- Synthesis and experimental study of improved double perovskite-type PrBaCo2O5+δ materials to produce oxygen-enriched CO2 for oxyfuel combustion application
Qiuwan Shena, Yuhang Jianga, Shian Lia,*, Guogang Yanga and Bengt Sundenb
aMarine Engineering College, Dalian Maritime University, China
bDepartment of Energy Sciences, Lund University, SwedenThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Perovskite oxides are promising oxygen carrier materials to produce O2/CO2 gas for oxyfuel combustion application. In this study, PrBaCo2O5+δ double perovskite oxide was prepared by the EDTA sol-gel method and first applied as oxygen carrier for oxygen production. The oxygen adsorption/desorption performance of PrBaCo2O5+δ perovskite was carrried out in a fixed-bed set-up. The operating factors of oxygen releasing performance were investigated in detail including adsorption temperature, desorption temperature, CO2 volumetric flow rate, and CO2 partial pressure. Due to the kinetic effects of gas-solid reactions, it is found that the adsorption and desorption temperature has greatly influence on the oxygen production performance. The surface morphology was observed by scanning electron microscope (SEM). In addition, the cyclic performance of PrBaCo2O5+δ under the optimal operating condition was examined. The oxygen production amount was reduced slightly from 110.9 mg/g perovskite to 99.2 mg/g after 8 cycles’ test. Therefore, the results indicated that PrBaCo2O5+δ has good stability and reactivity for cyclic use
Keywords: Double-Perovskite, PrBaCo2O5+δ, Oxygen desorption, Oxyfuel combustion
Billion tons of CO2 was let off into the air each year, which in part cause global warming and climate changes [1, 2]. New ways to reduce CO2 emissions need to be urgently developed to meet industrial demand. Oxyfuel combustion is also known as O2/CO2 cycle combustion, which uses the mixture of pure oxygen obtained from air separation and part of boiler exhaust gas, instead of air as oxidant in fossil fuel combustion [3]. In this way, the concentration of CO2 in the flue gas generated by boiler combustion can be up to 90%. Therefore, most of the CO2 in flue gas can be directly liquefied and recycled without separation. This can not only effectively reduce greenhouse gas emissions, but also effectively reduce reduce the emission of NOx and SO2 at the same time. Therefore, oxyfuel combustion technology is an effective way to reduce CO2 emission [4].
