- Preparation of anhydrous from red gypsum and effect of high strength gypsum on its properties
Changrong Liua, Lu Wanga, Hongbin Tana,b,*, Faqin Dongc, Xiaoling Maa and Feihua Yangd
aState Key Laboratory of Environment-friendly Energy Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang Sichuan 621010, China
bShaanxi Engineering Center of Metallurgical Sediment Resource, Shaanxi University of Technology, Hanzhong Shaanxi 723000, China
cKey Laboratory of Solid Waste Treatment and Resource Recycle, Ministry Education, Southwest University of Science and Technology, Mianyang Sichuan 621010, China
dState Key Laboratory of Solid Waste Reuse for Building Materials, Beijing Building Materirals Academy of Science Research, Beijing 100041, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Red gypsum waste comes from titanium dioxide production by sulphuric acid method. Anhydrite was prepared from the waste. The effects of calcined temperature on the properties of anhydrite were studied. The normal consistency of anhydrite decreased with the increase of calcined temperature, while the compressive strength firstly increase and then decrease. The effect of high strength gypsum on the properties of anhydrite was also studied. The normal consistency of sample decreased with the increase of high strength gypsum content, while the setting time firstly decrease and then increase, strength and density increased
Keywords: Red gypsum, Anhydrite, High strength gypsum, Properties
Titanium dioxide (TiO2) is widely used as a pigment in the manufacture of paints, papers and printing inks. Moreover, TiO2 is a promising photocatalyst for NOx reduction and water purification [1]. In general, TiO2 is produced by using the sulphuric acid method, which employs ilmenite (TiFe2O3) and sulphuric acid as raw material. Red gypsum (RG) emerges as a solid waste during the final stage of industrial process, where a low acidic liquid stream is treated by using lime or limestone [2]. Gypsum (CaSO4·2H2O) and amorphous ferric hydroxide (Fe(OH)3) are major components of the red gypsum [3]. Roughly, 5-6 tons of red gypsum is generated for every ton of TiO2 by using the method. Annually, 15 million tons of RG are generated in China [4].
