- Synthesis, crystal structure, and near-infrared reflective properties of ZnSxSe1-x nanopigments
Ti Zhanga,b, Yanmin Wanga,*, Shanjun Keb,* and Zhidong Pana
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this research, a series of near-infrared (NIR) reflecting nanopigments, based on ZnSxSe1-x(x = 1, 0.75, 0.55, and 0.35), have been synthesized via a coprecipitation reaction and subsequent calcination. Furthermore, their crystal structure, particle morphology, chromatic properties, and NIR reflectance have been investigated in detail; the results show that ZnSxSe1-x pigments have a cubic zinc-blende structure with a space group of F-43m(216), and the pigments’ microstructure presents the agglomerates composed of the spherical particles of different nanosizes. ZnSxSe1-x pigments exhibit not only colors ranging from ivory white to bright yellow but also significant NIR solar reflectance, ranging from 80.96% to 86.65%
Keywords: ZnSxSe1-x, Coprecipitation, Near-infrared reflectance, Cool pigment
Currently, due to the urban heat-island effect [1], temperatures in city centers are 3 oC–5 oC higher than in the surroundings. As the urban buildings absorb sunlight, the indoor temperatures increase, affecting people’s living comfort and increasing the building cooling energy consumption. Over the next three decades, the urban heat-island phenomenon is expected to become increasingly severe; at the same time, the energy consumption related to this phenomenon is estimated to increase with the deterioration of the ecological environment and the climate. Therefore, the implementation of urban heat-island mitigation strategies in the next few years will be crucial. In the past few decades, “cool pigments” with high solar reflectance have attracted a broad audience [2, 3]; furthermore, several inorganic pigments with high NIR reflectance have been developed to address this issue [4, 5].
