- Effect of Al modified Li4Ti5O12 anode/activated carbon cathode for advanced hybrid supercapacitors
Ye-Wan Yooa, In-Ho Imb,* and Seung-Hwan Leea,*
aDepartment of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
bDepartment of Electrical Engineering, Shin Ansan UniversityThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this paper, we successfully fabricated Al-modified Li4Ti5O12 in one-step, easily, simply, and quickly. The structural properties of Li4Ti5O12 by Al modification were favorable to electrochemical activity compared to pristine Li4Ti5O12, and thus, it was confirmed that electrochemical performances such as cell balancing and initial discharge capacitance were effectively improved. The optimized anode/cathode thickness was selected 70 μm/240 μm. Al modified Li4Ti5O12 realized high discharge capacitance of 61 F/g. Therefore, Al modification can be considered as one of the effective methods for the electrochemical performances of Li4Ti5O12 anodes for next-generation hybrid supercapacitors
Keywords: Li4Ti5O12, Al modification, Optimized anode/cathode thickness, Hybrid supercapacitors
Currently, with interest in electric vehicles, electric motorcycles, uninterruptible power supplies, and new and renewable energy, research on energy storage devices such as lithium-ion batteries, supercapacitors, and fuel cells is being actively conducted [1, 2]. Until now, lithium-ion batteries have been applied in most places due to their high energy density, but studies are being conducted to prevent safety accidents such as low power density, short lifespan, and fire and explosion. However, there is a limit to reliably solving these shortcomings in terms of the energy storage mechanism of the lithium-ion battery. Therefore, although the energy density is low, studies on high power density, infinite lifespan, and safe supercapacitors