Generally, there are five gypsum phase in CaSO4-H2O system, including seven various forms. They are dihydrate gypsum (CaSO4·2H2O), α- and β-hemihydrate gypsum (α-CaSO4·0.5H2O, β-CaSO4·0.5H2O), α- and β-anhydrite Ⅲ (α-CaSO4 Ⅲ, β-CaSO4 Ⅲ), Ⅱ anhydrite (CaSO4 Ⅱ) and Ⅰ anhydrite (CaSO4 Ⅰ) [5, 6].
The red gypsum could be used as calcined gypsum (β-hemihydrate gypsum) raw material, which calcined gypsum can be used to produce blocks or light-weight walls. But the ferric hydroxide impurity prevents the waste reuse due to it has bad effect on the normal consistency and compressive strength of calcined gypsum. As a result, RG waste is treated by outdoor stacking, which induces additional costs and raises environmental concerns [7].
α-hemihydrate gypsum (high strength gypsum) powder, with a low aspect ratio crystals, results in a paste with better injectability and mechanical properties, which are being widely utilized in ceramics, molding, binders, industrial arts and architecture and construction industry [8]. The CaSO4 II phase of anhydrite can be produced by high-temperature calcinations of byproduct gypsum. Under ambient conditions, CaSO4 II reacts very slowly with water. The ability of anhydrite to react with water, converting itself into gypsum, is the basis of its use as a construction material. The reactivity can be enhanced considerably by adding certain activators (e.g. Portland cement, CaO, K2SO4, et al.) [9].
Phosphogypsum can be used to produce an anhydrite and the impurities become inert when heated at elevated temperature [10]. The red gypsum can also prepare an anhydrite and the ferric hydroxide impurity maybe be also become inert. On the other hand, if high strength gypsum is used as anhydrite activator, the gypsum products have stable performance because they have same hydrate (gypsum, CaSO4·2H2O).
There is currently limited work reported on the CaSO4 II from red gypsum and activator of high strength gypsum. Moreover, the underlying mechanism has not been unveiled. In this work, the effects of calcined temperature and activator (high strength gypsum) on the anhydrite properties were studied and the underlying mechanism has been unveiled.
RG was provided by Chongqing Yugang titanium dioxide Co., Ltd, China. High strength gypsum was provided by Longyuan gypsum Co., Ltd, Sichuan, China. P. O 42.5R Portland cement was provided by Beichuan Zhonglian Cement Co., Ltd, China.
