- Quantitative study on erosion degree of bone china glaze by common acid reagent at different temperature
Wei Honga,*,#, Wen-jie Lib,d,*,#, Hui-chao Huangc, Xiao-wei Wenge, Yi-qin Zhangd, Xiao-hui Liud, Yan-hua Guob and Ya-bin Sub
aKey Laboratory of Mineral High Value Conversion and Energy Storage Materials of Liaoning Province, College of Materials Science and Engineering, Liaoning Technical University, Fuxin 123000, China
bTangshan Customs Comprehensive Technical Service Center, Tangshan 063000
cJiangxi Ceramic Testing Center, Jingdezhen 333000, China
dTechnology center of Shijiazhuang customs district, Shijiazhuang 050051, China
eTaizhou Institute of Product Quality and Safety Inspection, Taizhou 318000, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this experiment, we selected "Tangshan bone china" 10.5-inch white porcelain flat plate produced by five different enterprises as experimental samples to study the erosion of bone porcelain enamel by different kinds of acidic reagents at different temperatures. The specific experimental process was as follows: at different temperatures, 20% hydrochloric acid, 30% sulfuric acid, 100 g/L citric acid and 10% acetic acid were used to continuously erode the sample glaze for 10h, and the whiteness and 45º mirror direction gloss were measured every 2h. The results show that different acidic reagents at different temperatures have significant differences in the erosion characteristics and strength of bone porcelain glaze, and the corrosion resistance of products from different enterprises also have significant differences
Keywords: Corrosion resistance mechanism, Difference, Time and temperature effects, Whiteness index, Glossiness index
Bone china originated in Britain in the 18th century, and is now recognized as a high-grade porcelain, with the excellent quality of “thin as paper, transparent as mirror, sound as chime, white as jade” favored by consumers at home and abroad. Bone china is made from the ashes of herbivores, supplemented by inorganic nonmetallic materials such as kaolin, feldspar and quartz, and is fired through two processes of high-temperature pigment firing and low-temperature glaze firing. Bone china production technology was born in Tangshan in the early 1970s. After more than 40 years of strong development, Tangshan has formed the largest bone porcelain production base in Asia. “Tangshan bone porcelain” was also approved by the General Administration of Quality Supervision, Inspection and Quarantine as a geographical indication protection product in 2012 [1-3]. Unlike ordinary porcelain, the chemical stability of the glaze of bone china is relatively low. In daily life, when bone china is used to hold acidic food or condiments in the diet, imperfections of different depths will appear on the glaze, resulting in a decrease in its use value [4, 5]. In order to study the effect of acid reagent on the color and gloss of bone porcelain glaze, four kinds of acid solutions with different concentrations and temperatures were used to continuously act on five groups of samples, and their whiteness and glossiness were quantitatively tested every 2 hours to analyze the change trend of the two indexes [6].
Corrosion resistance mechanism of bone porcelain glaze
Relationship between bone china phase, whiteness and glossiness
Bone china is mainly composed of crystal phase, glass phase and gas phase, which is composed of tricalcium phosphate (β-C3P): 42-46V%; Calcium feldspar (CAS2): 34~39V%; Residual quartz (Q): < 3V%; Glass phase (G): 16~20V%; Stomata: 3~5%. Glaze gloss is based on the reflection effect of the sample surface and depends on phase composition and surface finish. Due to the different refractive index of each phase, refraction, reflection and diffraction of light must occur on the phase boundary when the light enters the ceramic mold [7]. If the glass phase composition of porcelain embryo is not uniform, the propagation of incident light will be disturbed and the scattering loss will be increased, which will lead to the decrease of glaze gloss and permeability of porcelain embryo. The whiteness of porcelain embryo is not only related to the optical properties of porcelain body, but also affected by stomata. Low stomata rate (<5%) and small stomata (<2.3 m) are the prerequisites for maintaining a higher whiteness of bone porcelain. Therefore, in order to form a reasonable microstructure, the ratio of raw materials, the fineness of the billet and the firing process should meet the requirements of the unique chemical properties of bone china [8].
Corrosion mechanism of acid solution on ceramic glaze
The glaze thickness of bone china is usually 0.2-1.0 mm, and the whiteness and mirror reflectance of the glaze are high. It has certain hardness and brittleness, and its chemical composition is not fixed, similar to that of vitreous solid solution.
