- Investigation of Al modification as cationic dopants in Ni-rich LiNi0.91Co0.06Mn0.03O2 cathode
Ye-Wan Yoo and Seung-Hwan Lee*
Department of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this paper, we have successfully prepared Al-doped Ni-rich LiNi0.91Co0.06Mn0.03O2 cathodes. The structural properties and electrochemical performances are studied according to Al cationic doping. It can be confirmed that the crystallinity and cation disordering of Ni-rich LiNi0.91Co0.06Mn0.03O2 were improved by Al doping. Based on such excellent structural quality, the electrochemical performance of Al doping LiNi0.91Co0.06Mn0.03O2 was superior to that of pristine LiNi0.91Co0.06Mn0.03O2. The Al doping Ni-rich NCM has an initial discharge capacity of 209.2 mAh g-1. In addition, it shows superior rate capability by showing capacity retention of 58.5% under a high rate of 6.0 C. Therefore, it can be judged that Al doping LiNi0.91Co0.06Mn0.03O2 can be applied to next-generation cathode for long-distance and fast-charging electric vehicles
Keywords: Al-doped Ni-rich LiNi0.91Co0.06Mn0.03O2, Excellent structural quality, Next-generation cathode, Long-distance and fast-charging electric vehicles
Recently, studies on energy storage devices such as lithium ion batteries, fuel cells, supercapacitors, hybrid supercapacitors and power semiconductors are being conducted. Among them, a lithium ion battery having a high energy density is commercialized as a power source for electric vehicles, energy storage devices, and mobile devices, and is the most widely used energy storage device.
Among various applications of lithium-ion battery, the development of lithium-ion battery for electric vehicles is important because the battery market for electric vehicles is predicted to grow the most rapidly. For example, a portable device contains one lithium ion battery, but a hybrid electric vehicle, a plug-in hybrid electric vehicle, and a battery electric vehicle contain 100 to 6000 or more lithium ion batteries.