Red gypsum was dried in an oven (105 ℃) and ground with a ball mill to obtain powder. Anhydrite was obtained by calcining the powder in muffle furnace at different temperatures for 3 h. After adding Portland cement/high strength gypsum and water into the anhydrite and mixing them evenly, the standard consistency, setting time and strength of samples were tested according to Chinese standard (building gypsum, GB/T 9776-2008).
The chemical composition of raw materials was measured by X-ray fluorescence spectrometer (Axios-Poly, PANalytical, Netherlands). The morphology was observed by scanning electron microscopy (TM-2000/4000, Hitachi, Japan). The phase analysis was carried out by using X-ray powder diffractometer (Smartlab, Rigaku, Japan), equipped with Cu Kα radiations (λ = 0.15406 nm). The compressive strength was measured by using a Micro computer controlled Electromechanical Universal Testing Machine (104C, Shenzhen Wance Testing Machine, China), under a loading rate of 0.02 kN/s.
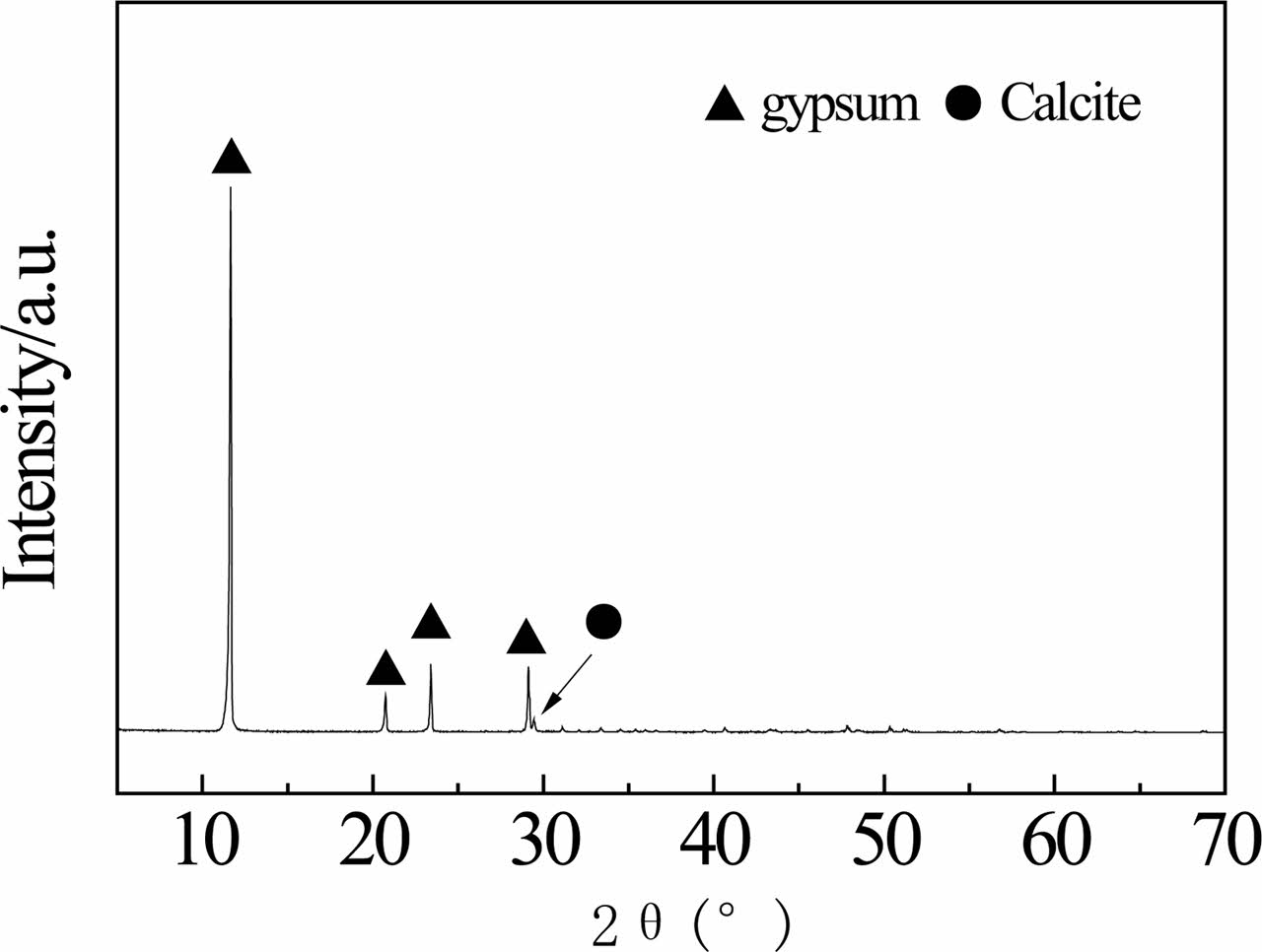
Chemical composition, X-ray diffraction (XRD) pattern and scanning electron microscopy (SEM) image of RG are presented in Table 1, Fig. 1 and Fig. 2, respectively. The RG contain 40.26 wt.% CaO, 37.46 wt.% SO3, 10.18 wt.% Fe2O3, 3.81 wt.% TiO2 and others. From structural viewpoint, the RG is mainly composed of gypsum (CaSO4·2H2O, PDF: 70-0982), calcite (CaCO3, PDF: 05-0586) and amorphous Fe(OH)3 phases. The calcite presents because some limestone didn't react completely, which acidic liquid stream was treated by using limestone to obtain RG. According to SEM image (Fig. 2), the gypsum crystals are fascicular, columnar and irregular shape, and some crystals present a rough surface. Fe(OH)3 particles (about 0.1 μm in diameter) agglomerate irregular shapes.
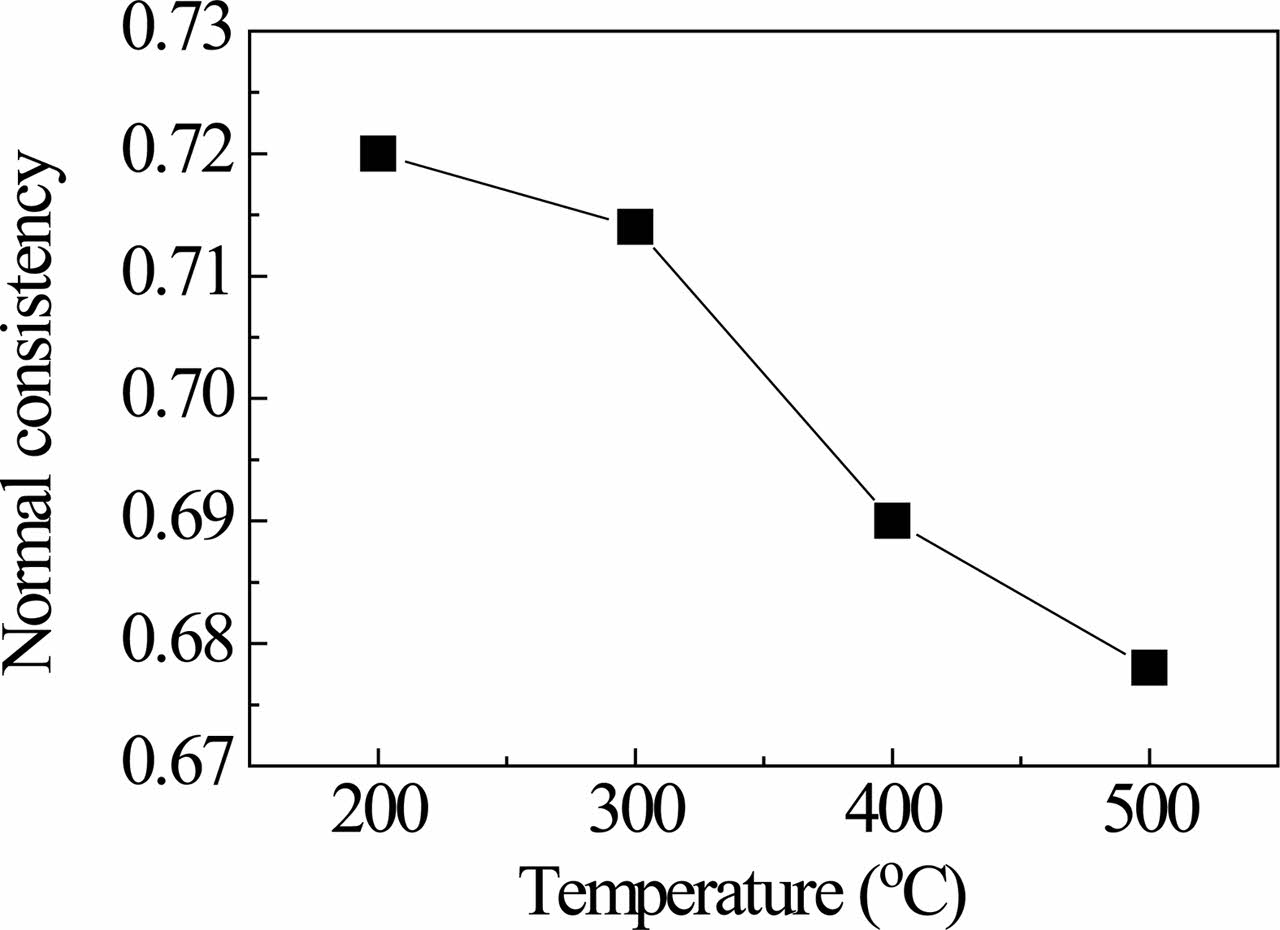
The normal consistency of red gypsum calcined at different temperatures is shown in Fig. 3. The normal consistency decreases with the increase of calcined temperature. The aggregate of iron hydroxide shrinks, the porosity decreases, and then absorption amount of water decreases with the increase of calcined temperature. Moreover, some iron hydroxide may transit into iron oxide, which further reduces its normal consistency. The decrease of normal consistency will reduce the addition amount of water, which can improve the strength of sample.