The corrosion of ceramic glaze by acidic substances is mainly to decompose the alkaline components in the glaze, so that Na+ ions are in a free state and H+ ions penetrate into the glaze layer. Although the cationic carrier and the corresponding electron neutralization force have not changed, the chemical composition of the glaze has changed. The corrosive effect of acid on the glaze begins with the replacement of OH- group with SiO44-. When the product appeared as ONa- group, the water molecule was adsorbed again, and the decomposition continued until the final complex Si4++O2-6H+8-xNa+X was formed [9]. In addition, the corrosive characteristic of acidic substances is the destruction and reorganization of the original network structure of the sample, and the difference of the shape of the corroded interface will have a certain impact on the corrosion effect [10, 11].
General technical requirements of "Tangshan bone china" [12]
Main Raw materials
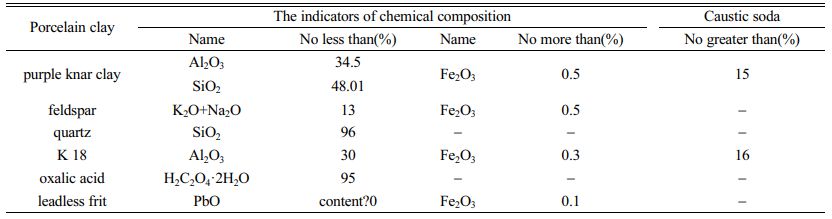
The raw material of the blank shall be china clay with each component content in accordance with the provisions of Table 1, and the raw material of the glaze shall be frit with B2O3 content of 8%~15%. The bone of herbivorous animals calcined at 1100 ℃~1250 ℃ with the content of 98% tricalcium phosphate was mixed into the raw material of bone china as natural bone carbon, and accounted for about 40%~50% of the raw material of bone china.
Processing requirements
Process flow
Tangshan bone china (white china): raw material processing → molding → high temperature pigment firing → low temperature glaze firing → white china.
Firing temperature
The temperature range of high-temperature element is 1240 ℃~1255 ℃.
The low-temperature glaze firing temperature ranges from 1100 ℃ to 1150 ℃.
Experimental samples and preparation
From the samples provided by five enterprises in Tangshan production area, two 10.5-inch white porcelain plates were selected as 10 samples to be tested. There is no crack or lack of glaze on the surface of the sample. Four pieces of porcelain with an area of about 2500 mm2~3000 mm2 are cut from the bottom of each sample as samples. All the samples were cleaned and placed in the electric blast drying oven at (100±5)℃ for 30 min, and then placed in the dryer for cooling for more than 2h. Then, they were transferred to a dryer and cooled for more than 2 hours. WSB-VI intelligent whiteness tester and KGZ-1C intelligent gloss tester were used to measure the whiteness and gloss of the glaze surface of the samples after drying.
Experimental steps
Experiment at room temperature
Under the conditions of ambient temperature (23±5) ℃, humidity (70±5)%RH and shading, 2.0 mL of 20% (mass fraction) hydrochloric acid solution, 30% (mass fraction) sulfuric acid solution, 100 g/L citric acid solution and 10% (volume fraction) acetic acid solution were transferred to the surface of five groups of bone china samples. The acid erosion time was 10 h±10 min. The whiteness of the glaze and the glossiness of the 45 mirror shall be measured every 2 hours. Before the test, the area to be tested shall be washed clean with distilled water and sucked dry with a fluorine-free filter paper.
Heating experiment
Install the test device as shown in Fig. 1, and place the bone china samples to be tested in a flat-bottomed reaction bottle. Fill a flat-bottomed reaction flask with 2000 mL of test solution at a level above the sample. Heat the test solution to 100 ℃. Start timing after acid boiling, boiling acid erosion time is set to 10 h±10 min. During the experiment, heating was stopped every 2 hours and samples were taken out to determine the whiteness and gloss of the glaze.