The one of the most important parameter in lithium-ion battery for electric vehicles is energy density. Among lithium-ion battery components, the cathode determines not only the price due to expensive raw materials such as Li, Ni and Co, but also the energy density of the lithium-ion battery. Therefore, studies on the electrochemical performances of various cathode candidates are being conducted.
One of two widely studied candidates, the Ni-rich NCM (Ni ≥ 80%) has higher energy density under actual operating conditions than Li- and Mn-rich NCM, and it has high economic feasibility due to low content of expensive lithium. One of the fatal disadvantages of Ni-rich NCM is the cycle performance degradation due to side reaction with electrolyte. Therefore, in this paper, we tried to improve the cyclability of Ni-rich LiNi0.91Co0.06Mn0.03O2 through bulk modification using Al.
To improve the electrochemical performances of pristine LiNi0.91Co0.06Mn0.03O2, Al doped LiNi0.91Co0.06- Mn0.03O2powders were fabricated by co-precipitation method. The Ni0.91Co0.06Mn0.03(OH)2 precursor was fabricated via using NiSO4·6H2O, MnSO4·H2O, CoSO4·7H2O, NH3·H2O and Na2CO3 aqueous solution.The NH4OH and NaOH solution was adapted as a precipitating agent. The spherical Ni0.91Co0.06Mn0.03 (OH)2 precursor was mixed with Li sources (LiOH·H2O) at the molar ratio of Li sources/Ni0.91Co0.06Mn0.03(OH)2 precursor was 1.05 : 1. Also 0.5 wt% Al2O3 powders were added as a cationic dopants. After that, the powders were calcined at 710 oC for 7 h and then sintered860 oC for 10 h under O2 atmosphere.