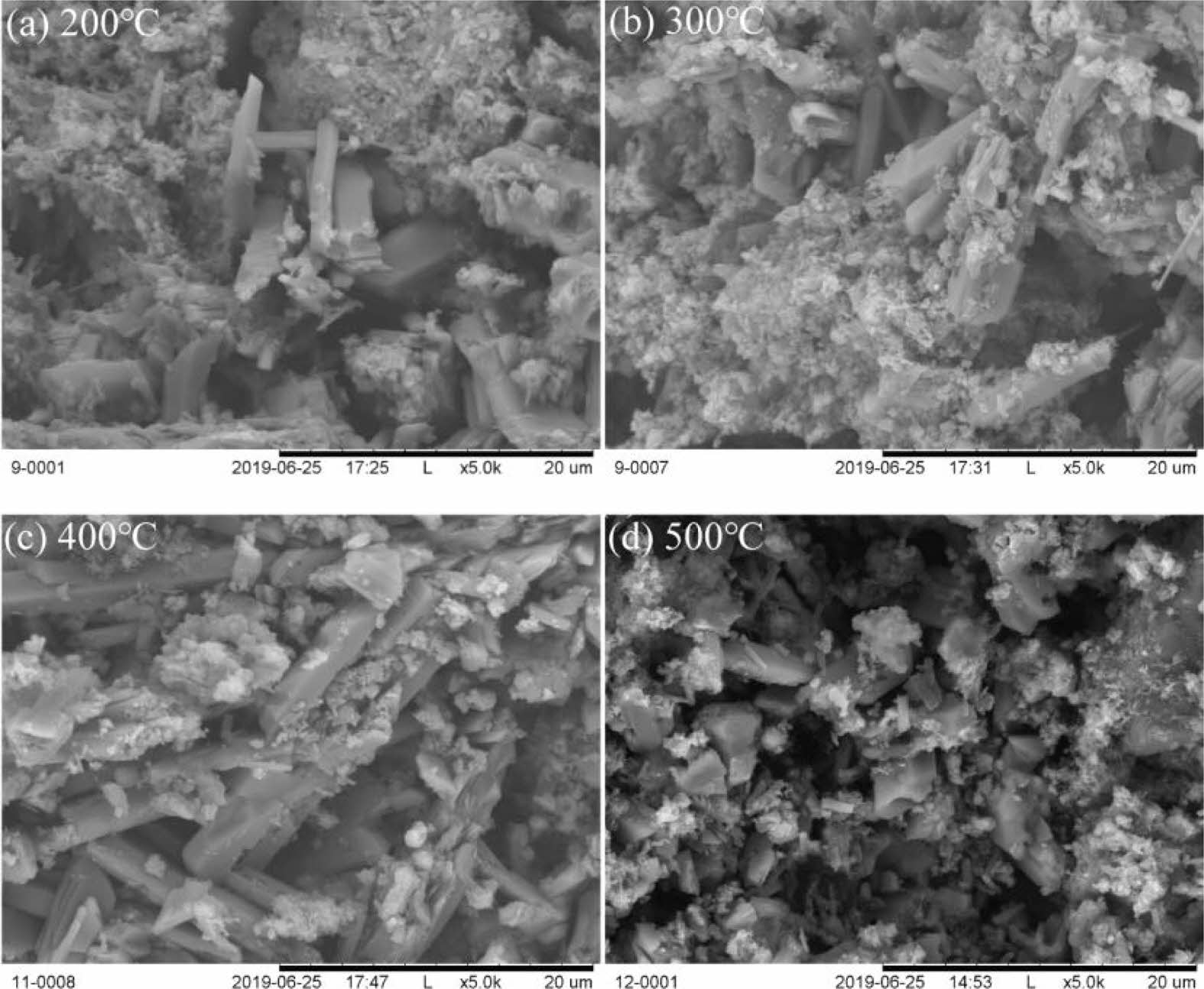
SEM images of red gypsum calcined at different temperatures are shown in Fig. 4. Columnar crystals with smooth surface are observed, but coarse crystals are not observed. The coarse crystals possibly rupture into small particles during the RG heat treatment. The morphology of crystals are long columnar after heat treatment at 200 ℃, 300 ℃ and 400 ℃, respectively, and short columnar and irregular crystals are observed after heat treatment at 500 ℃, which irregular crystals may generate from the fracture of long columnar gypsum. Moreover, with the increase of calcined temperature, the diameter of aggregates decreases, which can make normal consistency of sample decrease.
Under ambient conditions, the dehydration trans- formation of gypsum in industry is as follows:
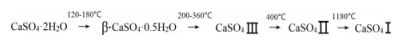
The β-hemihydrate gypsum (CaSO4·0.5H2O) is formed under ambient conditions at temperatures ranging from 120 °C to 180 °C. Further heating of bassanite above temperatures of 200 °C leads to soluble anhydrite (CaSO4 III). Continued heating above 400 °C, leads to less soluble anhydrite (CaSO4 II). The structures of bassanite and anhydrite III are considered to be very similar as both structures provide structural channels of 0.4 nm diameter. In the case of bassanite those channels are partially filled with H2O molecules and they are empty in case of anhydrite III [11]. Anhydrite III is easy to transform into hemihydrate gypsum by adsorbing water vapor in atmospheric environment. The CaSO4 II phase of anhydrite is the one that is thermodynamically stable up to 1180 °C and occurs naturally as the mineral anhydrite. Under ambient conditions, CaSO4 II reacts very slowly with water, which this mineral is also given as dead burnt gypsum [9].
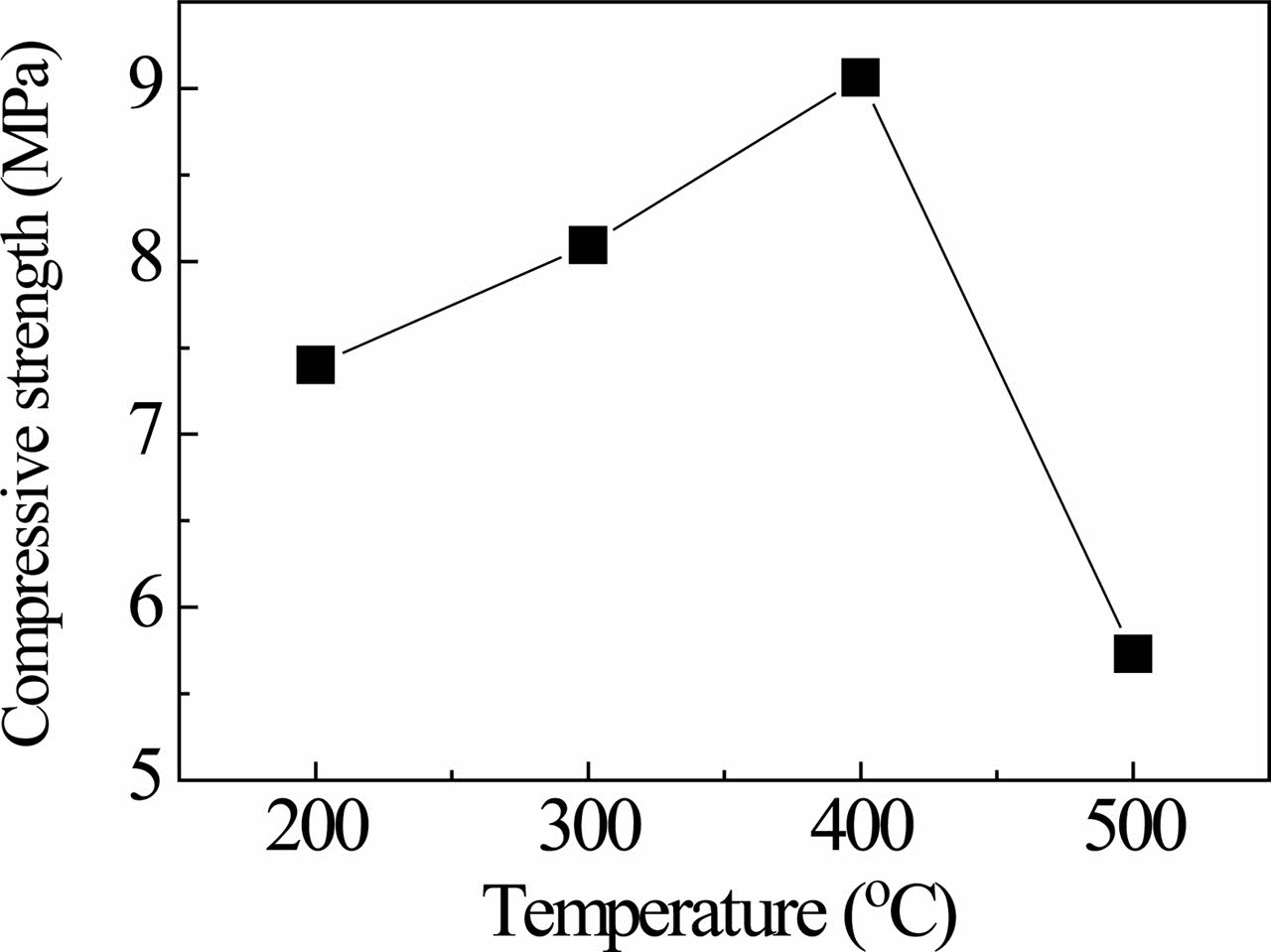
