Res. 333 (1996) 4-14.
-

- 36. C. Pironti, M. Ricciardi, O. Motta, Y. Miele, A. Proto, L. Montano, Toxics. 9 (2021) 224.
-

- 37. V. Sukiene, N. Von Goetz, A.C. Gerecke, M.I. Bakker, C.J.E. Delmaar, K. Hungerbühler, Environ. Sci. Technol. 51 (2017) 3269-3277.
-

- 38. V. Linares, M. Bellés, J.L. Domingo, Arch. Toxicol. 89 (2015) 335-356.
-

- 39. C.N. Perez, F. Carré, A. Hoarau-Belkhiri, A. Joris, P.E.G. Leonards, M.H. Lamoree, J. Environ. Chem. Eng. 10 (2022) 107421.
-

- 40. M. Eriksen, L.C.M. Lebreton, H.S. Carson, M. Thiel, C.J. Moore, J.C. Borerro, F. Galgani, P.G. Ryan, J. Reisser, PLoS One. 9 (2014) e111913.
-

- 41. L.C. Woodall, A. Sanchez-Vidal, M. Canals, G.L.J. Paterson, R. Coppock, V. Sleight, A. Calafat, A.D. Rogers, B.E. Narayanaswamy, R.C. Thompson, R. Soc. Open Sci. 1 (2014) 140317.
-

- 42. A.H. Iri, M.H.A. Shahrah, A.M. Ali, S.A. Qadri, T. Erdem, I.T. Ozdur, K. Icoz, Environ. Sci. Pollut. Res. 28 (2021) 63860-63866.
-

- 43. Y. Li, J. Yao, P. Nie, X. Feng, J. Liu, Chemosphere. 276 (2021) 128696.
-

- 44. F. Watteau, M.F. Dignac, A. Bouchard, A. Revallier, S. Houot, Front. Sustain. Food Syst. 2 (2018) 81.
-

- 45. E. Dümichen, A.-K. Barthel, U. Braun, C.G. Bannick, K. Brand, M. Jekel, R. Senz, Water Res. 85 (2015) 451-457.
-

- 46. M. Lapointe, J.M. Farner, L.M. Hernandez, N. Tufenkji, Environ. Sci. Technol. 54 (2020) 8719-8727.
-

- 47. B. Ma, W. Xue, C. Hu, H. Liu, J. Qu, L. Li, Chem. Eng. J. 359 (2019) 159-167.
-

- 48. M.T. Sturm, H. Horn, K. Schuhen, Water. 13 (2021) 675.
-

- 49. M. Rajeshwaran, B. Shreeram, T. Thangeeswari, D. Elil Raja, J. Ceram. Process. Res. 23 (2022) 459-465.
-

- 50. Z. Ge, S. Li, Y. Wu, Y. Sha, J. Sun, J. Tian, J. Ceram. Process. Res. 23 (2022) 685-693.
-

- 51. R. Souag, N.E. Kamel, D. Moudir, Y. Mouheb, F. Aouchiche, J. Ceram. Process. Res. 23 (2022) 304-311.
-

- 52. P. Satishkumar, N. Natarajan, J. Ceram. Process. Res. 23 (2022) 383-390.
-

- 53. S.D. Yudanto, S.A. Chandra, R. Roberto, D.P. Utama, V.O. Herlina, Lusiana, J. Ceram. Process. Res. 23 (2022) 287-291.
-

- 54. F. Zhan, H. Zhang, K. Xv, M. Zhu, Y. Zheng, P. La, J. Ceram. Process. Res. 23 (2022) 694-708.
-

- 55. B. Fryczkowska, L. Przywara, Desalin. WATER Treat. 214 (2021) 252-262.
-

- 56. A.R.P. Pizzichetti, C. Pablos, C. Álvarez-Fernández, K. Reynolds, S. Stanley, J. Marugán, Case Stud. Chem. Environ. Eng. 3 (2021) 100075.
-

- 57. L. Li, G. Xu, H. Yu, J. Xing, Sci. Total Environ. 627 (2018) 332-340.
-

- 58. M.E. Ersahin, Y. Tao, H. Ozgun, J.B. Gimenez, H. Spanjers, J.B. van Lier, J. Memb. Sci. 526 (2017) 387-394.
-

- 59. M. Lares, M.C. Ncibi, M. Sillanpää, M. Sillanpää, Occurrence, Water Res. 133 (2018) 236-246.
-

- 60. J. Talvitie, A. Mikola, A. Koistinen, O. Setälä, Water Res. 123 (2017) 401-407.
-

- 61. F. Liu, N. Nord, K. Bester, J. Vollertsen, Water. 12 (2020) 1085.
-

- 62. S. Kim, A. Sin, H. Nam, Y. Park, H. Lee, C. Han, Chem. Eng. J. Adv. 9 (2022) 100213.
-

- 63. T.J. Suhrhoff, B.M. Scholz-Böttcher, Mar. Pollut. Bull. 102 (2016) 84-94.
-

- 64. X. Liu, P. Sun, G. Qu, J. Jing, T. Zhang, H. Shi, Y. Zhao, J. Hazard. Mater. 407 (2021) 124836.
-

- 65. R. Zafar, S.Y. Park, C.G. Kim, Environ. Eng. Res. 26 (2020) 200412-0.
-

- 66. H. Hidayaturrahman, T.-G. Lee, Mar. Pollut. Bull. 146 (2019) 696-702.
-

- 67. I. Nabi, A.-U.-R. Bacha, K. Li, H. Cheng, T. Wang, Y. Liu, S. Ajmal, Y. Yang, Y. Feng, L. Zhang, IScience. 23 (2020) 101326.
-

- 68. A. Uheida, H.G. Mejía, M. Abdel-Rehim, W. Hamd, J. Dutta, J. Hazard. Mater. 406 (2021) 124299.
-

- 69. T.S. Tofa, F. Ye, K.L. Kunjali, J. Dutta, Catal. 2019, Vol. 9, Page 819. 9 (2019) 819.
-

- 70. K.B. Sundbæk, I.D.W. Koch, C.G. Villaro, N.S. Rasmussen, S.L. Holdt, N.B. Hartmann, J. Appl. Phycol. 30 (2018) 2923-2927.
-

- 71. M. Padervand, E. Lichtfouse, D. Robert, C. Wang, Environ. Chem. Lett. 18 (2020) 807-828.
-

- 72. T.M. Nolte, N.B. Hartmann, J.M. Kleijn, J. Garnæs, D. van de Meent, A. Jan Hendriks, A. Baun, Aquat. Toxicol. 183 (2017) 11-20.
-

 This Article
This Article
-
2022; 23(6): 934-945
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.934
- Received on Sep 1, 2022
- Revised on Oct 2, 2022
- Accepted on Oct 16, 2022
 Services
Services
- Abstract
introduction
sources of microplastics
environmental health effects
quantification of microplastics
microplastics removal technologies
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Sung Chul Yi a,b and Byoung-In Sang a,b
-
aDepartment of Chemical Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
bClean-Energy Research Institute, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
Tel : +82-2-2220-0481 (Prof. Yi), +82-2-2220-2328 (Prof. Sang)
Fax: +82-2-2298-5147 (Prof. Yi), +82-2-2220-4716 (Prof. Sang) - E-mail: scyi@hanyang.ac.kr, biosang@hanyang.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.