- Improving anti-glare properties for display glass of soda-alumino-silicate by two steps etching method
Heesu Woo and Seunggu Kang*
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Glass for display should show a low glare phenomenon in order to transmit characters and images clearly and quickly. In this study, after primary and secondary etching of the surface of the soda-alumino-silicate glass with buffered oxide etchant (BOE) and HF solution, the anti-glare properties of glass with the etchant concentration were analyzed. The rate of change of optical properties for the A-series specimens prepared with variable concentrations of the BOE solution in the primary etching step was larger than that of the B-series specimens obtained with the HF solution of varying concentrations in the second etching step. A protrusion with a size of about 25 nm was generated on the surface of the etched specimen, and the size increased with the etchant concentration. The protrusions created on the surface scatter and diffuse light incident on the glass, thereby lowering the reflectance and improving the anti-glare properties, while lowering the transparency, gloss, and haze properties of the glass.
The reflectance, transmittance, haze, and gloss values of the A-series specimens were 1.8-2.0%, 85%, 6.6-6.9%, and 95-103 GU, respectively; the values of the B-series specimens were 2.2%, 86-91%, 7.0-7.2%, and 103 GU, respectively. As a result of this study, the optimal etchant concentration condition to minimize the loss of other optical properties while suppressing the glare of glass was 16 to 17% of the BOE concentration in the A-series specimen, whereas the HF concentration of 10 to 11% in the B-series was confirmed
Keywords: Display glass, Glare, Acid etching, Protrusion, Reflectance
The readability of display glass is the ability to convey text and images clearly and quickly. Recently, as various display markets such as portable display devices, UV-blocking glass, and vehicle LCD expand, high readability is required for display glass [1-3]. The glare phenomenon refers to a phenomenon in which light incident on a glass surface is strongly reflected from the surface and annoys an observer's eyes. The glare phenomenon is one of the biggest causes of deterioration of the readability of display glass. In order to suppress the glare phenomenon of the display glass, therefore, it is necessary to reduce the amount of reflected light from the glass surface [4].
One of the methods of suppressing the glare phenomenon of display glass is to use an anti-reflective film of polymer material. However, the surface of the polymer film may be easily damaged or detached from the glass depending on external physical pressure or the passage of time, which deteriorates the readability of the glass [5-7].