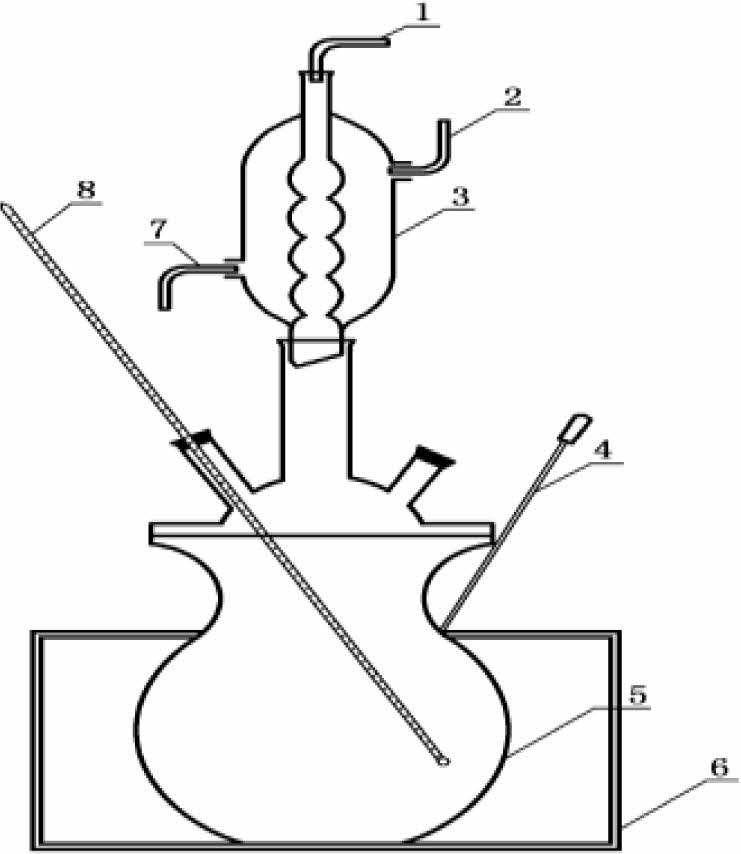
|
Fig. 1 Schematic diagram of heating experimental apparatus. (1-nitrogen pipe; 2 - water inlet; 3 - condenser pipe; 4 - Temperature sensors, 5 - Flat bottomed reaction bottles; 6 - electric heating circle; 7 - outlet; 8 - thermometer) |
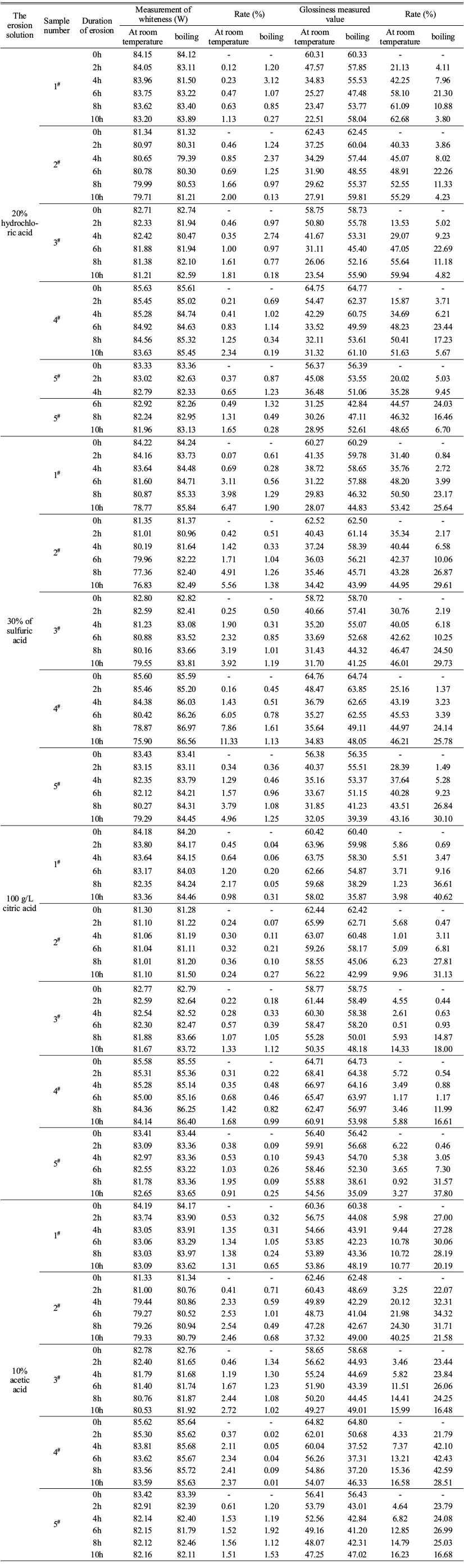
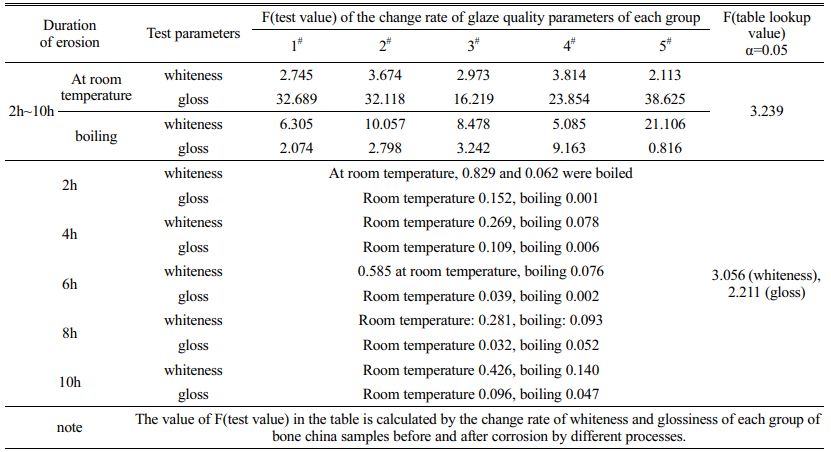
The glaze whiteness and glossiness of bone china samples eroded by room temperature acid and boiling acid for a certain time were measured, and the results were shown in Table 2. The F(test value) values of the correlated measured values of the five groups of samples are shown in Table 3 and Table 4.