The solar/NIR reflectance of inorganic pigments [6] are closely related to its color. White pigments, e.g., TiO2 [7] and ZnO [8], have excellent thermal reflection properties (total solar reflectance of approximately 85%). However, due to the poor stain resistance of white coatings, it is necessary to develop a variety of NIR reflective pigments with different colors that can simultaneously meet the aesthetics, antiglare, and anti- fouling properties, so as to meet people’s many needs for product functionality and practical beauty [9-19].
Recently, zinc seledide sulfide (ZnSxSe1-x) materials have emerged as promising materials for environmental and energy applications. ZnSxSe1-x is a continuous solid solution of two compounds, zinc sulfide [20-24] and zinc selenide, and its band-gap value shows a continuous change with the composition x. As a wide-bandgap II–VI semiconductor material with high photostability and luminescence quantum yield potential, ZnSxSe1-x has been widely studied in the fields of light-emitting devices, solar cells, sensors, and optical recording materials. However, there are still few reports on the preparation of ZnSxSe1-x as high NIR reflection inorganic pigments. Although our previous work found that ZnSxSe1-x/ZrSiO4 composite can be used as a cool pigment on ceramic tiles for energy saving, the formation mechanism of ZnSxSe1-x “cool pigment” is still unknown and the research on the color and the thermal performance of ZnSxSe1-x are not systematic enough.