Another way to enhance the anti-glare properties of display glass is to use a silicon or silica thin film etching process, which is currently being carried out in the semiconductor process [9, 10].
Methods used for thin film etching in the semiconductor process include dry ion etching and wet ion etching with chemical reactions. However, the dry etching method is not only disadvantageous in its low etching rate and high cost; but also has limitations in application to large-area specimens [11, 12].
On the other hand, wet etching has been mainly applied to mass production in ceramic processing due to its low cost and fast etching rate [3, 13-16]. In general, hydrofluoric acid is used in processing ceramic surfaces by wet etching [13]. Liu et al. [17] fabricated aggregated light-scattering particles in the AG coating layer in order to increase the outer haze, so to decrease the reflectance of the layer. D. J. Monk et al. [18] studied an etching mechanism for thin silicon dioxide films in hydrofluoric acid solutions; and analyzed the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications. K. Wen et al. [19] used a mixed solution of HF and H2SO4 for optimizing the test parameters of the leaching process for extracting gallium from a high-silica-content flue dust formed in corundum production.
Kim et al. [20] studied the leaching behavior of CaO-MgO-SiO2-Li2O glass ceramics as a function of the CaO/MgO ratio using an HF solution. Barboux et al. [21] researched depending parameters for smooth or frosted surfaces of glass or silica using hydrofluoric acid solutions. They found that the surface of frosted glass exhibits a peculiar morphology with a crystal-like aspect characterized by well-facetted lattice planes, and the frosting effect could be explained by the competition of two reactions; the dissolution of the silica network by hydrofluoric acid leads to the formation of H2SiF6. Also, they claimed that the precipitation of the weakly soluble ammonium fluorosilicate (NH4)2SiF6 forms a passivating layer that blocks the dissolution.
Putting these findings together, the mechanism of erosion of glass by hydrofluoric acid can be summarized as follows; hydrogen fluoride (HF) is most frequently used for glass etching. Fluoride ions (F-) released from dissociation in HF react with SiO2, the main compound in display glass, to form crystalline compounds containing SiO2. A part of the crystal phase generated is decomposed into SiF62- and 2H+ ions. At this time, the dissociated metal ion M+, one of the components of the glass, reacts with SiF62- to form various crystal phases in the form of M2SiF6.