As shown in Table 2, the enamel whiteness and 45º mirror glossiness of each group of bone china samples changed to different degrees after being continuously eroded by acid solutions of different kinds and different temperatures for 2 to 10h. Sulfuric acid to the destruction of the bone porcelain glaze effect than hydrochloric acid, lead to group 1#~5# sample whiteness decreased respectively 6.47%, 5.56%, 3.92%, 11.33%, 4.96% and 1.13%, 2.00%, 1.81%, 2.34%, 1.65%. Under the conditions, 100 g/L citric acid and 10% acetic acid are weak acidic, and the corrosion of bone porcelain glaze is relatively small, and the corrosion time lasts 8h~10h. Whiteness by respectively 2.17%, 0.36%, 1.33%, 1.68%, 1.95% and 1.38%, 2.54%, 2.72%, 2.41%, 1.56%. In boiling state, the high temperature accelerates the diffusion and infiltration of H+ ions into the glaze layer, and ONa- group is constantly generated, and the erosion reaction cannot be sustained after a certain period of accumulation. Due to the strong ability of boiling sulfuric acid to provide H+ to water molecules, the time corresponding to the peak corrosion strength of bone china glaze is not more than 2h, and the damage caused in a short time is relatively slight, only -0.61%, -0.51%, -0.50%, -0.45%, -0.36%; About 4h to 6h later, the corrosion of hydrochloric acid basically stops, and the damage degree is relatively high: -3.12%, -2.37%, -2.74%, -1.14%, -1.32%. Although citric acid and acetic acid corrosion time is slightly longer, about 6h to 8h, but the damage intensity of the glaze is not as good as hydrochloric acid. With the continuous replenishment process of the blank glaze, the porosity and pore size of the bone porcelain embryo decreased continuously. The scattering loss and absorption loss of light change accordingly, so that the diffuse reflection characteristics of glaze are improved, and the measured whiteness value is increased correspondingly and the increase rate is increasing with the extension of time. The whiteness increased by 1.90%, 1.38%, 1.19%, 1.61%, 1.25% and 0.31%, 0.27%, 1.12%, 0.99%, 0.25% after 8h and 2h corrosion by sulfuric acid and citric acid in boiling state, respectively.