In order to measure the electrochemical performances, the LiNi0.91Co0.06Mn0.03O2 cathode was prepared using LiNi0.91Co0.06Mn0.03O2 powder (96 wt%), polyvinylidene fluoride (2 wt%) as a binder and Super P (2 wt%) as a conductive additive. For making slurry, the mixture was blended with N-methyl-pyrrolidinone (NMP) solvent. Afterward, slurry was coated on aluminum-foil and dried at 100 oC for 24 h in a vacuum oven to eliminate the NMP solvent. The CR 2032 coin cells were prepared with lithium foil as an anode in Ar-gas filled glove box. The dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and ethylene carbonate (EC) (1 : 1 : 1 by volume) with 1M LiPF6 were selected as an electrolyte.
In order to compare the morphologies and structural properties, field emission scanning electron microscopy (FE-SEM, Hitachi S-4800) and X-ray diffraction (XRD, X'pert MPD DY1219) were measured. The initial charge-discharge capacities, rate performances, long-term cycle performances are estimated via electrochemical equipment (TOSCAT-3100, Toyo system).
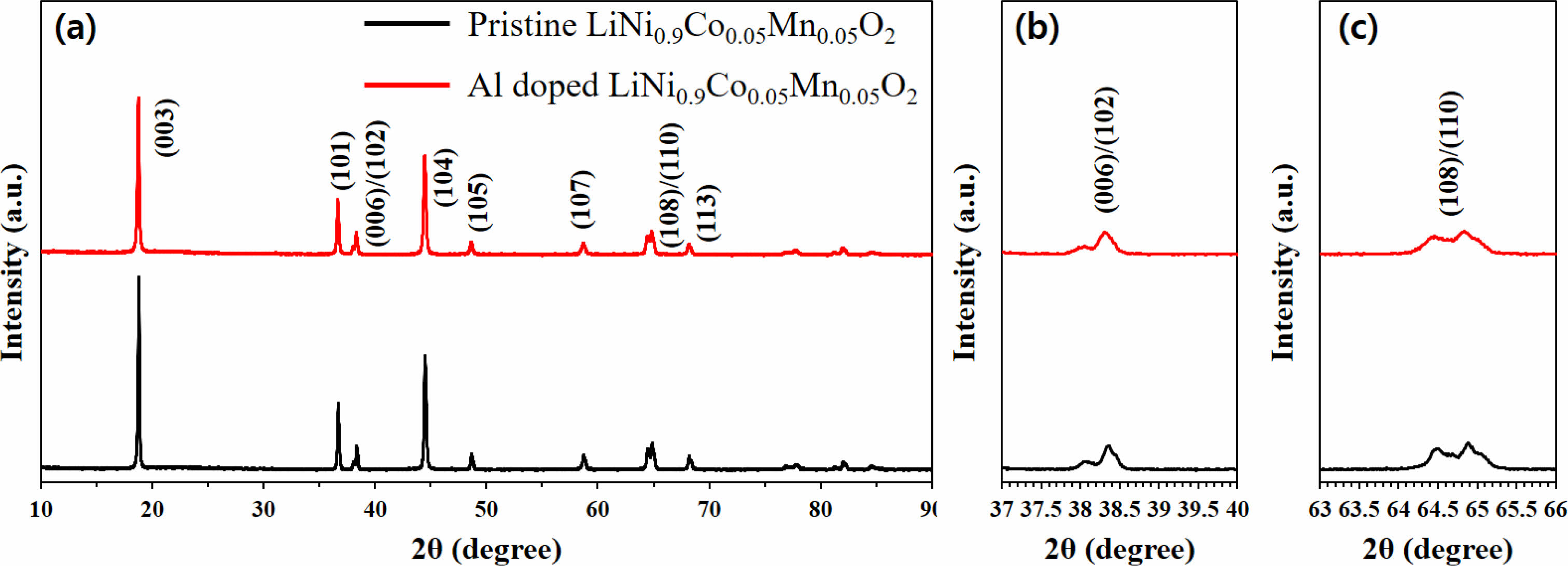
Fig. 1 shows the XRD patterns of Al-doped and pristine LiNi0.91Co0.06Mn0.03O2. Both samples show typical layered hexagonal α-NaFeO2 structure with R-3m space group. Also, no additional phases were observed for the both samples. Therefore, it can be inferred that both samples are well crystallized. The good separation of (006)/(102) and (108)/(110) peaks means excellent layered structure. It is well known that the I(003)/I(104) ratio is a key parameter to determine the degree of cation disordering, which is one of the negative factor for electrochemical performances of Ni-rich NCM. If the (003)/(104) peak ratio value is lower than 1.2, the cation ordering is considered poor. In case of pristine LiNi0.91Co0.06Mn0.03O2, the value of I(003)/I(104) value is 1.35, which is also higher than 1.2, indicating good cation ordering. The Al-modified LiNi0.91Co0.06Mn0.03O2 possess a higher I(003)/I(104) value of 1.54 than the pristine sample. Therefore, it can be confirmed that the cation ordering is effectively improved by the Al doping into the pristine LiNi0.91Co0.06Mn0.03O2.
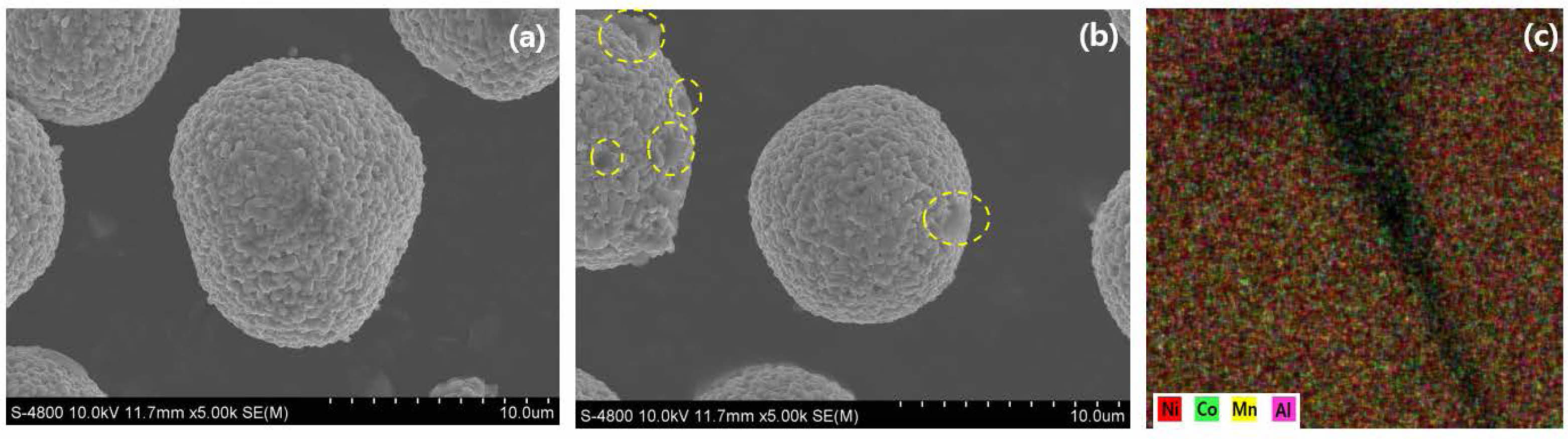