In this paper, we have synthesized a series of NIR-reflecting nanopigments based on zinc seledide sulfide (ZnSxSe1-x, x = 1, 0.75, 0.55, and 0.35) and a solid solution whose composition changes with the x com- ponent ratio[25] via a coprecipitation reaction and subsequent calcination. Furthermore, we have analyzed the crystal structure, valence state, morphology, chro- maticity, and NIR reflective properties of the same.
Materials and methods
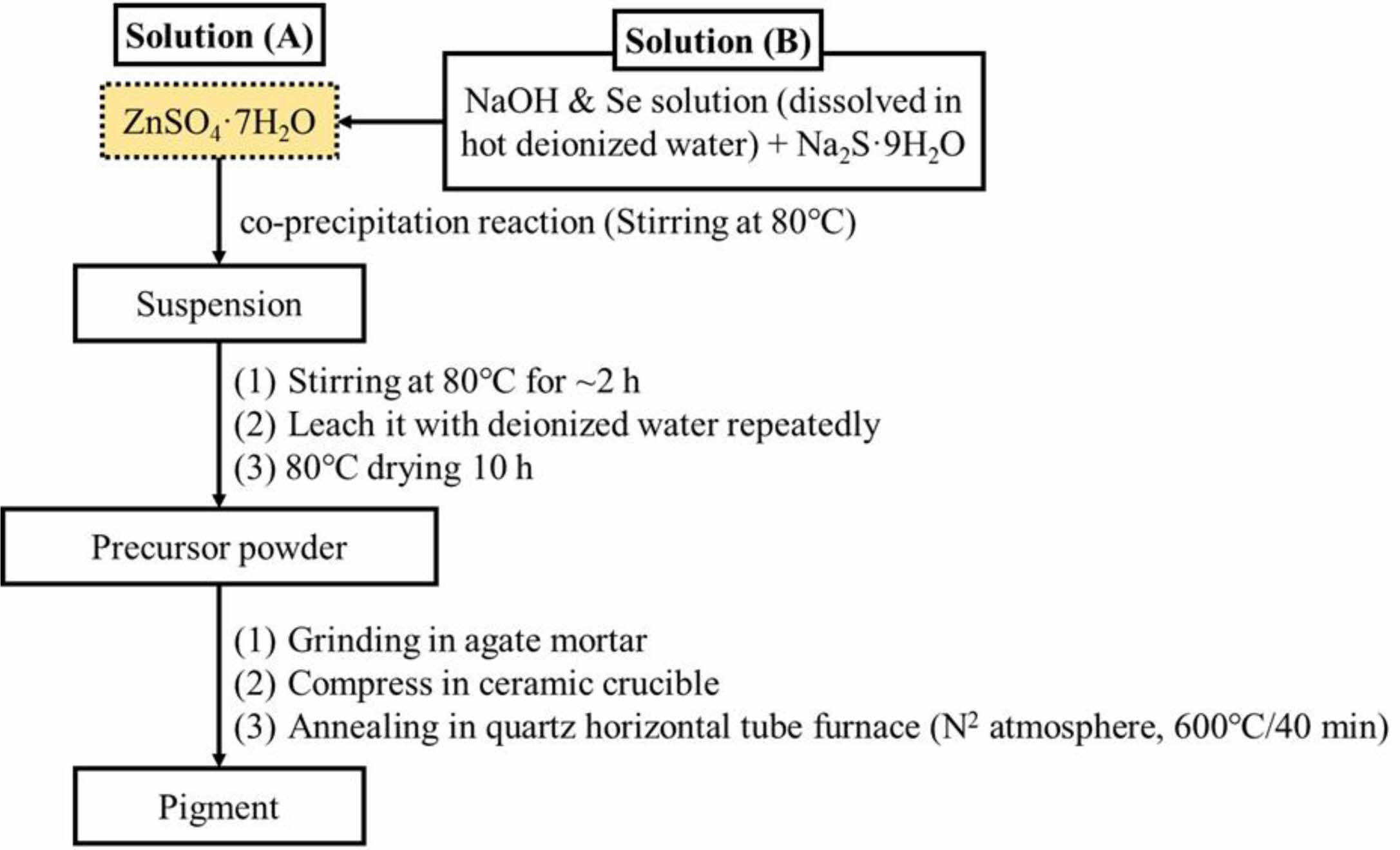
ZnSxSe1-x pigments (x = 1, 0.75, 0.55, and 0.35) have been synthesized by acoprecipitation reaction and subsequent calcination (Fig. 1) using ZnSO4·7H2O (99.5%), Na2S·9H2O (98%), Se (99%), and NaOH (97%) as starting materials, which have been supplied by the Sinopharm Group Co. Ltd., China. Regarding the compositions of ZnSxSe1-x precursors, the x value is noted to change (Table 1).
Characterizations
Crystalline phases of samples were examined through an X-ray diffractometer (PW-1710, Philips Co. Ltd., The Netherlands); the elements’ valence state was determined using an X-ray photoelectron spectroscopy (ESCALAB 250Xi, Thermo Fisher Scientific Co. Ltd., USA); the samples’ morphology was examined through a scanning electron microscope (EVO-18, Carl Zeiss AG, Germany); the particle size distributions were determined by a laser diffraction particle size analyzer (BT-9300S, Bettersize Instruments Ltd., China); specific surface areas of samples were determined based on the nitrogen gas adsorption principle (BET) (Flowsorb III 2310, Micrometrics Co. Ltd., USA); and the samples’ color was determined on a reflection differential colori- meter (Color Premier 8200, X-Rite Incorporated, USA). Furthermore, the diffuse reflectance of samples was measured through a UV–vis-NIR spectrophotometer (LAMBDA 950, PerkinElmer Co. Ltd., USA), using BaSO4 as a reference. The solar reflectance in the NIR region (R*) can be calculated by:
