- Synthesis of mesoporous zeolite catalysts from kaolin via low-temperature tailoring and its applications
Pranav Sankarana, Kannan Kandasamya,*, Aravindh Rajagopalanb and Boopathy Raja Chenniappanc
aDepartment of Chemical Engineering, Kongu Engineering College, Erode
bDepartment of Chemical and Material Sciences, Amirta Viswa Vidyapeetham, Coimbatore
cSolid State Electronics(SEES), Zacatanco, CInvestav, Mexico CityThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Kaolin clay mineral is transformed into cage-type catalysts with acid sites through a hydrothermal process at three different temperatures namely 100, 125, and 150 °C using N-propyl amine at three different time durations between 24 to 48 hours. The catalysts were supported with silicon dioxide. Supported and unsupported catalysts were characterized by SEM, TEM, FTIR, BET Surface area Analysis, and TGA. The catalysts synthesized indicated the presence of nearly uniform-shaped particles with good surface area and thermal stability. The acid site concentration in the catalysts is improving upon synthesis at low temperature and limited time duration. The catalysts were found to be effective in improving the selectivity for the cyclization of citronellal to isopulegol
Keywords: Kaolin, Mesoporous catalysts, Hydrothermal synthesis, Organic amines, Sustainability
Organic synthesis aided by catalysts is a pivotal field of study, which has its impact on various applications like production of fine chemicals, petrochemical processes and bio-fuel synthesis [1]. Materials like Zeolites find applications in a large variety of catalytic reactions. They exhibit excellent shape-selective behavior because of distinct microporous structure, organized acid sites and impressive thermal stability [2]. The spatial confinement effects enable these materials to act as excellent catalysts and adsorbents [3]. Zeolite materials like ZSM-5, MCM 41 are tailored to achieve excellent crystallo-graphic architecture, so that the shape-selectivity of the catalysis is controlled [4].
The synthesis of zeolites has been experimented using various precursors, surface directing agents and templating agents [5]. Hydrothermal synthesis is a promising technique for synthesis of various zeolite materials with optimized porous structure and acid sites [6]. This method has also been applied for re-crystallization of multiple zeolites into composite catalysts and such catalysts indicate good performance in organic synthesis reactions [7]. However, such synthesis techniques have sustainability issues due to usage of synthetic precursors, templating agents and high temperature processing.
In order to make the synthesis process eco-friendly, it is essential to utilize sustainable precursors, green templating agents and low temperature processing [8]. Natural materials and wastes that are rich in silica and alumina are experimented for zeolite synthesis to eliminate the usage of synthetic silica and alumina.
