- Influence of graphene oxide on the heat resistance of high strength concrete
Guangming Meng, Yuwu Sui*, Shu Liu, Qingbo Tian, Xinling Cui and Yuejian Wu
School of Materials Science and Engineering of Shandong Jianzhu University, Jinan 250101, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The effect of Graphene Oxide (GO) on the thermal properties of high-strength concrete has been less studied. The mechanical properties, compactness, mineral composition and microstructure of the high-strength concrete with GO addition were examined by heat treatments at different temperatures. The results showed that the enhancement of the residual flexural strength with GO addition was greater than that of the residual compressive strength; GO addition had a significant effect on the mechanical properties of concrete treated at less than 500 ℃; Differential Scanning Calorimeter (DSC) test showed that the high-strength concrete with GO addition required more heats for its decomposition after 500 ℃; The internal pores/cracks of the high strength concrete with GO addition were reduced after the heat treatment at a high-temperature because the producing of new crystals can improve the residual properties of the high-strength concrete
Keywords: Graphene oxide, Compactness test, High strength concrete, Heat resistance
Concrete is a main preparation material for current buildings and has good mechanical properties, but it has structural defects and durability problems [1]. In addition to this, the heat resistance property of concrete is also required paying attention due to the high frequency of building fires.
Concrete undergoes a series of physico-chemical reactions as the temperature increases. After 60 °C~90 °C treatment of cementite, the internal free water evaporates and the hydrated calcium silicate gel (C-S-H) goes through a polymerization and a lattice shrinkage [2, 3]; at about 100 °C ettringite becomes dehydrated and decomposes [4]; at 100 °C~170 °C, gypsum begins to decompose and physically bound water begins to evaporate [5]; at 180 °C, C-S-H dissociates [6]; at 200 °C~300 ℃, the chemically adsorbed water inside the caement commences to evaporate [7]. At about 500 °C, calcium hydroxide decomposition into calcium oxide and water happens [8,9]. At 600 °C~750 °C, C-S-H is decomposed to produce β-C2S [3]; at about 750 °C, calcite decomposes and C-S-H begins to decompose and gradually disappears [10]. There are fewer studies on the thermal performance of concrete. Most methods to improve the heat resistance of concrete are addition inorganic substances in concrete [11, 12].
Graphene oxide (GO) is widely used as a new two-dimensional nanomaterial with good hydrophilicity and surface activity due to its surface containing carboxyl [13-15], hydroxyl and epoxy groups. The main preparation method of GO is the Hummers method, which has a short preparation time and a high degree of oxidation [16]. GO has shown good thermal properties in other materials [17, 18], while GO research on concrete has mostly focused on mechanical properties [19-21], with less research on thermal properties.