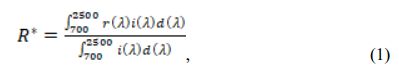
where r(λ) is the spectral reflectance (W·m−2), and i(l) is the standard solar spectrum (W·m−2·nm−1) obtained from the ASTM standard G159-98.
|
Fig. 1 Flowchart for synthesis procedure of ZnSxSe1-x pigments. |
Powder X-ray diffraction analysis
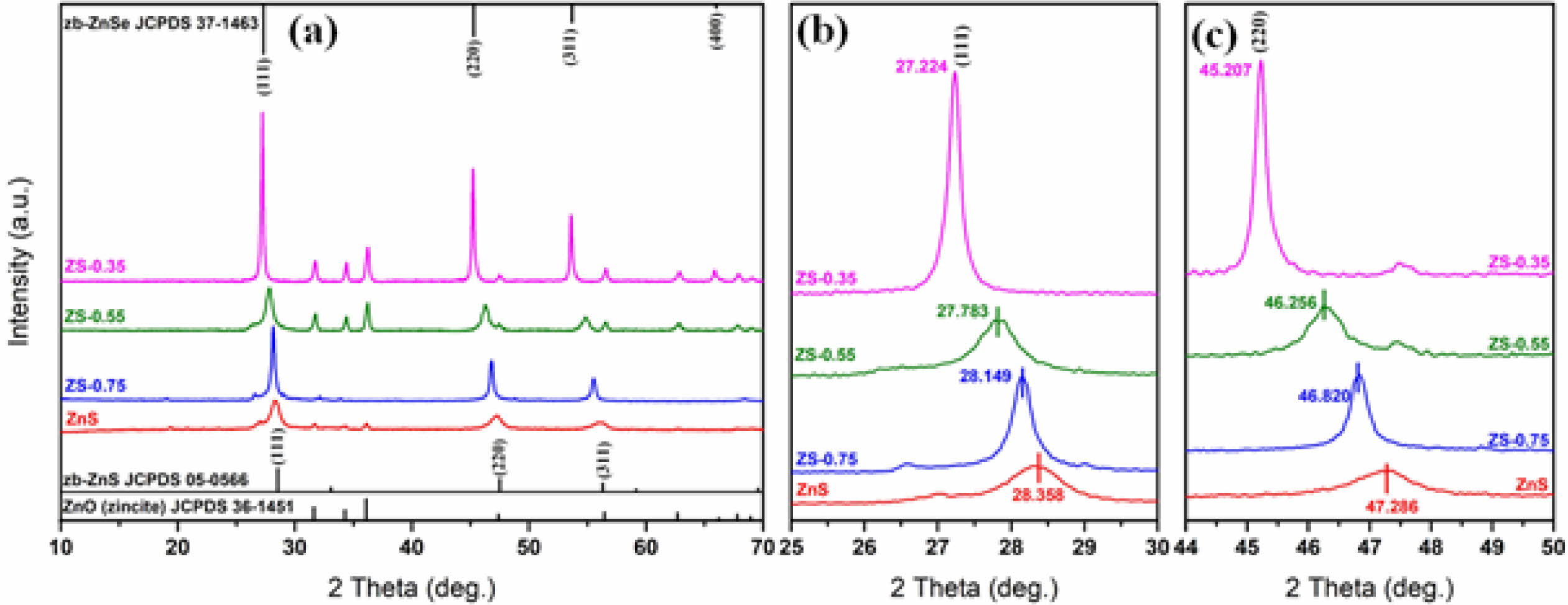
Fig. 2(a) shows XRD patterns of ZnSxSe1-x (x = 1, 0.75, 0.55, and 0.35) composite pigments. Comparing the standard JCPDS File of ZnS (JCPDS card No. 05-0566) with that of ZnSe (JCPDS card No. 37-1463), we found that they have the same cubic zinc-blende (zb) structure with a space group of F-43m(216). The zb-ZnS shows characteristic features at 28.6o, 33.1o, and 56.3o, corresponding to (111), (220), and (311) planes, respectively. Similarly, zb-ZnSe shows characteristic features at 27.2o, 31.5o, and 45.2o, respectively, corres- ponding to the same planes. As the Se content further increases, the samples begin to show peaks of zb-ZnSe (JCPDS card No. 37-1463, cell parameters of a = 5.669 Å). Fig. 2(b) and Fig. 2(c) show the shifts of the Bragg reflections (111) and (220) of ZnSxSe1-x powders; these are due to changes in the cell volume of ZnSxSe1-x pigments. The ionic radius of Se2− (1.91 Å) is greater than that of S2− (1.84 Å) on the tetrahedral sites. When S2− is substituted by Se2−, the bond length of Zn‑Se is less than that of Zn‑S. On the contrary, when the Se content in ZnSxSe1-x increases, (111) and (220) planes shift toward lower 2θ values, increasing the lattice parameters.
Valence state analysis
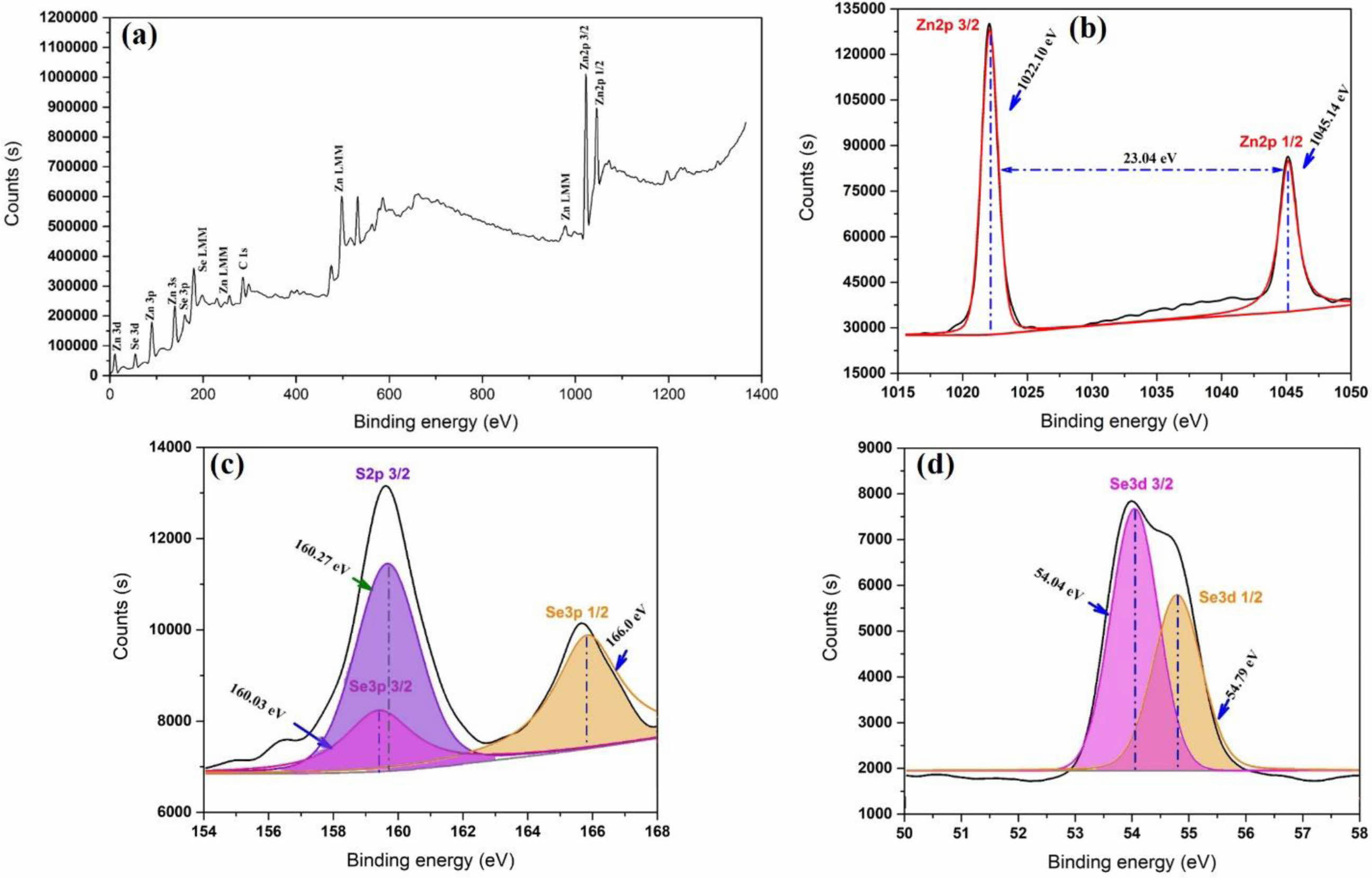