are in progress. Recently, in order to improve the low energy density of the existing supercapacitor, interest in a hybrid supercapacitor using a lithium ion battery and an electrode of a supercapacitor one by one is increasing [3-5]. The hybrid supercapacitors are designed to combine the advantages of supercapacitors and lithium-ion batteries.
Among the various components, it is the anode that affects the electrochemical performances of hybrid supercapacitors. Among various anode candidates such as TiO2, H2Ti12O25, Li4Ti5O12, Nb2O5, α-Fe2O3, LiCrTiO4, MoO2 and Li4Ti5O12 is the most promising anode candidate because it possesses almost infinite lifetime by zero-strain [1-3]. However, since Li4Ti5O12 is also an insulator with low conductivity, studies such as morphology control, doping, and coating are being conducted to improve it. Among them, doping is the easiest and most effective way to definitely improve electrochemical performances [3, 6]. Therefore, studies have been reported to improve the electrochemical performance by doping cations such as Cr, Ni, Sr, Ta and La and anions such as F and Br.
In this paper, an Al modified Li4Ti5O12 anode was fabricated using Al2O3, one of the most economical materials among various dopants, to fabricate a hybrid supercapacitor.
Granuel-Li4Ti5O12 powder was prepared via spray drying method with precursor slurry and afterwards sintered in air at 790 oC for 8 h. The precursor slurry was fabricated using Li2CO3 and TiO2. The molar ratio of Li2CO3 and TiO2 was 4:5. The powder was mixed in ethyl alcohol for 20 h with ZrO2 ball for homogeneous mixing. The slurry was atomized at 205 oC via a two-fluid nozzle with atomizing pressure (3.8 kg/cm2). The commercial activated carbon was selected as a cathode of hybrid supercapacitors. For Al-modified Li4Ti5O12, the Li4Ti5O12 and Al2O3 are mixed in a weight ratio of 99.5 to 0.5, and heat treatment was performed at 790 °C for 3 h.