This combustion technology requires a high con- centration of oxygen. At present, cryogenic process is the only commercial operation mode that can provide large-scale oxygen production [5]. However, the large investment and high energy consumption are the main bottle-necks for this technology. Perovskite-type materials as high temperature oxygen carriers are envisaged to produce oxygen for oxyfuel combustion application [6]. This new technology can reduce 35% cost on oxygen production which can also cut the energy penalty by 50% when applied in an oxyfuel power plant [2].
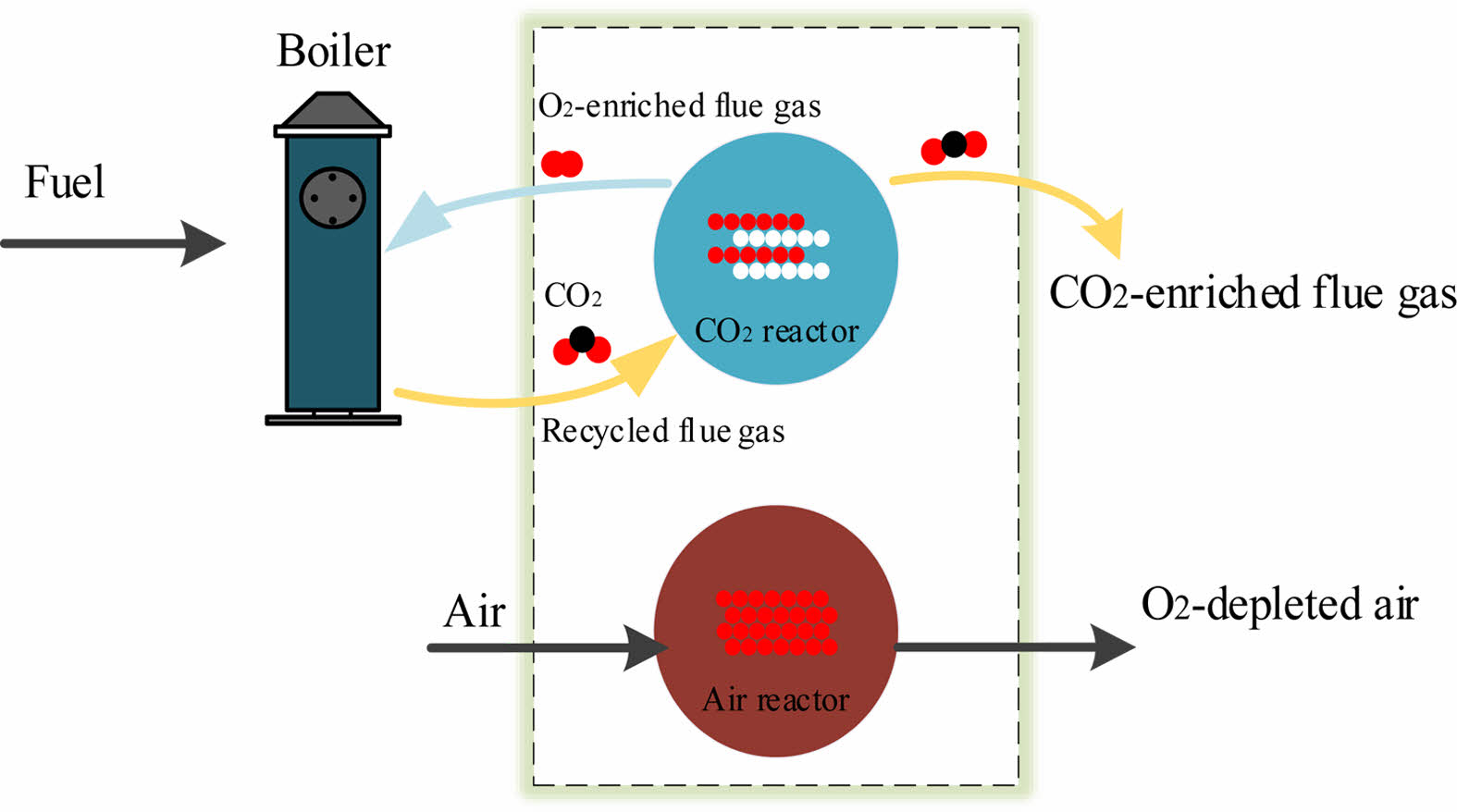
Perovskite-type oxides can absorb oxygen from air at high temperature, which can be used as the oxygen carrier for oxyfuel combustion [7]. Our group has reported a procedure which used perovskite oxides as oxygen carriers to directly produce O2/CO2 mixed gas at high temperature [2]. This O2/CO2 production process consists of two steps, as shown in Fig. 1.
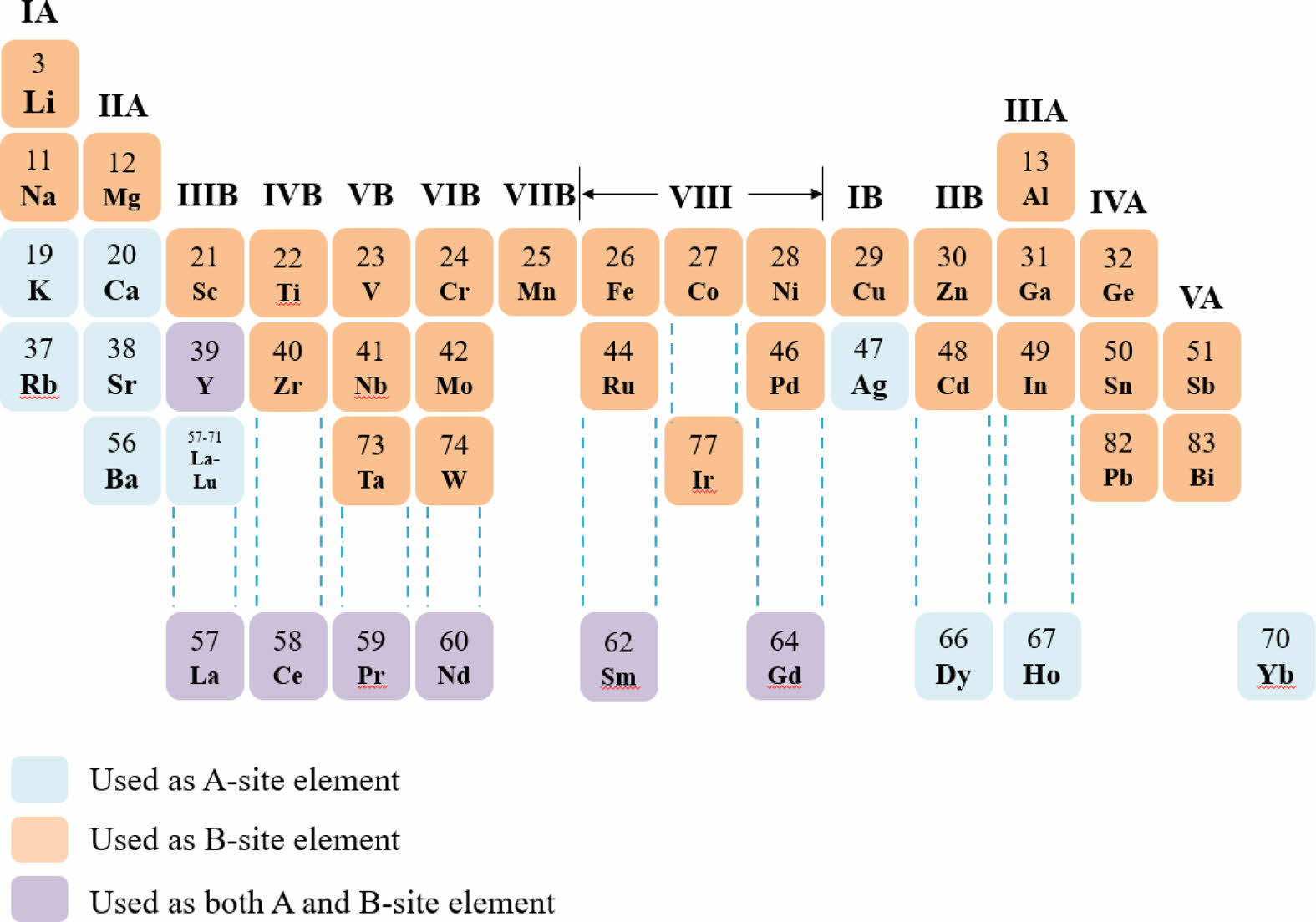
For a ideal cubic structure perovskite oxide, the value of t is about 1. The A-site is generally a rare earth or alkaline earth element ion, while the B-site is a transition element ion (Fig. 2). To form the perovskite structure, the radii of the A-site and B-site ions need to satisfy the formula.
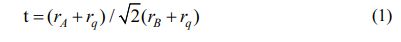
where t is tolerance factor (0.77
rB is the ion radii of the B-site cation
rq is the ion radii of the oxygen anion
The A and B positions can be radius similar metal ions which are partially substituted to keep their crystal structure basically unchanged, so it is theoretically an ideal sample for studying the catalyst surface and catalytic performance [8]. Because of the unique crystal structure, perovskite-type oxides can be used in many fields such as solid fuel cell, solid electrolyte, sensor, high temperature heating material and solid resistor [9-11].
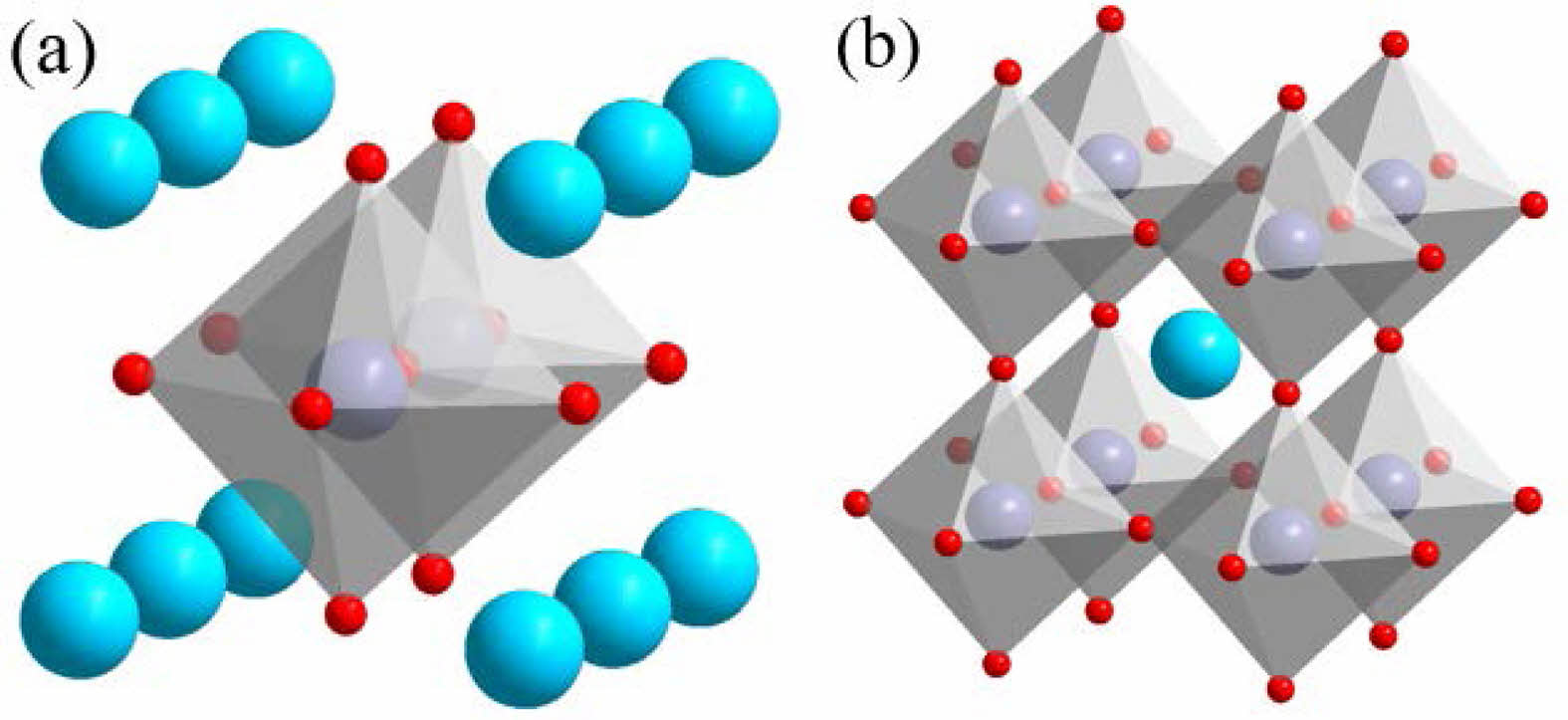
In the past decade, double perovskite materials with a formula of AA'B2O5+δ (A as the rare earth element, A′ as the alkaline earth element, B as the transition metal element) have attracted much more attention (Fig. 3) [12]. When the B-site transition metal ions of double perovskite are replaced, oxygen vacancies or defects will be formed due to the change of transition metal oxidation price state. Therefore, the adsorption and desorption