Fig. 3 shows the XPS spectra of the ZnSxSe1-x(x = 0.35) pigment, while Fig. 3(b) shows the high-resolution Zn 2p electron region. The binding energy interval 1022.1 and 1045.14 eV, and that between Zn 2p3/2 and Zn 2p1/2 are characteristic of a Zn2+ oxidation state [26]. The chemical state of S 2p is shown in Fig. 3(c). The peak at 160.27 eV is ascribed to S 2p3/2, which is in agreement with the 160–164 eV scope attained for S in the sulfide phase [27]. The locations of the major peaks (54.04 and 54.79 eV) represent Se 3d3/2 and Se 3d1/2, respectively, indicating that element Se mainly exists as a chemical state of Se2− [28], as shown in Fig. 3(d).
Particle size and morphological analysis
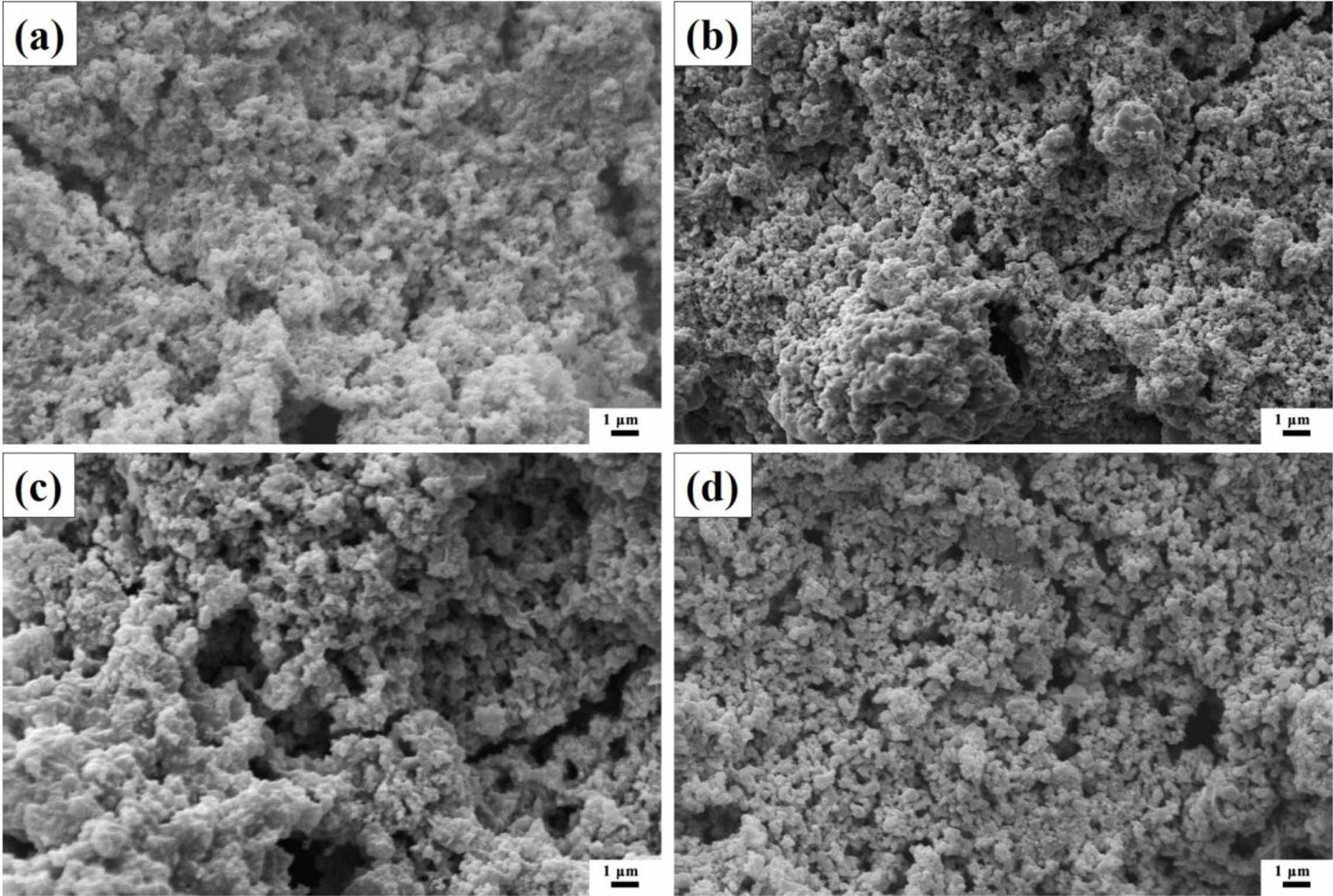
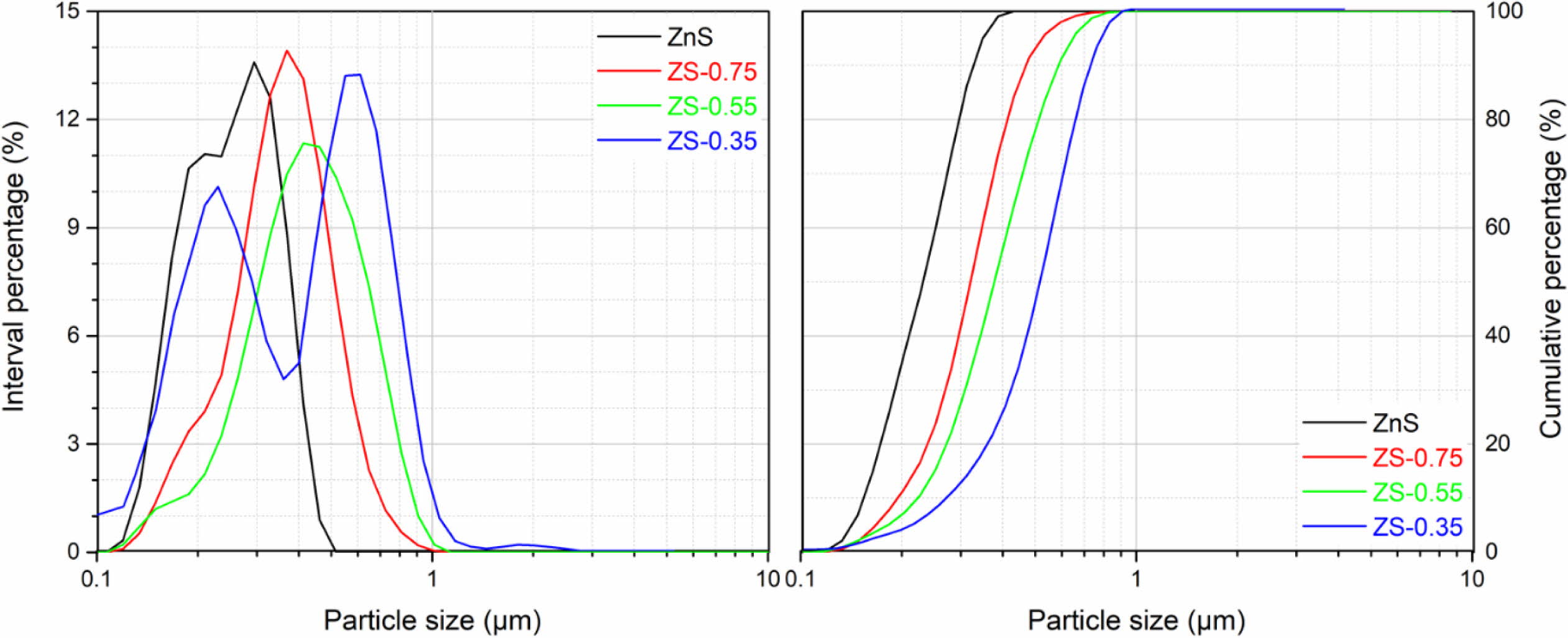
Fig. 4 shows the SEM micrographs of ZnSxSe1-x pigments. The result shows that the increase of Se content in ZnSxSe1-x does not significantly change the samples morphology, which are granular and have a relatively uniform size distribution (see Fig. 5). However, the samples’ microstructure presents agglomerates composed of spherical particles of different nanosizes.
The specific surface areas of ZnSxSe1-x pigments are shown in Table 2. According to the BET test results, the specific surface area of the ZnS sample is 8.14 ± 0.03 m2/g. As the x value decreases, the specific surface area of ZnSxSe1-x pigments gradually decreases to 5.27 ± 0.03 m2/g (x = 0.35).
Chromatic properties analysis
Fig. 6shows photographs of as-synthesized ZnSxSe1-x pigments with different component ratios (x = 1, 0.75, 0.55, and 0.35) by calcination in an N2 atmosphere at 600 oC. The visual color of calcined powders varies from ivory white to bright yellow with the variation of the component ratio.
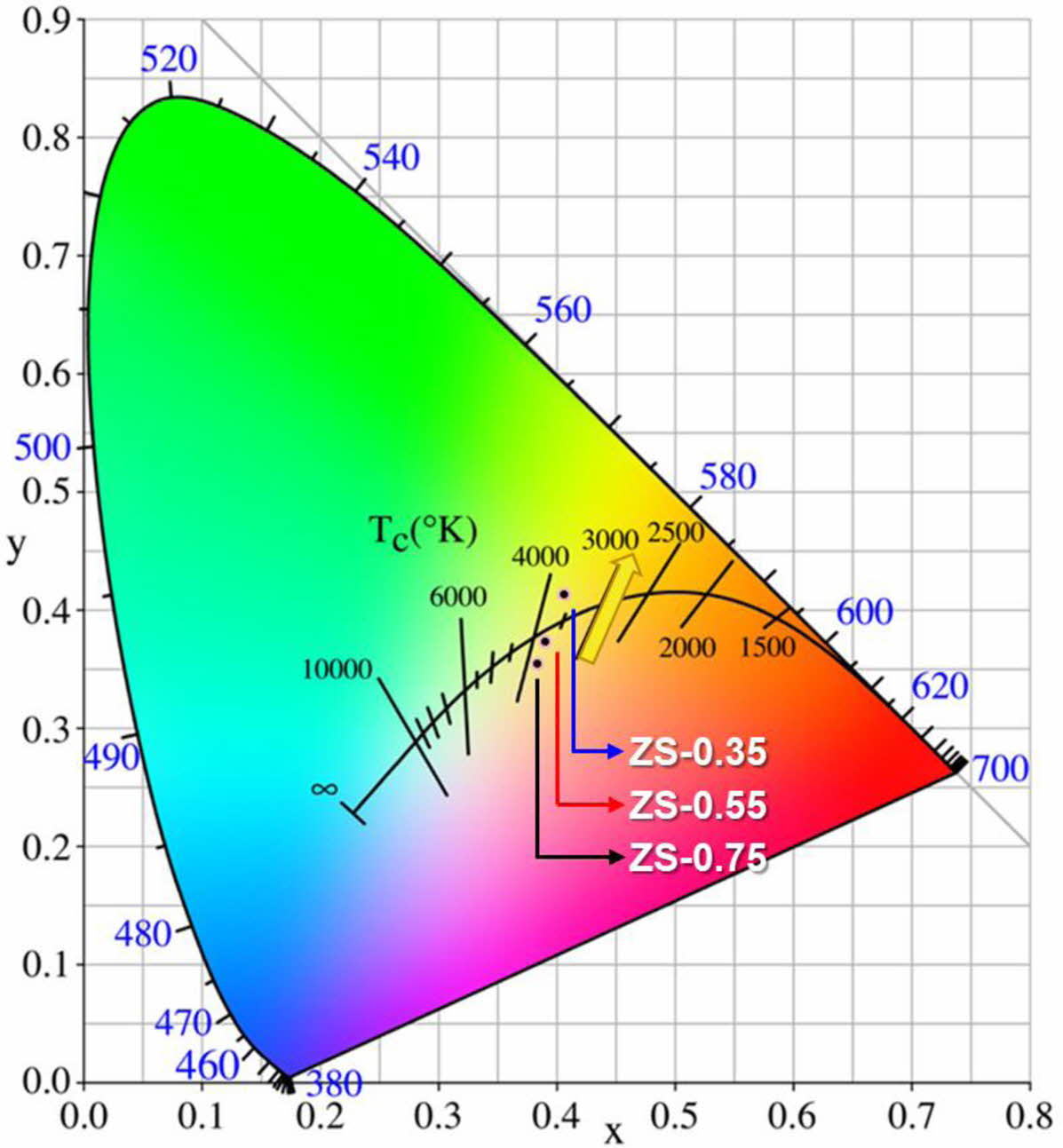
Fig. 7 shows the chromaticity coordinates of ZnSxSe1-x pigments: these are gradually close to yellow with increasing Se content, which is consistent with the fact that the samples’ color changes from ivory white to bright yellow.