The formed M2SiF6 compound covers a part of the glass surface, and unlike glass components, these compounds are not soluble in HF solution or water. Therefore, the surface not covered by M2SiF6 is continuously etched by the HF solution, while the covered portion remains on the glass surface, forming irregular and uneven projections. The surface protrusions formed in this way have a great influence on the viewer's recognition of characters or images transmitted through the display according to their size or arrangement [18, 22-24].
The protrusion created on the glass surface lowers the reflectance of the glass and suppresses the glare phenomenon, but it is accompanied by deterioration of transparency, gloss, and haze characteristics, so it is inevitable to adjust the trade-off between these optical characteristics. In this experiment, therefore, irregular protrusion patterns were formed on the glass surface through a wet etching process, and the effect of these patterns on the anti-glare properties of the glass was analyzed. In particular, in this experiment, the primary etching was performed using a buffered oxide etchant (BOE) solution, which is mainly used in the semiconductor etching process, and then the secondary etching was performed with an HF solution to effectively control the anti-glare properties of the glass. While most of the previous papers analyzed the anti-glare properties with one cleaning process, this paper improved the anti-glare properties of glass substrates by using two types of etchants and concentrations as variables as well as through two cleaning processes.
In this experiment, the glass surface was etched using a buffered oxide etchant (BOE) solution. The composition of the BOE solution was NH4F: HF: H2O = 36: 15: 49 (vol %). In this experiment, distilled water was added to the BOE solution to make the concentration of 15 to 18%. The glass substrate used was ‘G’ glass 3 (Corning Co.) of a square shape having a thickness of 1 mm and a side of 30 mm. The XRF analysis of the chemical composition of the glass substrate showed SiO2 61.8%, Al2O3 16.9%, Na2O 12.8%, and alkali and alkaline earth metal oxides such as MgO, K2O, and CaO were 8.5% in total, indicating that the glass was a typical soda-alumino-silicate.
After holding the tip of the substrate with Teflon tongs for etching the glass substrate, the substrate was completely immersed in the etching solution filled in the ultrasonic cleaner. Etching was primarily performed with BOE solution for 10 minutes, washed with distilled water, and secondly performed with HF solution for 10 minutes. The specimens prepared by changing the concentration of the BOE solution in the primary etching step were called A-series, and the specimens etched by HF solution of varying concentration in the second etching step were called B-series. The reason for performing the etching twice is that; it was difficult to control various optical properties integrally with only one etching in the preliminary experiments [25]
.