The Al-modified Li4Ti5O12 anode was produced by the subsequent process: to prepare a slurry, anode powder, conductive Super P (carbon black) and polyvinylidene fluoride (PVDF) binder were blended with a weight ratio of 85:6:9. The N-Methyl pyrrolidone (NMP) was added for slurry formation. The weights of cathode and anode were 2.8 g and 3.2 g, respectively. The blended slurry was cast on Al foil with 110 mm thickness and then dried at 100 oC to eliminate the NMP. The Al foil was compressed with 75-85 mm thickness. After that, the hybrid supercapacitor was dried in a vacuum oven for 40 h to eliminate the moisture and impregnated with a 1.7 M LiBF4 solution in dimethyl carbonate (DMC):ethylene carbonate (EC).
The crystallinity and microstructure of the pristine and Al modified Li4Ti5O12 particles were measured via X-ray diffraction (XRD) and field emission scanning electron microscope (FESEM). The electrochemical performances were confirmed by an Arbin BT 2042 in the voltage range of 0-2.8 V.
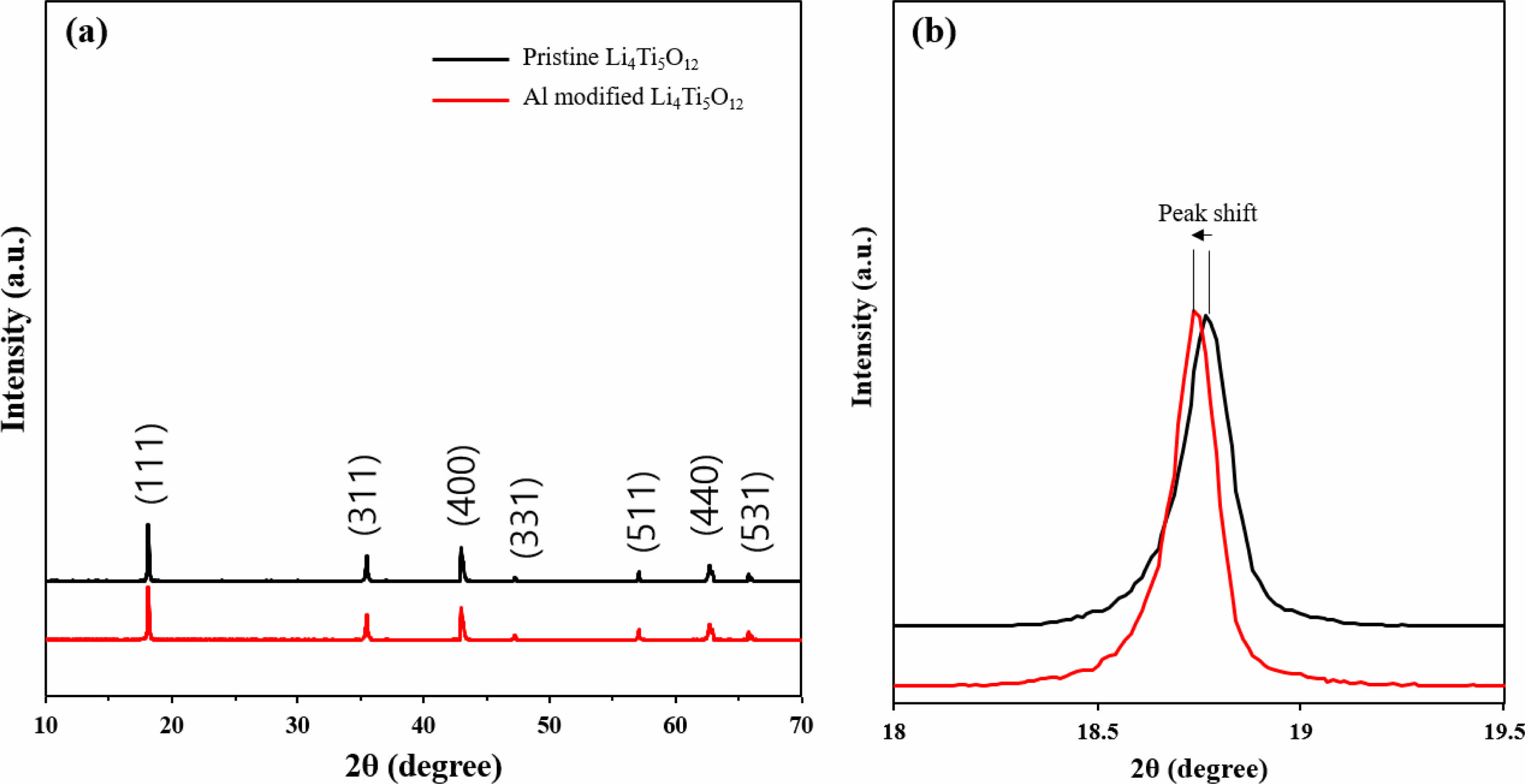
Figure 1 shows the XRD patterns of Al-modified Li4Ti5O12. The (111), (311), (400), (331), (511), (440) and (531) peaks with cubic spinel structure (Fd3m space group) are located at 18.8, 35.2 43.4, 47.7, 57.2, 62.9 and 66.3, respectively (JCPDS file no. 26-1198) [4]. This result is consistent with the typical Li4Ti5O12 peaks. Also, no significant secondary peaks is confirmed. As a result, it can be inferred that Al-modified Li4Ti5O12 is successfully synthesized. As shown in Fig. 1(b), we can confirm that the (111) peak of Al-modified Li4Ti5O12 is shifted to a lower angle compared to pristine Li4Ti5O12, which indicates successful modification of Al. The some of Al3+ (0.52 Å) of Al-modified Li4Ti5O12 leads to reduction/oxidation of Ti4+/Ti3+, indicating charge compensation. It can change the lattice plane of Li4Ti5O12 since the Ti4+ and Ti3 have different ionic radii of 0.68 Å and 0.76 Å, respectively. The bigger lattice parameters spacing via Al substitution, resulting in rapid lithium ion transfer.