High-strength concrete is sensitive to temperature due to its dense structure and high strength, and is prone to crumbling at high temperatures, for which heat resistance is an important property [22]. To study the effect of GO on the heat resistance of high-strength concrete, mineral admixtures and GO were added to mortar in certain proportions to prepare high-strength concrete, what’s more, it was thermal treated at 250 ℃, 500 ℃, 750 ℃ and 900 ℃, then, the effect of GO on its heat resistance properties was tested by X-ray Diffractometer (XRD), DSC, Thermogravimetric Analysis (TGA), Scanning Electron Microscope (SEM), Ultrasonic Non-Destructive Testing.
Materials
The GO used in the experiment is a monolayer powder produced by KNA Carbon New Materials Co, Ltd. with earthy brown color, its flake diameter is between 0.2 μm and 10 μm, its purity is 96%, its main chemical compositions contain 48% carbon, 45% oxygen and < 3% sulfur. The Fig. 1 shows the Fourier Transform Infrared Spectrometer (FTIR) spectra of GO, where O-H, C-H, C=C groups at 3434.63, 2813.52, 1590.46 cm-1, respectively, which indicates that GO has high activity. Fig. 2
The high strength concrete was generated by cement, fly ash, mineral powder, sand, water and water reducing regent. P.O 42.5 cement produced by Shanshui Group, the primary fly ash produced by Henan Platinum Run Casting Materials Co., Ltd. with a density of 2.55 g/cm3 and a bulk density of 1.12 g/cm3, the S95 mineral powder produced by Henan Platinum Run Casting Materials Co., Ltd. with a density of 2.9 g/cm3 are utilized. The chemical compositions of the above materials are shown in the Table 1. The sand used is the standard sand produced by Xiamen Aisiou Standard Sand Co. Ltd. The water reducing agent is an ordinary polycarboxylic acid with water reduction rate of 25%~35%. The experimental water is tap water.
The prepared high-strength concrete was thermally treated at 250 ℃, 500 ℃, 750 ℃ and 900 ℃, then its mechanical properties, mineral composition and microstructure, etc. were tested.
Recipe of the high strength concrete
Referring to the concrete design standards and the results of the relevant literature, the test design water-cement ratio is 0.25, the matching ratio is shown in Table 2, the specific amount of each group of materials is shown in Table 3.
Test procedure and methods
Concrete preparation, fluidity and curing