For A-series specimens, the concentration of BOE solution was changed to 16-18% in the primary etching step, while in the second etching step, the concentration of HF solution was fixed at 10%. For B-series specimens, the concentration of BOE solution was fixed at 15% in the primary etching step, but the concentration of HF solution was changed to 6-15% in the second etching step.
Measurement of haze and gloss of the etched glass was performed with a Glossmeter (Rhopoint), and reflectance and light transmittance were obtained in the visible light wavelength using a spectrophotometer (UV-VIS, Cary 5000, Varian). The roughness of the surface was measured using atomic force microscopy (XE150, PSIA).
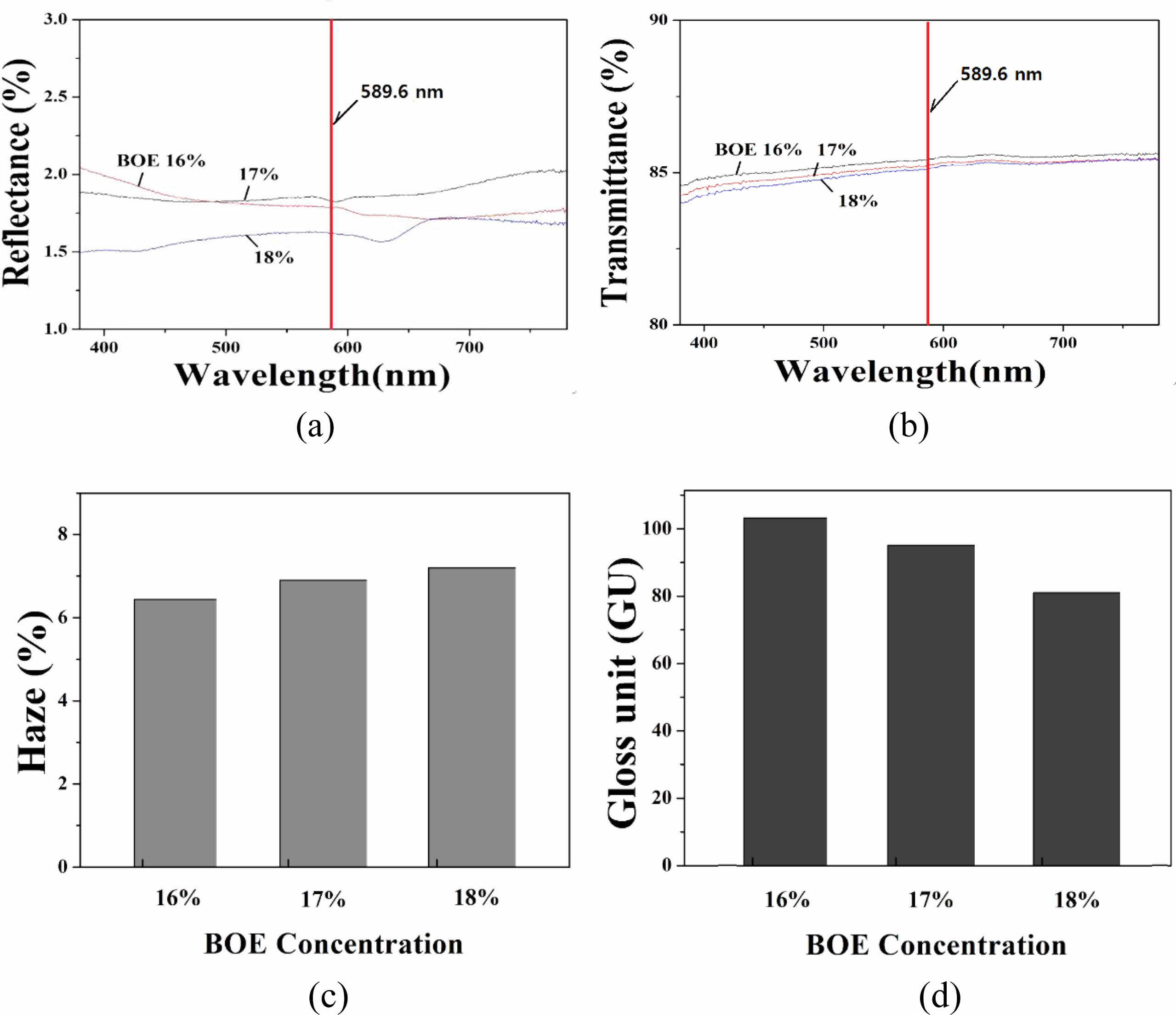
The measurement results of reflectance, transmittance, haze, and gloss for A-series specimens are shown in Fig. 1. When glass is etched with a solution containing F- ions, the eluted glass components, and F- ions generally react to form various crystalline phases, which accumulate on the surface of the specimen to form protrusions. When the etching time elapses, the etching continues in the portion where the protrusions do not occur, and the surface roughness increases.
Due to these phenomena, the glass surface diffuses and scatters incident light, thereby changing the various optical properties of the glass. First, lower the reflectance of the glass. Since the glare phenomenon is the reflection of harsh and dazzling light from the display glass, the anti-glare characteristic must be lowered first. At the same time, the transparency and gloss of the glass are lowered by the etching process, and the haze is increased, thereby reducing the original advantages of the glass.
In Fig. 1, the reflectance tends to decrease with the concentration of BOE solution in the primary etching step. When the concentration of the BOE solution was 16 to 17%, the reflectance showed a value of 1.75 to 1.70% for a light of 589.6 nm (sodium D line), but when the concentration was 18%, the reflectance was lowered to 1.60%. The transmittance of all specimens showed a high value of 85% or more for the light of 589.6 nm. As the concentration of the BOE solution increased, the transmittance decreased, but the rate of change was not significant. As the concentration of the BOE solution increased, the haze value of the etched glass increased and the gloss decreased. Gloss is a value indicating the degree of brightness of a smooth and shiny glass surface.
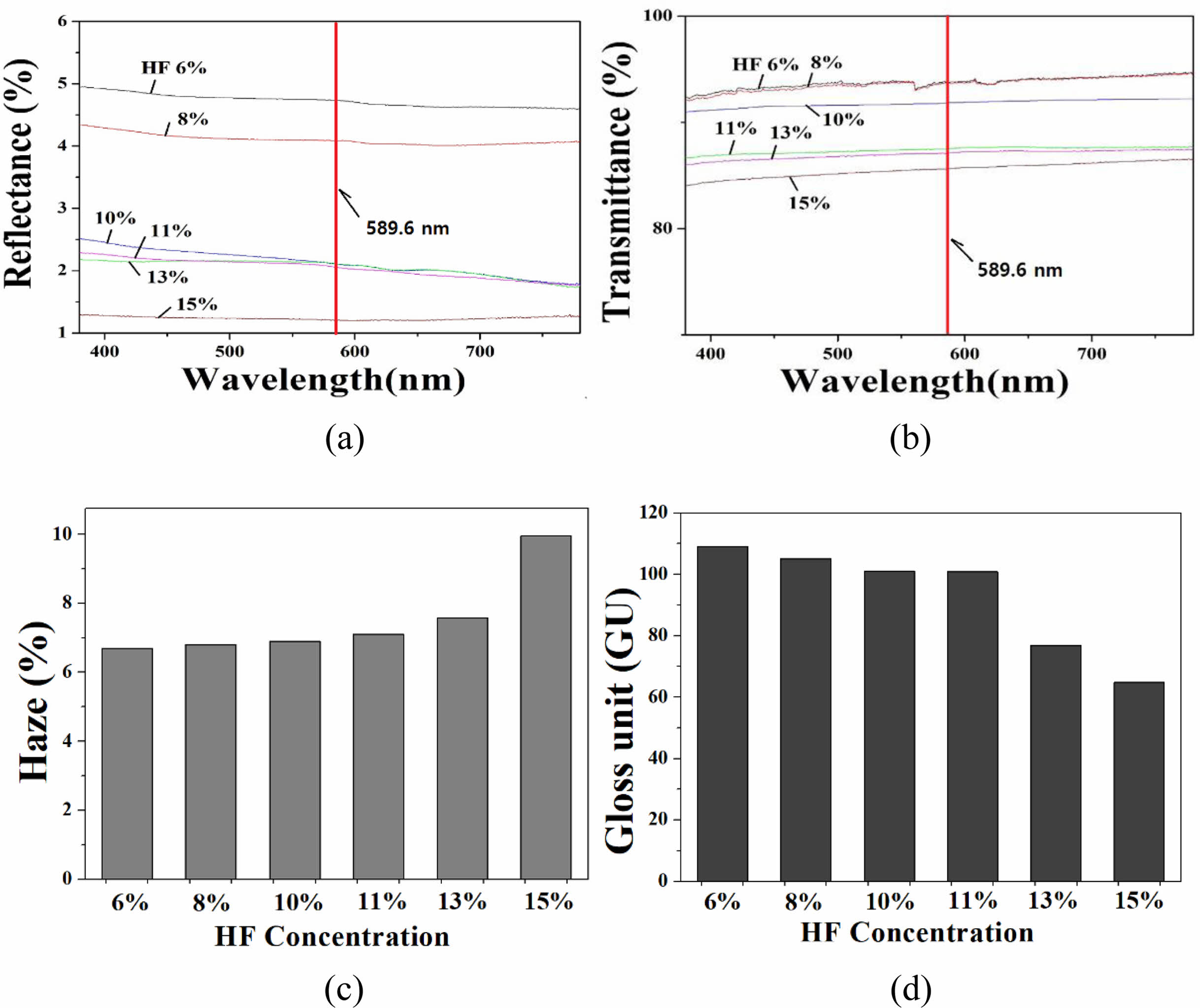
The measurement results of reflectance, transmittance, turbidity; and gloss for B-series specimens are shown in Fig. 2. The reflectance decreased with increasing HF solution concentration; when the HF concentration was 6%, the reflectance was 4.7% at the light of 589.6 nm wavelength, but when the concentration was 15%, it was lowered to 1.2%.