properties of oxygen can be changed. Double perovskite PrBaCo2O5+δ (PBCO) has attracted much attention as potential cathodes for SOFCs thanks to its superior oxygen-ion transport capability [13, 14]. Due to its special crystal structure, PrBaCo2O5+δ have been also found to exhibit a large capacity for reversible oxygen absorption and desorption [15]. Thus double perovskite is a promising material for the O2-enriched CO2 gas production for oxyfuel combustion.
However, there are limited studies about PrBaCo2O5+δ (PBCO) used as potential oxygen carrier for oxyfuel combustion application. The objective of this study is to evaluate the oxygen adsorption/desorption performance of PBCO double perovskite. Especially, a thorough study of the effects of the reaction conditions on the oxygen production properties of synthesized PBCO double perovskite are inverstigated.
|
Fig. 1 Schematic diagram of producing O2/CO2 by perovskitetype oxides. |
|
Fig. 2 Common elements in the ABO3-type perovskite oxides. |
|
Fig. 3 Schematic diagram of double perovskite A2B2O5+δ cell: (a) monolayer cell (b) bilayer cell. |
PBCO synthesis
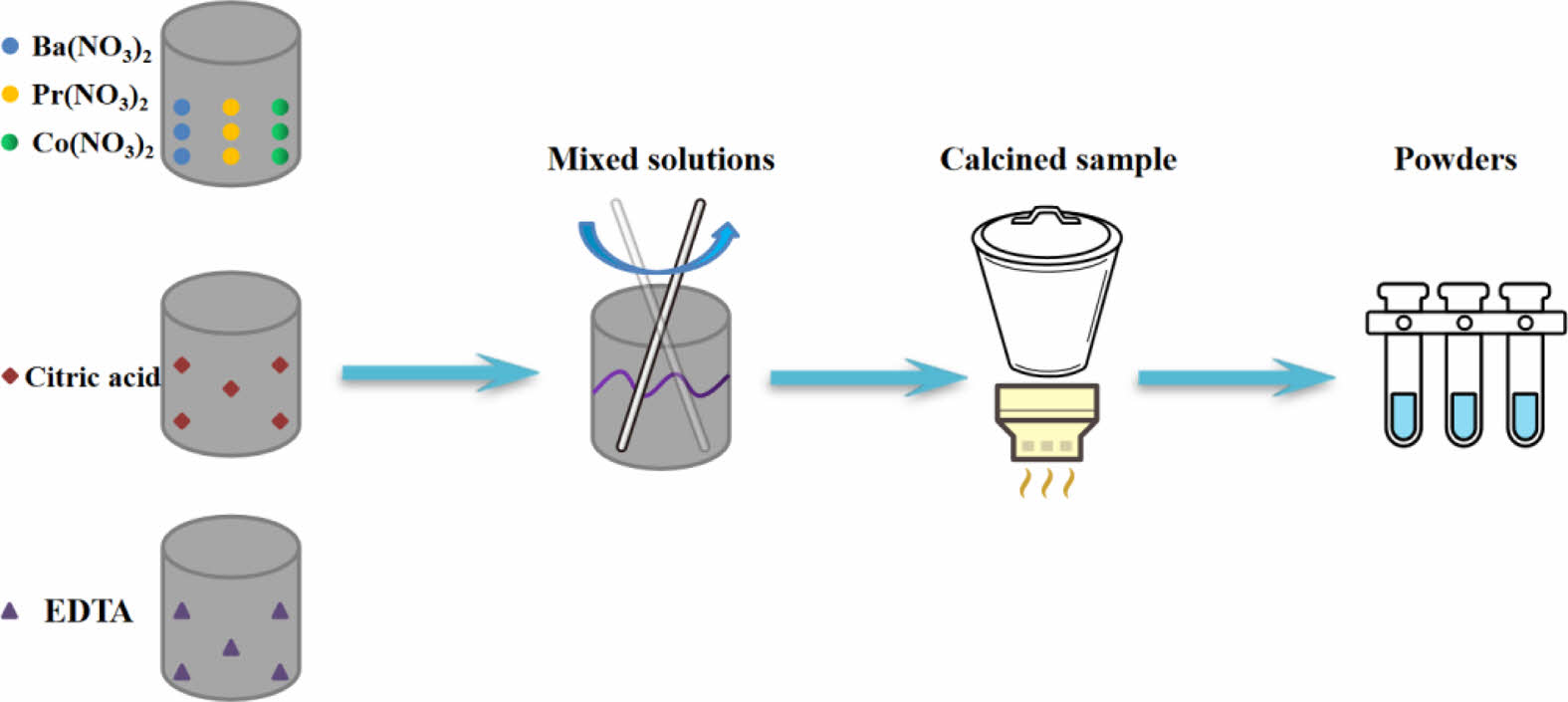
PBCO was preperaed by EDTA sol-gel method as shown in Fig. 4. Metal nitrates, Ba(NO3)2(AR, Aladdin), Pr(NO3)2(AR, Aladdin) and Co (NO3)2·6H2O(AR, Aladdin) were used as the raw materials and all were of analytical purity. Metal nitrate, citric acid and EDTA are added to the beaker at a ratio of 1:1.5:1. The specific preparation steps are shown in details in our previous study [2, 3].
Oxygen production experiments
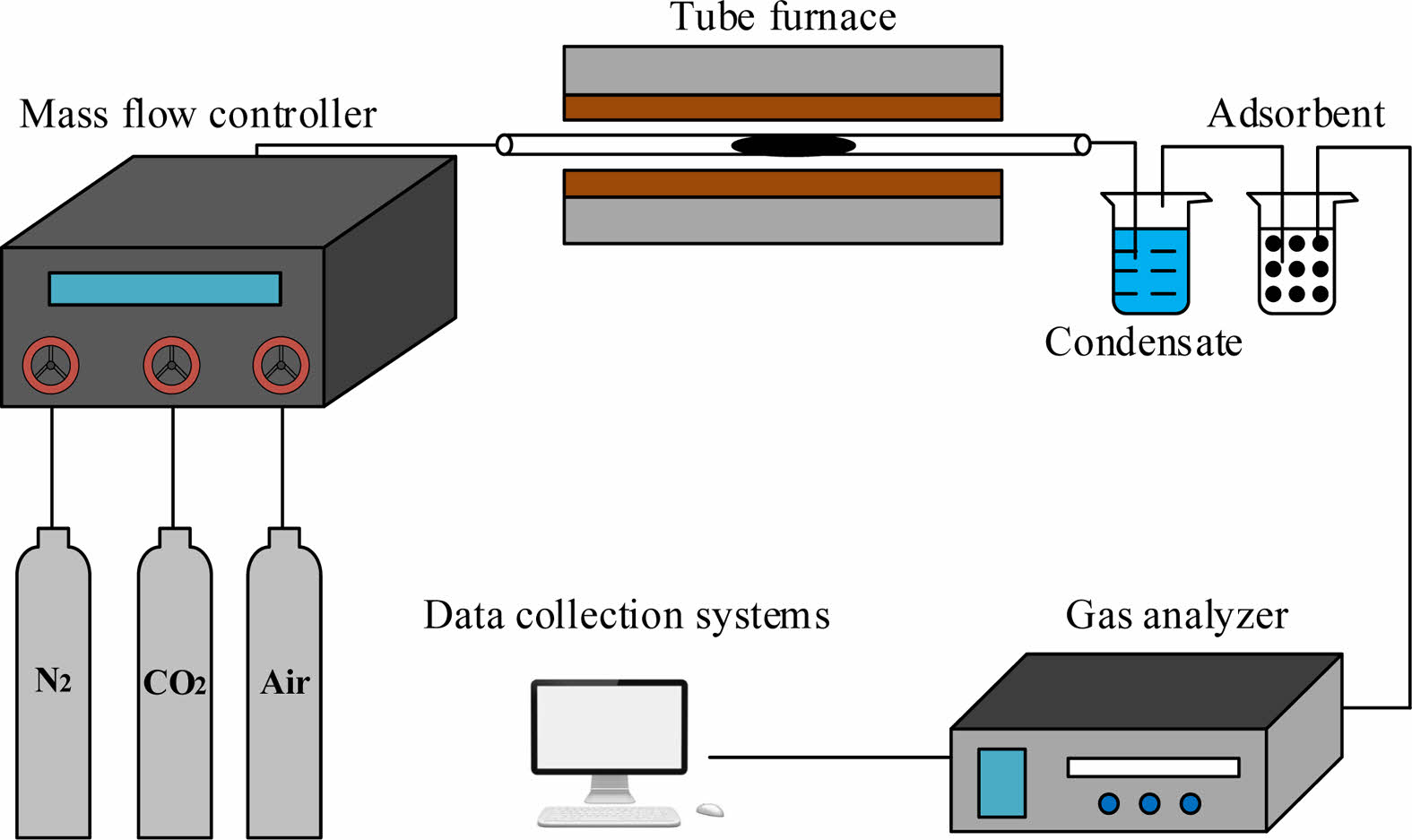
The oxygen adsorption/desorption experimental process have been presented in details in the previous works (Fig. 5) [1, 2]. 1 g of sample is tightly rolled with quartz cotton and placed in the center of the quartz tube so that it can fully contact with the gas.
In the oxygen adsorption process, the perovskite is heated to a given adsorption temperature. Air and nitrogen were adopted as the feed and purge gas, respectively. In the desorption stage, the sample was exposed under a flow of CO2. After a adsorption/desorption experiment is completed, the tube furnace is adjusted to the preset temperature of the adsorption step for the following cyclic test.
The oxygen concentration of the outlet gas was recorded during the experiment, and the oxygen release quantities was calculated according to the formula:

where MO2: Oxygen release amount
∑SCO2: Sum of oxygen concentration measured by gas analyzer
f: Flow rate of desorption gas CO2
mO2: Molar mass of oxygen molecules
m: Sample quality to be placed in a tubular furnace
Through the formula, the amount of released oxygen can be calculated for 1 g of the test sample, which is convenient for comparison of different perovskite properties.
Characterization
Particle morphology of the sample was observed by scanning electron microscopy (SEM, SUPRA 55 SAPPHIRE).
|
Fig. 4 Flow diagram of the preparing process of PBCO powders. |
|
Fig. 5 Experimental system diagram. |
Adsorption temperature
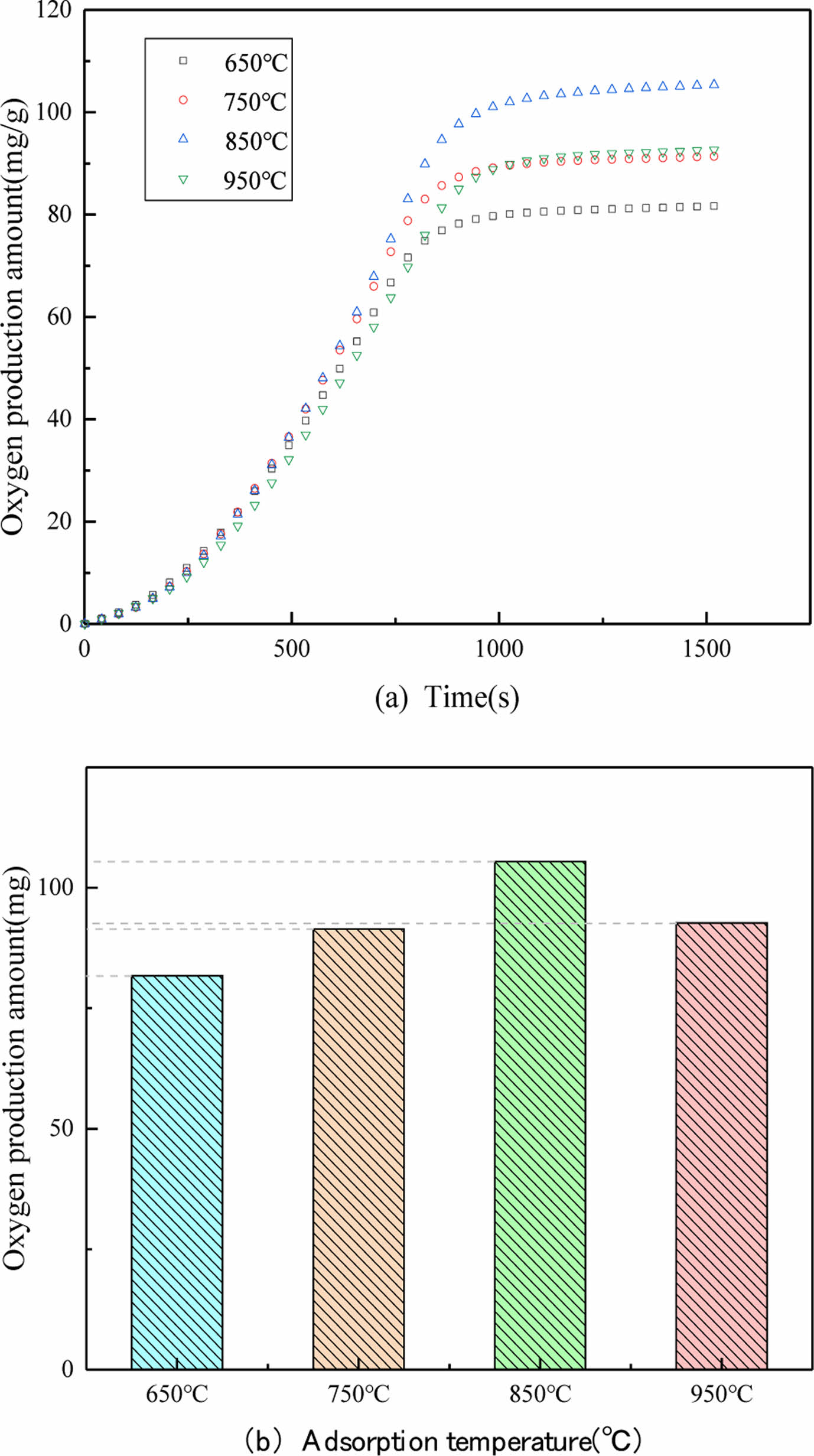
Fig. 6(a) and 6(b) present the comparation of oxygen release performance by PBCO at different adsorption temperatures (650 ℃, 750 ℃, 850 ℃, 950 ℃), respectively. The remaining operating conditions are determined as follows according to our previous study [1]. The performance of oxygen desorption PBCO is improved with the increase of temperature, reaching a relatively ideal value at 850 °C and then decreasing. It can be clearly seen that the PBCO produced 110.9 mg/g of oxygen when the adsorption temperatures is 850 °C. This is because the adsorption capacity of PBCO to oxygen in the adsorption phase is influenced by kinetics below 850 °C. With the increase of the adsorption temperature of PBCO, the quality of the provided oxygen production increased remarkably. Due to thermodynamic constraints, the adsorption process is regarded as exothermic at temperatures above 850 °C and high temperature will lead to the sintering of perovskite, which affect the oxygen desorption performance [4]. Oxygen cannot be adsorbed at high temperatures, thus 850 °C has been determined as the optimal adsorption temperature.
Desorption temperature
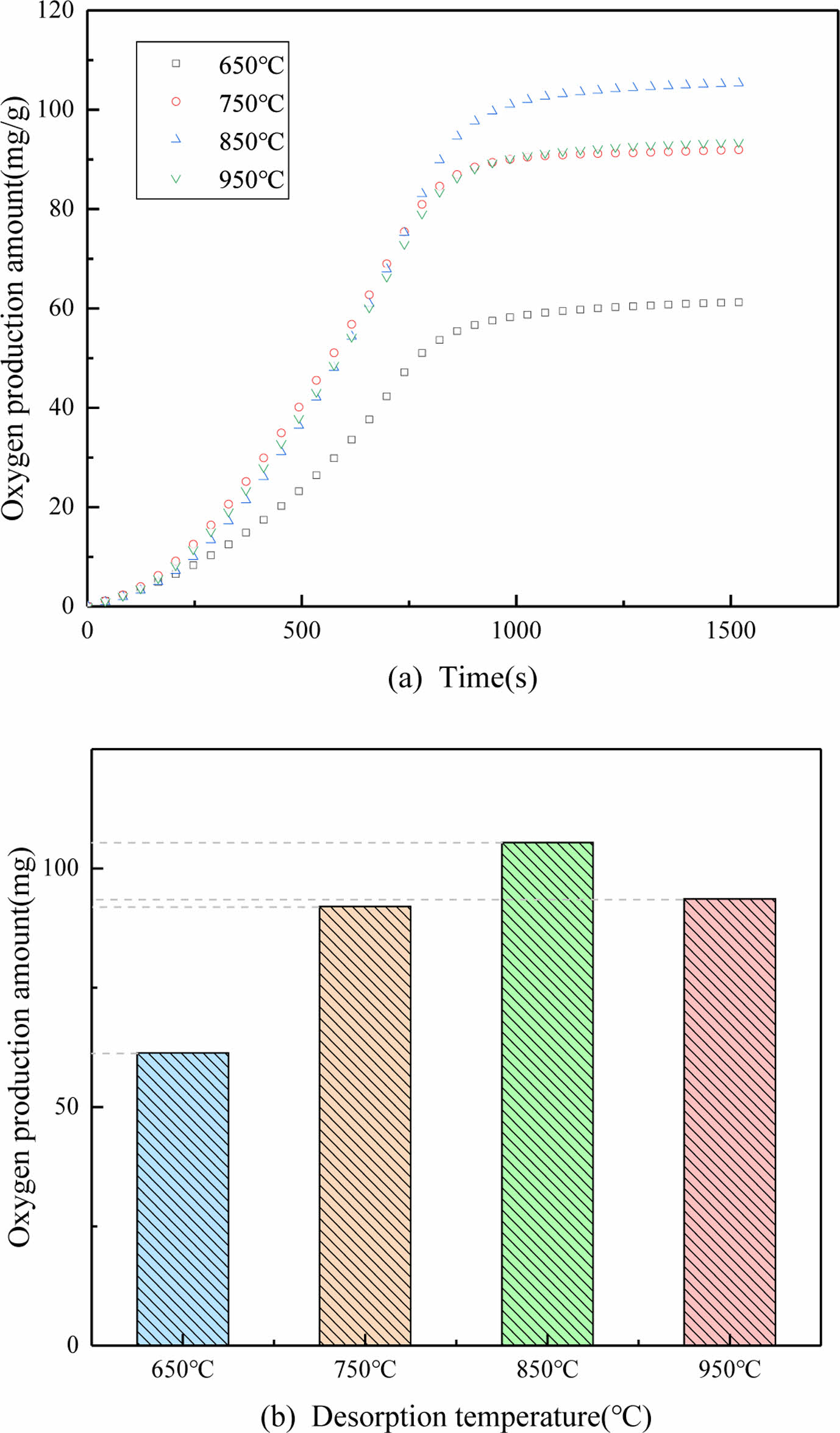
It can be seen from the Fig. 7 that the oxygen production of PBCO will increase with the increase of the desorption temperature. Oxygen production rates and amount reach a minimum of 650 °C. Then temperature rises to 850 °C, the reaction rate was increased significantly. The production of oxygen was improved nearly double. The results further indicated