GO has hydrophilic functional groups and is prone to agglomeration in water [23], so it cannot be directly added into the stirring mixer, and it is necessary to disperse GO in water by ultrasonic. The ultrasonic dispersion time and power will affect the dispersion of GO in water [20], and this experiment used a 100 W ultrasonic dispersion instrument with a water bath to disperse for 10 min. After dispersion, GO was immediately added to the planetary mixer, and the mixing was completed according to the national standard GB/T 17671-1999 “Test method for cementitious sand strength (ISO method)” [24].
The fluidity test was carried out by calculating the average value of the diameter in both directions through the fluidity test, the result was taken as an integer in millimeters. In accordance with the national standard GB/T 17671-1999 “cementitious sand strength test method (ISO method)” for curing [24], the temperature of water was kept at 20 ℃ ± 1 ℃, curing 28 d.
Heat resistance test
After the specimens were maintained in water for 28 d, the test cuboids were taken out, blown with a hair dryer until the surface was dry, and then put into an oven with the temperature set to 65 ℃ for drying 48 hours. After drying, the specimen block was put into a CNC high-temperature furnace and fired from room temperature to the test temperature (250 ℃, 500 ℃, 750 ℃, 900 ℃), then held for 30 min and 90 min, respectively. After the holding time, it was removed with crucible tongs and cooled naturally to room temperature. After being left for 1 d, subsequent performance tests were carried out.
Main tests
The infrared absorption spectrum of GO was tested with a Bruker TENSOR II Fourier Transform Infrared Spectrometer (FTIR). The heat (ΔQ and ΔH) variations of the samples were analyzed by Differential Scanning Calorimeter (DSC) model Q20. The thermal stability and component changes of the samples were studied with a simultaneous thermal analyzer Q600 (TGA). A Bruker D8 advance X-ray Diffractometer (XRD) was used to characterize the physical phase and crystal structure of the samples. The sample microscopic morphology and hydration products were analyzed by EVO 18 analytical Scanning Electron Microscope (SEM) from Zeiss, Germany. The compressive and flexural strengths of the specimens were tested with a Tianjin TYE-300D universal pressure tester. The results were obtained to calculate the average value, and the calculation was accurate to 0.1 MPa. The ZBL-U510 type non-metallic ultrasonic detector is used to detect cracks and internal structural defects and compactness of the test block. If certain parameters are judged to be abnormal (abnormal sound time, abnormal amplitude), the existence of uncompacted areas and cavities inside the concrete can be determined in conjunction with the waveform condition.
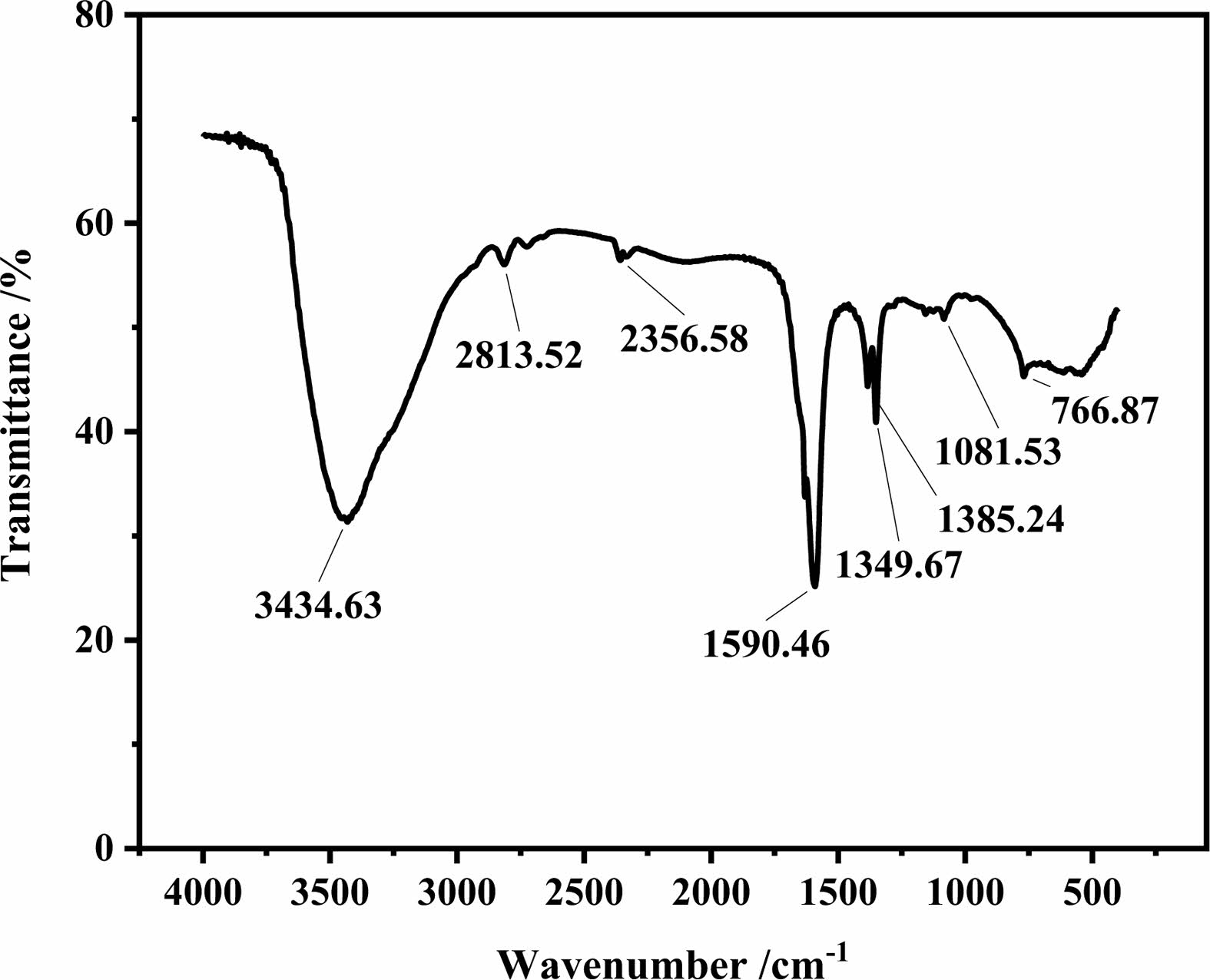
|
Fig. 1 FTIR spectrum of GO. |
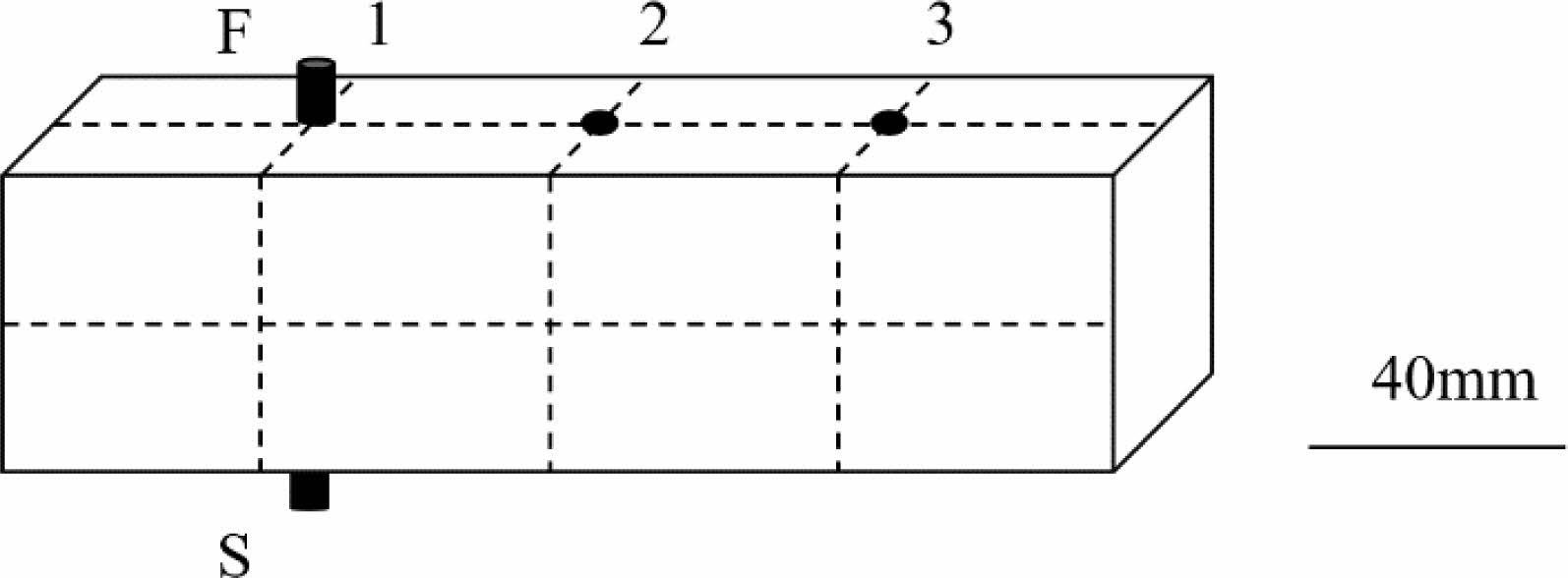
|
Fig. 2 Illustration of the compactness measure for specimen by a nonmetallic ultrasound detector (1-3 vertical direction; F-the transmitting transducer; S-the receiving transducer). |
|
Table 1 Composition analysis of cement, fly ash and mineral powder for test /%. |