The compressive strength of samples from red gypsum calcined at different temperatures and added 5 wt% Portland cement as activator is shown in Fig. 5. The strength of the samples firstly increases and then decreases. The calcined temperature increases, the normal consistency of the samples decrease, and the density of the samples increase, resulting in the increase of strength. But the strength of sample calcined at 500 ℃ gets worse because activity of anhydrite decreases with the increase of calcined temperature. The hydration kinetics of pure synthetic orthorhombic anhydrite depends on the temperature of preparation, mechanical activation by grinding and the nature of foreign cations in the solution used as chemical activators [12]. And the strength of samples depends on the degree of hydration. Although the activity of anhydrite can be improved by adding alkaline activators, such as sodium sulfate, potassium sulfate, aluminum potassium sulfate, high treatment temperature will increase energy consumption. In present work, the calcination temperature is selected as 400 ℃.
X-ray diffraction patterns of red gypsum calcined at 400 ℃ and aged for different time are shown in Fig. 6. The crystal phases of samples are anhydrite (CaSO4, PDF: 37-1496, Orthorhombic, Cell: 6.9933×7.0017× 6.2411<90×90×90>) and calcite (CaCO3, PDF: 05-0586). The anhydrite was aged in atmospheric condition for 2 months, and the phase did not change because anhydrite II is stable. In addition, comparing with Fig. 1, the diffraction peak of calcium carbonate in this sample is strong, which is mainly due to the anhydrite has weak peak intensity. By the way, the decomposition temperature of carbonate is about at 900 ℃. Moreover, the diffraction peaks of Fe(OH)3 or Fe2O3 are not observed possibly because them are still amorphous phases.
Portland cement can be used as activator of anhydrite and improve its water resistance. But the hydration rate of Portland cement is slower than anhydrite. After gypsum plaster setting, Portland cement continues to react with gypsum to form ettringite and lead to plaster volume expansion, which affects the stability of gypsum products. To the stability of gypsum products from anhydrite added Portland cement, further research continues to define these dependencies.
The normal consistency of samples contained different content high strength gypsum is shown in Fig. 7. With the added content increases of high strength gypsum, the standard consistency decreases because high strength gypsum has low standard consistency.