An innovative approach to use rice husk ash as the silica source and waste aluminum cans as alumina source for zeolite synthesis is reported. Acid and alkali treatment is used to transform these precursors into nano-zeolites. The above zeolite has been effectively utilized for adsorption of Cobalt, Copper and Zinc Ions [9]. Alkali fusion has been used for synthesis of Zeolite A from fly-ash, which was effective in adsorption of Chromium [10]. However, alkali/acid fusion has limited impact on generation of mesopores and acid sites in the prepared zeolites. Therefore, the above mentioned strategy is not suitable for preparation of shape selective catalysts. Clay minerals like kaolin have alumino-silicate structure incorporating substituted minerals. They exhibit excellent thermal stability enabling them to be used in catalytic processes [11]. HS zeolite was synthesized using natural kaolin via hydrothermal transformation and it was used as membrane [12]. Bentonite which has similar properties, is also largely used in pollution abatement [13]. Both Kaolin and Bentonite can be transformed into catalysts and adsorbents by mechano-chemical synthesis.
Kaolin based catalysts were utilized for de-butylation reaction and they indicated good selectivity for the synthesis [11]. Kaolin is also found to exhibit good surface area and it has been applied as a catalyst carrier for oxidative desulfurization using vanadium pentoxide catalyst [12]. Tungsten and molybdo-phosporic acid were impregnated on acid treated kaolin and the catalyst was used for biginelli reaction [13]. Kaolin was transformed into a catalyst for acetalization of glycerol by acid activation. Here, the synthesized catalysts indicated slightly unorganized morphology and very small pore size. However, the yield of solketol from the catalysis is found to improve upon acid activation [14].
From the above works, it is evident that kaolin exhibits intrinsic catalytic performance, surface properties. However, it is crucial to transform the clay mineral into well organized structures with mesopores like zeolites to apply them for complex reactions like biofuel synthesis, methanation of Carbon dioxide, etc. Hydrothermal synthesis has been familiar in synthesis of zeolite materials from different kinds of precursors. Zeolite A has been prepared by hydrothermal method using fly-ash as a starting material [15] Siliceous mudstone was utilized to prepare ZSM-5 using hydrothermal synthesis [16]. This technique is also successful in synthesis of zeolites from kaolin minerals. Zeolite 4A was synthesized from kaolin using hydrothermal and one pot fusion method with sodium hydroxide and aluminum hydroxide as agents. It is indicated that the silica-alumina proportions can be adjusted to achieve high purity of zeolites [17]. N type zeolites have been synthesized using alkali-based hydrothermal treatment, and the zeolites exhibited good ion-exchange capabilities [18].