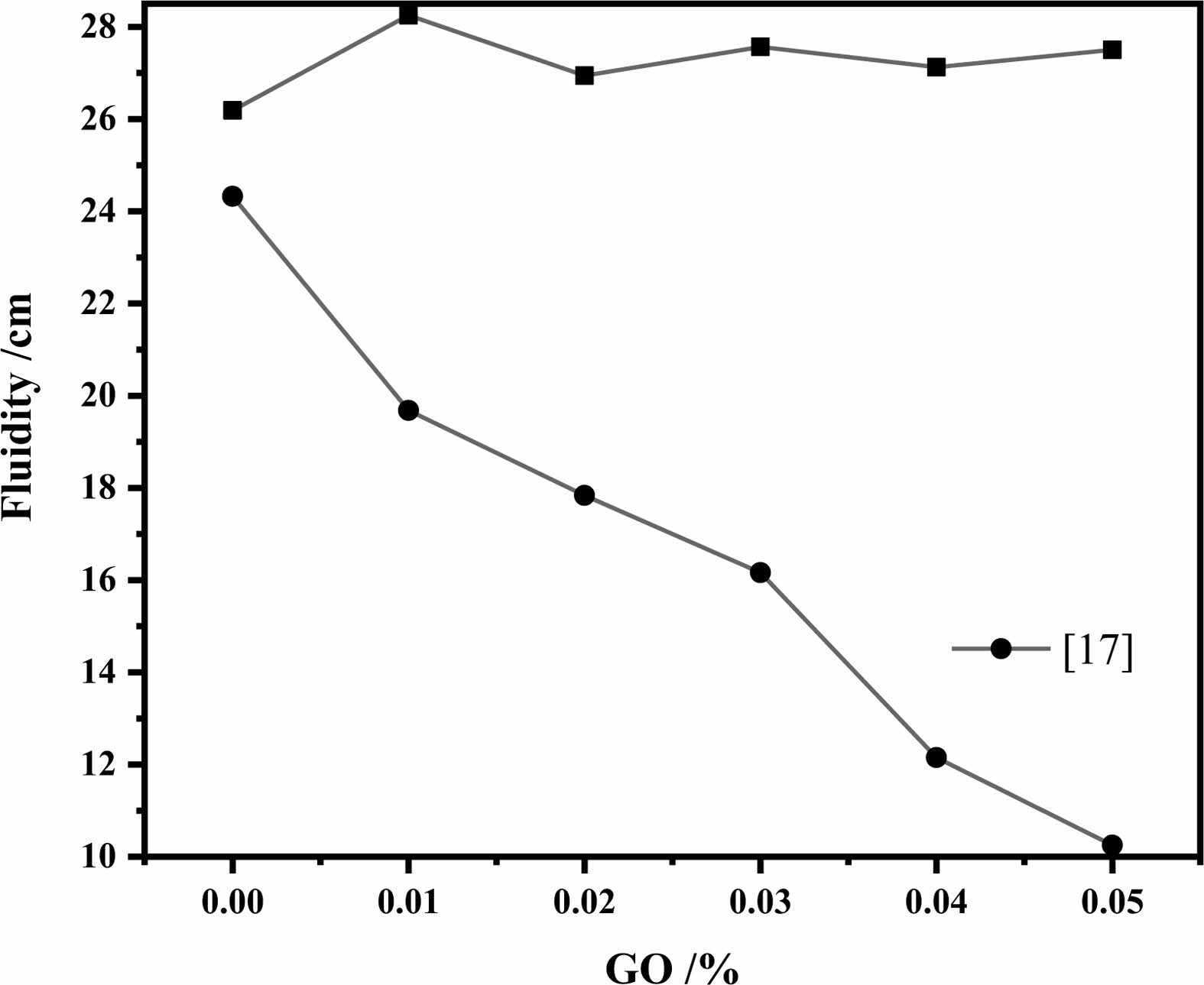
Effect of GO on the Fluidity of high-strength concrete
From the test data (Fig. 3), it is obtained that the mortar obtains better fluidity after adding water reducing agent, and the fluidity does not decrease with the increase of GO addition, but remains stable. Without the addition of water reducing agent GO decreases the fluidity of mortar [19, 20], and the more the addition, the more obvious the decrease of fluidity. The polycarboxylic acid water reducing agent forms a layer of common charge on cement particles to ensure the required fluidity of GO cement samples, promotes the electrostatic repulsion between them, and the water released by cement flocculation compensates the water absorbed by GO [25], improves the fluidity and overcomes the high specific surface area of GO and the binding of part of free water by active oxygen-containing functional groups.
Changes of concrete at high temperatures
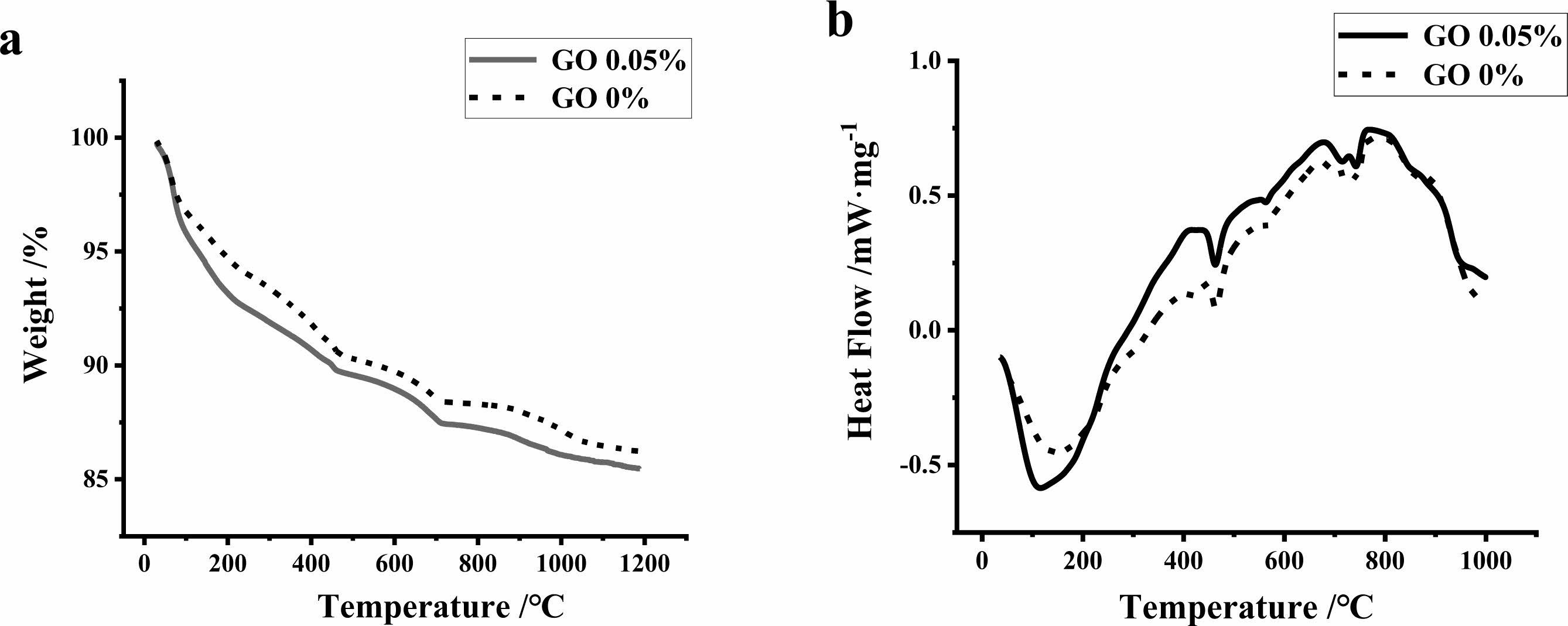
The change in mass of high-strength concrete with increasing temperature is shown in Fig. 4(a), where the mass of high-strength concrete containing GO is lost more rapidly with increasing temperature, and the mass loss of specimens at 400 ℃ without GO and with GO is 8.22% and 9.31%, respectively, which indicates that GO can enhance the concrete hydration. Three distinct heat peaks and one insignificant heat absorption peak were observed for both the concrete with and without GO in Fig. 4(b), the same position of each peak in the figure indicats that the addition of GO does not affect the decomposition temperature of various hydration products in the high temperature.
The first absorption peak appears below 400 ℃, at which the evaporation is mainly the heat absorption of the bound water in the hydration products and the free water inside the specimen. From the decreasing speed of the curve, the evaporation of free water and bound water is faster after adding GO. The water vapor changes the microstructure of the specimen during the evaporation process, and the water vapor exerts a certain pressure on the microporous walls inside the specimen, leading to microcracks and serious surface spalling, which is more obvious in ultra-high performance concrete [26]. The second heat absorption peak appears at 400 ℃~500 ℃, which is related to the fact that some of the hydration products will be dehydroxylated and Ca(OH)2 is decomposed. The curves with GO addition above and below 500 ℃ were significantly higher than those without GO addition, which indicated that GO was effective in improving the heat resistance of the specimens and that more heat was required to be absorbed during the decomposition of the components (Fig. 4). The third heat absorption peak occurs near 570 °C, at which the quartz sand undergoes a transformation from b quartz to a quartz. This produces some lattice expansion, and although the quartz transformation is a reversible process, the expansion is retained on cooling [27]. The fourth heat absorption peak occurs at 700 °C to 800 °C, where the decomposition of CaCO3 and C-S-H mainly occurs at this temperature.