The transmittance showed a tendency to gradually decrease as the HF concentration increased: at 6% HF, the transmittance of light with a wavelength of 589.6 nm was 94%, but at 15% HF, it was lowered to 86%. The transmittance of the B-series specimen group all showed a high value of 86% or more. The haze value showed a tendency to increase as the HF etching concentration increased: the haze value was 6.6% at 6% HF, but 10.0% when etched with HF 15%. The gloss showed a tendency to decrease as the concentration of HF increased: the gloss was 109 GU when the concentration of HF was 6%; and decreased to 65 GU when the concentration was 15%.
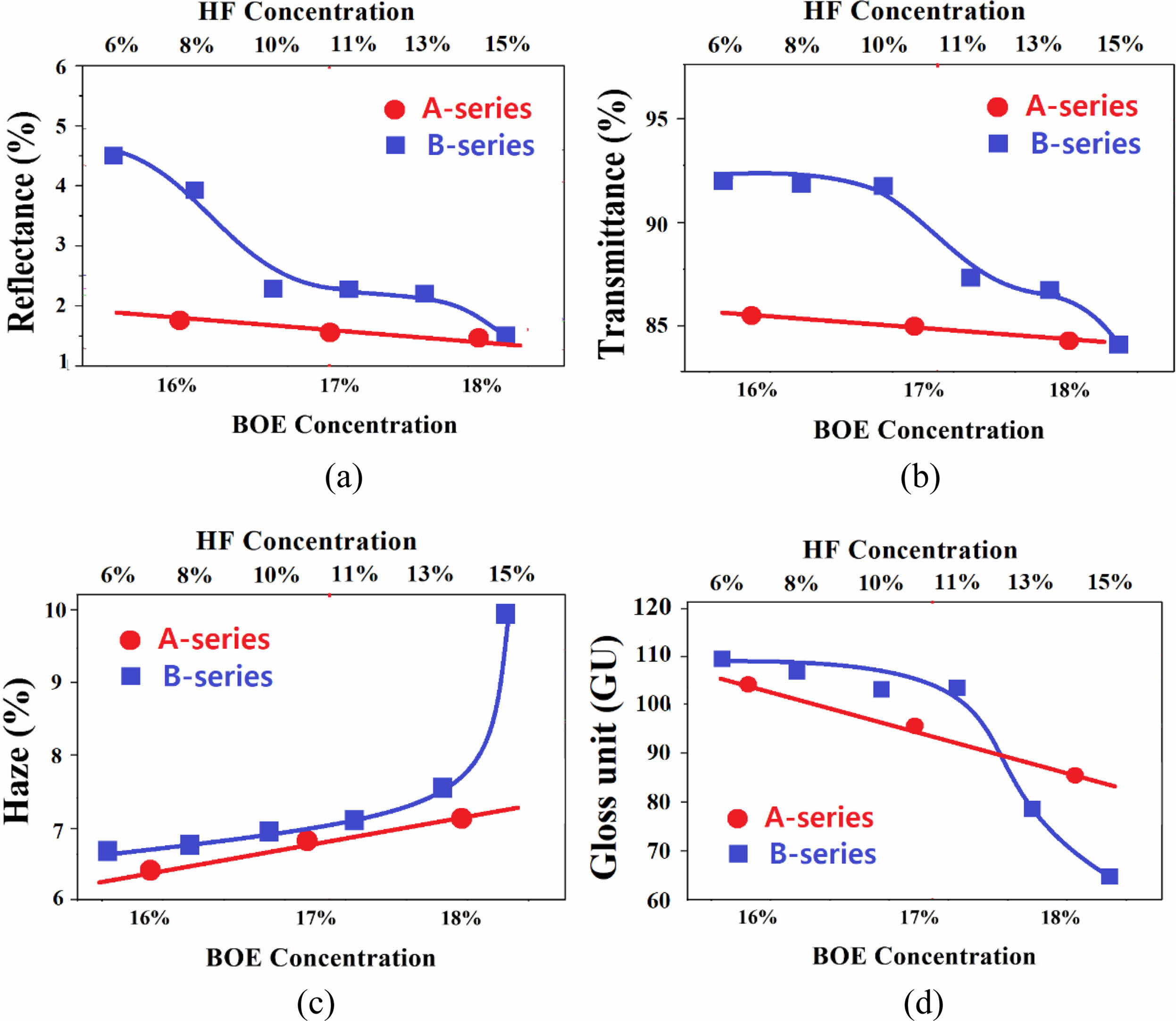
Fig. 3 shows a comparison of the rate of change of optical properties of A- and B-series specimens. As a result, the optical property change rate was larger in the B-series specimens controlling the HF concentration than in the A-series controlling the BOE concentration. This difference is judged by the difference in the degree of surface morphology formed by the HF and BOE solutions and the difference in the surface crystal phase. As described above, etching suppresses glare by lowering the reflectance of the glass, but it is accompanied by a decrease in transparency, an increase in the haze, and a decrease in gloss. Therefore, in selecting the etching conditions showing the optimal anti-glare properties, it is inevitable to adjust the trade-off between these optical properties.
The A-series specimens showed better anti-glare properties as the reflectance was lower than that of the B-series specimens in the BOE concentration range of 16 to 18%. Also, the haze increasing and gloss decreasing rate with the BOE concentration of A-series specimens was smaller than that of the B-series specimens, making it relatively easy to control the properties. On the other hand, B-series specimens etched with HF 11-13% concentration showed appropriate anti-glare properties. In particular, B-series has the advantage that the gloss value is generally higher than that of the A-series specimens. However, the haze of the B-series is still higher than that of the A-series specimens, so a solution is needed from a process point of view.
In the BOE solution, NH4F is present in addition to HF, which contains F- ions that can etch the glass. Since ions of H+ and NH4+ exist in addition to F- ions in these complex solutions, the number of ions and ion activity are considered to have an effect on the etching rate, the size, and distribution of the protrusions, and the protrusion formation rate [26-29]. Although the rate of change of optical properties was larger in the B-series specimens than in the A-series in this study, accurate analysis of this result requires additional research on the factors mentioned above.
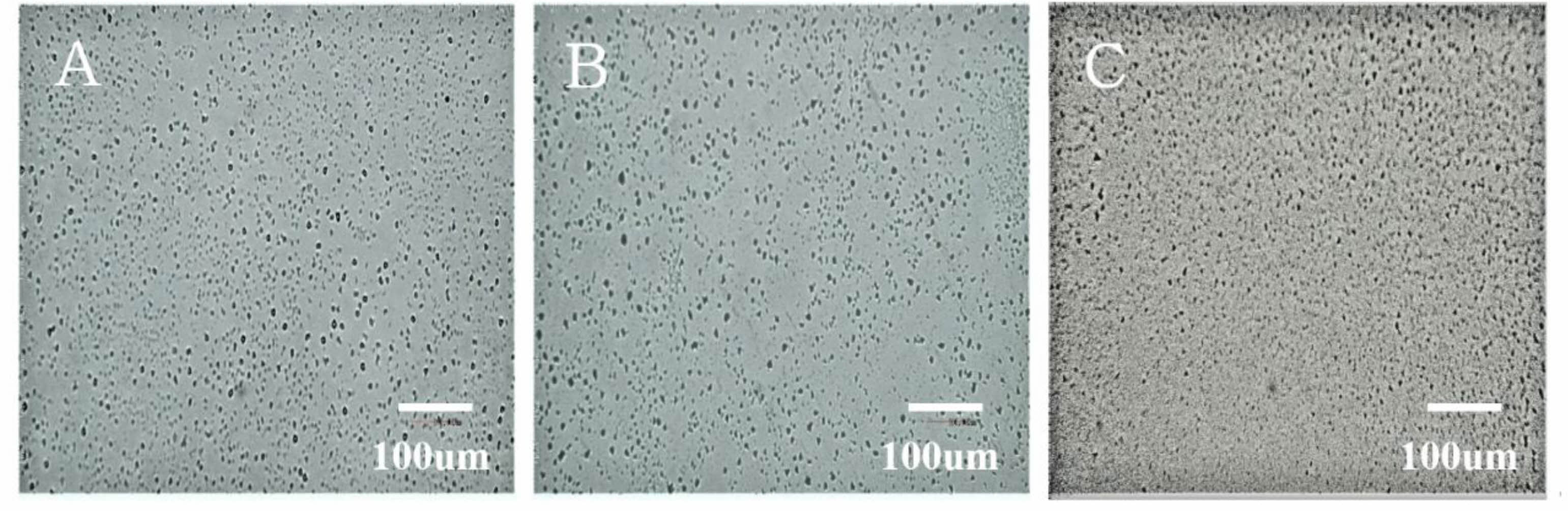
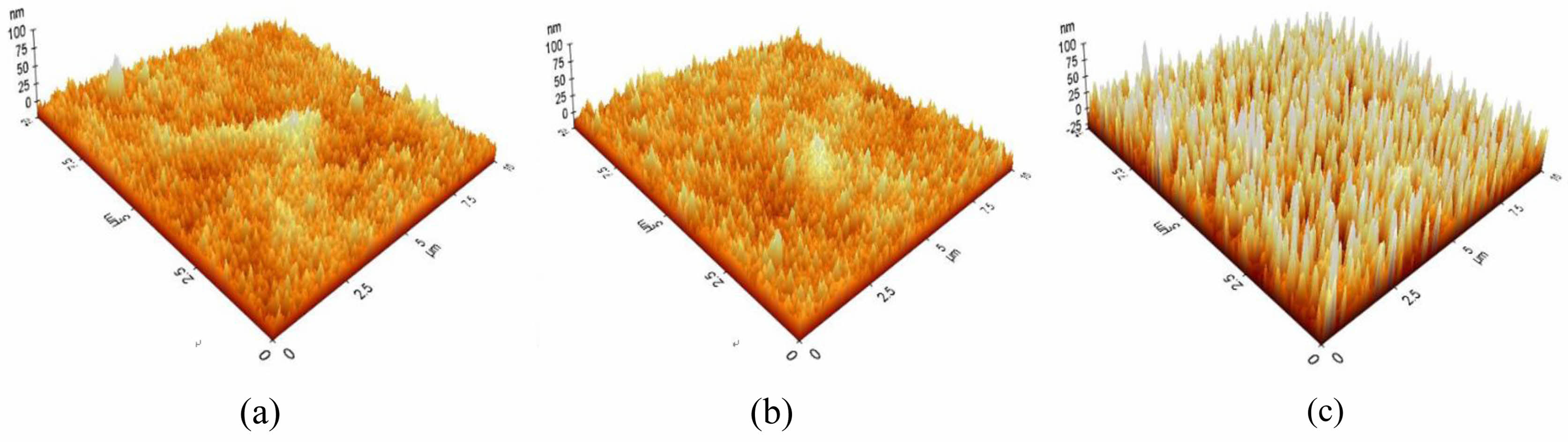