Zeolites synthesized through such methods lack sufficient cage formations to catalyze organic reactions. Zeolite-Beta was prepared using hydrothermal synthesis without templating agents. However, sodium hydroxide and ammonium nitrate were used for the synthesis. The catalysts indicated presence of micro and mesopores with beta cages aiding the esterification of benzoic acid [19]. It is understood that ammonium nitrate is responsible for the acid site creation in the catalyst. Keeping in mind about the devastating explosion of ammonium nitrate in Lebanon, the usage of this chemical substance is disputable. It is also a non-eco friendly approach to synthesize the zeolite catalyst [20].
It is also essential to optimize the temperature for the synthesis of zeolite to reduce the environmental impact. The usage of microwave synthesis, in situ crystallization and direct crystallization are suggested [21]. Organo-nitrogen compounds have been used in the synthesis of zeolites from silica resources like fly-ash under low temperatures [22]. This reduced temperature for synthesis considerably decreases the emissions and energy consumption making the synthesis more sustainable.
The above research works indicate the possibility of eliminating synthetic pre-cursors for zeolite synthesis using kaolin. Green synthesis routes suggest usage of alkali fusion for zeolite synthesis. But this method doesn’t produce active catalysts with acid sites and cages. Usage of unsafe mesoporogens is also not advisable. The presented research work attempts to produce zeolite catalysts under low temperature using kaolin and organic mesoporogens. The physico- chemical characteristics of the catalysts are compared with zeolite 4a and zeolite beta prepared by sustainable methods, to strike a balance in sustainable approaches in catalyst synthesis.
Chemicals
For the catalyst preparation, the precursor Kaolin is purchased from Ashapura minechem Ltd. India. The organic surface directing agent N-propyl amine is procured from Loba chem. India. Silicon dioxide used as support for the catalyst and pyridine which is used for FTIR analysis are purchased from Nice chem. India. Citronellal and Hexane was purchased from TCI chemicals, India.
Catalyst and supported catalyst preparation