Effect of GO dosing on mechanical properties of concrete at different temperatures
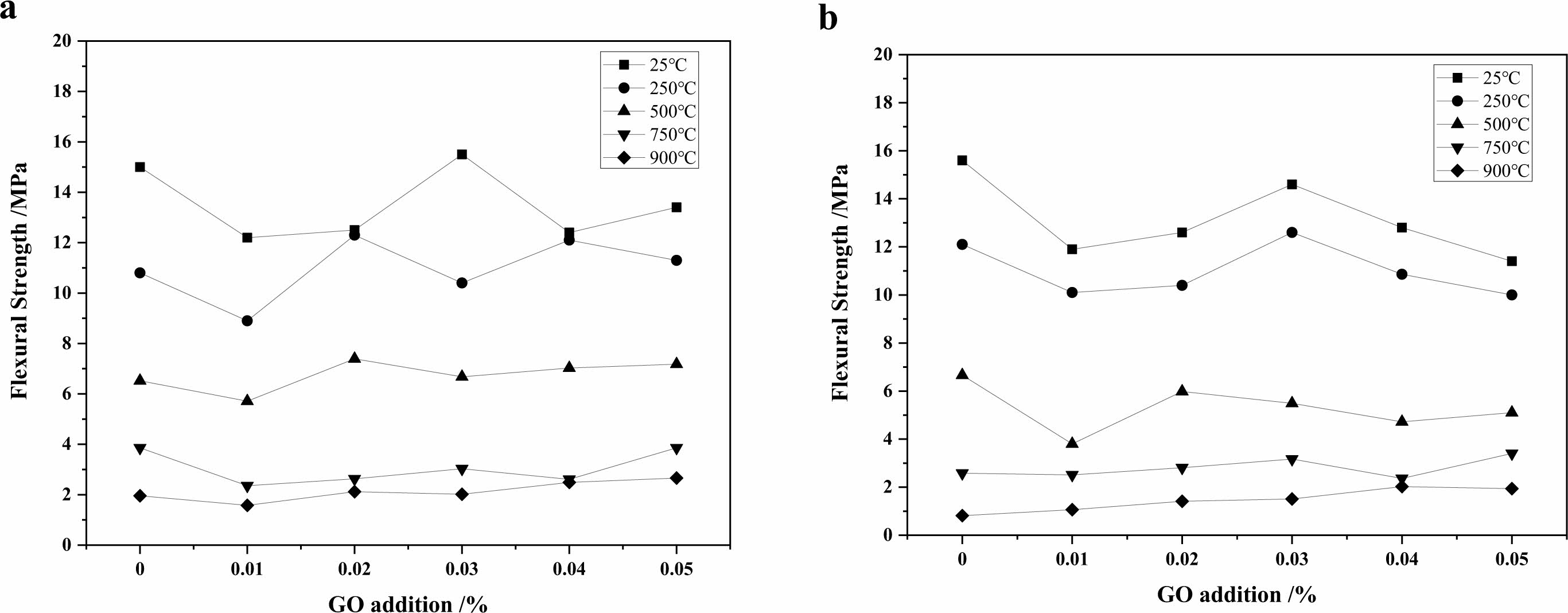
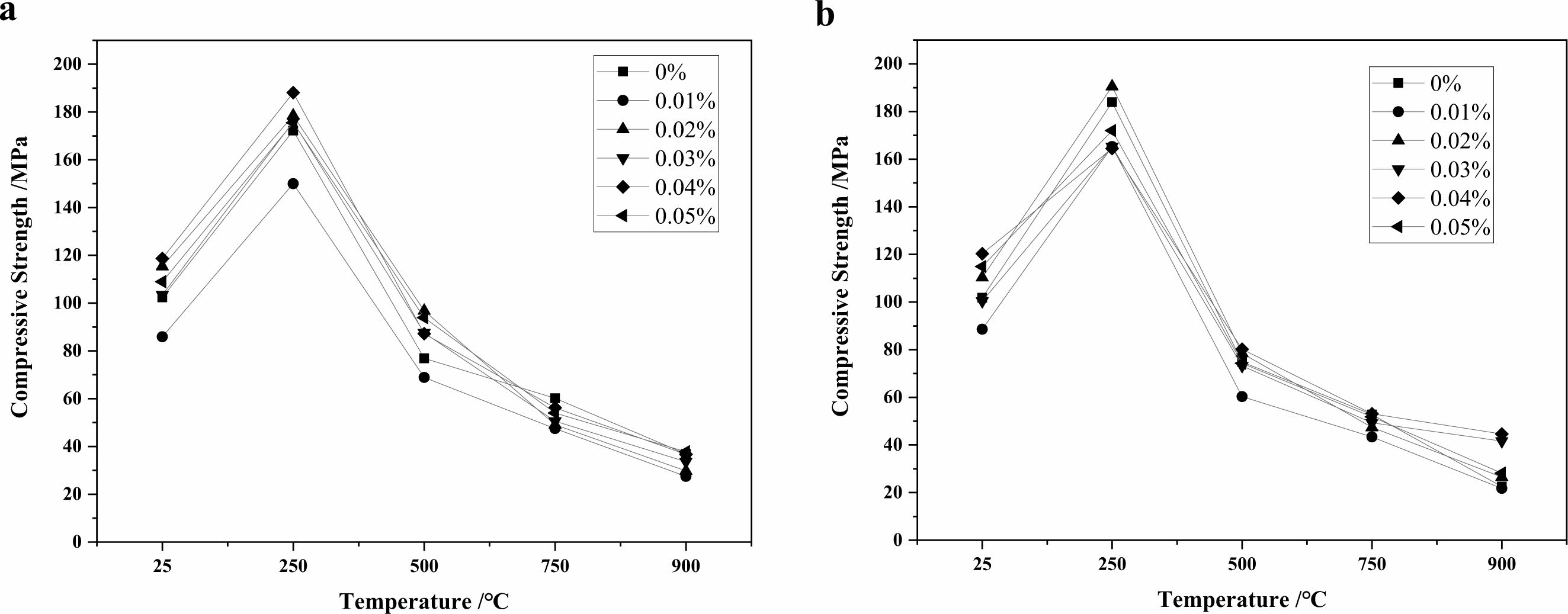
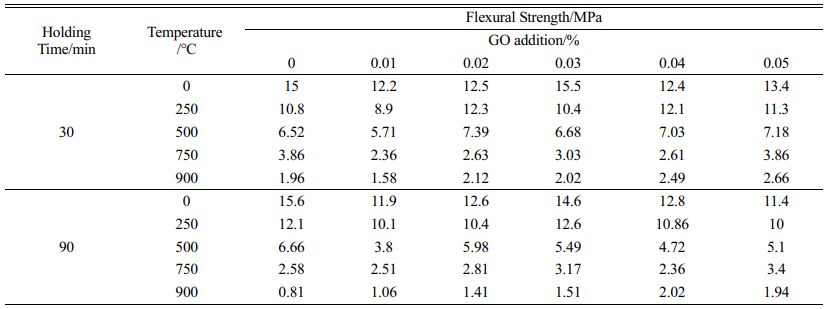
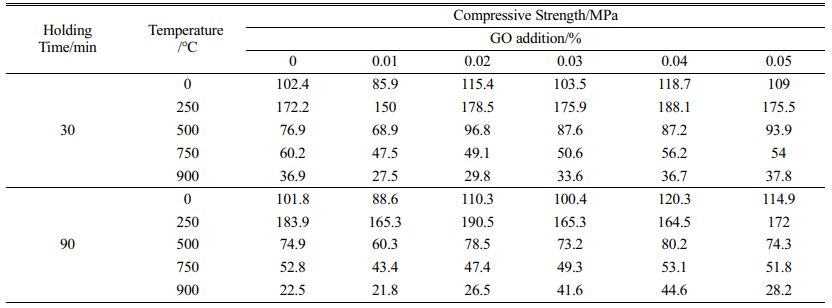
The results of compressive and flexural tests of high strength concrete with GO were conducted after heat treatment at 250 ℃, 500 ℃, 750 ℃ and 900 ℃ with different admixtures as shown in Fig. 5, 6 and Table 4, 5. The compressive strength increase is about 10% when 0.02%~0.05% GO is added to the concrete, and the strength of the specimens enhances with the increase of GO admixture at 250 ℃. The effect is most pronounced when the admixture is 0.02%. This is because at this temperature, the free water and part of the bonded water inside the specimen are evaporated by the high temperature, which overcomes the decrease of the strength caused by the decrease of the van der Waals force between the aggregate particles due to the presence of water molecules in the cementitious material, and makes the strength of the specimen increase [28]; at 500 ℃, the strength of the concrete decreases obviously, but the strength of the GO addition specimen is about 20% higher than that of the specimen without GO. After heating at 750 ℃ and 900 ℃, the C-S-H in concrete has been completely decomposed, and the flexural and compressive strengths of concrete are greatly reduced, less than half of the strength of unsintered specimens, and the effect of GO cannot be reflected. In a comprehensive view, the addition of GO helps to improve the mechanical strength of concrete after heat treatment below 500 ℃, and the improvement of flexural strength is better than compressive strength, and the admixture of GO has the best effect at 0.03%~0.04%.
Concrete structural compactness testing
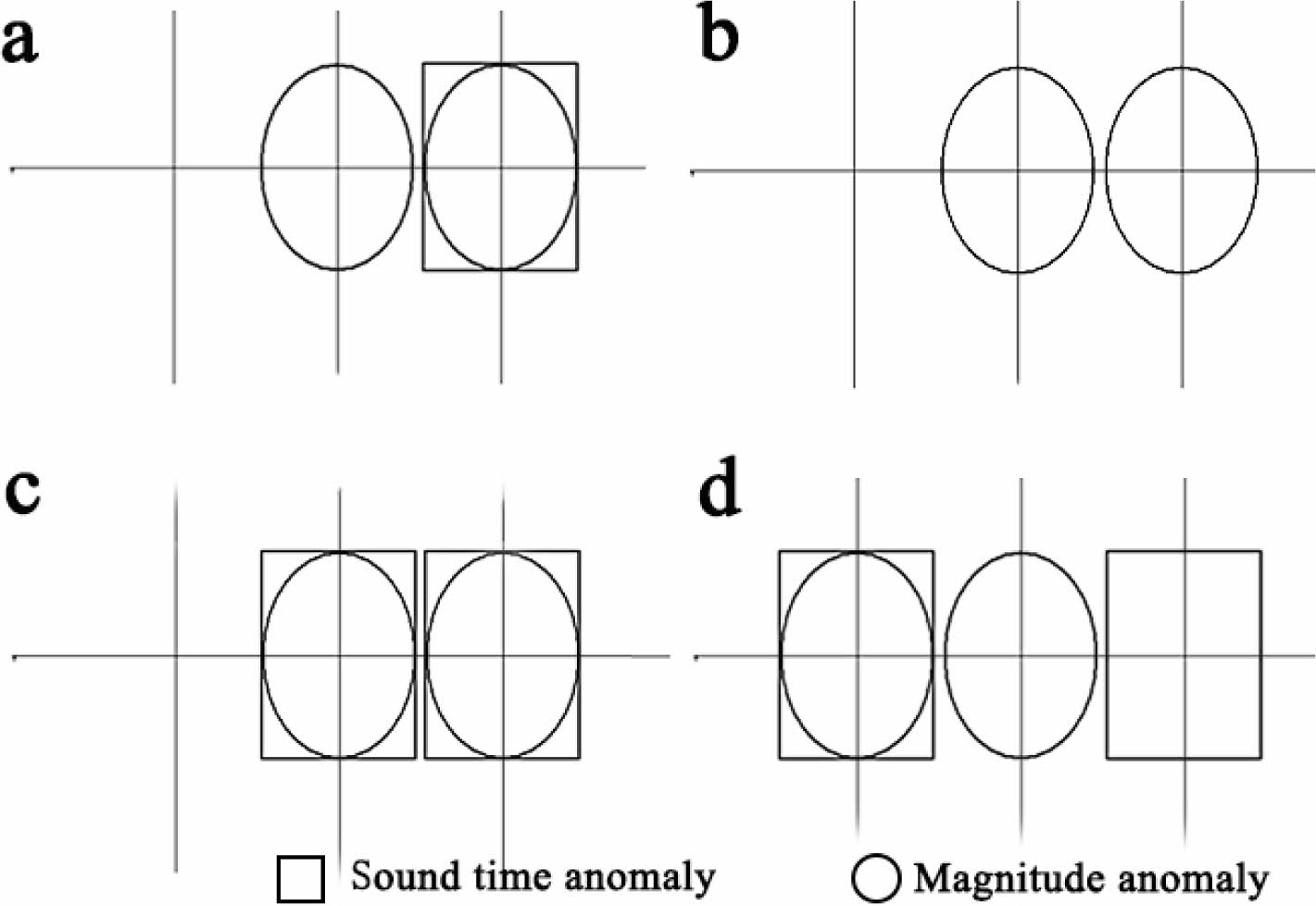
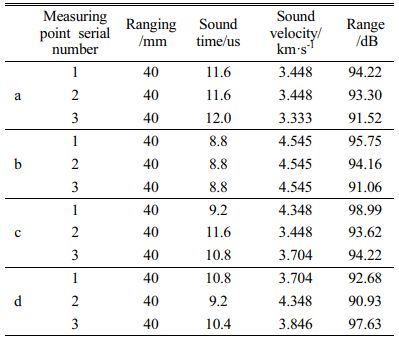
Denseness detection using ultrasonic nondestructive testing instrument, the use of different reflection signal transmission time difference to the probe, can check the internal defects of the components, where the box represents the sound time abnormal, the ellipse represents the amplitude abnormal, the more abnormal signal appears represents the more obvious defects, the detection results are shown in Fig. 7, the test results are shown in Table 6.
The concrete without GO addition has more abnormal values before sintering and more abnormal values after sintering at 750 °C, indicating poor compactness and more voids. The specimens with GO addition have less abnormal values and still had less abnormal values after sintering than the specimens without GO addition. This indicates that GO can improve the compactness and reduce the porosity of cementitious materials.