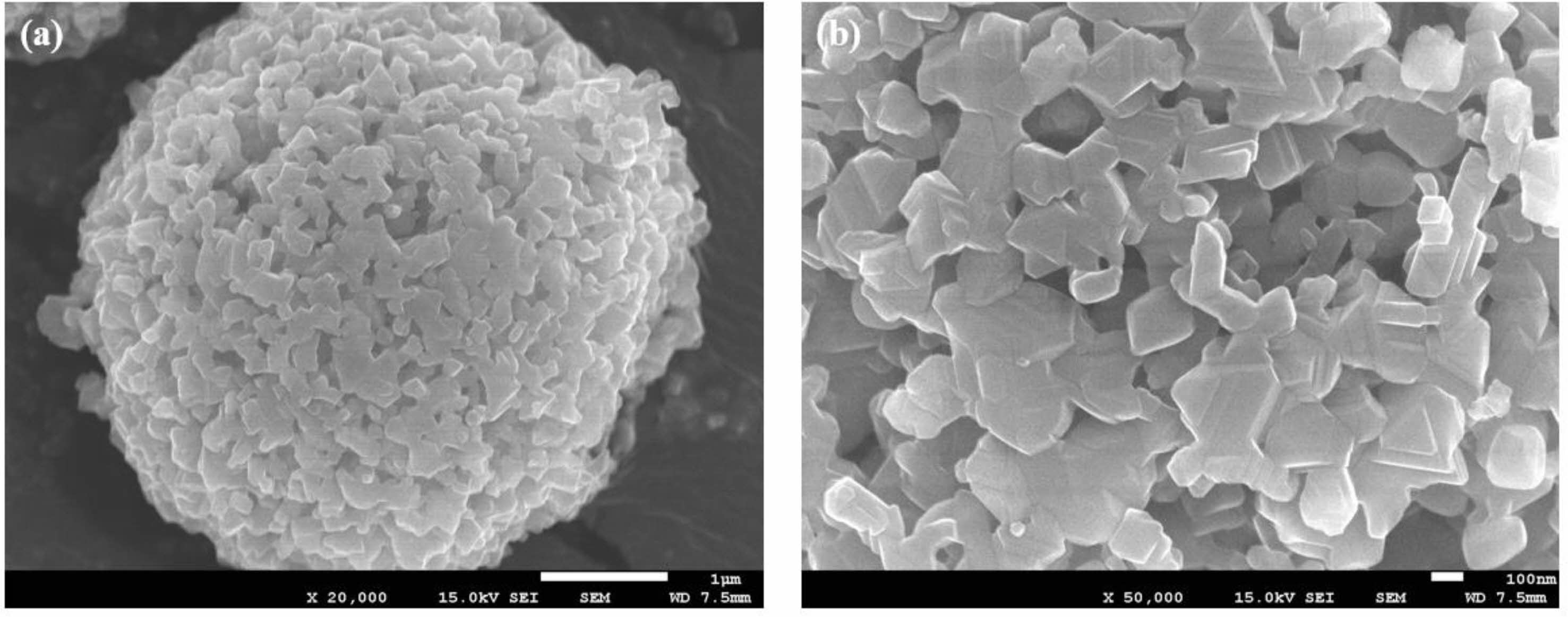
Figure 2 shows the microstructure and morphology of Al-modified Li4Ti5O12. Al-modified Li4Ti5O12 has a spherical shape like typical Li4Ti5O12, and the size of secondary particles is about 4.8 μm. The secondary particles consist of numerous primary particles with 300-500 nm. In general, such porous structure of Al-modified Li4Ti5O12 in with numerous pores is thought to facilitate fast and smooth movement of lithium ions and electrons due to sufficient wetting of the electrolyte [5, 6].
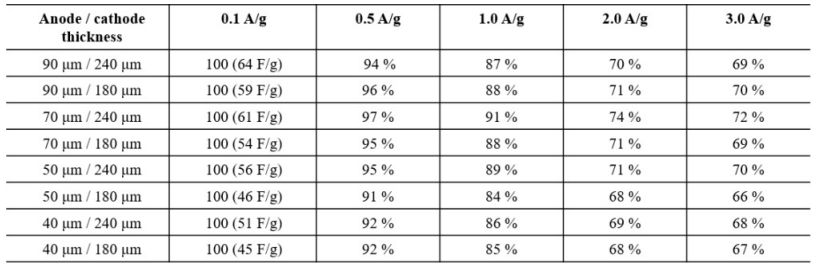
In order to measure the electrochemical performances of the hybrid supercapacitor with Al-modified Li4Ti5O12 anode, the discharge capacitance retention with the various electrode thickness, as shown in Table 1. The capacity retention tends to decrease as the electrode thickness increases. When the current density of 0.1 A/g, the capacitance is proportional to the electrode thickness. It can be explained by sufficient movement of lithium ions and electrons. However, it can be confirmed that the capacitance decreases at high current density (1.0 A/g and 2.0 A/g). It is closely related to the relatively slower kinetics of lithium ions in the anode than BF4- ions in the cathode, which move relatively faster. Therefore, only the upper part of the anode participates in the electrochemical reaction. In the cathode, capacitance retention increases with thickness regardless of current density. As a result, the 90 um anode and 240 um cathode have the highest capacitance at 0.1 A/g, but have low capacitance retention at high current density. Therefore, 70 um of anode and 240 um of cathode were selected as thicknesses showing the optimal electrochemical performance (72 % at 3.0 A/g) [1, 2].