Fig. 2 shows the microstructures of (a) Al-doped, (b) pristine LiNi0.91Co0.06Mn0.03O2 and (c) EDS mapping of Al-doped LiNi0.91Co0.06Mn0.03O2. It can be seen that there is no big difference between both samples. Both samples have a similar secondary particle size of 11.5 μm and the same spherical shape with porous structures. The size of primary particle sizes, constituting the secondary particles, are in the range from 100 to 200 nm for both samples. Based on these, it was confirmed that Al doping did not clearly affect particle morphology or size. The significant difference between the both samples is the surface chemistry. In the case of the xx-modified sample, the surface is clean, but in the case of the pristine sample, it can be confirmed that there are impurities such as LiOH and Li2CO3 on the surface, as indicated by the yellow circle. These materials are due to the low structural stability of the pristine sample. In many previous papers, it has been reported that performance degradation is caused by existence of unwanted lithium compounds on the Ni-rich cathode surface. As shown in Fig. 2(c), the successful Al modification can be confirmed through EDS mapping. In the case of the Al modified sample, the chemical stability of the Ni-rich NCM can be explained by the Al substitution.
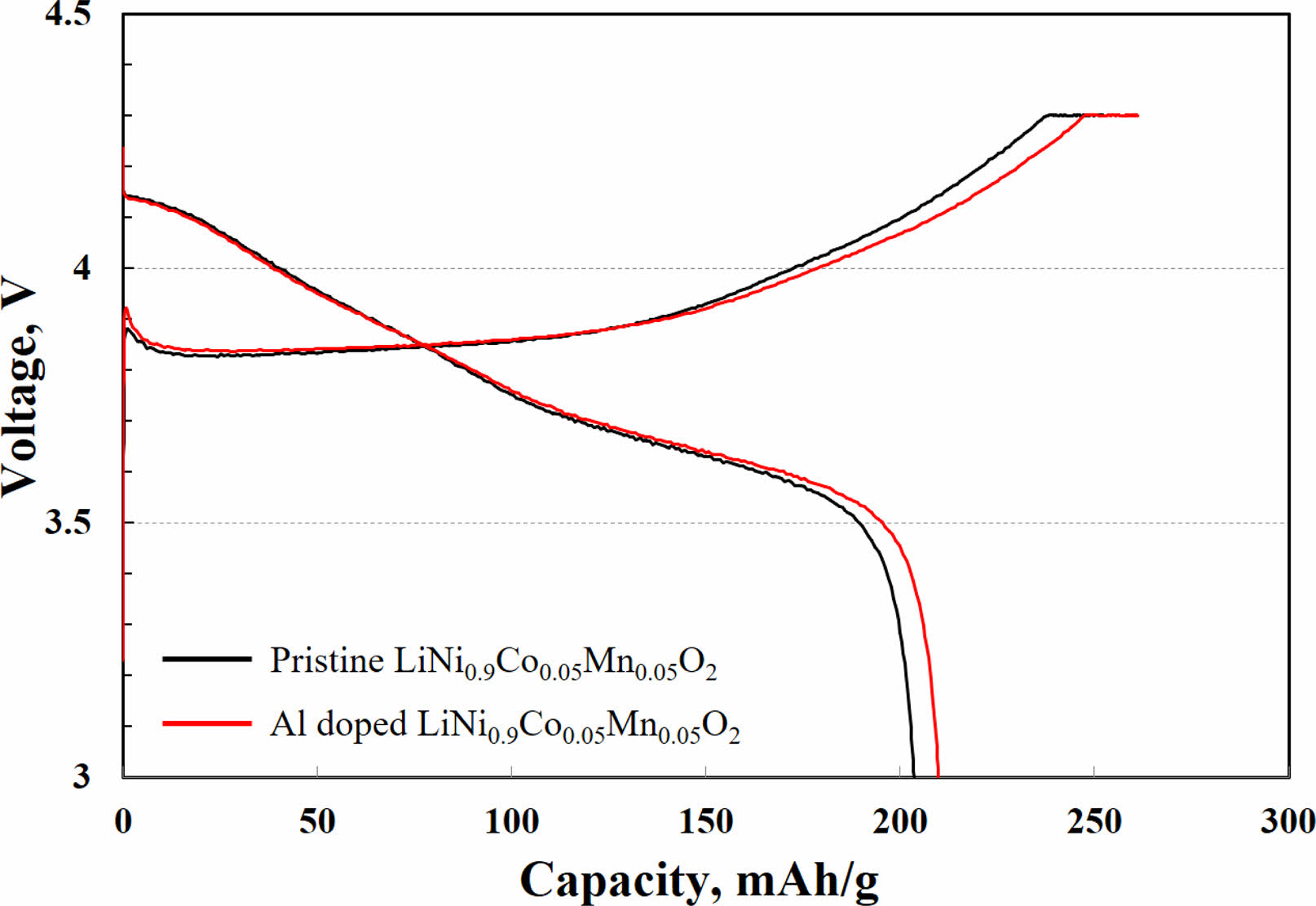
To confirm the commercial potential, both samples for measuring the electrochemical performance were prepared to have a high mass loading per are of about 15.4 mg/cm2. Fig. 3 shows the initial charge-discharge curves of Al-doped and pristine LiNi0.91Co0.06Mn0.03O2 in the voltage range from 3.0 V to 4.3 V at 0.1 C at 25 oC. The voltage plateaus, meaning the charge/discharge process, with no significant difference between the both samples, which are the same as that of the typical Ni-rich NCM sample. The specific charge and discharge capacity of Al doped LiNi0.91Co0.06Mn0.03O2 are 