Cage type catalysts were prepared by hydrothermal method using an Stainless steel autoclave provided with a Teflon vessel. The technique is chosen as it is capable of giving out hierarchial structures similar to zeolites [23]. Hydrothermal synthesis is carried out in Teflon lined stainless steel reactor at temperatures of 100 °C and 150 °C for times of 24 and 48 hour. Kaolin calcined at 300 °C for 3 hours is mixed with template at a ratio of 2:1. The resultant slurry is fed into the Teflon vessel and the vessel is sealed in the stainless hydrothermal reactor. The reactor is heated to the required temperature in a muffle furnace for the stipulated time span. The catalyst slurry is withdrew from the reactor, dried at 100 °C and calcined. The support with catalyst is dried for 6 hours to remove the water at 100 °C and calcined for 2 hours at 500 °C. The synthesized catalysts were named according to the time and temperature of synthesis as shown in the table below.
All the catalysts and supported catalysts were characterized by Scanning Electron Microscopy (SEM) (Sigma ZEISS at 5 kV with gold sputtering) and Transmission Electron Microscopy (TEM) (JEOL JEM 2100) to understand the morphology. The SEM and TEM analyses were done at PSG College of Technology, Coimbatore. Further, the pore geometry and surface area were determined by Brunauer-Emmett-Teller (BET) (MicroTrac-Belsrop Max) analysis. The thermal stability of the catalysts were understood by Thermo-gravimetric Analysis (TGA) (TGA2 SF/1100). The presence of acid sites on the catalysts were evaluated by Pyridine adsorbed FTIR (SHIMADZU IR Affinity-1S) analysis. The BET, TGA and FTIR analyses were carried out at Amirta Viswa Vidyapeetham.
Morphological characterization of catalyst
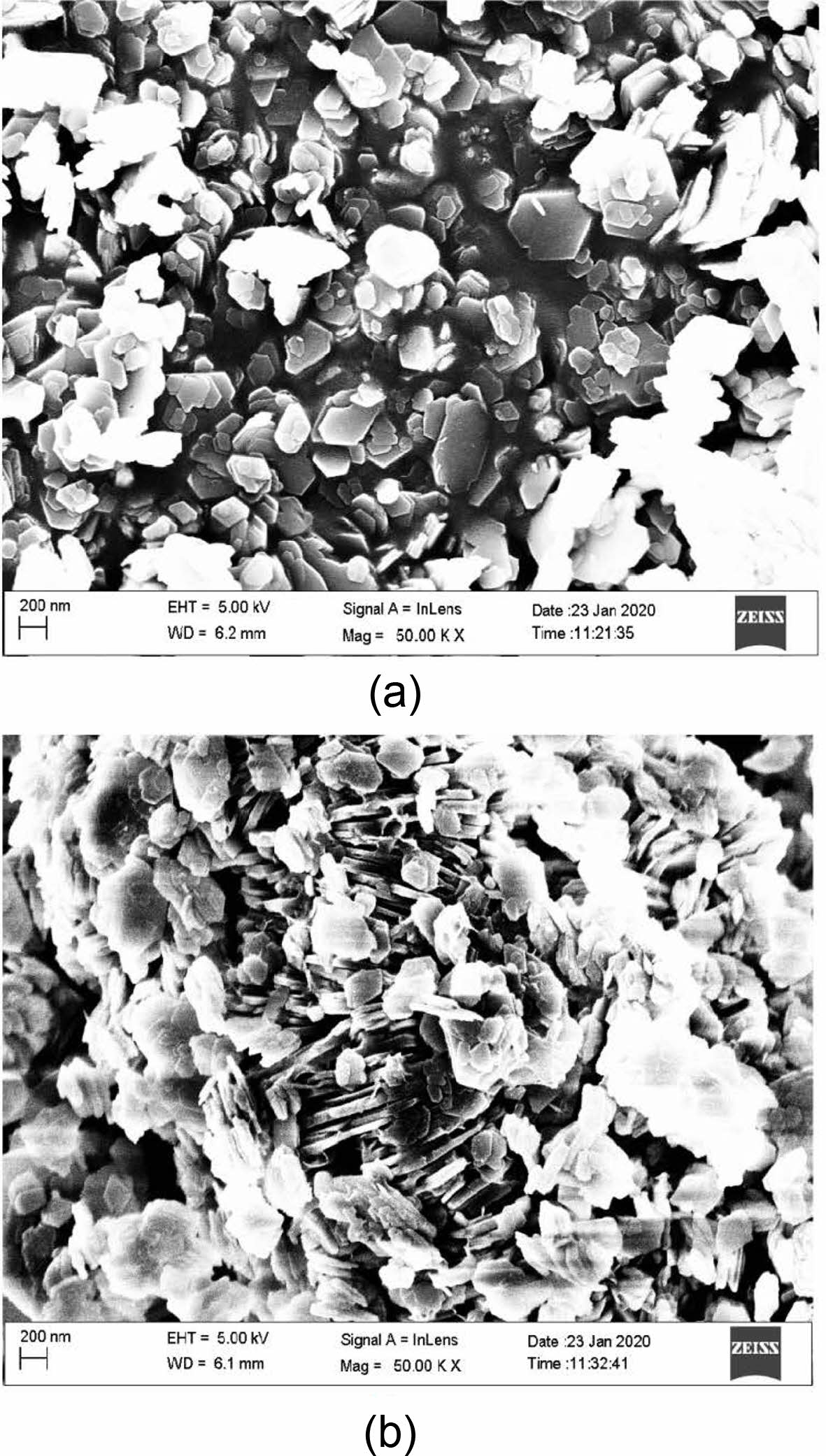
The SEM images of the catalysts synthesized at different temperatures are presented. The sample synthesized at low temperature (100 ℃) and shorter duration (24 hours) indicated more uniform cluster formations, as shown in Fig. 1(a). The cluster showed formation of hexagonal structures, which could be hexagonal cages. On the other hand, the sample synthesized at 150 ℃ and 48 hours showed larger and smaller hexagonal clusters with random orientations. This is shown in Fig. 1(b). Therefore, it is evident that the uniformity of clusters formed is disturbed by excess heating.
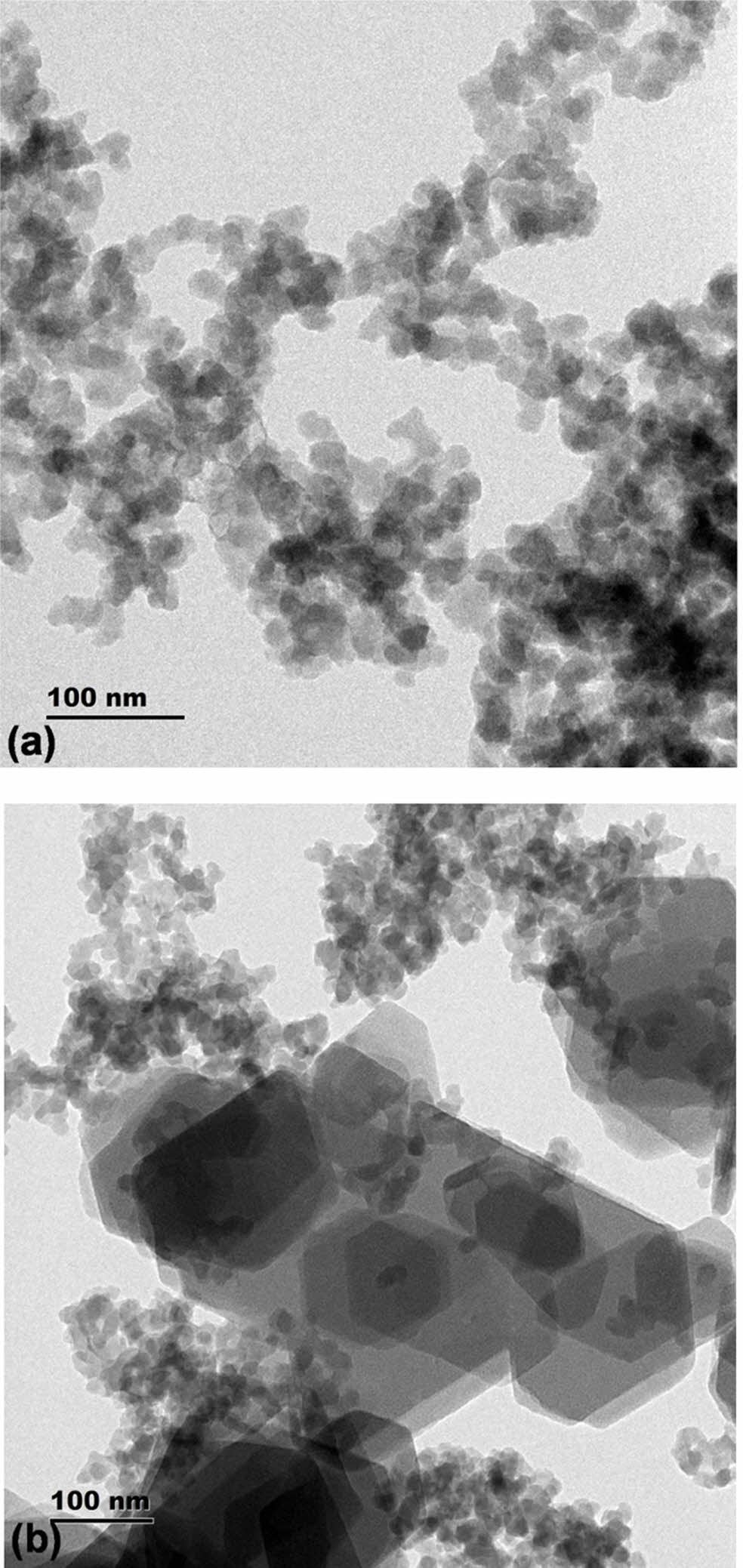
The transmission electron microscopy images indicated the presence of distinct hexagonal cages aggregated as clusters. It was also evident that the clusters are present one above other, which is indicated by the dark zones. The catalysts showed presence of similar clusters which are aggregated one above other. The cages were found to be hexagonal with different sizes. This once again indicates the presence of hierarchical cages. The sample synthesized at low temperature indicated more uniform sized clusters, while the sample synthesized at high temperature indicated the presence of large and small clusters as shown in Fig. 2(a) and (b). Thus, the uniformity of cages is strongly influenced by temperature of the synthesis.