The results of observation of the surface microstructure of the A-series specimen with an optical microscope are shown in Fig. 4. As the concentration of the BOE solution increased, the number of uneven protrusions on the surface increased. The roughness of the etched A-series specimen measured by AFM is shown in Fig. 5. There were many protrusions with a height of 25 nm on the surface of the BOE 16% specimen. It can be seen that the size and height of the protrusions increased with the concentration of the BOE solution, and the height of the protrusions grew to about 70 nm in the BOE 18% etched specimen. It was confirmed that the formation of these protrusions lowered the reflectance of the glass and expressed anti-glare properties.
The photographs of the exterior building seen through the B-series glass manufactured with two different etchant concentrations are shown in Fig. 6. As shown in Fig. 3, the specimen etched with HF 15% concentration had a very low reflectance value of 1.2%, but haze was very high at 9.9%, the gloss was as low as 65 GU, and transparency was also the lowest among all etched specimens at 84% due to heavy etching. When an observer takes a picture by placing the specimen in front of the camera lens and looking at the building, as in picture (b), the building would be appeared blurry.
On the other hand, the specimen etched with HF 15% concentration had the reflectance of rather high at 2.2%, and the haze of not high at 6.8%. Allow the gloss was high at 103 GU, and the transmittance was also very high at 92%. If you look at the building through this specimen, the landscape of the building looks very clean as in photo (a).
In this study, two ions NH4+ and H+ ions were used in combination to etch glass and improve anti-glare properties; nevertheless, experiments on the activity and concentration of each of the two cations have not been conducted. We think, however, that these factors are important in forming protrusions on the glass surface. Therefore, a quantitative analysis is required through additional experiments in the future.
|
Fig. 1 Optical properties of A-series specimen : (a) reflectance (b) transmittance (c) haze and (d) gloss. The concentration of BOE solution varied in the range of 16-18% in primary etching step. |
|
Fig. 2 Optical properties of B-series specimen : (a) reflectance (b) transmittance (c) haze and (d) gloss. The concentration of HF solution varied in the range of 16-18% in secondary etching step. |
|
Fig. 3 The rate change of optical properties of A- and B-series specimens as a function of etchant concentration. |
|
Fig. 4 hotographs of the surface for A-series specimen taken with an optical microscope. The concentration of BOE solution is; (a) 16% (b) 17% and (c) 18%. |
|
Fig. 5 AFM images showing surface roughness of A-series glass etched as a function of BOE concentration; (a) 16% (b) 17% (c) 18%. |