251.4 and 209.2 mAh g-1, respectively. Such high charge and discharge capacities of Al doped LiNi0.91Co0.06Mn0.03O2 are because unwanted materials (lithium compounds) are formed on the surface of the pristine LiNi0.91Co0.06Mn0.03O2 by reaction with the electrolyte. These lithium compounds have relatively low lithium ion and electronic conductivity. Therefore, the movement of lithium ions and electrons becomes difficult. As a result, the charge and discharge capacities of pristine LiNi0.91Co0.06Mn0.03O2 are 249.6 and 204.7 mAh g-1, respectively, which are lower than those of Al doped LiNi0.91Co0.06Mn0.03O2.
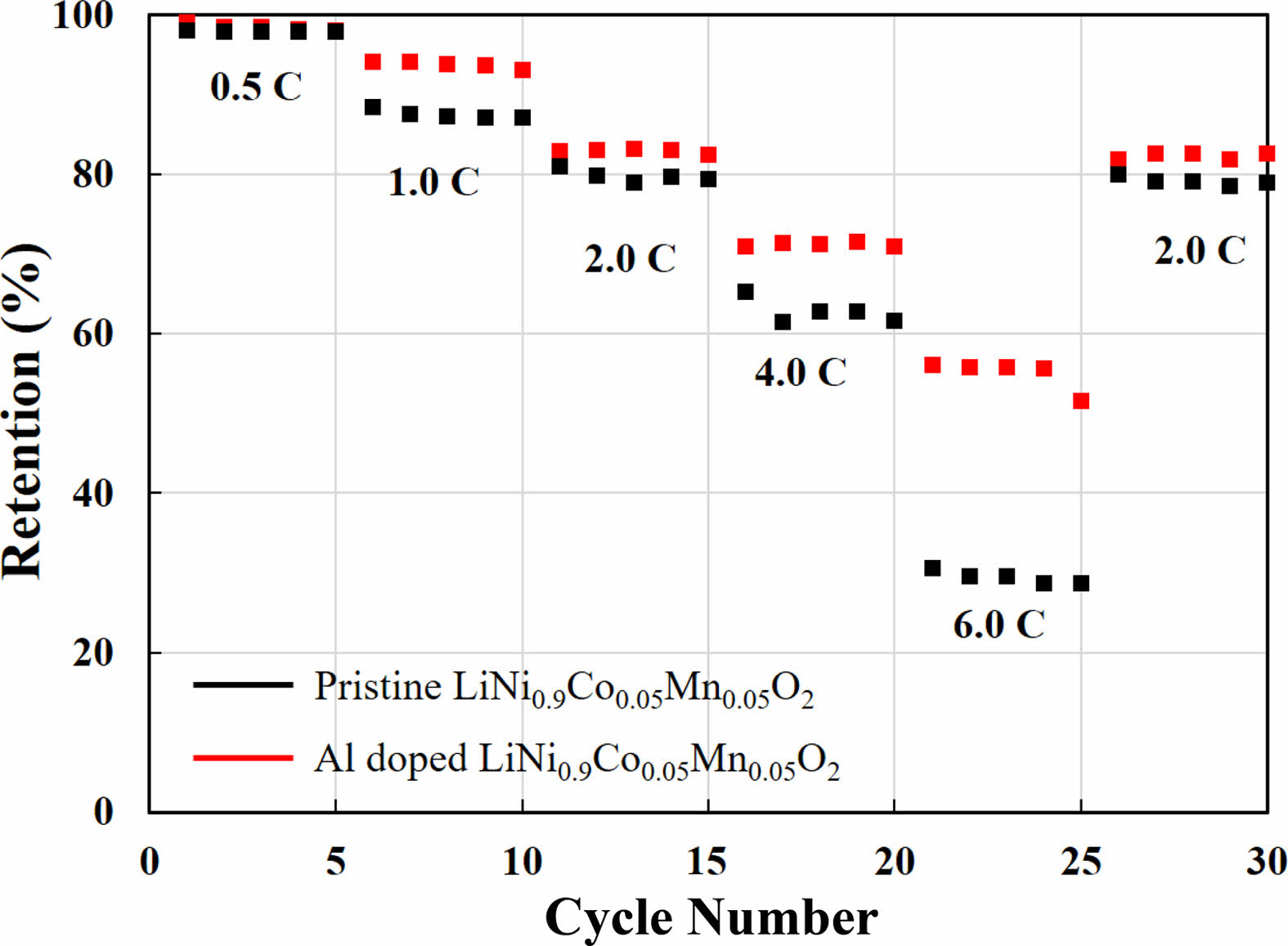
Fig. 4 shows the rate performances of Al-doped and pristine LiNi0.91Co0.06Mn0.03O2 in the current density range from 0.5 C to 6.0 C. Under low current densities such as 0.5 C, it can be seen that the capacity retentions of both samples are almost the same. This means that the movement of lithium ions and electrons is possible regardless of the surface chemistry at low current densities. In both samples, capacity retentions are decreased in proportion to current densities from 0.5 C to 6.0 C. However, the pristine sample decreases much faster compared to the Al doped sample. The capacity retentions of Al-doped samples are 99.1, 92.5, 82.5, 72.3 and 58.5% at 0.5 C, 1.0 C, 2.0 C, 4.0 C, and 6.0 C. And when the current density return to 2.0 C again, the recovery of capacity retention is excellent. However, the capacity retentions of the pristine sample are poor than those of the Al-doped sample (99.0, 90.7, 80.4, 64.7 and 31.2% at 0.5 C, 1.0 C, 2.0 C, 4.0 C, and 6.0 C). In addition, it can be seen that the recovery is also lower when the current density returns to 2.0 C. The lithium diffusion coefficients of Al-doped and pristine samples are 5.04 × 10-12 and 3.41 × 10-12, respectively. The higher value allows for fast and smooth Li-ion movement, resulting in superior