- Synthesis of in-situ encapsulated ZnSxSe1-x/ZrSiO4 composite as cool pigment on ceramic tiles for energy saving
Ti Zhanga,b, Yanmin Wanga,*, Shanjun Keb,* and Zhidong Pana
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
An in-situ encapsulated ZnSxSe1-x@ZrSiO4 composite as a cool pigment on ceramic tiles was synthesized via soft mechano-chemical (MC) pretreatment of ZrSiO4 precursors, high-shear homogeneous dispersing and subsequent sintering. The properties of ZnSxSe1-x@ZrSiO4 composite cool pigment and solar near-infrared (NIR) reflective ceramic tiles coated with the cool pigment were investigated by X-ray diffraction (XRD), Fourier transfer infrared spectroscopy (FTIR), thermogravimetry and differential scanning calorimetry (TG-DSC), scanning electron microscopy (SEM), ultraviolet-visible-near-infrared (UV-VIS-NIR) spectroscopy and infrared irradiation image analysis. The results show that compared to ceramic tiles uncoated (R* = 60.07%), solar NIR reflective ceramic tiles coated with ZnSxSe1-x@ZrSiO4 composite cool pigment have a higher solar reflectance (R* = 87.61%), indicating that the ceramic tiles coated with the cool pigment have a superior NIR reflectivity. The surface temperature of ceramic tiles coated with ZnSxSe1-x@ZrSiO4 composite is 7.2 oC lower than that of ceramic tile uncoated with the cool pigment. In addition, the energy saving of ceramic tiles coated the cool pigment can be achieved based on the energy simulation of solar NIR reflective ceramic tiles for ‘cool roof’ application in subtropical climate by a software named Design Builder 4.6.0.015
Keywords: Zircon in-situ encapsulated, ZnSxSe1-x cool pigment, Near-infrared reflectance, Solar NIR reflective ceramic tile, Energy savings, Emission reductions
Using ‘cool roof’ [1] products instead of conventional roofs in urban constructions provides less absorption of heat and keeps the building’s interior cool. The existing polymer materials dominate the cool roof product applications, e.g., cool coating [2-5] and cool waterproof membrane [6-8]. The industrial process of these polymer materials is simple and easy to operate. However, their weather resistance and anti-aging performance become a challenge.
Ceramic tiles coated with a cool pigment enamel layer could give an innovative approach [9-13]. This approach may complement the cool roof products [14]. Ceramic-based cool roof products [15-17] (e.g., ceramic tiles, clay roof tiles, etc.) possess high mechanical properties, fire resistance, and excellent chemical and physical durability [18]. Conventional ceramic tiles [19, 20] usually consist of three layers, i.e., ceramic substrates, thin engobe and pigmented overglaze [21, 22]. To meet the application demand for cold roof products with different colors and decorative effects, multicolor inorganic pigments with a high NIR reflectance were added into the ceramic glaze to prepare cool ceramic tiles [23-27]. The dense glaze layer on the surface of ceramic tiles has a certain antifouling ability, which can reduce the surface pollution caused by weathering and scaling.
Ferrari et al. [28] prepared a white ceramic tile with a high total solar reflectance (TSR) by coating ZrSiO4 glaze on the surface of ceramic substrates. They found that the TSR values of ceramic tiles are related to its microstructure, mineral composition and surface roughness. Levinson et al. [29] proposed a manufacturing method to prepare multi-colored overglaze and coating onto the clay tile surfaces as non-white surface ceramic tiles with a high NIR reflectance using clay tiles as base materials. The cool ceramic tile products can be industrially produced via only adjusting the formula of ceramic tiles. Therefore, it is more cost-effective to prepare cool ceramic tile products by coating near-infrared reflective glaze on the surface of ceramic substrates. The development of cool ceramic tiles will become a promising research field.
In this paper, the application and energy saving of potential cool ceramic pigments (e.g. zircon in-situ encapsulated ZnSxSe1-xcool pigment) on ceramic tiles were evaluated. The annual energy and emission savings of applying solar NIR reflective ceramic tiles in subtropical regions were simulated by a software named Design Builder based on the dynamic energy consumption simulation.
Materials
Tetraethyl orthosilicate (TEOS, 98% purity), zirconium oxychloride (ZrOCl2·8H2O, 98% purity), lithium fluoride (LiF, 99% purity), zinc sulfate heptahydrate (ZnSO47H2O, 99.5% purity), copper sulfate pentahydrate (CuSO4·5H2O, 99% purity), indium sulfate (In2(SO4)3, 99% purity), sodium sulfide nonahydrate (Na2S9H2O, 98% purity), selenium (Se, 99.9% purity, Umicore Group, Belgium), sodium hydroxide flakes (NaOH, 97% purity) were used as raw materials. Chemicals were purchased from Shanghai Macklin Biochemical Co. Ltd., China.