Both SEM and TEM images confirm formation of uniform shaped hexagonal cages. In comparison with the zeolite beta catalyst synthesized without any templating agents, these catalysts show uniform cluster formation [17]. On the other hand, Zeolite 4a synthesized using one pot fusion using Sodium hydroxide and aluminum hydroxide indicated better uniform sized clusters [18]. Therefore, the usage of n-propyl amine as templating agent is effective in creation of cages. These cages are molecular sieving zones and they can act as facilitators of selectivity. Further, the effect of n-propyl amine on acid site creation has to be investigated. Also, the surface and pore characterization can give insight about the ability of catalyst to exhibit shape-selectivity.
Surface acidity of the catalyst
Pyridine adsorbed Fourier Transform Infra-red Spectroscopy has been profound for analysis of catalytic sites and cage structures. The acid site concentration of the catalyst is determined by SHIMADZU IR Affinity-1S with a wavelength of 400 to 4000 cm-1. The prepared catalyst was calcined at 600 °C for 3 hours and 2 grams of catalyst was taken for pyridine adsorption. The catalyst was loaded in a closed container and the surface of the catalyst was wetted with pyridine. The catalyst with the closed container was heated in hot air oven up to 60 °C for 20 minutes to remove physisorbed pyridine from the surface. The dried samples were withdrawn, powdered and used for analysis [24].
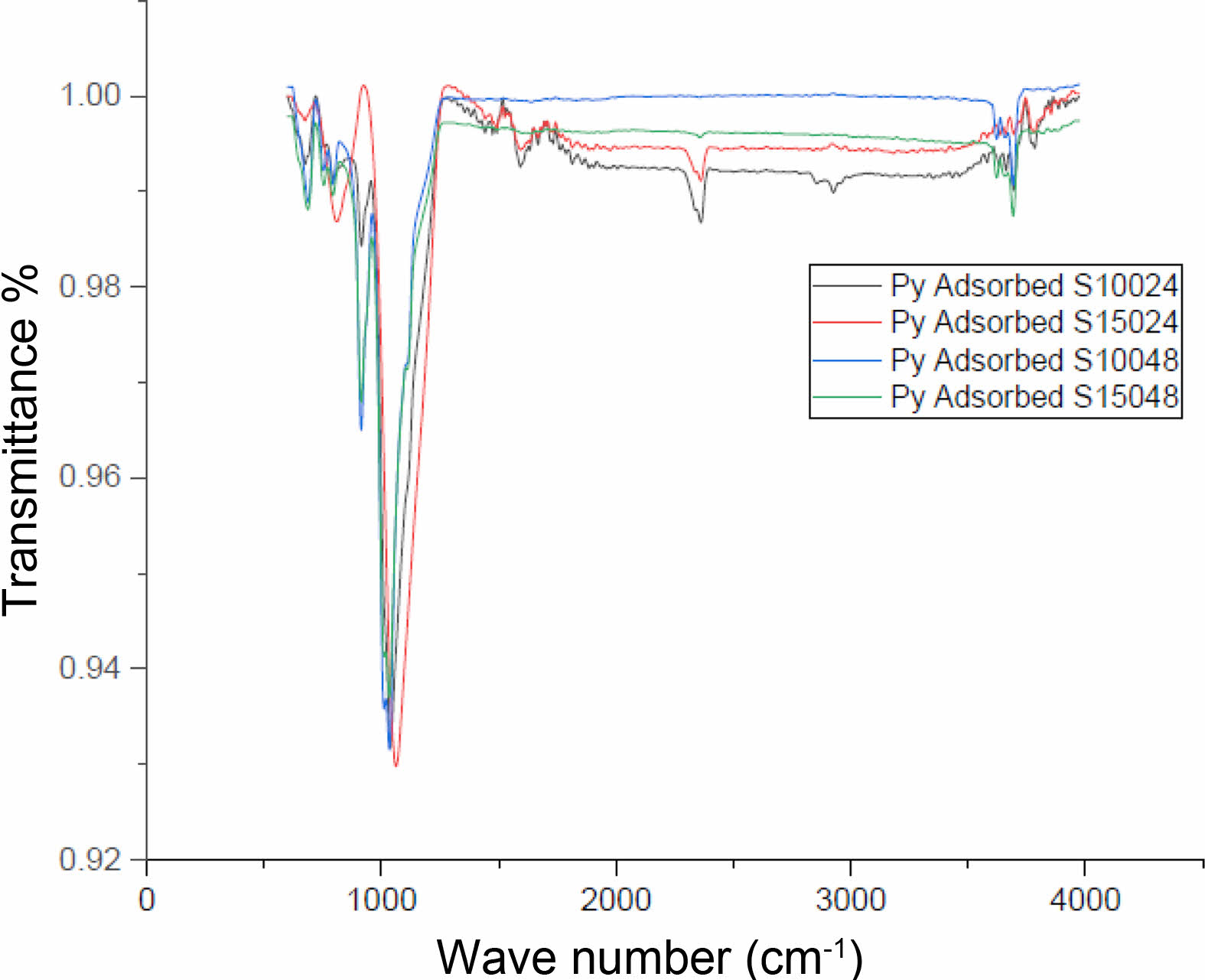
The transmittance plots of kaolin and the synthesized catalysts are indicated in Fig. 3. The catalyst synthesized at higher temperature (150 ℃) and longer time (48 hours) indicated very minimal elongation and stretching of transmittance plots. This could be because of sintering of clay materials due to interaction with the pillaring agent for longer duration at high temperature. The availability of molecules for pyridine adsorption and interaction with IR radiation is low. On the other hand, the catalyst synthesized for 24 hours 100 ℃ indicated transmittance spectra with more elongation and stretching. Catalyst synthesized at 150 ℃ for 24 hours also indicated similar spectra. Hence, it is understood that catalyst synthesis for longer duration may not be improving the catalytic properties. The catalyst shows the acid sites in 1400 to 1700 cm-1. The peak at 1480 cm-1 shows the Lewis acid site and the peak at 1590 cm-1 shows the Bronsted acid site. The both catalysts which are prepared at 24 hours shows the maximum acid site concentration but the catalyst prepared at 48 hours shows negligible acid sites. The increase in Bronsted acid site is because of the dealumination of kaolin during the synthesis of catalysts. Bronsted acid sites are associated with low energy organic synthesis reactions like isomerization, cyclization, etc. All the spectra indicated presence of sodalite cages. Hence, the synthesized materials are resembling zeolite structures and have good potential to be applied as catalysts. Fig. 4