that the PBCO desorption reaction time was 2min30s. The oxygen desorption reaction of PBCO is a gas-solid reaction. Due to the kinetic effects of gas-solid reactions, oxygen desorption reactions are associated with temperature [7]. However, when the temperature increases to 950 °C, the amount of O2 production is declined. Thus 850 °C was used as the optimal desorption temperature for PBCO in the following experiments.
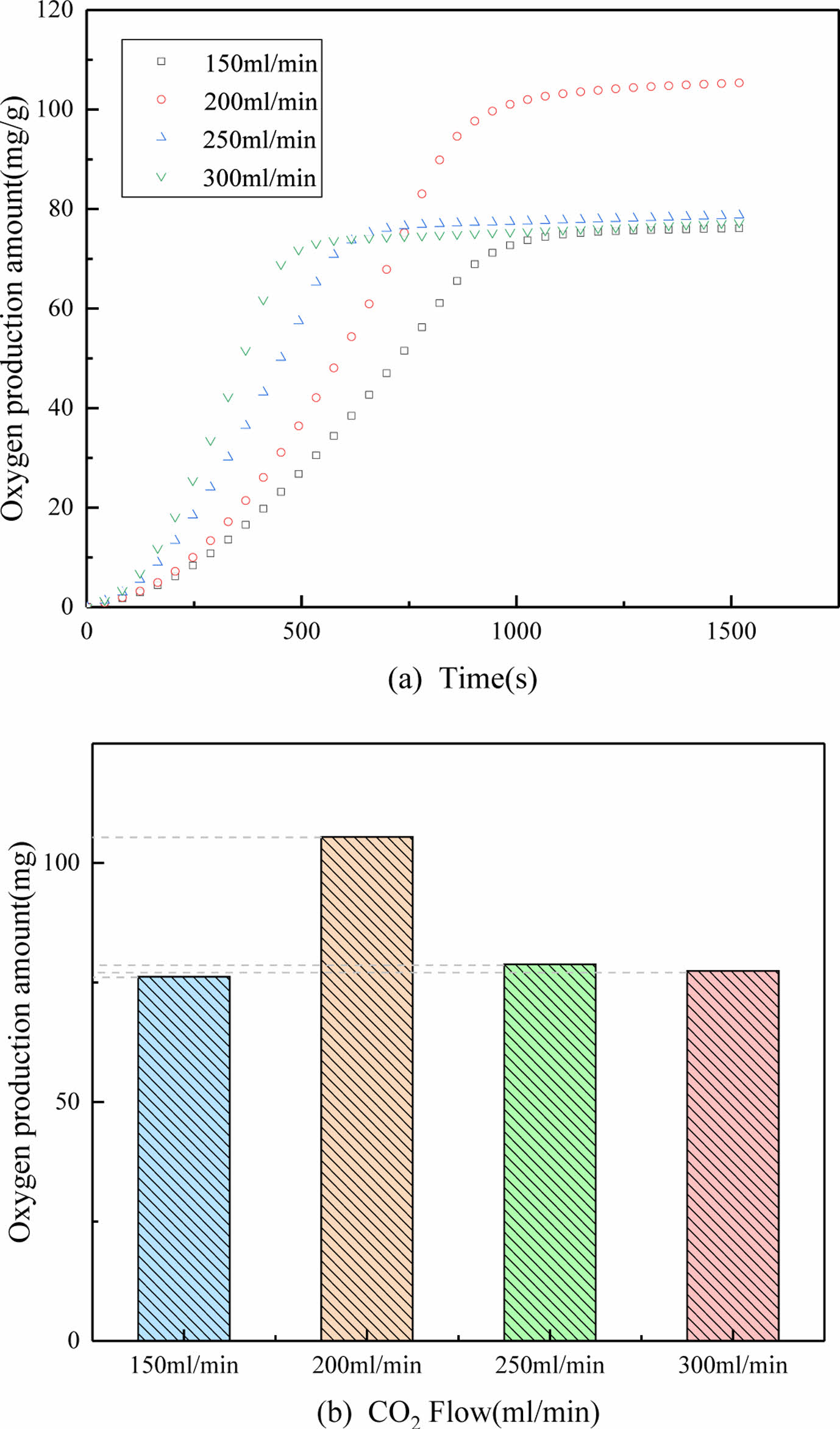
CO2 volumetric flow rate
The comparison of oxygen release performance at different CO2 volumetric flow rate conditions can be seen in Fig. 8. The oxygen production amount values are also displayed in Fig. 8. As a general trend, with the increase of CO2 flow rate, the desorption rate of PBCO increases. The results show that the production of oxygen was increased when the rate of CO2 gas increases from 150 ml/min to 200 ml/min. The desorption time of the sample is about 17 mins at 150 ml/min, while it is only 8 mins at 300 ml/min.The oxygen production reached at the highest at 200 ml/min and the final oxygen production amount was 110 mg. When the CO2 flow rates was increased to 300 ml/min, the PBCO did not react adequately due to the excessive flow. Thus it can be concluded that the optimal CO2 volumetric flow rate is 200 ml/min.
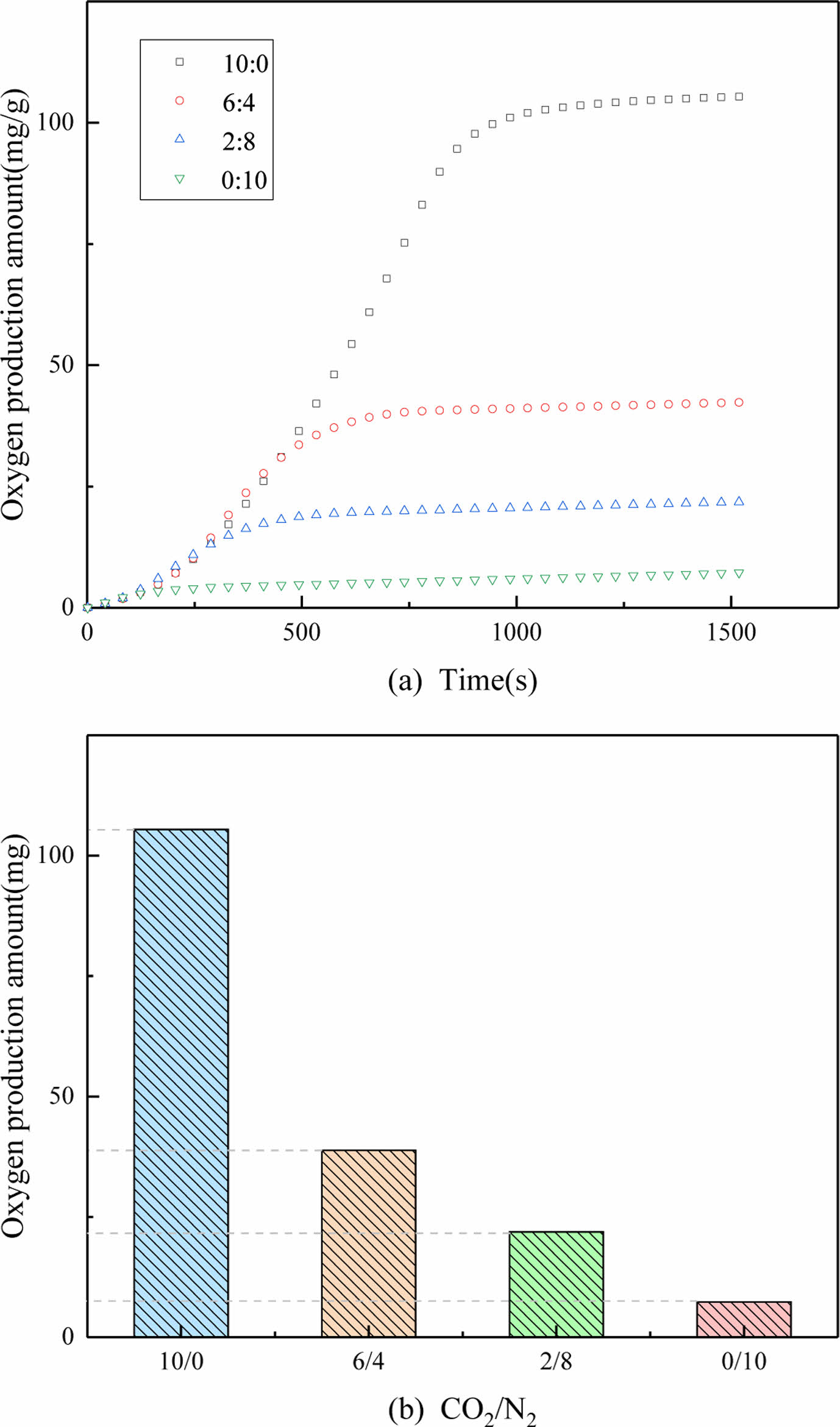
CO2 partial pressure
The partial pressure of carbon dioxide has an important influence on the carbonation reaction between perovskite and CO2. The oxygen desorption performance of PBCO can be seen in the Fig. 9 (the partial pressures of N2 and CO2 are 10:0, 6:4, 2:8 and 0:10 , respectively). As can be seen from Fig. 9(a), the fluctuation of oxygen desorption performance is very small in the reaction time of 1500 s. With the increase of CO2 volume, the production of oxygen increases gradually. When the ratio of N2/CO2 increases from 6:4 to 10:0, the production of oxygen increases significantly. This is because perovskite reacts with CO2 to produce more oxygen than desorbed oxygen in low oxygen and high nitrogen atmosphere. Therefore, the higher the partial pressure of carbon dioxide, the more oxygen is produced.
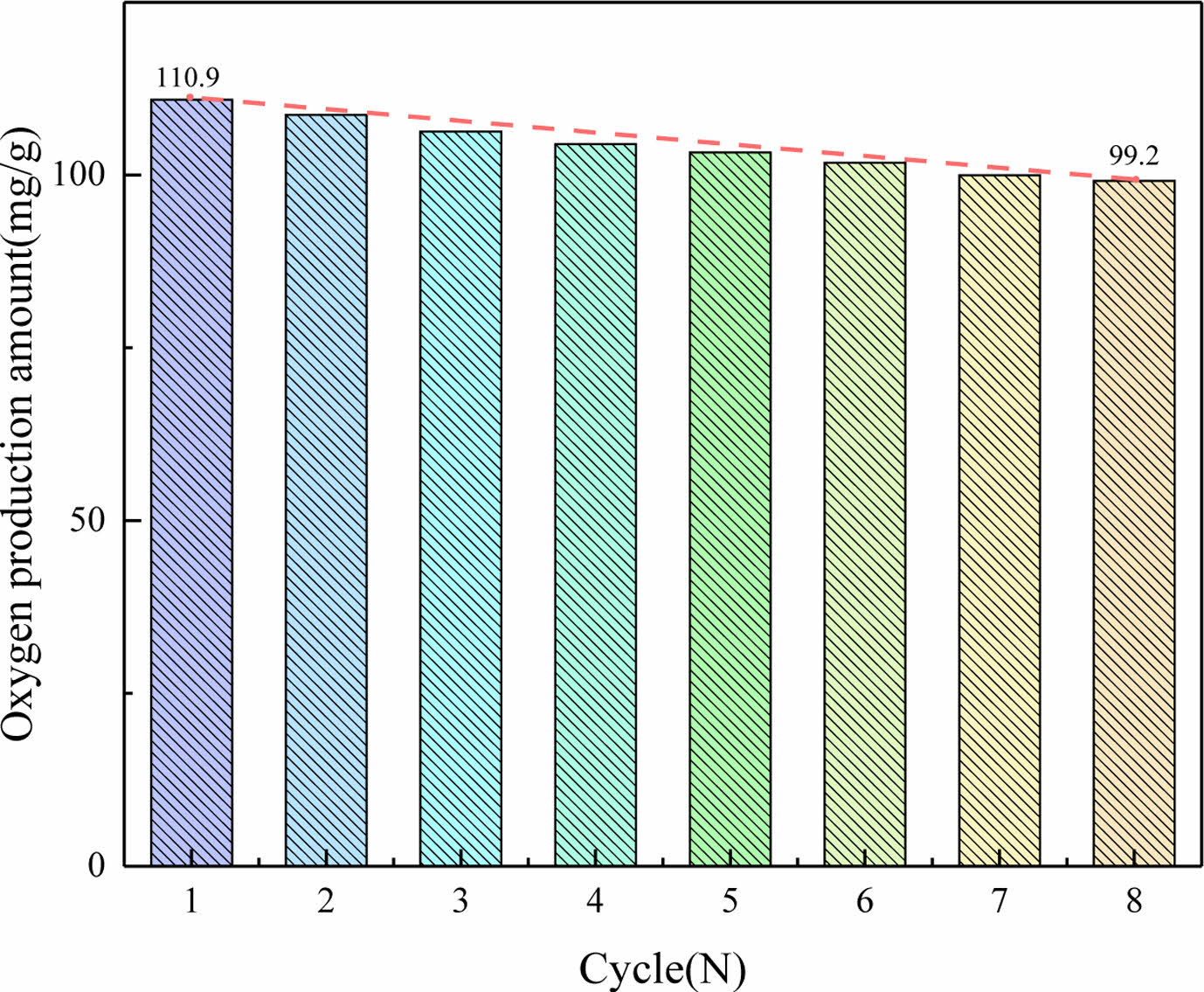