Synthesis of in-situ encapsulated ZnSxSe1-x@ZrSiO4 composite cool pigment
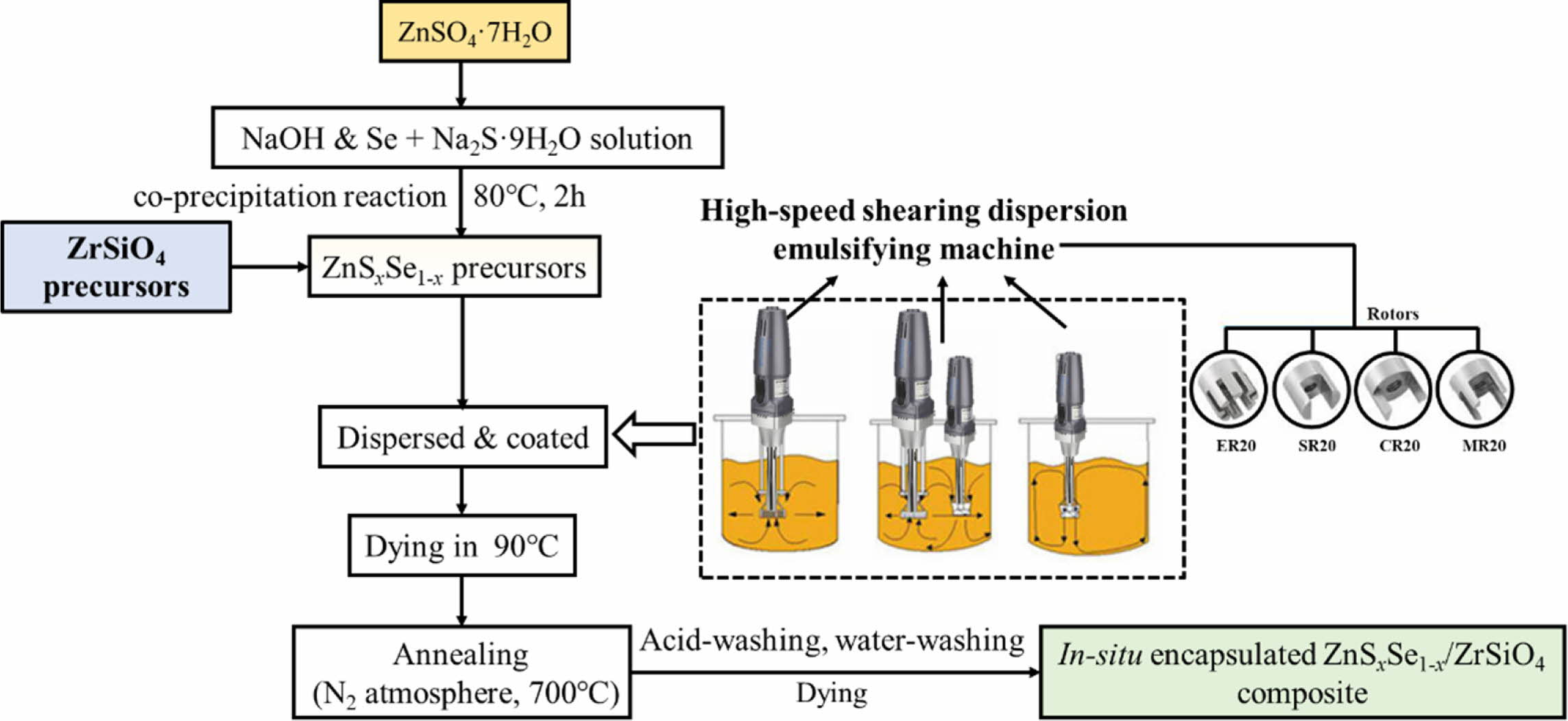
Zircon-based in-situ encapsulated pigment (i.e., ZnSxSe1-x@ZrSiO4 composite) was prepared by soft mechano-chemical pretreatment of ZrSiO4 precursors [30],high-shear homogeneous dispersing and subsequent sintering. Fig. 1 shows the preparation process of ZnSxSe1-x@ZrSiO4 compositeas a cool pigment.
For preparation of ZnSxSe1-x@ZrSiO4 (labeled as ZS@ZrSiO4) compositecool pigment, ZnSxSe1-x (labeled as ZS) precursor particles were synthesized via co-precipitation reaction, the optimum process of ZnSxSe1-x precursor synthesis was described in a previous work [31] and the atomic ratio of Zn:S:Se is 1.00:0.35:0.65. Afterwards, ZnSxSe1-x precursor particles were mixed with soft mechano-chemical pretreated ZrSiO4 precursors in a model D-500 high-shear homogeneous dispersing emulsifier (Wiggens Co., Ltd., Germany) at a rotational speed of 20,000 rpm. ZS@ZrSiO4 precursors were calcined in N2 atmosphere at 700 oC for 60 min.
Preparation of solar NIR reflective ceramic tiles
The preparation process of solar NIR reflective ceramic tiles consists of two steps, i.e., ceramic substrates preparation and solar NIR reflective glaze coating on ceramic substrate surfaces.
The ceramic substrate comprises ceramic body and ceramic engobe with the formula according to GB/T 4100-2015 (Ceramic Tile), PRC. The sintering process of solar NIR reflective ceramic tiles is a double-firing process (i.e., the ceramic substrates are sintered at 1,100~1,200 oC, and then the ceramic tiles with solar NIR reflective glaze are sintered at 1,050~1,150 oC). The chemical compositions of ceramic bodies and engobe are shown in Tables A.1 and A.2.
Solar NIR reflective glaze comprises low-temperature frit and NIR reflective pigment (i.e., ZnSxSe1-x@ZrSiO4 composite). The chemical composition of low-temperature frit is shown in Table A.3. ZnSxSe1-x@ZrSiO4 compositecool pigment and low-temperature frit were put in an agate mortar and mixed evenly (the mass ratio of pigment to frit is 10:90), then a certain amount of anhydrous ethanol was added into and mixed with above powder to form a slurry and coated on the ceramic substrate. After drying overnight, the solar NIR reflective ceramic tiles were calcined at 1,050 oC for 30 min.