Table 1
Pore and surface characteristics
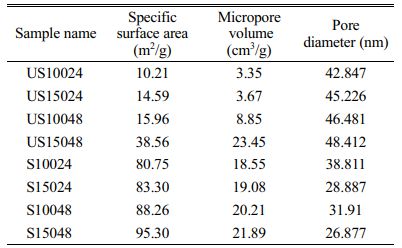
The pore size and surface area of the raw catalyst and supported catalyst is determined by Nitrogen adsorption method. The nitrogen is adsorbed by 0.3 grams of catalyst and support at 77 K temperature. The surface area is determined using BET method and the nature of the material is determined using the adsorption-desorption plot. The catalyst materials synthesized indicated formation multi-layer adsorption with increase in pressure. Also, significant capillary condensation is witnessed in all the catalysts. From the adsorption-desorption hysteresis loop it is identified that the catalyst materials are mesoporous. The surface area and pore characteristics are shown in the Table 2 below. Table 3
In comparison with the zeolite beta synthesized without template, the surface area created for the catalysts is significantly low [17]. But the pore volume created in the catalysts is considerably high. In case of shape-selective catalysis, the influence of porous pathway is more important than the surface area of the catalyst. The collision behavior of the reacting species with the pore wall is more crucial in determining the selectivity. Taking the pore diameter and volume into consideration, the catalyst synthesized using n-propyl amine has sufficient pore volume to catalyze organic synthesis reactions and improve the selectivity.
Thermal stability analysis of the catalyst
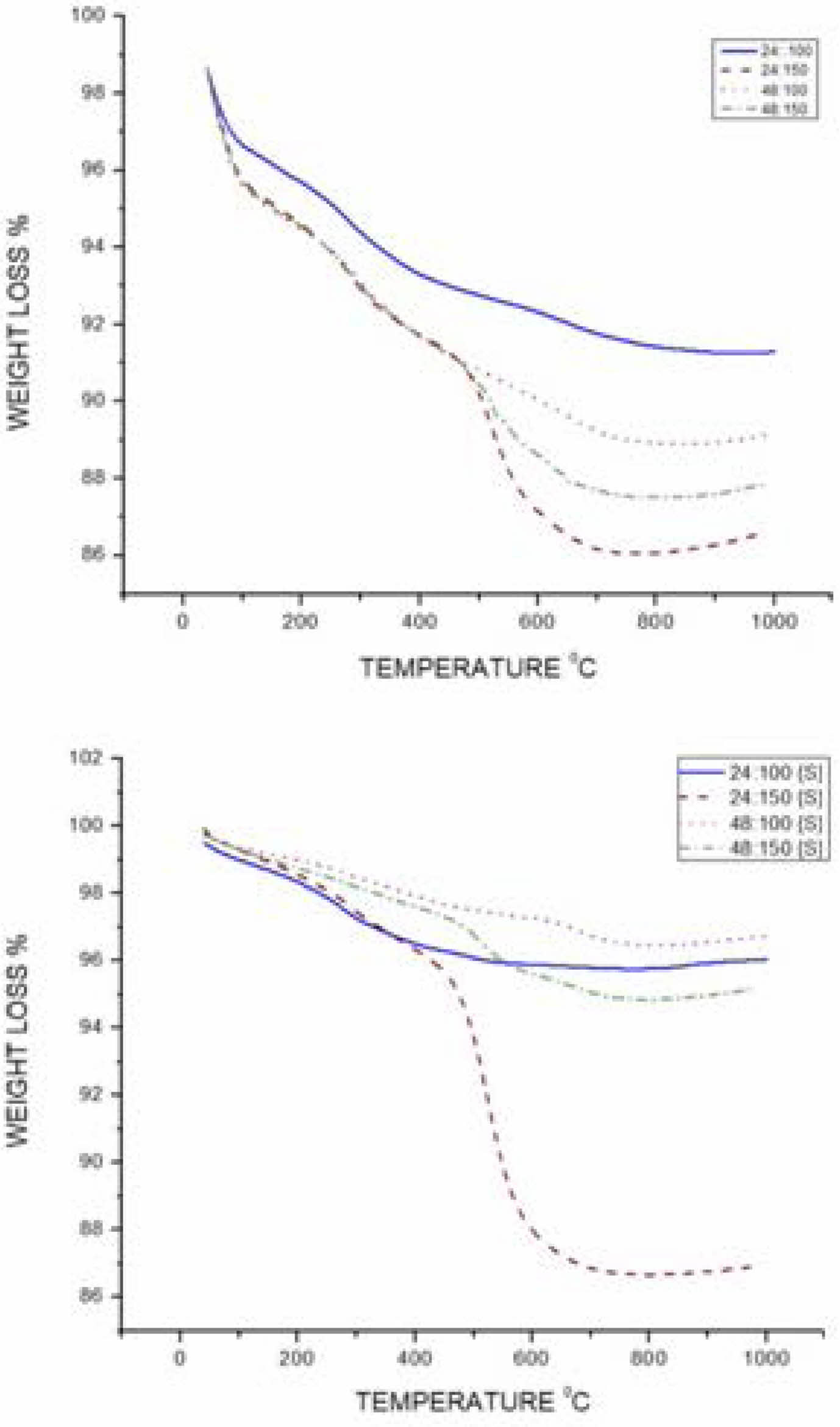
The thermal stability of raw catalyst and support catalyst is determined by TGA by TA instruments in the temperature range of 1100 °C. Nitrogen is used as a purge gas. 2 grams of sample is taken and nitrogen is purged into the chamber from room temperature. Fig. 3 shows the percentage weight loss of kaolin while increase the temperature up to 1000 °C. The graph indicates the weight loss from 40 °C to 200 °C, the water present in both raw and support catalyst is vaporized. There is a drop in weight loss at 500 °C is due to the removal water content at which replace the Alumina during dealumination. At higher temperature the 24 hours and 100 °C sample in both support and raw catalyst shows minimum amount of weight loss. In support catalyst, all the four catalyst drops nearly 2-5% loss. The prepared clay based zeolite can with stand up to 500 °C and its support withstand for 600 °C.
From the thermal stability analysis of the supported and unsupported catalysts, it is clear that the supported catalysts are more suitable for high temperature reactions. Whilst, the unsupported catalysts can be used to bring out low temperature organic synthesis reactions like cyclization, isomerization, etc.
Effect of catalytic properties on cyclization