Cycle capacity of novel PBCO
PBCO is used as an oxygen carrier to provide a stable mixture of gases for oxyfuel combustion, so it should have excellent cycling characteristics. The oxygen desorption amounts for continuous 8 cycles demonstrated in Fig. 10 were obtained from Eq. (3). As can be seen in Fig. 10, the PBCO sample still has excellent oxygen release capability after cyclic experiments. The amount of oxygen released was stabilized at 99.2 mg/g after cyclic testing. Although the oxygen desorption performance of PBCO decreased with the increase of cycle times, the decreasing trend of oxygen release was smoothened. Compared with single perovskite BaCo0.6Ni0.4O3-δ, the oxygen desorption capacity of PBCO perovskite is significantly improved [7]. It is concluded that PBCO has excellent cycling performance and is an excellent oxygen carrier to provide stable circulating gas for oxyfuel combustion.
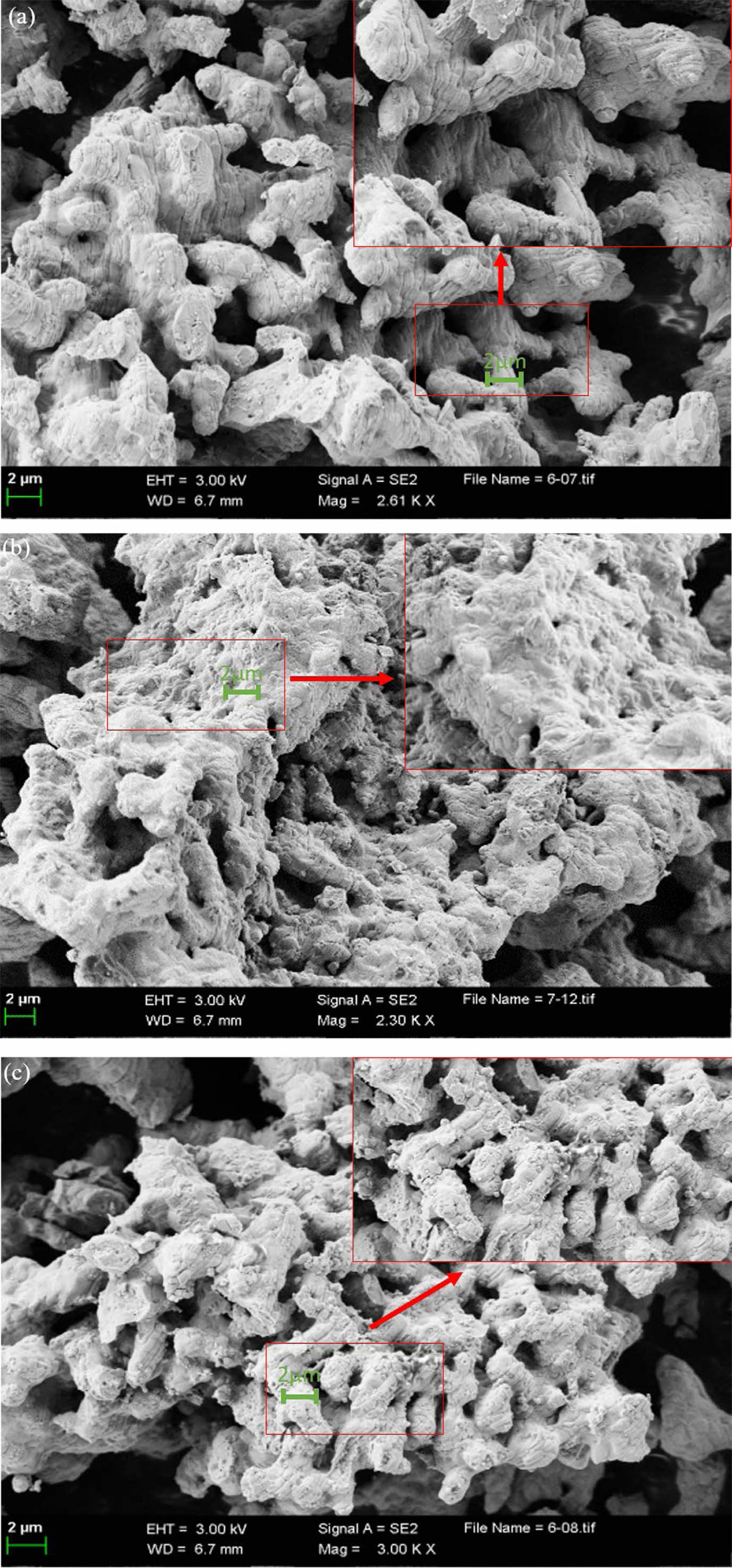
SEM analysis
Fig. 11 dispalys the SEM images of the fresh PBCO samples, the samples after 8 cycles' reacted and the reverted products of PBCO after 8 cycles. It can be seen from the fresh sample that the morphology of PBCO is porous and rough. The fresh PBCO presents a fluffy porous network structure. As shown in the Fig. 11b, the structure of PBCO becomes massive and irregular. This is because after 8 adsorption and desorption cycles of perovskite, the structure of perovskite disappears and becomes massive. It can be seen from the Fig. 11c that PBCO restores porous and sparse components. After recycling, the appearance of perovskite hardly changes and returns to porous structure. Therefore, perovskite is expected to have strong recycling capacity.
|
Fig. 6 Oxygen release performance of PBCO at diffirent adsorption temperature: (a) Penetration curve. (b) Oxygen production diagram. |
|
Fig. 7 Oxygen production amount of PBCO at diffirent desorption temperature: (a) penetration curves, (b) oxygen production quality diagram. |
|
Fig. 8 Oxygen production amount of PBCO at diffirent CO2 flow rate: (a) penetration curves, (b) oxygen production quality diagram. |
|
Fig. 9 Oxygen production amount of PBCO in diffirent CO2 partial pressure: (a) oxygen concentration curves. (b) oxygen production amount. |