Energy simulation
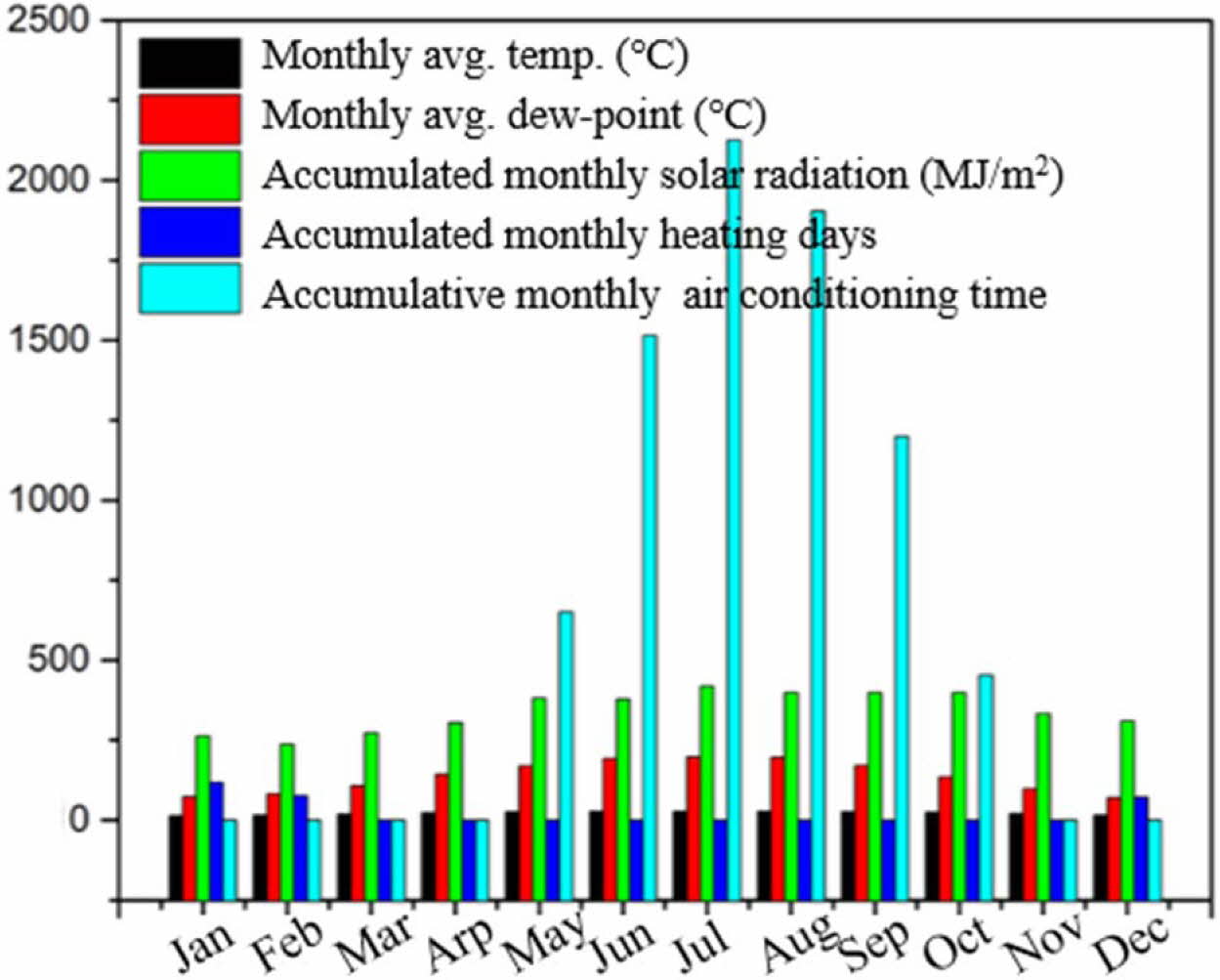
The simulations were performed in subtropical climate (see Fig. 2) by using a graphical user interface for EnergyPlus which named Design Builder 4.6.0.015.
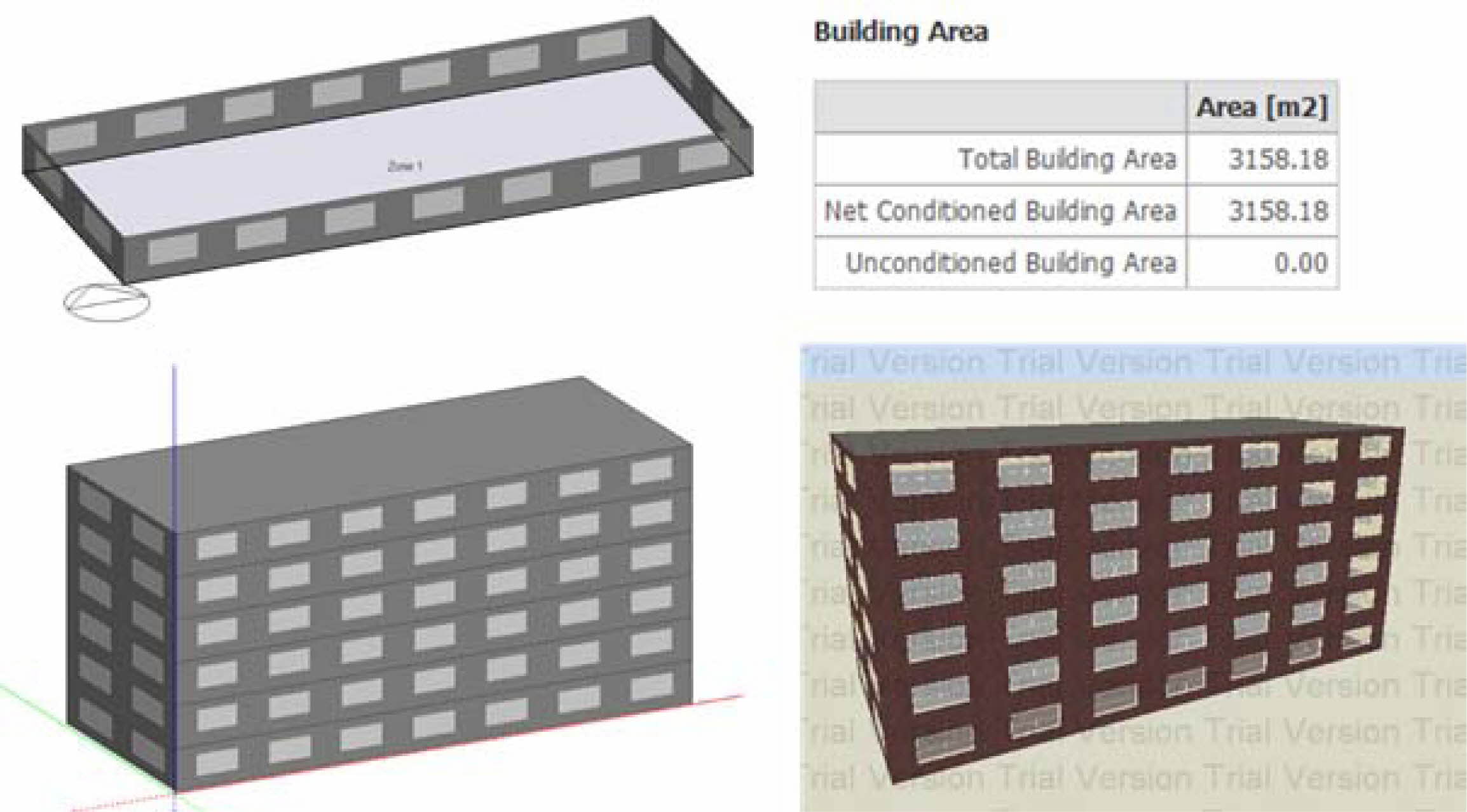
A six-story residential house (see Fig. 3) was consi- dered for simulation. The total building area is 3,158.18 m2. The prototype was modeled with two different roof albedos, representing as-prepared solar NIR reflective ceramic tiles (solar radiation absorption coefficient ρ = 0.3) and cement wall (ρ = 0.7), respectively. Geography and climate of Guangzhou, China see Table 1. Assumptions of parameters used to compute energy cost, and emission savings see Table 2.