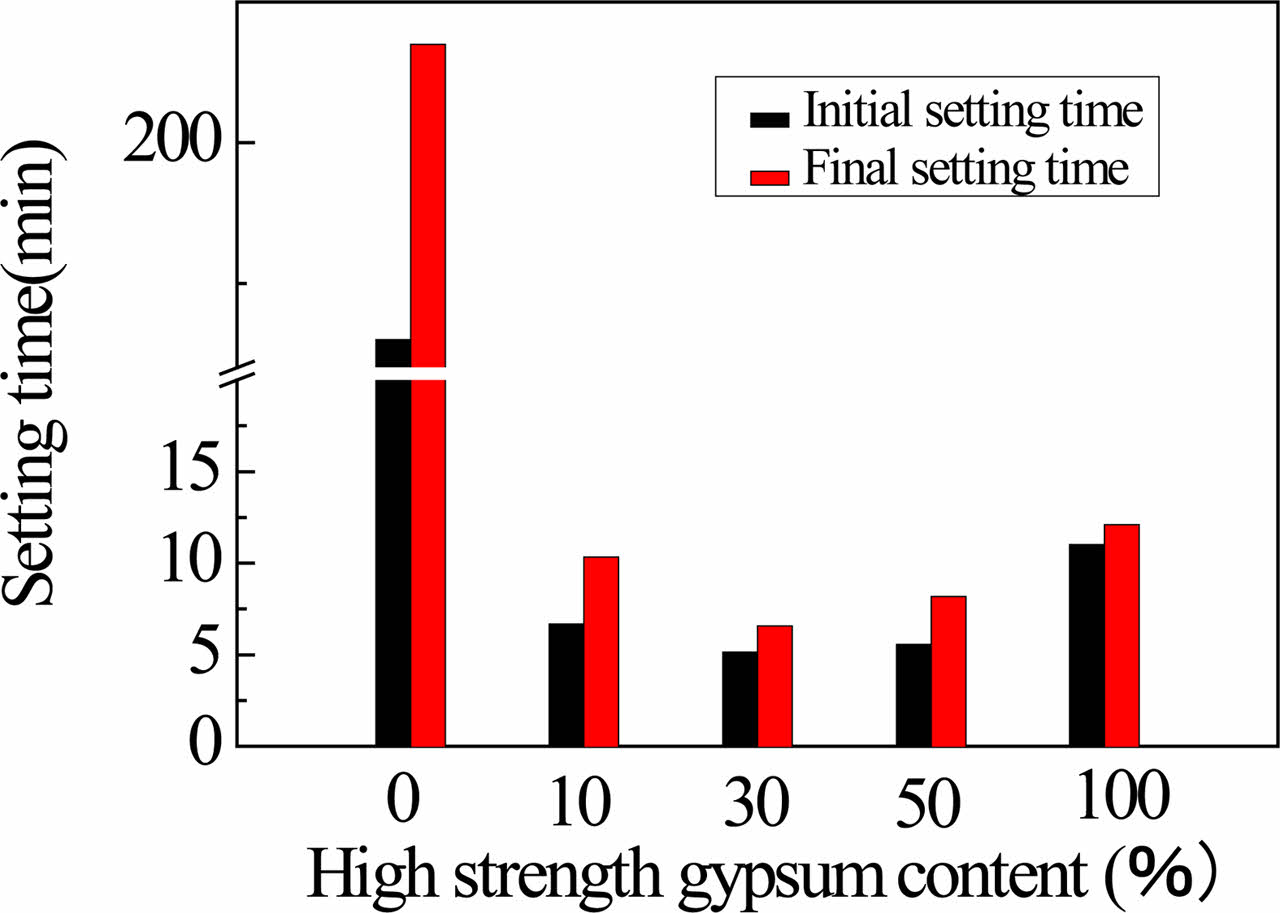
The setting time of samples contained different content high strength gypsum is shown in Fig. 8. With the added content of high strength gypsum increases, the setting time firstly decreases and then increases. The initial and final setting times of anhydrite are 130 and 235 min, respectively, because it reacts very slowly with water. The initial and final setting times of the sample from anhydrite with added 30 wt.% high strength gypsum are 5.2 and 6.6 min, respectively, which sample has shortest setting time in these samples because the Ca2+ from high strength hydration improves anhydrite activation, and high strength gypsum has high hydration activity. With added content of high strength gypsum increase, the setting time increases because high strength gypsum has long setting time.
The compressive strength of samples contained different content high strength gypsum is shown in Fig. 9. With the added content of high strength gypsum increase, the strength of samples increases because high strength gypsum has high strength (25 MPa). Compressive strength of samples from anhydrite and anhydrite added 30% high strength gypsum are 2.3 and 9.9 MPa, respectively. The sample with anhydrite added 30% high strength gypsum is economic to produce blocks or light-weight walls.
The SEM images of samples contained different content high strength gypsum are shown in Fig. 10. It can be clearly observed that the microstructure of samples have been significantly altered due to the presence of different content high strength gypsum. With the added content of high strength gypsum increasing, thin columnar crystals increases and a dense microstructure has been formed due to the interlocking of these plate-like and needle-like crystals. The hydration of anhydrite and high strength gypsum are dissolution–nucleation–growth process, in which nucleation was the more concerned step [11]. High strength gypsum firstly dissolves and forms nucleation, and then anhydrite dissolves. Thin gypsum crystals are observed because a lot of nucleation from high strength gypsum. According to Fig. 10(a) and Fig. 4(b), they samples have coarse crystals because they samples contain a little of nucleation.
|
Fig. 1 X-ray diffraction pattern of RG. |