rata performance. This is due to the low-conductivity materials created on the surface of the pristine sample, mentioned earlier. Conversely, in the case of the Al modified sample, low conductivity lithium compounds are not generated and the surface is clean due to the stronger bonding force by Al doping into the cathode. Therefore, it is considered that Al modified sample has relatively high capacity even at high current density.
|
Fig. 1 XRD pattern of Al doped and pristineLiNi0.9Co0.05Mn0.05O2. |
|
Fig. 2 FESEM images and EDS mapping of Al doped and pristine LiNi0.9Co0.05Mn0.05O2. |
|
Fig. 3 Initial charge-discharge profiles of Al doped and pristine LiNi0.9Co0.05Mn0.05O2 at 0.5 C (1 C = 210 mAh g-1) |
|
Fig. 4 Rate performance of Al doped and pristine LiNi0.9Co0.05- Mn0.05O2. |
In this paper, we have successfully prepared Al -doped LiNi0.91Co0.06Mn0.03O2. The Al -doped LiNi0.91- Co0.06Mn0.03O2 has a well ordered layered structure compared to the pristine LiNi0.91Co0.06Mn0.03O2. Based on this, Al -doped LiNi0.91Co0.06Mn0.03O2 exhibits relatively higher electrochemical performances. This can be explained by the fact that the Al dopant can effectively inhibit the unwanted reaction with the electrolyte based on its strong bonding with Ni, Co and Mn. As a result, the structure of the Al -doped LiNi0.91Co0.06Mn0.03O2 does not collapse during charging and discharging process, and the original structure is well maintained. Therefore Al-doped LiNi0.91Co0.06Mn0.03O2 can be regarded as a promising way to improve the electrochemical performances of pristine NCM.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1055979). Following are results of a study on the "Leaders in INdustry-university Cooperation +" Project, supported by the Ministry of Education and National Research Foundation of Korea.
- 1. J. W. Seok, J. Lee, T. Rodgers, D.H. Ko, and J.H. Shim, Trans. Electr. Electron. Mater. 20 (2019) 548-553.
-