Characterizations
The crystal structure of samples were determined by an X-ray diffractometer (XRD, PANalytical B.V., the Netherlands) with Cu Kα radiation (λ = 1.5406 Å). The Fourier transform infrared spectra of samples were recorded on a Fourier infrared spectrometer (FTIR, Thermo Fisher Scientific Co. Ltd., USA) in a frequency range of 400-4,000 cm-1. The thermal properties of samples were measured by a model STA449C/3/MFC/G thermogravimeter and differential scanning calorimeter (TG-DSC, Netzsch Instruments Ltd., Germany) in air at a heating rate of 10 oC/min with α-Al2O3 as a reference. The microstructures of samples were characterized by a scanning electron microscope (SEM, Carl Zeiss AG, Germany), equipped with energy-dispersive X-ray analysis. The L*a*b* color parameters of samples were measured by a reflection differential colorimeter (X-Rite Co., USA). The UV-vis-NIR diffuse reflectance spectra of samples were acquired in a high-resolution double-monochromator spectrophotometer (PerkinElmer Co. Ltd., USA). The NIR solar reflectance (R*) was calculated in accordance with the ASTM standard G159-98,
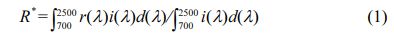
where r(λ) is the spectral reflectance (W·m-2) and i(λ) is the solar spectral irradiance (W·m-2·nm-1) obtained from the ASTM standard G159-98.
For evaluating thermal performance of the solar NIR reflective ceramic tiles, an infrared thermal camera (FLIR Systems Inc., USA) was used to observe the surface temperature distribution of ceramic tiles. Fig. A.1 shows the experimental device to measure the temperature difference of ceramic tiles.
|
Fig. 1 Preparation process of ZnSxSe1-x@ZrSiO4 composite cool pigment. |
|
Fig. 2 Standard annual weather data in subtropical climate. |
|
Fig. 3 Design Builder simulation building model. |
|
Table 1 Summer and winter average temperatures and solar irradiance of subtropical climate |
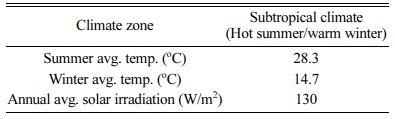
|
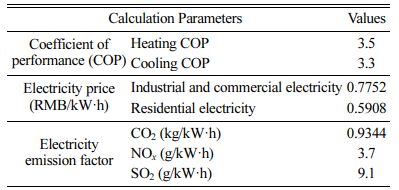
Table 2 Assumptions of parameters used to compute energy cost, and emission savings from heating and cooling load savings |
ZnSxSe1-x@ZrSiO4 composite cool pigment
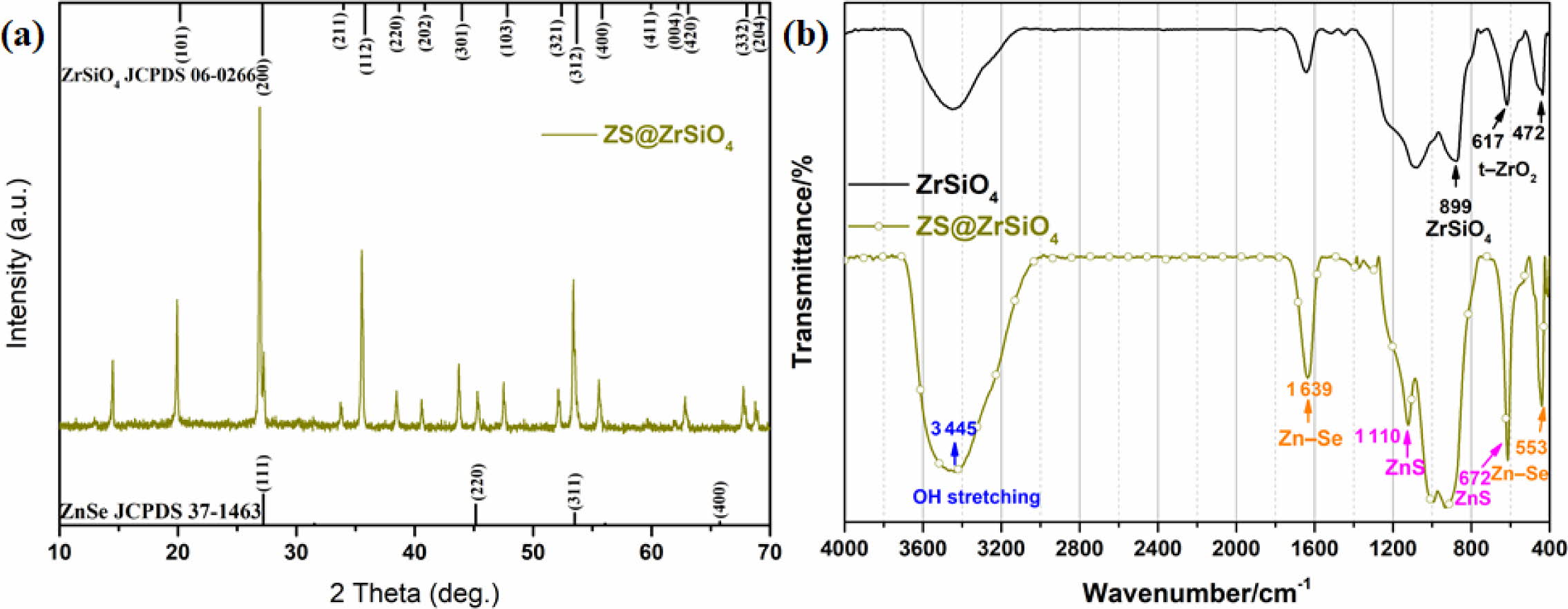
Fig. 4 shows XRD patterns and FTIR spectra of ZS@ZrSiO4 compositecool pigment sintered at 700 oC. The diffraction peaks of ZS@ZrSiO4 composite mainly consist of zircon phase (ZrSiO4, JCPDS card 06-0266) with high crystallinity degree, and a small amount of ZnSe (JCPDS card 37-1463) are detected (see Fig. 4(a)). It indicates that the coating process does not change the crystalline structure of ZnSxSe1-x pigment (i.e., ZnSe, cubic zinc-blende structure, space group of F-43m(216)) [31].