45º mirror glossiness and whiteness are optical indicators of specular reflection and diffuse reflection of enamel, respectively. Their sensitivity to acid chemical corrosion is different, and the variation range of glossiness of samples after the same time is usually much greater than that of whiteness. Therefore, the effects of strong acid and weak acid at the same temperature and the same acid solution at different temperatures on the glossiness of bone china are obviously different.heir sensitivities to acid chemical corrosion are different, and the variation amplitude of the sample gloss is usually much larger than the whiteness after the same time of action. For example, the damage degree of strong acid to glaze gloss at room temperature is very different from its damage degree to glaze gloss at boiling state. Although hydrochloric acid and sulfuric acid always increase luster loss in the erosion process of 10h. The corrosion strength increases to -62.68%, -55.29%, -59.94%, -51.63%, -48.65% and -53.42%, -44.95%, -46.01%, -46.21%, -43.16%, respectively. However, the corrosion strength decreases continuously after 6h and 4h, respectively. The boiling hydrochloric acid could reduce the glaze gloss of each group to the lowest within 6h, and the valley value was about 1/3 to 1/2 of the glaze gloss decrease of using hydrochloric acid at room temperature. But since the beginning of 6h, gloss has been increasing, and luster growth and its early decline is basically the same. The damage degree of enamel gloss caused by sulfuric acid at high temperature is similar to that at room temperature. After 8h continuous erosion, the gloss loss of the sample increases greatly. In addition, the acidity coefficient of citric acid and acetic acid at 25 ℃ is relatively small, which is 3.15 Ka and 4.75 Ka, respectively. The action mode and erosion degree of citric acid on bone porcelain glaze are far different from strong acid. For example, in the erosion cycle of 4h~6h, weak acid has the effect of improving glaze luster from beginning to end, but when it increases for 6h, it begins to decrease, and the increase and decrease amplitude is basically the same. The damage of boiling citric acid and acetic acid to gloss was similar to that of sulfuric acid and hydrochloric acid, and the drop of gloss loss caused by acetic acid was larger than that of hydrochloric acid.
The data in Table 3 reveal that the acid resistance of bone china products produced by different enterprises varies with the raw material of blank glaze and production process. When the glaze surface of the five groups of samples was corroded by strong and weak acid at room temperature for 2h~10h, the whiteness change rate F(test value) of the second and fourth samples was greater than F(table value), showing poor homogeneity. The gloss changes of the five groups of samples showed significant differences at the same time. In addition, the whiteness and glossiness measured every 2 hours fluctuated slightly with the continuous and slow progress of acid etching. Table 4 shows that the temperature change of hydrochloric acid has a relatively small effect on the whiteness of the enamel of bone china, but a great effect on the direction gloss of the 45º mirror. The influence of sulfuric acid, citric acid and acetic acid on specular and diffuse reflection of glaze is closely related to temperature change. Because of the weak acidity of acetic acid, the corrosion effect of acetic acid on the enamel surface of bone china is significantly different at room temperature and boiling state, and the influence of temperature change on whiteness is greater than that on gloss. In addition, due to the different water absorption rate and tricalcium phosphate content of products from different enterprises, the internal microstructure and optical properties of the glaze are different. Therefore, under different temperature acid corrosion, glaze change trend is different. For example, the whiteness of the samples in group no. 4 showed an increasing and decreasing trend respectively after being eroded by room temperature and boiling acetic acid for 2h~8h, which was different from other samples.
|
Table 2 Determination and change rate of whiteness and glossiness of enamel of bone china samples before and after acid etching. |
|
Table 3 F(test value) of whiteness and glossiness measured after the glaze of bone china samples was eroded by acid at different temperatures. |
|
Table 4 The F(test value) of whiteness and glossiness measured after the glaze of bone china samples was eroded by different acid solutions. |
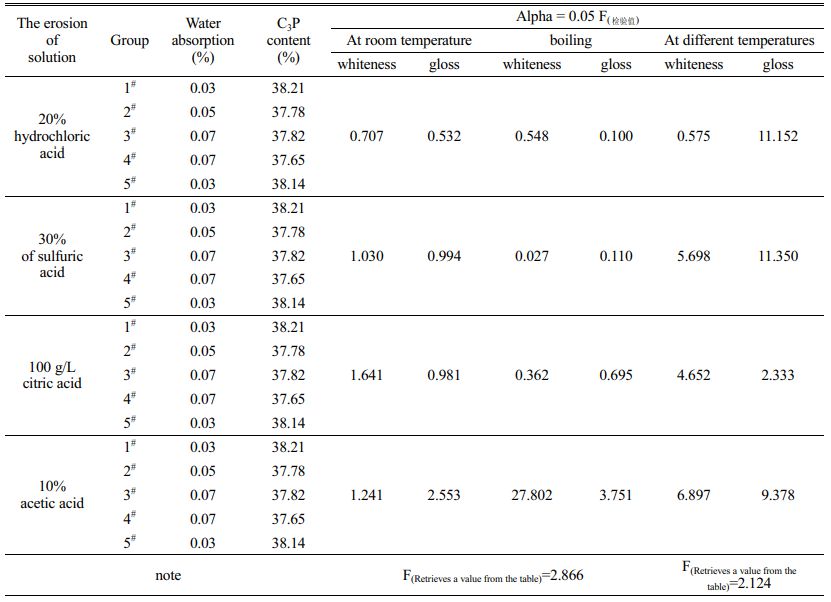
The erosion mode, effective action time and influence degree of the same acidic solution are different at room temperature and boiling state. At room temperature, the weakening effect of strong acid on whiteness and glossiness lasts longer, and the effective action time and erosion intensity of weak acid are both weaker than that of strong acid. In boiling state, because of H+ ion diffusion infiltration process to the glaze layer is accelerated, the surface of the vitreous body continuously formed silicate protective film. The acid etching reaction is difficult to continue after a certain time of accumulation, but it causes a large loss of 45º mirror gloss. The measured value of whiteness can be increased with the decrease of porosity during the filling process.