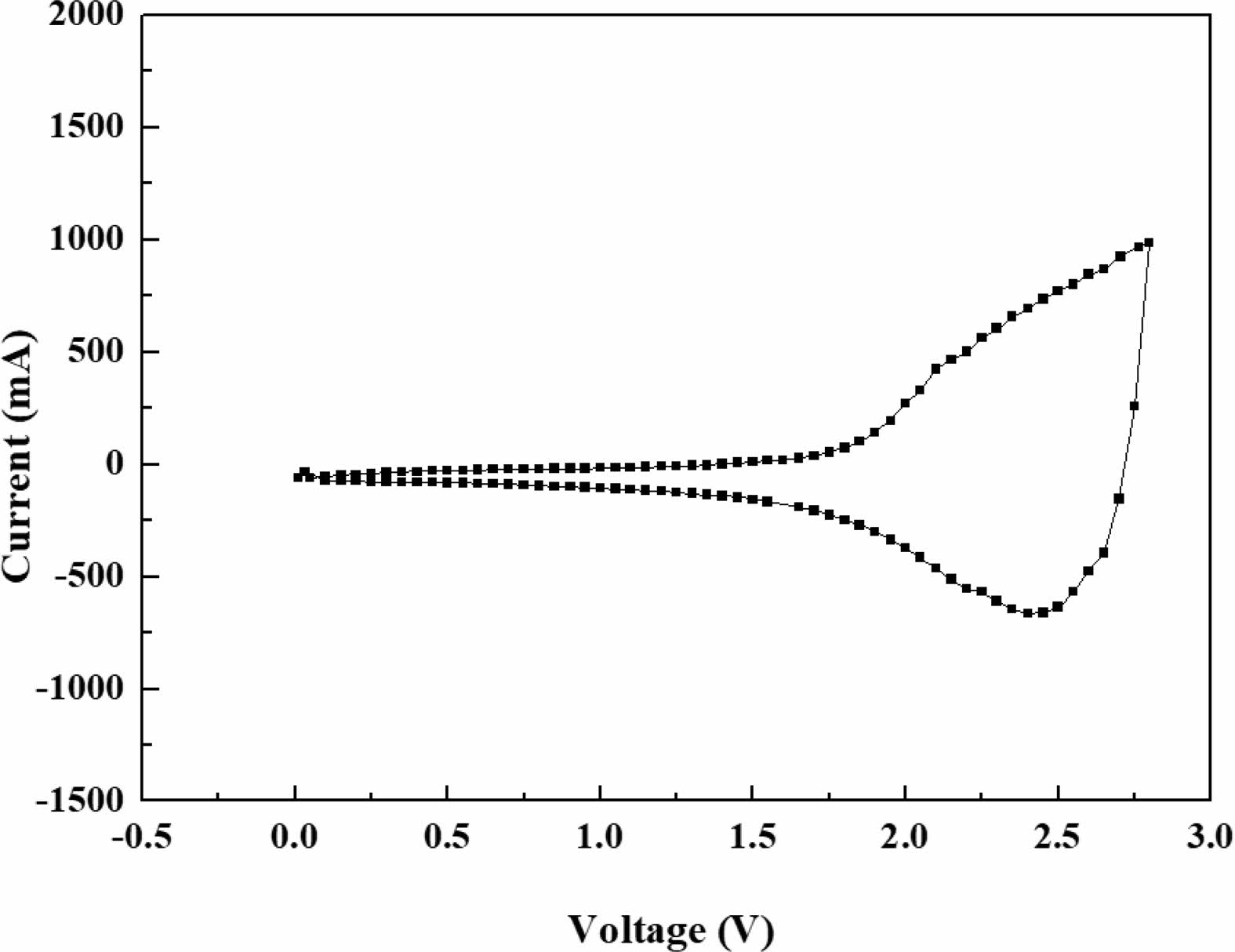
Figure 3 shows the cyclic voltammetry (CV) curves at a scan rate of 10 mV/s in the voltage of 0-2.8 V since working voltage of activated carbon (cathode) is 4.3 V and Al-modified Li4Ti5O12 (anode) is 1.5 V. The shape of CV curve is similar to the previously reported hybrid supercapacitors. The movement of lithium ions at the anode and BF4- ions at the cathode can be confirmed from the CV curve. Below a voltage of 1.5 V, a small and similar amount of charging and discharging occurs only by lithium ions. The BF4- ions at the cathode do not affect the capacitance. Therefore, it is called the “mirror effect” [7, 8]. Above 1.5 V, both lithium ions and BF4- ions participate in electrochemical activity. Among them, BF4- ion is a major factor and lithium ion is a minor factor for capacitance. The oxidation and reduction peaks of the hybrid supercapacitors are about 2.52 V and 2.35 V, respectively. Such small polarization of the oxidation and reduction peaks indicates the excellent reversibility of the hybrid supercapacitors [9, 10]. In the CV curve, the inner area is proportional to the total capacitance of hybrid supercapacitor. Therefore, it can be inferred that Al modified Li4Ti5O12 can possess higher capacitance than pristine Li4Ti5O12. This is because Al modified Li4Ti5O12 has greater electrical conductivity compared to pristine Li4Ti5O12.
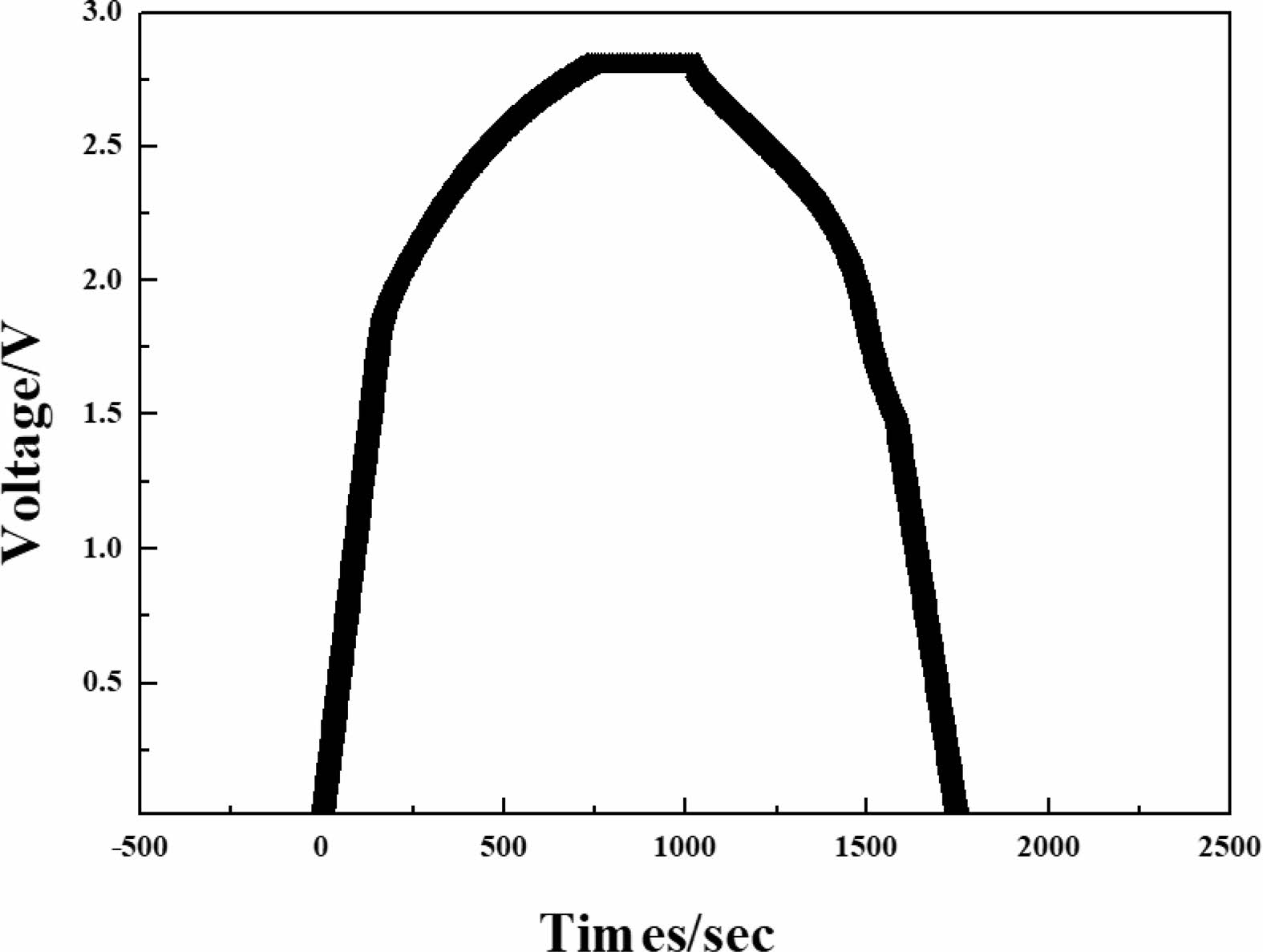
Figure 4 shows the initial charge-discharge curves of the hybrid supercapacitor with Al modified Li4Ti5O12 anode/activated carbon cathode. The specific capacitance of the hybrid supercapacitor can be calculated using following equation [11, 12]:

where ‘C’ is the capacitance, ‘q’ is the charge, ‘ΔV’ is the potential range, ‘m’ is the mass of the active materials, ‘i’ is the current, and ‘t’ is discharge time. The discharge capacitance of the hybrid supercapacitor with Al modified Li4Ti5O12 anode/activated carbon cathode is 61 F/g at current density of 0.1 A/g. This capacitance is higher than that of conventional hybrid supercapacitors. This is related to the internal resistance called IR drops, which can be calculated as [13, 14]:

where ‘Vcharge’ is the end charge voltage, ‘Vdischarge’ is the starting discharge voltage and ‘I’ is the current. The IR drop value of hybrid supercapacitor is 0.19 Ω at 0.1 A/g. It is well known that IR drop can affect the electrochemical performances. This is because IR drop is related to the different mechanism of both Faradaic process on anode and non-Faradaic process on cathode [15]. It is judged that the electrochemical properties have a positive effect by the low IR drop of Al modified Li4Ti5O12.
|
Fig. 1 XRD pattern of Al modified Li4Ti5O12. |
|
Fig. 2 FESEM images of Al modified Li4Ti5O12. |
|
Fig. 3 CV curves of Al modified Li4Ti5O12. |
|
Fig. 4 nitial charge-discharge profiles of Al modified Li4Ti5O12. |
|
Table 1 Discharge capacitance retention of Al modified Li4Ti5O12 with different anode/cathode thickness. |
In this study, we successfully synthesized Al-modified Li4Ti5O12. In addition, an asymmetric hybrid supercapacitor was fabricated using an Al-modified Li4Ti5O12 anode and an activated carbon cathode, and electrochemical performances such as cell balancing and initial discharge capacity were measured. Al-modified Li4Ti5O12 displayed the identical crystal structure and morphology as Li4Ti5O12. To optimize of electrochemical performances, capacitance retention was calculated with various thickness of anode and cathode. In addition, high initial discharge capacity and low IR drop were obtained through Al modification. As a result, we can conclude the Al modification as one of the methods that can compensate for the shortcomings of Li4Ti5O12 anode.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1055979).
- 1. J.H. Lee, H.K. Kim, E. Baek, M. Pecht, S.H. Lee, Y.H. Lee, J. Power Sources 301 (2016) 348-354.
-