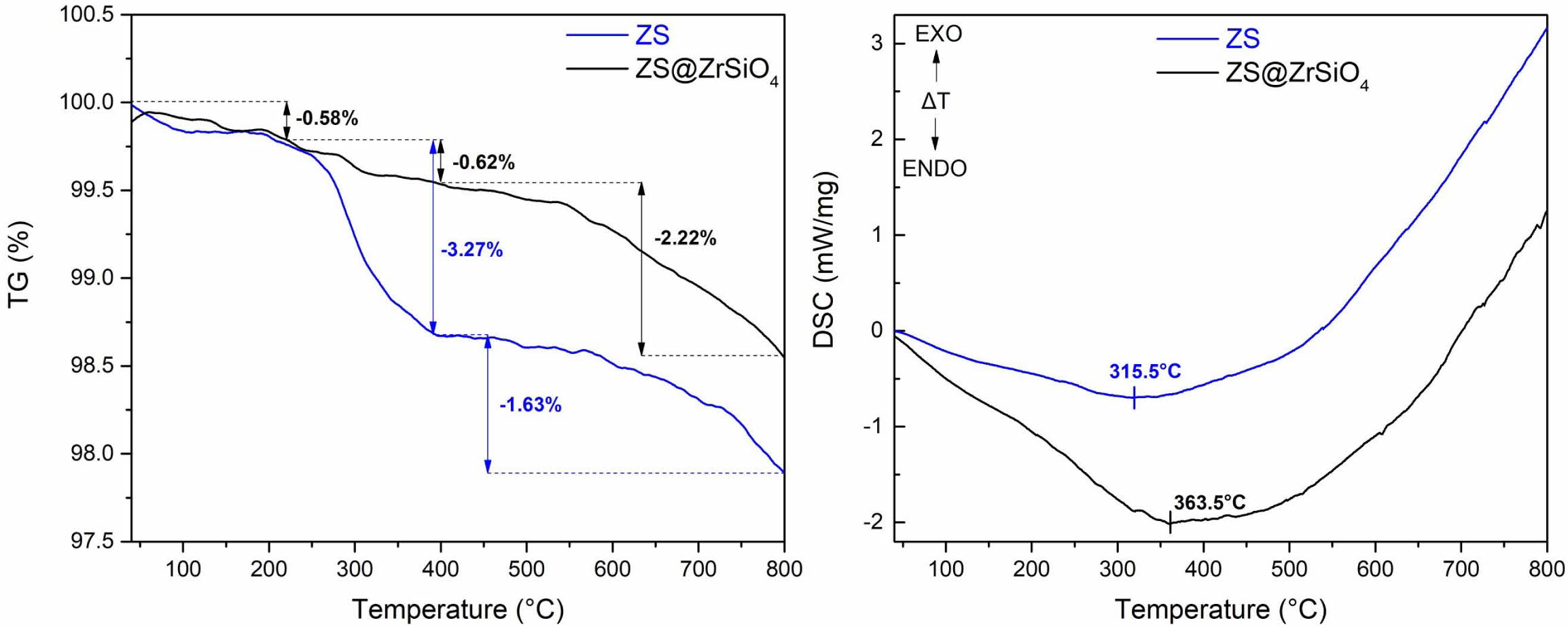
Fig. 4(b) shows the FT-IR spectra of ZS@ZrSiO4 composite. The bands located at 899 cm-1 are ascribed to Si-O-Zr characteristic vibrations [32], which is similar to the FTIR spectrum of ZrSiO4. Besides, the characteristic major peaks of ZnS appear at 1,110 cm-1 and 672 cm-1 [33], and the bands appeared at 553 and 1,639 cm-1 belong to Zn-Se vibrations [34], indicating that the ZS@ZrSiO4 compositecool pigment are effectively coated by ZrSiO4. The thermal stability of ZnSxSe1-x@ZrSiO4 composite is superior to ZnSxSe1-x pigment [31, 35] (see Fig. 5) for ceramic glazes and tiles.
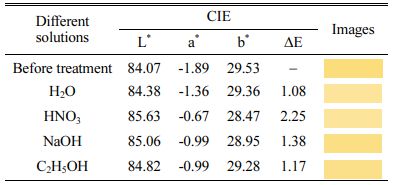
The mechanism diagram for the formation of ZrSiO4 in-situ encapsulated ZnSxSe1-x pigment see Fig. A.2. The chemical and coloration stability of ZS@ZrSiO4 compositecool pigment were tested according to the PRC National Standard GB/T 3810.13-2016 [36]. The as-prepared compositecool pigments were soaked in H2O and 6 wt.% HNO3, NaOH, C2H5OH, respectively. The color coordinates of ZS@ZrSiO4 compositecool pigments treated in different solutions see Tables 3. The ∆E represented the total color difference between untreated and treated pigments.
Ceramic tile coated with ZS@ZrSiO4 composite cool pigment
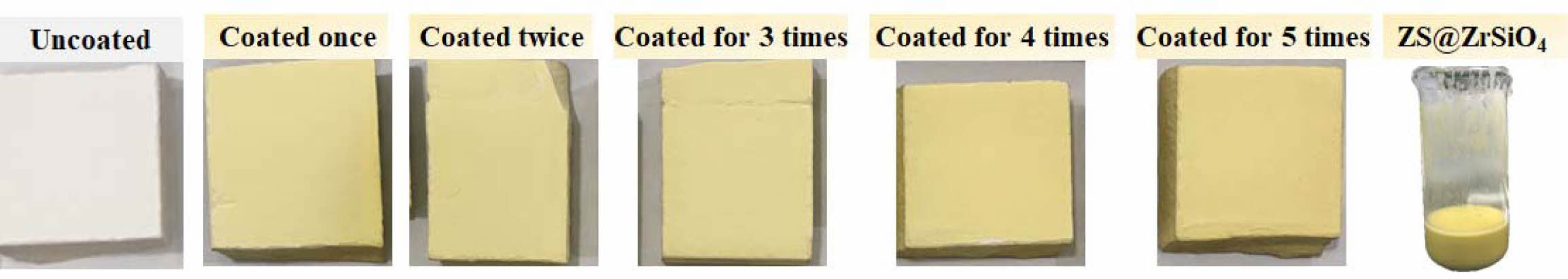
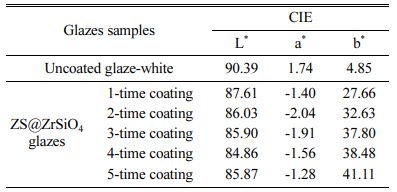
Photographs of as-prepared solar NIR reflective ceramic tiles see Fig. 6. All enameled samples with ZS@ZrSiO4 compositecool pigment exhibit a homo- geneous yellow hue, and the yellow chromaticity of samples enhanced with the increasing of coating times. It means that the as-prepared ZS@ZrSiO4 compositecool pigment has high thermal stability and tinting strength, which makes them suitable for ceramic decoration.