Changes in mineral composition of concrete after high temperature treatment
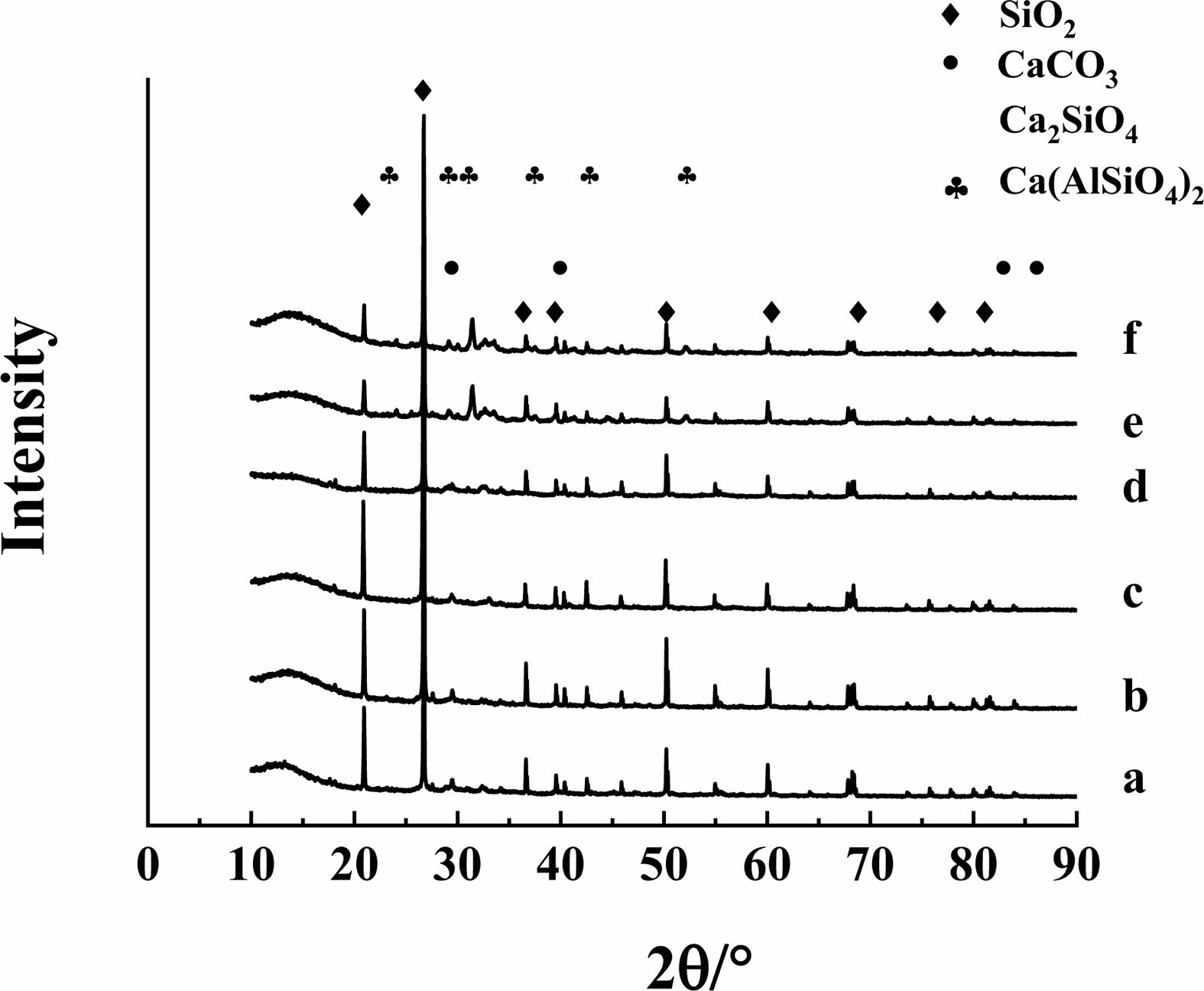
The samples of concrete without GO and with 0.05% GO treated at 500 ℃ and 900 ℃ were examined for mineral composition using XRD, and the results were shown in Fig. 8.
The position of the peaks measured for each specimen is basically in the same position, and the peak height of specimen with GO is slightly higher than that of specimen without GO, which indicates that GO can promote the hydration process of cement, but does not change the type of hydration products. After sintering at 900 ℃, new crystals of calcium silicate aluminate (Ca2Al(AlSiO7)) are generated in both samples, and the peaks of both samples are basically in the same position, indicating that GO does not have much influence on the generation of the new crystals.
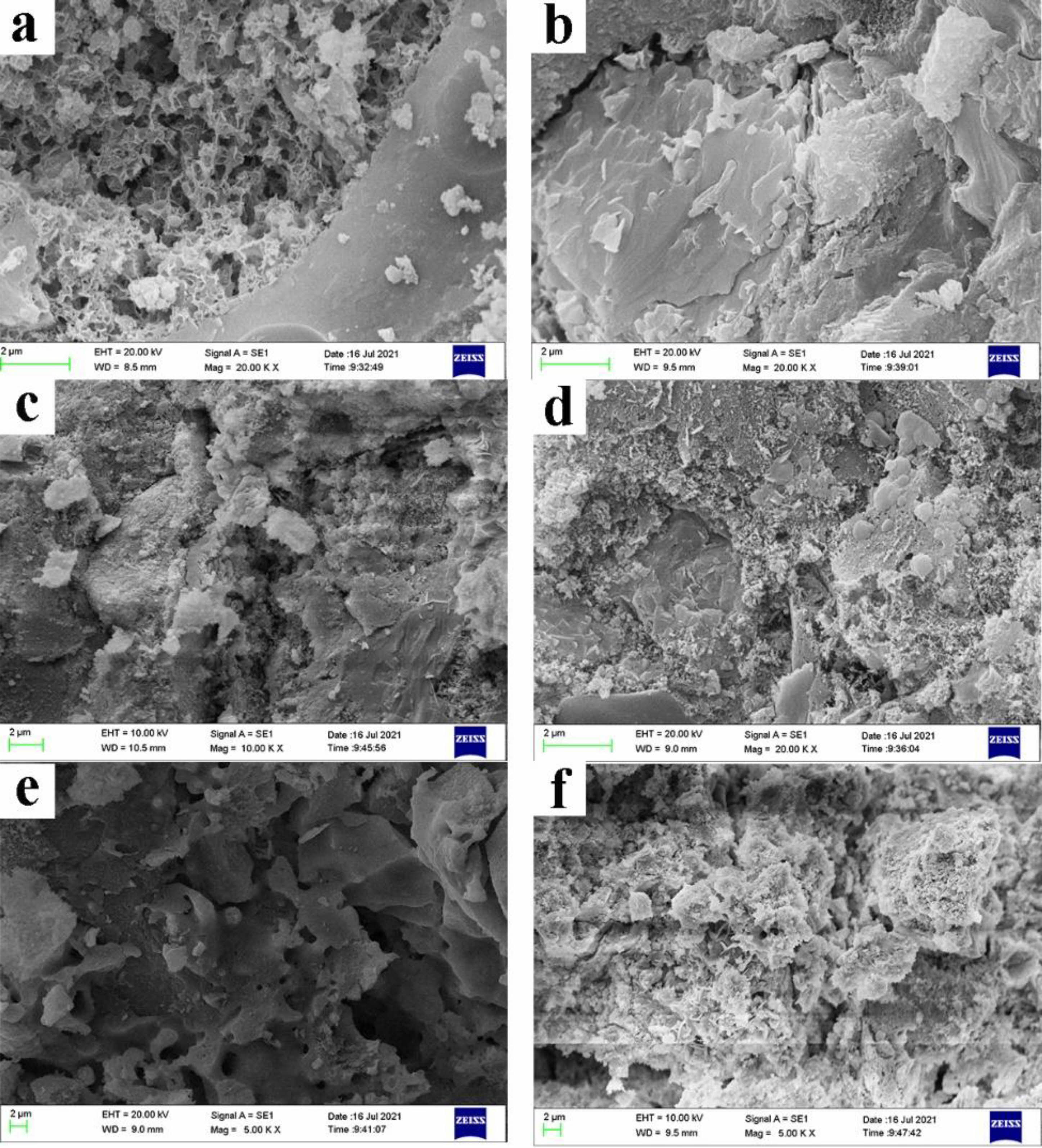
Concrete micromorphological analysis
The concrete without GO and with 0.05%GO treated samples after 500 ℃ and 900 ℃ were examined for microstructure using SEM (Fig. 9(a), (b)). The specimens without GO have many pores inside with fine crystals scattered on the surface and a sparse structure. The specimens with GO have petal-like crystals with dense structure and no obvious pores and cracks, and more dense structure, which is consistent with the results obtained from other literatures [19, 29]. This indicates that the addition of GO improves the crystal morphology of cementitious materials, and these petal-like crystals can reduce the tiny cracks generated internally and the pores, thus improving the mechanical properties of the specimens. GO acts as a nucleation-growth site during the hydration process of cement [30], forming a COO-Ca-COO network structure with free Ca2+ [31], and can enter the pores and cracks formed by the hydration products and change the growth direction and morphology of the surrounding crystals, thus improving the compactness and reducing the porosity [18].
After sintering at 500 ℃, some fine and dense pores appear in the unadulterated GO concrete, while some fine crystals can be seen on the surface, as can be obtained from Fig. 9(c). 500 ℃ heat treatment decomposes Ca(OH)2 in concrete into CaO and water, and the structure will be further destroyed. The petal-like crystals of GO-doped concrete without obvious pores and cracks can be seen in Fig. 9(d).
The concrete added with GO showed many irregular cracks and large pores in Fig. 9(e), (f). The reason is mainly after the heat treatment at 900℃, the internal C-S-H and CaCO3 of concrete will decompose due to high temperature and generate CaO, CO2 and other substances, making the internal structure appear pores and cracks [2]. This indicates that the bridging effect of GO is still effective at high temperature, which can reduce the strength loss of specimens and improve the heat resistance of specimens to a certain extent.