|
Fig. 2 SEM image of RG |
|
Fig. 3 Normal consistency of red gypsum calcined at different temperatures. |
|
Fig. 4 SEM images of titanium gypsum calcined at different temperatures. |
|
Fig. 5 Compressive strength of samples from red gypsum calcined at different temperatures and added 5 wt.% Portland cement as activator. |

|
Fig. 6 X-ray diffraction patterns of gypsum calcined at 400 ℃ and aged for different time. |

|
Fig. 7 Normal consistency of samples contained different content high strength gypsum. |
|
Fig. 8 The standard consistency of samples contained different content high strength gypsum. |

|
Fig. 9 Compressive strength of samples contained different content high strength gypsum. |

|
Fig. 10 The SEM images of samples contained different content high strength gypsum. |
The anhydrite is prepared by sintering RG at different temperature. The normal consistency of anhydrite decreases with the increase of calcination temperature. The strength of the samples firstly increases and then decreases, which samples are obtained from the anhydrite by adding 5 wt% Portland cement as activator. The calcination temperature is selected as 400 ℃, which anhydrite has high hydration activity and sample has high strength (9.062 MPa).
With the added content of high strength gypsum increases in anhydrite, the standard consistency of sample decreases, the setting time firstly decreases and then increases, the compressive strength increases. The initial and final setting times of the sample from anhydrite with added 30 wt% high strength are 5.2 and 6.6 min, respectively, which the sample has shortest setting time in these samples. And the compressive strength of the sample is 9.9 MPa, which sample is economic to produce blocks or light-weight walls.
This work was supported by the Research Fund of the Sichuan Science and Technology Program of China (2020YFS0334), Natural Science Foundation of Southwest University of Science and Technology (19zx7130) and and State Key Laboratory of Solid Waste Reuse for Building Materials (SWR-2021-001).
- 1. M.Z. Borhan, T.Y. Nee, J. Nanostructure Chem. 5 (2015) 71-76.
-