|
Fig. 6 View of the building observed through the B-series glass. (a) HF 10% (haze = 6.8%), (b) HF 15% (haze = 9.9%). |
In this study, to improve the anti-glare properties of soda-alumino-silicate glass, the glass surface was etched by two steps with HF and buffered oxide etchant (BOE) solution, and the optical and surface properties of the glass were analyzed accordingly. A protrusion with a size of about 25 nm was generated on the surface of the etched specimen, and the size increased with the etchant concentration. Due to the generated protrusions, the reflectance of the glass was reduced, but the transparency, gloss, and haze properties deteriorated.
The rate of change of optical properties of the A-series specimens in which the concentration of the BOE solution was varied in the primary etching step was larger than that of the B-series specimens etched with HF solution of varying concentration in the secondary etching step. This difference is thought to be due to the effect of the concentration and activity of various ions from the BOE solution on the etching rate, the protrusion formation rate, and the size and distribution of the formed protrusions. A more definitive analysis requires further studies.
The reflectance, transmittance, haze, and gloss values of the A-series specimens prepared in this study were 1.8-2.0%, 85%, 6.6-6.9%, and 95-103 GU, respectively, and for the B-series specimens, 2.2%, 86-91%, 7.0-7.2%, and 103 GU respectively. Etching conditions with excellent anti-glare properties were BOE concentration of 16-17% in A-series and HF concentration of 10-11% in B-series. If the haze value can be further reduced through precise surface morphology control for glass specimens in the future, the etched glass fabricated in this way can be applied to smart display materials in various fields.
This work was supported by Kyonggi University Research Grant 2019.
- 1. S. Kim, J. Lee, and B. Jeon, J. Korean Inst. Electr. Electron. Mater. 30[12] (2017) 800-805.
-