|
Fig. 3 Effect of GO content on Fluidity |
|
Fig. 4 TGA and DSC analysis diagram of specimens with or without GO (a-TGA; b-DSC). |
|
Fig. 5 Effect of GO on flexural strengths of concrete treated at different temperatures (a-holding time of 30 min; b-holding time of 90 min). |
|
Fig. 6 Effect of GO on compressive strengths of concrete treated at different temperatures (a-holding time of 30 min; b-holding time of 90 min). |
|
Fig. 7 Compactness test of concrete structure (without heat treatment: a - GO 0%, b - GO 0.05%; after heat treatment at 750 °C, c - GO 0%, d - GO 0.05%). |
|
Fig. 8 Concrete XRD test results (a-0%-25℃; b-0.05%-25℃; c0%-500℃; d-0.05%-500℃; e-0%-900℃; f-0.05%-900℃. Take a-0%-25℃ as an example, where a is the number, 0% is the GO addition, and 25℃ is the treatment temperature). |
|
Fig. 9 SEM of samples (a-0%-25℃; b-0.05%-25℃; c-0%-500℃; d-0.05%-500℃; e-0%-900℃; f-0.05%-900℃). |
The influence of GO on the heat resistance of high strength concrete was studied, and the slump values, mechanical strengths, compactness space, microstructure and mineral composition are detected. The conclusions are demonstrated, as followed:
(1) Polycarboxylic acid water reducing agent can stabilize the fluidity of concrete with GO and reduce the adverse effect of GO on the fluidity of concrete.
(2) GO can improve the mechanical properties of cementitious composites after sintering, and the improvement of residual flexural strength of high strength concrete with GO after high temperature treatment is greater than that of concrete without GO. GO addition has a significant effect on the mechanical properties of concrete treated at less than 500 ℃, which can improve the heat resistance of concrete, but for the mechanical properties of concrete treated at 750 ℃ and 900 ℃ heating, GO addition has a weak effect.
(3) From the DSC/TGA analysis, the weight loss of specimens without and with GO at 400 ℃ was 8.22% and 9.31%, respectively, which indicates that GO increases the hydration of high-strength concrete. At around 500 ℃, more heat is required for the decomposition of hydration products with GO addition, indicating that GO improves the heat resistance of cementitious materials.
(4) After the addition of GO, the internal pores of cementitious materials are reduced, no obvious cracks are produced after sintering, and more crystals appear after high temperature sintering to improve the residual properties. After 900 ℃ heat treatment the concrete appeared new mineral composition.
The Project is sponsored by the Shandong Province Key R&D Program (Public Welfare) 2019 (Grant Number: 2019GSF109108); The authors also thank to the help from Dr. Fukun Ma
- 1. S.H. Eom, S.S. Kim, J.B. Lee, S.H. Park, J. Ceram. Process. Res. 21[2] (2020) 1669.
-