The physical and chemical properties of enamel, body water absorption, tricalcium phosphate content, internal porosity and glass of bone china products are different due to the different raw and auxiliary materials formula and production process of different enterprises, so that the corrosion resistance of each product is obviously different. Therefore, it is suggested that enterprises optimize the formulation and process to further improve the corrosion resistance of the products according to consumers' demand for the quality of bone porcelain glaze.
This work is supported by the Basic Scientific Research Project of Education Department of Liaoning Province (General Project) (LJKMZ20220668) and the Industry-university Cooperative education program of Ministry of Education (220504210095831) and the the Natural Science Foundation of China (21872119, 22072127) and the Scientific Research Funding Project of the Educational Department of Liaoning Province of china (LJKQZ2021158) and the Science and Technology Project of AQSIQ(2017IK286) and the University-enterprise Cooperation Project of Liaoning Technical University (300221143).
The authors of the paper referenced above, have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
- 1. L. Wenjie, J. Huida, and X. Wenchao, Ceram. 6 (2017) 36-42.
- 2. S.C. Jeong, S.H. Ann, and K.W. Nam, J. Ceram. Process. Res. 18[7] (2017) 506-520.
- 3. X.D Spiliotis, K.I. Ntampegliotis, V.G. Karayannis, and G.A. Papapolymerou, J. Ceram. Process. Res. 16[1] (2015) 11-17.
- 4. L. Wenjie, L. Xuejun, and X. Wenchao, Ceram. 5 (2017) 32-40.
- 5. W. Liting and W. Yangfang, J. Ceram. Process. Res. 20[3] (2019) 216-221.
- 6. V.G. Martins, C. Cavion, J. Tonet, L.S. Miola, C.A. Perottoni, and J.E. Zorzi, Bol. Soc. Esp. Ceram. V (2020) 00-00.
-

- 7. W. Chao and Z. Li, J. Chinese Ceram. Soc. 34[11] (2015) 3157-3161.
- 8. H. Qiu-ju, L.U. Sh-ling, P.E.I. Ya-jing, L.I. Yu-ling, and Z.H.A.O. Rui-ting, Science of cultural relic protection and archaeology 26[2] (2014) 16-21.
- 9. C. Aifen, China Ceram. Industry 3 (1997) 26-27.
- 10. G. Ziqiang, L. Songhua, W. Yuhou, S. Yong, S. Jian and T. Junxing, J. Ceram. Process. Res. 23[5] (2022) 685-693.
-

- 11. S.C. Jeong, S.H. Ahn, and K.W. Nam, J. Ceram. Process. Res. 18[7] (2017) 506-520.
- 12. Z. Yin, K. Deshuang, G. Changjun, K. Shuanghua, and Y. Yuquan, China Ceram. 47[7] (2011) 16-18.
 This Article
This Article
-
2022; 23(6): 823-830
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.823
- Received on Apr 13, 2022
- Revised on Aug 14, 2022
- Accepted on Sep 3, 2022
 Services
Services
- Abstract
introduction
experiment
results and analysis
conclusion
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Wei Hong a, Wen-jie Li b,d
-
aKey Laboratory of Mineral High Value Conversion and Energy Storage Materials of Liaoning Province, College of Materials Science and Engineering, Liaoning Technical University, Fuxin 123000, China
bTangshan Customs Comprehensive Technical Service Center, Tangshan 063000
dTechnology center of Shijiazhuang customs district, Shijiazhuang 050051, China
Wenjie Li
Tel: +86 18712817961 Fax: +86 031166709916
Wei Hong
Tel: +86 15232366454 Fax: +86 04185110098 - E-mail: hongwei20160211@126.com, 26282946@qq.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.