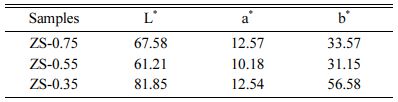
Table 3 shows the influence of the x component ratioon L*, a*, and b* ZnSxSe1-x parameters. As the x value decreases, brightness (L*) increases from 67.58 to 81.85. The yellow chromaticity (b*) of the samples depends on the degree of Se2− substituting S2−. With the increase of Se content, the b* value consistently increases from 33.57 to 56.58, indicating that the yellowness of the sample is enhanced.
NIR reflectance
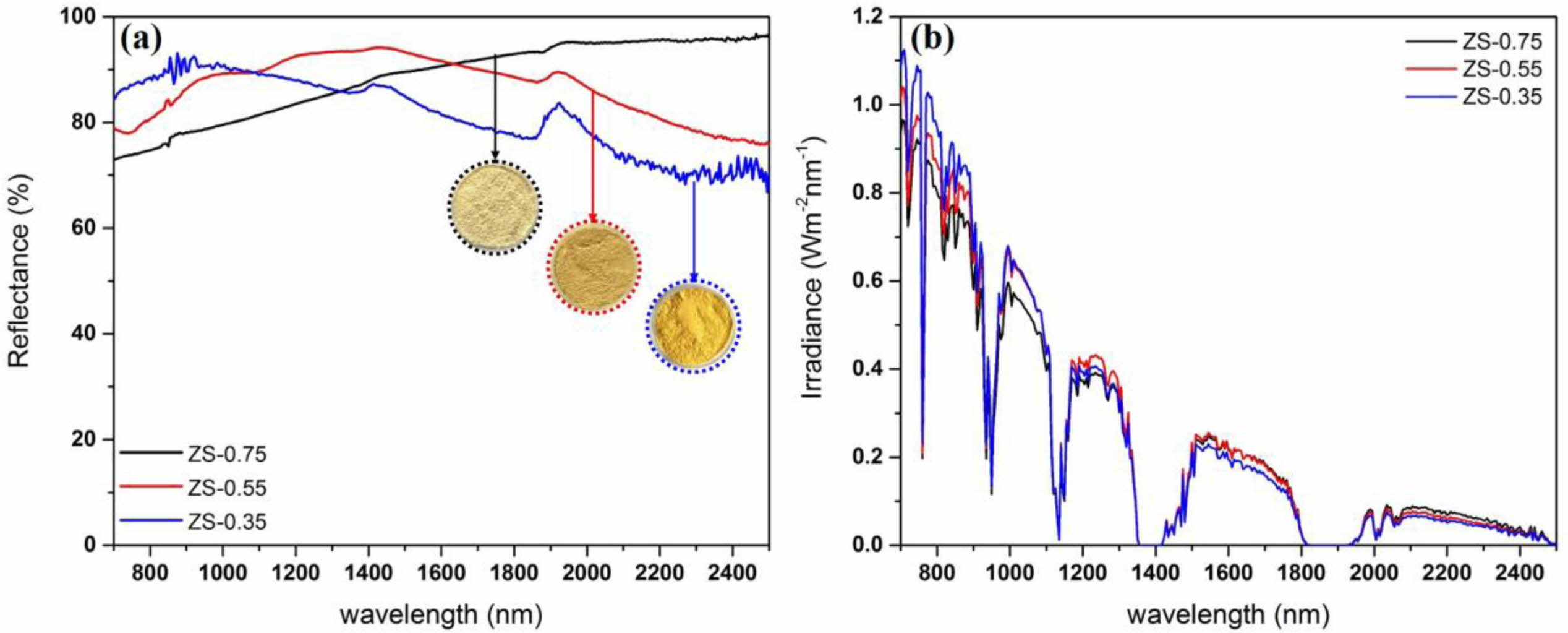
Fig. 8 shows NIR reflectance and solar reflectance of ZnSxSe1-x pigments. The former are greater than 80% in the wavelength range of 700–2,500 nm (Fig. 8(a)). With the increase of Se content, the R* of ZnSxSe1-x pigments increased from 80.96% to 86.65%, while the TSR increased from 65.30% to 71.1% (Fig. 8(b)).
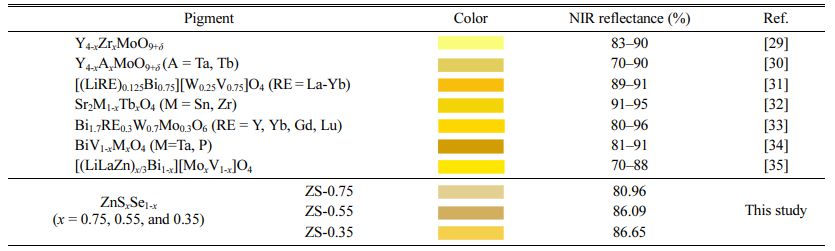
Table 4 shows the comparison of color and NIR reflectance value of ZnSxSe1-x pigments, taking into account data from past researches [29-35]. The ZnSxSe1-x (x = 0.75, 0.55, 0.35) pigments show a good color rendering and excellent NIR reflection performance. With the increase of Se content, the yellowness of the ZnSxSe1-x pigments increases significantly along with the NIR reflectance.
|
Fig. 2 (a) XRD patterns of ZnSxSe1-x (x = 1, 0.75, 0.55, and 0.35) pigments; (b) Bragg reflection shifts for (111) plane; and (c) for (220) plane |
|
Fig. 3 XPS spectra of (a) Survey scan; (b) Zn 2p; (c) S 2p; and (d) Se 3d for the ZnSxSe1-x (x = 0.35) pigment. |
|
Fig. 4 SEM micrographs of ZnSxSe1-x pigments: (a) ZnS; (b) ZS-0.75; (c) ZS-0.55; and (d) ZS-0.35. |
|
Fig. 5 Particle size distributions of ZnSxSe1-x pigments. |