- 2. M.J. Gazquez, J.P. Bolivar, F. Vaca, R. García-Tenorio, A. Caparros, Cem. Concr. Compos. 37 (2013) 76-81.
-

- 3. S.M. Perez-Moreno, M.J. Gazquez, and A.G. Barneto, J.P. Bolivar. Thermochimica Acta 552 (2013) 114-122.
-

- 4. J. Zhang, Y. Yan, Z. Hu. Constr. Build. Mater. 171 (2018) 109-119.
-

- 5. D. Han, G. C. Lee, G. Y. Lee, J. Ceram. Process. Res. 17[2] (2016) 129-137.
- 6. K.-H. Cho, J.-W. Ahn, and S.-C. Ur, J. Ceram. Process. Res. 18[9] (2017) 659-665.
- 7. M.J. Gázquez, J.P. Bolívar, R. García-Tenorio, F. Vaca, J. Hazard. Mater. 166 (2009) 1429-1440.
-

- 8. B. Guan, L. Yang, and H. Fu, et al. Chem. Eng. J. 174 (2011) 296-303.
-

- 9. T. Sievert, A. Wolter, and N.B. Singh, Cem. Concr. Res. 35 (2005) 623-630.
-

- 10. M. Singh, M. Garg, Cem. Concr. Res. 30 (2000) 571-577.
-

- 11. S. Seufert, C. Hesse, F. Goetz-Neunhoeffer, J. Neubauer, Cem. Concr. Res. 39 (2009) 936-941.
-

- 12. M. Murat, A.E. Hajjouji, and C. Comel, Cem. Concr. Res. 17[4] (1987) 633-639.
-

 This Article
This Article
-
2022; 23(6): 778-782
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.778
- Received on Nov 5, 2021
- Revised on Mar 9, 2022
- Accepted on Mar 22, 2022
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hongbin Tan
-
aState Key Laboratory of Environment-friendly Energy Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang Sichuan 621010, China
bShaanxi Engineering Center of Metallurgical Sediment Resource, Shaanxi University of Technology, Hanzhong Shaanxi 723000, China
Tel/Fax: +86 816 2419022 - E-mail: hb-t@163.com







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.