The color coordinates of solar NIR reflective ceramic tiles see Table 4. The L* values of uncoated glaze-white are 90.39, indicating that the as-prepared ceramic substrates have excellent whiteness. In the enameled samples with ZS@ZrSiO4 composite, the b* value of enameled samples rises from 27.66 to 41.11 with the increasing of coating times, and the color of all of the enameled samples are brighter yellow.
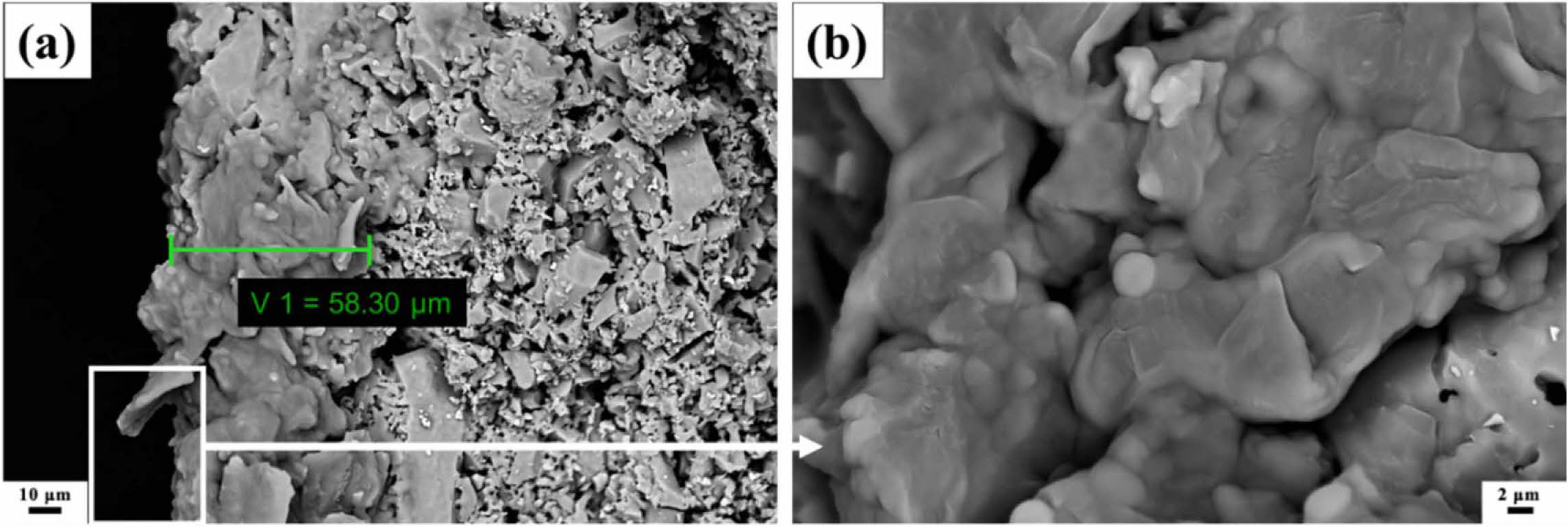
The cross-section of enameled sample with ZS@ ZrSiO4 compositecool pigment and coated for 3 times (i.e., ZS@ZrSiO4 glaze-3) was observed by SEM, and the thickness of glaze layer is 50-60 μm (see Fig. 7(a)). Fig. 7(b) shows the surface of ZS@ZrSiO4 glaze-3 sample. The glaze sample surface is rough and possesses a small amount of pin-hole defects.
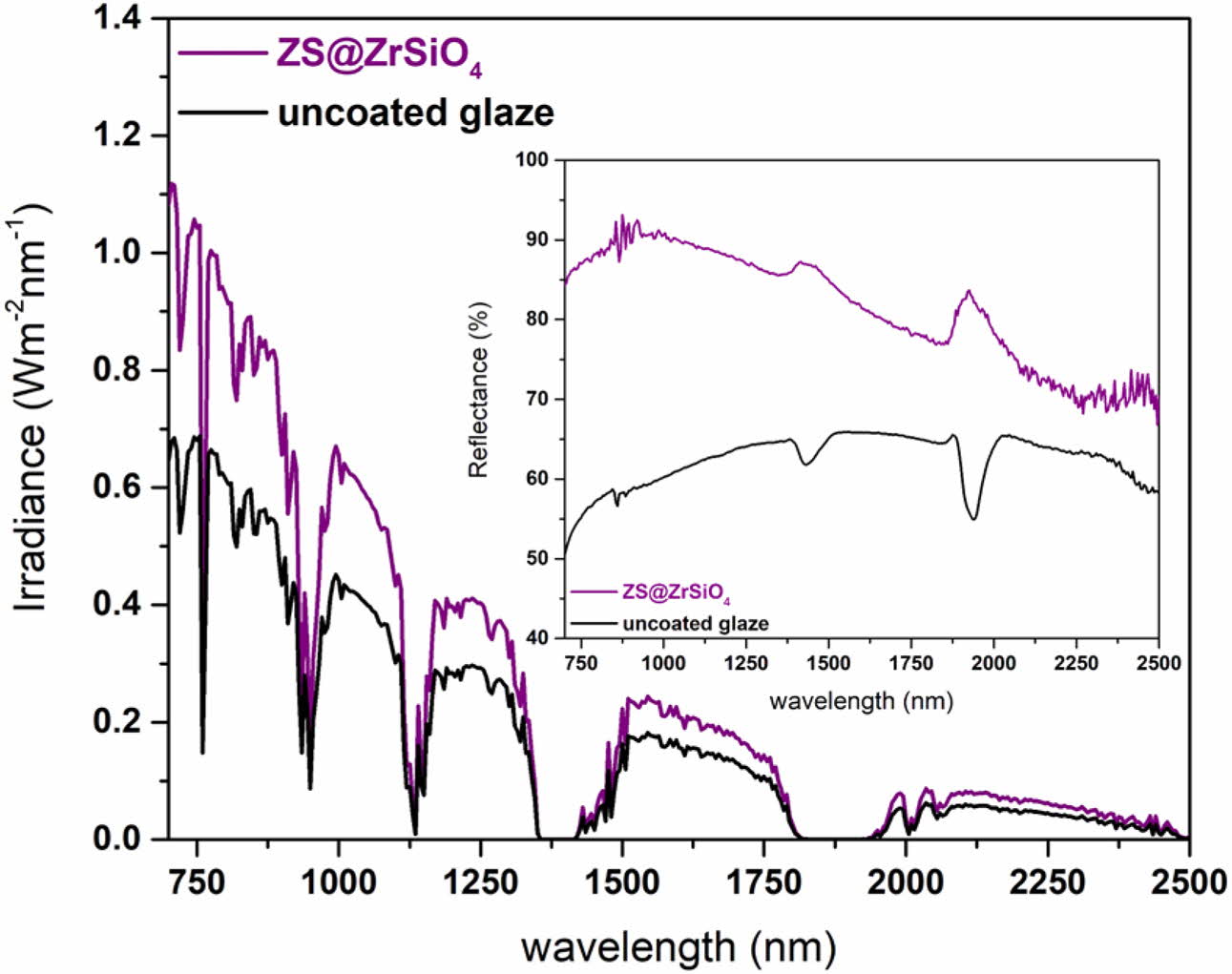
To evaluate the thermal insulation performance of solar NIR reflective ceramic tiles, Fig. 8 shows the comparison of NIR reflectance of the ceramic substrates (i.e., uncoated glaze-white) and enameled samples with compositecool pigment (i.e., ZS@ZrSiO4 glaze-3).