|
Fig. 6 As-synthesized ZnSxSe1-x pigments. |
|
Fig. 7 Chromaticity coordinates of ZnSxSe1-x pigments. |
|
Fig. 8 (a) NIR reflectance and (b) NIR solar reflectance of ZnSxSe1-x pigments. |
|
Table 4 Comparison of color and NIR reflectance value of ZnSxSe1-x pigments, taking into account data from past researches |
To develop the color-turntable inorganic pigments with high NIR reflectance, this paper synthesizes nanopigments based on ZnSxSe1-x(x = 1, 0.75, 0.55, and 0.35) via a coprecipitation reaction and subsequent calcination. The visual color of calcined ZnSxSe1-x powder varies from ivory white to bright yellow with the variation of the component ratio. Moreover, the samples of ZnSxSe1-x (x = 0.75, 0.55, and 0.35) possess higher NIR reflectance in the range of 80.96–86.65 at 700–2500 nm. As the x value decreases, brightness (L*) increases from 67.58 to 81.85. With the increase of Se content, the yellow chromaticity (b*) value consistently increases from 33.57 to 56.58, indicating the excellent yellow properties of ZS-0.35 pigment. Based on the NIR reflectance and various color hues obtained, the ZnSxSe1-x nanopigments could have an application as excellent candidates for “cool pigments.”
This work was supported by the China Postdoctoral Science Foundation (No. 2021M690722) and Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110487).
- 1. M. Ullah, H.J. Kim, J.G. Heo, D.K. Roh, and D.S. Kim, J. Ceram. Process. Res. 20 (2019) 86-91.
-