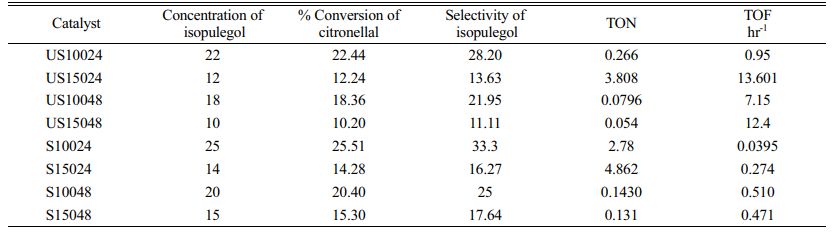
The reaction mixture withdrawn at different durations were characterized by GC-MS from Perkin Elmer clarus 680, with Helium as carrier gas. The column was operated at 65 °C and raised up to 300 °C. Reaction parameters namely yield, conversion and selectivity after 30 minutes were calculated. The turn over frequency and its number was determined for unsupported and supported catalyst. The number is higher for 24 hour sample than 48 hour sample which implies that the rate of reaction is higher in 24 hour sample. The frequency implies the catalytic cycles per time and the results shows the 24 hour sample have the minimum amount of catalytic cycles which is because of its higher surface area. The Raw catalyst shows lesser amount of selectivity than support because of lesser thermal stability of cages present in catalyst also the catalyst which is prepared at 24 hours and 100 °C shows 28.2% of selectivity at 30 min for the conversion of 22.44% of isopulegol which direct us that the cages in this catalyst is more stable than other. Similarly, the support of this catalyst shows 33.3% of selectivity for the conversion of 25.51% of isopulegol.
|
Fig. 1 Scanning electron microscopy images of catalysts prepared at different temperatures. |
|
Fig. 2 Transmission electron microscopy images of the catalysts synthesized at different temperatures. |
|
Fig. 3 Acid site concentration of pyridine adsorbed catalyst. |
|
Fig. 4 TGA of raw and supported catalyst. |
|
Table 3 Turn over frequency of raw and supported catalyst with its selectivity over cyclization of citronellal. |
The presented work is an attempt to identify a sustainable approach to synthesizing cage-type acid catalysts using a very less quantity of n-propyl amine as a templating agent. The formations of distinct cages were evident from the microscopic analysis. The synthesized catalyst was also supported with silica and it was witnessed that the thermal stability and surface area of the catalyst have improved. The surface acidity of the catalyst shows distinct Lewis and Bronsted acid sites. The catalysts synthesized using an organic templating agent showcased better catalytic properties than Zeolite 4A [17] and Zeolite beta [18]. Therefore the experimental work is successful in synthesizing effective catalysts with balanced emissions. Further, the catalysts are applied for the cyclization of citronellal and the selectivity of the reaction is considerably good. This indicates that the prepared zeolite-type catalysts are the potential to be used in catalytic reactions.
This project is supported by Seed Grant Research Scheme, Kongu Engineering College (RefNo: KEC/R&D/SGRS/09/2020).
The authors declare no competing financial interests.
- 1. W.J. Yoo, H. Ishitani, Y. Saito, B. Laroche, and S. Kobayashi, J. Org. Chem. 85 (2020) 5132-5145.
-