Clearly, the NIR reflectance of ceramic substrates is significantly lower in the NIR wavelength range of 1,850-2,050 nm. Because the white engobe coated on the ceramic substrate surface is a matte glaze, which has lower reflectivity compared with gloss glaze or transparent glaze [28].
The NIR solar reflectance of ZS@ZrSiO4 glaze-3 is 87.61%, higher than that of uncoated glaze-white (R* = 60.07), indicating that solar NIR reflective yellow ceramic tiles have excellent NIR reflectivity (see Table 5).
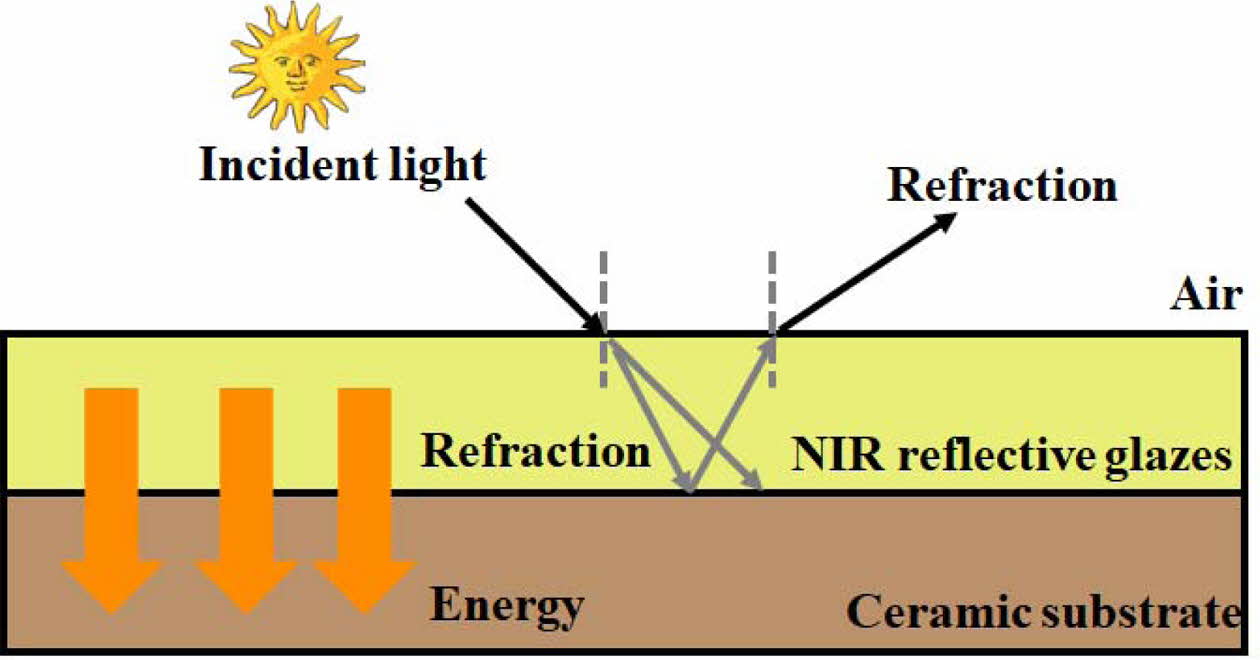
The radiant range of light energy or electromagnetism from the sun is wide (300-2,500 nm), therefore, there is only a part of the solar spectrum that can be seen by human eyes. The light irradiated on an object occurs various phenomena, such as surface reflection, selective absorption or transmission.
Suppose the initial light energy in the plane perpendicular to the propagation direction of light (i.e., Light fluence rate) is IO, the transmitted light energy of the object illuminated by light is IT, and the reflected light energy is IR, absorbed light energy is IA, then there is


where A, T, and R are absorption coefficient, trans- mission coefficient and reflection coefficient, respectively.