|
Fig. 10 Cyclic tests of PBCO. |
|
Fig. 11 SEM images of PBCO powders: (a) fresh sample. (b) after 8 cycles' reacted sample. (c) reverted sample after 8 cycles. |
In this study, the double perovskite PrBaCo2O5+δ with excellent performance was prepared by the EDTA sol-gel synthesis method and firstly applied to produce oxygen for oxyfuel combustion application. The oxygen production properties of the PrBaCo2O5+δ were investigate in a fixed-bed system. The optimal operation conditions for PrBaCo2O5+δ were studied in-depth. The main conclusions are as follows:
1. The experimental results show that the adsorption/desorption temperature has the greatest influence on the oxygen desorption performance of perovskite. The optimal reaction conditions of PrBaCo2O5+δ to oxygen production are as below: adsorption temperature was 850 ℃; desorption temperature was 850 ℃; the volume proportion of CO2 and N2 was determined to be 10:0; CO2 volumetric flow rate was confirmed 200 ml/min.
2. The higher the partial pressure of carbon dioxide, the more oxygen is produced. This is because perovskite reacts with CO2 to produce more oxygen than desorbed oxygen in low oxygen and high nitrogen atmosphere.
3. The proposed novel double perovskite PrBaCo2O5+δ provided excellent performance, the O2 production of PrBaCo2O5+δ can still reach 99.2 mg/g after 8 cycles. SEM results show that after 8 cycles, after recycling, the appearance of perovskite hardly changes and returns to porous structure.
4. In summary, the synthesized PBCO powders shows excellent O2 production performance which may be a potential oxygen carrier material for oxyfuel application. Moreover, this simple synthetic method can be extended to other double perovskite oxide systems for more applications.
This work was supported by the Dalian City Innovative Support Program for High-Level Talents (No.2019RQ036), Postdoctoral Research Foundation of China (No.2019M651094), Natural Science Foundation of Liaoning Province (No. 2020-HYLH-38).
The authors declare no conflict of interest.
- 1. Q.W. Shen, Y.H. Jiang, S.A. Li, X.R. Lv, G.G. Yang, and B. Sunden, Int. J. Energy Res. 44 (2020) 6991-6999.
-