- 2. H. Wang, L. Wang, and F.S. Xiao, ACS Cent. Sci. 6 (2020) 1685-1697.
-

- 3. Y. Chai, W. Dai, G. Wu, N. Guan, and L. Li, Acc. Chem. Res. 54 (2021) 2894-2904.
-

- 4. Y. Shen, Z. Qin, S. Asahina, N. Asano, G. Zhang, S. Qin, Y. Ma, Z. Yan, X. Liu, and S. Mintova, J. Mater. Chem. A 9 (2021) 4203-4212.
-

- 5. C. Li, M. Moliner, and A. Corma, Angew. Chem. 57[47] (2018) 15330-15353.
-

- 6. C. Liu, Y. Long, and Z. Wang, Chinese J. Chem. Eng. 26 (2018) 2070-2076.
-

- 7. W. Jin, J. Ma, H. Ma, X. Li, and Y. Wang, J. Solid State Chem. 267 (2018) 6-12.
-

- 8. A. Maghfirah, M.M. Ilmi, A.T.N. Fajar, and G.T.M. Kadja, Mater. Today Chem. 17 (2020) 100348.
-

- 9. E.A. Abdelrahman, Y.G. Abou El-Reash, H.M. Youssef, Y.H. Kotp, and R.M. Hegazey, J. Hazard. Mater. 401 (2021) 123813.
-

- 10. U. Rentsennorov, B. Davaabal, B. Dovchin, and J. Temuujin, J. Ceram. Process. Res. 22[2] (2021) 232-239
-

- 11. A. Kumar and P. Lingfa, Mater. Today: Proc. 22 (2020) 737-742.
-

- 12. M. Kazemimoghadam, J. Ceram. Process. Res. 22[2] (2016) 978-984.
- 13. M.M. Palanisamy and K. Kandasamy, J. Ceram. Process. Res. 21[1] (2020) 75-85.
-

- 14. R. Ismail, W. Almaqtri, and M. Hassan, Chem. Int. 7[1] (2021) 21-29.
- 15. U. Rentsennorova, B. Davaabala, B. Dovchinb, and J Temuujina, J. Ceram. Process. Res. (2021) 22[2] 232-239.
-

- 16. H.T. Tuana, I. Baeb, Y. Jangb, S. Chaeb, Y. Chaeb, and D. Suhra, J. Ceram. Process. Res. (2010) 11[2] 204-208.
- 17. C. Huang, P. Wu, Y. Guo, and Y. Guo, Microporous and Mesoporous Mater. 306 (2020) 110415.
-

- 18. D.S. Aher, K.R. Khillare, L.D. Chavan, and S.G. Shankarwar, RSC Adv. 11 (2021) 2783-2792.
-

- 19. I. Zahid, M. Ayoub, B.B. Abdullah, M.H. Nazir, Zulqarnain, M.A. Kaimkhani, and F. Sher, Sustainability 13 (2021) 2631.
-

- 20. S.K. Kirdeciler and B. Akata, Adv. Powder Technol. 31 (2020) 4336-4343.
-

- 21. Y. Yue, X. Guo, T. Liu, H. Liu, T. Wang, P. Yuan, H. Zhu, Z. Bai, and X. Bao, Microporous and Mesoporous Mater. 293 (2020) 109772.
-

- 22. S. Ur Rehman, R. Ahmed., K. Ma, S. Xu, M.A. Aslam, H. Bi, J. Liu, and J. Wang, Ecotoxicol. Environ. Safety 210 (2021) 111834.
-

- 23. Y. He, S. Tang, S. Yin, and S. Li, J. Clean. Prod. 306 (2021) 127248.
-

- 24. A. Valechha, S.K. Singh, A.S. Al-Fatesh, S. Rayalu, and N. Labhsetwar, Current Science (00113891) 119[1] (2020) 123-128.
-

 This Article
This Article
-
2022; 23(5): 666-671
Published on Oct 31, 2022
- 10.36410/jcpr.2022.23.5.666
- Received on Mar 18, 2022
- Revised on May 14, 2022
- Accepted on Jun 4, 2022
 Services
Services
- Abstract
introduction
materials and method
results and discussion
conclusion
- Acknowledgements
- Declaration
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kannan Kandasamy
-
Department of Chemical Engineering, Kongu Engineering College, Erode
Tel : +91-9159192731 Fax: +04294-220087 - E-mail: kannank@kongu.ac.in






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.