- 2. H. Yu, C. Hsiao, and W. Liu, Measure. Sci. Tech. 17[8] (2006) 29-36.
-

- 3. S. Triana, Kusumandari, and R. Suryana, J. Phys. : Conference Series, 776 (2016) 1-4.
-

- 4. J. Souk, S. Morozumi, F. Luo, and I. Bita, in “Flat Panel Display Manufacturing” (John Wiley & Sons Press, 2018) p. 1.
-

- 5. S. Kim, J. Hwang, and B. Jeon, J. Korean Inst. Elec. Electron. Mater. Eng. 28[9] (2015) 607-614.
-

- 6. F. Ezz-Eldin, T. Abd-Elaziz, and N. Elalaily, J. Mater. Sci. 45 (2010) 5937-5949.
-

- 7. L. Wong, T. Suratwala, M. Feit, P. Miller, and R. Steele, J. Non-Cryst. Solids 355[13] (2009) 797-810.
-

- 8. H. Prokschel and G. Nagorsen, J. Electrochem. Soc. 139 (1992) 521-524.
-

- 9. G. Spierings, J. Mater. Sci. 28 (1993) 6261-6273.
-

- 10. H. Park, D. Kim, J. Jung, D. Pham, A. Le, J. Cho, S. Hussain, and J. Yi, Heliyon 4[10] (2018) 835-836.
-

- 11. D. Shin and J. Eo, J. Ceram. Process. Res. 6[4] (2005) 345-350.
- 12. T. Kim, K. Leea, and Y. Junga, Y. Kima, H. China, H. Yoonb, J. Kangb, and B. Ryua, J. Ceram. Process. Res. 10[6] (2009) 848-850.
- 13. J. Kim, M. Lee, T. Lim, J. Hwang, E. Kim, and S. Kim, J. Ceram. Process. Res. 11[2] (2010) 259-262.
- 14. H. Lee, B. Kim, Y. Kim, M. Kim, S. Kim and S. Lee, J. Ceram. Process. Res. 21[5] (2020) 547-551.
-

- 15. D. Lee, Y. Pyun, K. Son, J. Choung, J. Lee, S. Son and W. Park, J. Ceram. Process. Res. 10[1] (2009) 1-5.
- 16. H. Lee, B. Kim, Y. Kim, M. Kim, S. Kim, and S. Lee, J. Ceram. Process. Res. 21[5] (2020) 547-551.
-

- 17. B. Liu and Y. Teng, J. Colloid Interf. Sci. 350[2] (2010) 421-426.
-

- 18. D. Monk, D. Soane, and R. Howe, Thin Solid Films, 232[1] (1993) 1-12.
-

- 19. K. Wen, F. Jiang, X. Zhou, and Z. Sun, T. Nonferr. Metal. Soc. 28[9] (2018) 1862-1868.
-

- 20. Y. Kim and S. Kang, J. Ceram. Process. Res. 14[2] (2013) 172-175.
- 21. P. Barboux, A. Laghzizil, Y. Bessoles, H. Deroulhac and G.Trouvé, J. Non-Cryst. Solids 345-346 (2004) 137-141.
-

- 22. W. Chen, X. Sun, S. Wang, S. Lee, and B. Teo, J. Phys. Chem. B, 109 (2005) 10871-10879.
-

- 23. N. Piret, R. Santoro, L. Dogot, B. Barthélemy, E. Peyroux, and J. Proost, J. Non-Cryst. Solids 499 (2018) 208-216.
-

- 24. H. Totsuka, I. Sato, H. Yamamoto, and M. Anma, J. Soc. Inf. Display 10 (2002) 381-387.
-

- 25. H. Woo, J. Kim, and S. Kang, J. Nanosci. Nanotechno. 21[3] (2021) 1937-1942.
-

- 26. H. Park, J. Cho, J. Jung, P. Duy, A. Le and J. Yi, J. Korean PVs. Soc. 5 (2017) 75-82.
- 27. J. An, Y. Shi, Z. Liu, R. Cui, T. Sun, T. Chen, J. Wang, X. Xu, J. Wang, J. Huang, X. Li, and C. Wu, in Proceedings of ISES World Congress, May 2007, edited by M. Anzeigen (Springer Berlin Heidelberg Press, 2007) p. 1051.
-

- 28. Y. Tiec, J. Rigaudiere, and C. Pizzetti, in “Cleaning Technology in Semiconductor Device Manufacturing VI” (The Electrochemical Society, Inc., 1999) p. 377.
- 29. J. Ikhmayies, J. Energ. Syst. 1[3] (2017) 120-128.
-

 This Article
This Article
-
2022; 23(6): 862-868
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.862
- Received on May 26, 2022
- Revised on Nov 30, 2022
- Accepted on Dec 2, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seunggu Kang
-
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon, Korea
Tel : +82-31-249-9767 Fax: +82-31-249-9774 - E-mail: sgkang@kgu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.