- 2. M. Malik, S.K. Bhattacharyya, S.V. Barai, Constr. Build. Mater. 270 (2020) 121398.
-

- 3. X. Cong, R.J. Kirkpatrick, Cement. Concrete Res. 25[6] (1995) 1237-1245.
-

- 4. I. Hager, Bul. Pol. Acad. Sci. Tech. Sci. 61[1] (2013) 145-154.
-

- 5. T.G. Nijland, J.A. Larbi, Heron 46[4] (2001) 253-264.
- 6. U. Schneider, U. Diederichs, C. Ehm, Nucl. Eng. Des. 67[2] (1982) 245-258.
-

- 7. Y. Ichikawa, G.L. England, Nucl. Eng. Des. 228[3] (2004) 245-259.
-

- 8. E. Menendez, L. Vega, C. Andrade, J. Therm. Anal. Calorim. 110[1] (2012) 203-209.
-

- 9. E. Gallucci, X. Zhang, K.L Scrivener, Cement. Concrete Res. 53 (2013) 185-195.
-

- 10. C. Alonso, L. Fernandez, J. Mater. Sci. 39[9] (2004) 3015-3024.
-

- 11. I. Grubega, B. Markovic, A. Gojevic, J. Brdarić, Constr. Build. Mater. 184 (2018) 473-484.
-

- 12. A. Zai, S. Salhotra, Mater. Today: Proceed. 33[2] (2020) 1733-1740.
-

- 13. T.V. Khai, P.T. Trang, L.N. Long, L.V. Thang, T.D. Chau, V.V. Dat, and M.T. Phong, J. Ceram. Process. Res. 22[4] (2021) 425-435.
-

- 14. H.G. Na, Y.J. Kwon, S.Y. Kang, W. Kang, M.S. Choi, J.H. Bang, T.K. Jung, C. Lee and H.W. Kim, J. Ceram. Process. Res. 17[6] (2016) 523-531.
- 15. T.V. Khai, D.S. Kwak, Y.J. Kwon, S.S. Kim, K.B. Shim and H.W. Kim, J. Ceram. Process. Res. 14[3] (2013) 355-362.
- 16. T. Nakajima, Y. Matsuo, Carbon 32[3] (1994) 469-475.
-

- 17. Z.J. Cao, W. Liao, S.X. Wang, H.B. Zhao, Y.-Z. Wang, Chem. Eng. J. 3611245 (2019) 1254.
-

- 18. F. Fang, S.Y. Ran, Z.P. Fang, P. Song, H. Wang, Comp. B 165 (2019) 406-416.
-

- 19. S. Ting, Xi'an: Shaanxi University of Science and Technology (2015).
- 20. C.J. Liu, X.C. Huang, Y.Y. Wu, X. Deng, Z. Zheng, Constr. Build. Mater. 288[12] (2021) 123059.
-

- 21. Y.W. Sui, S. Liu, C. Ou, Q. Liu, G. Meng, Constr. Build. Mater. 276[10] (2021) 122229.
-

- 22. D. Han, J. Ceram. Process. Res. 17[6] (2016) 550-554.
- 23. S. Chuah, W.G. Li, S.J. Chen, J.G. Sanjayan, W.H. Duan, Constr. Build. Mater. 161 (2018) 519-527.
-

- 24. National Bureau of Quality and Technical Supervision, in “Cement Mortar Strength Inspection Method (ISO Method) GB/T 17671” (China Standards Press, 1999)
- 25. L. Zhao, L.X. Guo, C. Ge, Q. Li, L. Guo, X. Shu, J. Liu, Comp. B Eng. 113[3] (2017) 308-316.
-

- 26. B. Qu, A.F. Jimenez, A. Palomo, A. Martin, J.Y. Pastor, Materiales de Construcción, 70[337] (2020).
-

- 27. Z. Zhongkui, S. Qingzhou, Z. Puqing,. Mater. Eng. [10] (2016) 25-27.
- 28. L. Shoulong, Y. Ling, Northwest University of agriculture and forestry science and technology (2014).
- 29. D. Lijuan, Xi'an: Shaanxi University of science and technology (2017).
- 30. S. Ghazizadeh, P. Duffour, N.T. Skipper, M. Billing, Y. Bai, Cement Concr. Res. 99[122] (2017) 116-128.
-

- 31. W. Yixuan, Xi'an: Xi'an University of technology (2019).
 This Article
This Article
-
2022; 23(6): 831-838
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.831
- Received on Apr 29, 2022
- Revised on Jul 1, 2022
- Accepted on Jul 25, 2022
 Services
Services
- Abstract
introduction
materials and test methods
results and analysis
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yuwu Sui
-
School of Materials Science and Engineering of Shandong Jianzhu University, Jinan 250101, China
Tel : +86 13864183731 Fax: +86 0531 86367282 - E-mail: herrsui@sdjzu.edu.cn







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.