- 2. A. Synnefa, M. Santamouris, and K. Apostolakis, Sol. Energy 81 (2007) 488-497.
-

- 3. M. Suwan, P. Premjit, P. Thavorniti, P. Kidkhunthod, and S. Supothina, J. Ceram. Process. Res. 18 (2017) 10-15.
- 4. P. Meenakshi and M. Selvaraj, Sol. Energy Mater. Sol. Cells 174 (2018) 530-537.
-

- 5. Y. Shi, M. Zhong, Z. Zhang, and D. Wang, Ceram. Int. 43 (2017) 5979-5983.
-

- 6. J.H. Lee, H.J. Hwang, J.W. Kwon, J.H. Kim, K.T. Hwang, and K.S. Han, J. Ceram. Process. Res. 20 (2019) 127-132.
-

- 7. R. Azizi, S. Rasouli, N.P. Ahmadi, A.J.J. Kolaei, and M. Azizie, J. Ceram. Process. Res. 13 (2012) 164-169.
- 8. T.N. Rao, I. Hussain, Riyazuddin, and B.H. Koo, J. Ceram. Process. Res. 20 (2019) 411-417.
- 9. L.L. Wang, X.X. Liu, X.P. Li, X.F. Wang, L.N. Feng, and X.R. Hou, J. Ceram. Process. Res. 22 (2021) 240-245.
-

- 10. J. Zhang, Y.M. Park, X.Y. Tan, M.K. Bae, D.J. Kim, T.H. Jang, M.S. Kim, S.W. Lee, and T.G. Kim, J. Ceram. Process. Res. 20 (2019) 589-596.
-

- 11. S. Jamshidi, S. Rasouli, B. Janipour, and B. Mirhadi, J. Ceram. Process. Res. 16 (2015) 667-673.
- 12. W. Chatjuthamanee, R. Muanghlua, B. Boonchom, and N. Vittayakorn, J. Ceram. Process. Res. 13 (2012) 713-716.
- 13. B. Tanisan and S. Turan, J. Ceram. Process. Res. 12 (2011) 462-467.
- 14. S. Rasouli, M. Valefi, S.J. Moeen, and A.M. Arabi, J. Ceram. Process. Res. 12 (2011) 450-455.
- 15. S. Rasouli, J. Ceram. Process. Res. 12 (2011) 668-672.
- 16. K.-R. Pyon, K.-S. Han, and B.-H. Lee, J. Ceram. Process. Res. 12 (2011) 279-288.
- 17. Y.Z. Halefoglu and E. Kusvuran, J. Ceram. Process. Res. 11 (2010) 92-95.
- 18. N. Gurbuz, E. Coskun, and E. Ozel, J. Ceram. Process. Res. 11 (2010) 184-190.
- 19. H.S. Lee and B.H. Lee, J. Ceram. Process. Res. 9 (2008) 286-291.
- 20. S.D. Yoon, J.W. Yun, and Y.H. Yun, J. Ceram. Process. Res. 21 (2020) 479-487.
-

- 21. X. Tian, Z. Chen, J. Wen, Y. Du, J. Hu, S. Wang, H. Peng, J. Li, and Y. Peng, J. Ceram. Process. Res. 18 (2017) 116-121.
- 22. K. Liu, W. Song, Y. Xu, J. Li, and Z. Wang, J. Ceram. Process. Res. 19 (2018) 146-149.
- 23. W.K. Jung, J.W. Hong, and D.H. Choi, J. Ceram. Process. Res. 22 (2021) 86-90.
-

- 24. M.R. Bodke, Y. Purushotham, and B.N. Dole, J. Ceram. Process. Res. 16 (2015) 601-604.
- 25. P. Kannappan and R. Dhanasekaran, J. Cryst. Growth 401 (2014) 691-696.
-

- 26. M. Danilson, M. Altosaar, M. Kauk, A. Katerski, J. Krustok, and J. Raudoja, Thin Solid Films 519 (2011) 7407-7411.
-

- 27. M. Tsega, F.B. Dejene, and D.-H. Kuo, J. Alloys Compd. 642 (2015) 140-147.
-

- 28. Y. Wu, Y. Zhang, Y. Sui, Z. Wang, S. Lv, M. Wei, Y. Sun, B. Yao, X. Liu, and L. Yang, Ceram. Int. 44 (2018) 1942-1950.
-

- 29. S. Chen, M. Cai, and X. Ma, J. Alloys Compd. 689 (2016) 36-40.
-

- 30. L. Yuan, A. Han, M. Ye, X. Chen, C. Ding, and L. Yao, Sol. Energy 163 (2018) 453-460.
-

- 31. T.R.A. Thara, P.P. Rao, A.K.V. Raj, and T.S. Sreena, Sol. Energy Mater. Sol. Cells 200 (2019) 110015.
-

- 32. A.K.V. Raj, P. Prabhakar Rao, S. Divya, and T.R. Ajuthara, Powder Technol. 311 (2017) 52-58.
-

- 33. B. Huang, Y. Xiao, H. Zhou, J. Chen, and X. Sun, ACS Sustain. Chem. Eng. 8 (2018) 10735-10741.
-

- 34. L. Sandhya Kumari, P. Prabhakar Rao, A. Narayana Pillai Radhakrishnan, V. James, S. Sameera, and P. Koshy, Sol. Energy Mater. Sol. Cells 112 (2013) 134-143.
-

- 35. T.R. Aju Thara, P.P. Rao, S. Divya, A.K.V. Raj, and T.S. Sreena, ACS Sustain. Chem. Eng. 5 (2017) 5118-5126.
-

 This Article
This Article
-
2022; 23(4): 415-420
Published on Aug 31, 2022
- 10.36410/jcpr.2022.23.4.415
- Received on Dec 16, 2021
- Revised on Mar 5, 2022
- Accepted on Mar 7, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yanmin Wang a, Shanjun Ke b
-
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, China
Tel : +86 20 87114883 Fax: +86 20 87110273 - E-mail: wangym@scut.edu.cn (Y. Wang), sjkescut@163.com (S.







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.