- 2. Z. Chen, J. Wang, D. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T.C. Sum, J. Lin, and Z. Shen, Scientific Reports 6 (2016) 25771.
-

- 3. S.J. Sim, S.H. Lee, B.S. Jin, and H.S. Kim, Scientific Reports 9 (2019) 8952.
-

- 4. S.J. Sim, S.H. Lee, B.S. Jin, and H.S. Kim, Scientific reports 10 (2020) 11114.
-

- 5. S.H. Lee, B.S. Jin, and H.S. Kim, Scientific reports 9 (2019) 17541.
-

- 6. T. Sattar, S.H. Lee, B.S. Jin, and H.S. Kim, Scientific reports 10 (2020) 8562.
-

- 7. S.J. Jo, D.Y. Hwang, and S.H. Lee, ACS Appl. Energy Mater. 4 (2021) 3693.
-

- 8. D.Y. Hwang, S.J. Sim, B.S. Jin, H.S. Kim, and S.H. Lee, ACS Appl. Energy Mater. 4 (2021) 1743.
-

- 9. Y. Xia, J. Zheng, C. Wang, and M. Gu, Nano Energy 49 (2018) 434-452.
-

- 10. S.H. Lee, K.Y. Kim, and J.R. Yoon, NPG Asia Materials 12 (2020) 28.
-

- 11. J. Zahnow, T. Bernges, A. Wagner, N. Bohn, J.R. Binder, W.G. Zeier, M.T. Elm, and J. Janek, ACS Appl. Energy Mater. 4 (2021) 1335-1345.
-

- 12. Y. Zhou, Z. Hu, Y. Huang, Y. Wu, and Z. Hong, J. Alloys. Comp. 888 (2021) 161584.
-

- 13. A. Habibi, M. Jalaly, R. Rahmanifard, and M. Ghorbanzadeh, J. Alloys. Comp. 834 (2020) 155014.
-

- 14. Y. Zhend, N. Xu, S. Chen, Y. Liao, G. Zhong, Z. Zhang, and Y. Yang, ACS Appl. Energy Mater. 3 (2020) 2837-2845.
-

- 15. P. Vanaphuti, S. Bong, L. Ma, S. Ehrlich, and Y. Wang, ACS Appl. Energy Mater. 3 (2020) 4852-4859.
-

 This Article
This Article
-
2022; 23(5): 566-569
Published on Oct 31, 2022
- 10.36410/jcpr.2022.23.5.566
- Received on Sep 19, 2021
- Revised on Dec 29, 2021
- Accepted on Jan 10, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seung-Hwan Lee
-
Department of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
Tel : +82-42-280-2414 - E-mail: shlee@dju.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.