- 2. H. Park, D. Kim, Trans. Electr. Electron. Mater. 22 (2021) 80-90.
-

- 3. B.G. Lee, S.H. Lee, J. Power Sources 343 (2017) 545-549.
-

- 4. S.H. Lee, K.Y. Kim, J. R. Yoon, NPG Asia Materials 12 (2020) 28.
-

- 5. S.H. Lee, J.M. Kim, Mater. Lett. 228 (2018) 220-223.
-

- 6. S.H. Lee, J.M. Kim, Energy 150 (2018) 816-821.
-

- 7. S.H. Lee, I.H. Im, Mater. Lett. 231 (2018) 38-42.
-

- 8. S.H. Lee, B.S. Jin, H.S. Kim, B.G. Lee, Inter. J. Hydro. Energy 44 (2019) 3013-3020.
-

- 9. H.K. Kim, S.H. Lee, Energy 109 (2016) 506-511.
-

- 10. H.J. Choi, J.H. Kim, H.K. Kim, S.H. Lee, Y.H. Lee, Electrochim. Acta 208 (2016) 202-210.
-

- 11. M. Secchiaroli, S. Calcaterra, R. Marassi, M.W. Mehrens, S. Dsoke, ChemElectroChem 7 (2020) 1631-1643.
-

- 12. Y. Chikaoka, E. Iwama, S. Seto, Y. Okuno, T. Shirane, T. Ueda, W. Naoi, M.T.H. Reid, K. Naoi, Electrochim. Acta 368 (2021) 137619.
-

- 13. M. Khairy, W.A. Bayoumy, K.F. Qasim, E.E. Shereafy, M.A. Mousa, Materials Science and Engineering: B 271 (2021) 115312.
-

- 14. J. Zhang, S. Wang, G. Xu, J. Alloy. Comp. 856 (2021) 158110.
-

- 15. M. Uceda, H.C. Chiu, R. Gauvin, K. Zaghib, G.P. Demopoulos, Energy Storage Materials, 26 (2020) 560-569.
-

 This Article
This Article
-
2022; 23(6): 774-777
Published on Dec 31, 2022
- 10.36410/jcpr.2022.23.6.774
- Received on Sep 17, 2021
- Revised on Nov 26, 2021
- Accepted on Nov 29, 2021
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- In-Ho Im b and Seung-Hwan Lee a
-
aDepartment of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
Tel : +82-31-490-6055 Fax: +82-31-495-7828
bDepartment of Electrical Engineering, Shin Ansan University
Tel : +82-33-250-6265 Fax: +82-33-259-5545 - E-mail: iminho@sau.ac.kr, shlee@kangwon.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.