- 2. Q.W. Shen, Y. Zheng, S.A. Li, H.R. Ding, Y.Q. Xu, C.G. Zheng, and M. Thern, J. Alloy. Compd. 658 (2016) 125-131.
-

- 3. Q.W. Shen, Z.C. Shao, Y.H. Jiang, S.A. Li, G.G. Yang, N.B. Huang, and X.X. Pan,J. Ceram. Process. Res. 22 (2021) 576-583.
-

- 4. Q.W. Shen, Y.H. Jiang, S.A. Li, G.G. Yang, H.P. Zhang, Z.G. Zhang, and X.X. Pan, J. Sol-Gel Sci. Technol. 99 (2021) 420-429.
-

- 5. J. Hwang and K. Lee, J. Ceram. Process. Res. 21 (2020) 148-156.
-

- 6. S.A. Li, R.Q. Wei, Y.H. Jiang, Q.W. Shen, G.G. Yang, and N.B. Huang, J. Ceram. Process. Res. 21 (2020) 64-68.
-

- 7. Y.H. Jiang, Q.W. Shen, S.A. Li, G.G. Yang, and N.B. Huang, New J. Chem. 44 (2020) 6003-6009.
-

- 8. N. Mohammadi, M.I. Hossain, A.D. Ebner, and J.A. Ritter, Ind. Eng. Chem. Res. 55 (2016) 10758-10770.
-

- 9. F. Abedini, S.A. Hosseini, A. Niaei, D. Salari, M. Abbasi, and S. Marmarshahi, Int. J. Green Energy 15 (2018) 20-27.
-

- 10. M. Ijaz, A. Shoukat, A. Ayub, H. Tabassum, H. Naseer, R. Tanveer, A. Islam, and T. Igbal, Int. J. Green Energy 17 (2020) 1022-1035.
-

- 11. N.Y. Yu, M.M. Nair, and N. Mahinpey, Can. J. Chem. Eng. 97 (2019) 2131-2136.
-

- 12. Y.H. Wan, Y.L. Xing, Y.H. Li, D.M. Huan, and C.R. Xia, J. Power Sources 402 (2018) 363-372.
-

- 13. S.L. Pang, X.N. Jiang, X.N. Li, Q. Wang, and Z.X. Su, J. Power Sources 204 (2012) 53-59.
-

- 14. L. Jiang, F. Li, T. Wei, R. Zeng, and Y.H. Huang, Electrochim. Acta 133 (2014) 364-372.
-

- 15. H.S. Hao, L. Zheng, Y.F. Wang, S.J. Liu, and X. Hu, J. Rare Earths 25 (2007) 275-281.
-

 This Article
This Article
-
2022; 23(5): 611-616
Published on Oct 31, 2022
- 10.36410/jcpr.2022.23.5.611
- Received on Feb 24, 2022
- Revised on Mar 19, 2022
- Accepted on Mar 21, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Shian Li
-
Marine Engineering College, Dalian Maritime University, China
Tel : +86-13190160896 Fax: +0411-84728659 - E-mail: lishian@dlmu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.