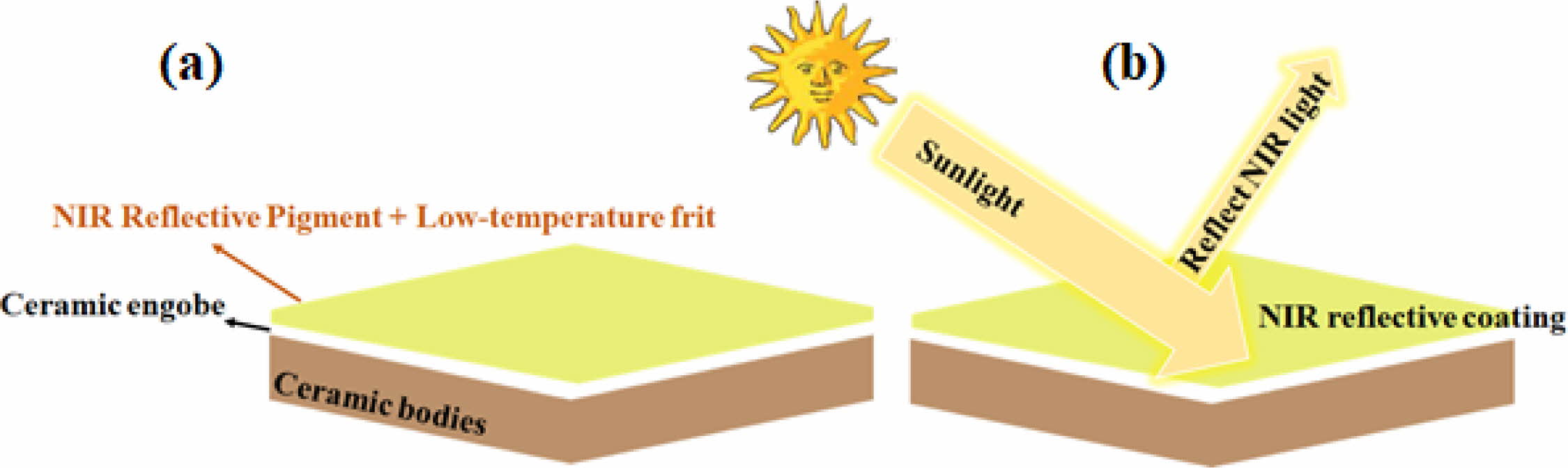
In a ceramic structure, the transmission effect is negligible (T ≈ 0) when light incidents from the air. Therefore, the intensity of light reflection on ceramic glaze surface determines the light energy entering the tile (see Fig. 9). Shining on the surface of the ceramic glaze is followed by the increasing heat energy conducted into the building. This achieves the conversion of optical phenomena into thermodynamic process.
Schabbach et al. [9] investigated the TSR and optical properties of pigmented glazed ceramic tiles. The results show that, there is high absorption of radiation in the near-infrared region for some glazes, which means most of the ceramic tiles in use show ‘non-cool’ surfaces [37]. However, the solar radiation reflecting can be achieved by coating NIR reflective glaze layer on ceramic substrates.
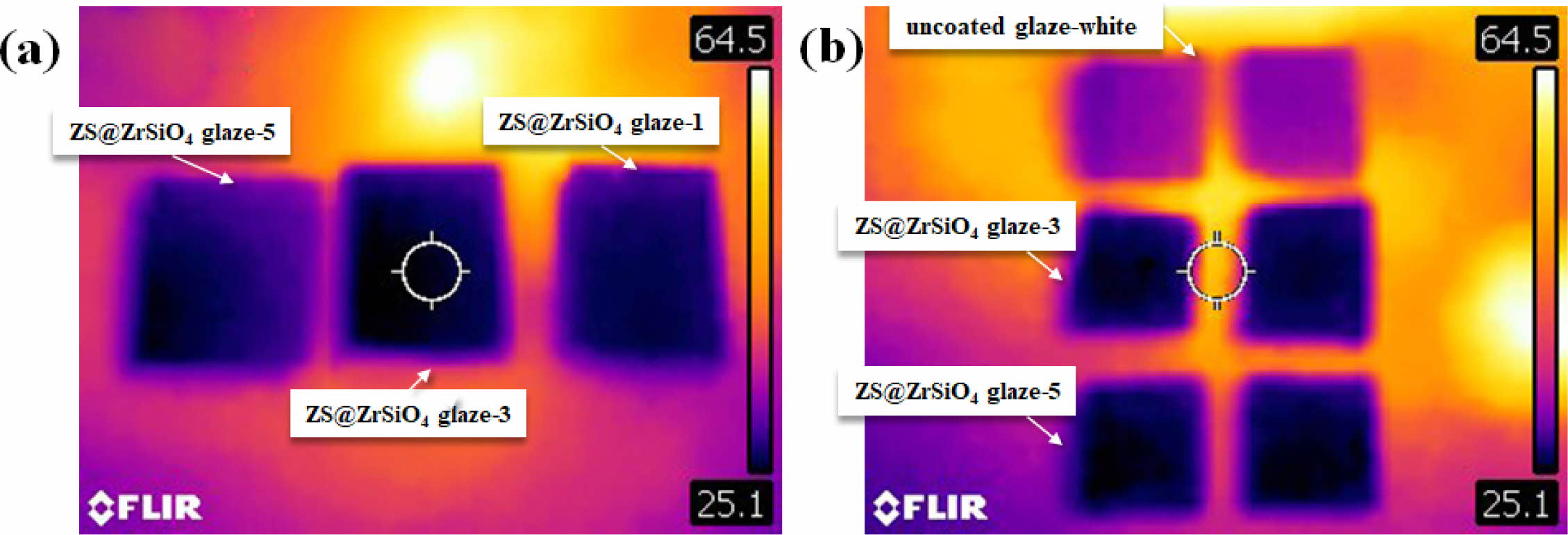
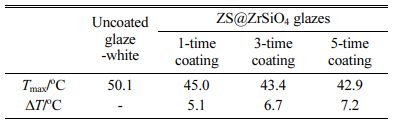
For thermal performance analysis, the solar NIR reflective ceramic tiles were exposed to infrared lamps, and the temperature distribution of the coating surface was visually depicted (see Fig. A.1). As shown in Fig. 11(a), the surface temperature of enameled samples with ZS@ZrSiO4 compositecool pigment decrease with the increasing of coating times, indicating the outstanding thermal insulation performance of solar NIR reflective ceramic tiles.
Compared with enameled samples with ZS@ZrSiO4 compositecool pigment, the uncoated glaze-white sample absorbed the most infrared radiation after exposing to the radiation for 10 min, resulting in the surface temperature rising to 50.1 oC (see Fig. 11(b)).
Libbra et al. [37] found that common ceramic tiles have a relatively low solar reflectance (i.e., smaller than 0.30-0.40), which means, it is necessary to optimizing the NIR reflective properties of ceramic tiles, especially the pigmented enamel layer on the ceramic substrates.
According to the temperature difference of 5.1-7.2 oC for enameled samples with ZS@ZrSiO4 compositecool pigment (see Table 6), solar NIR reflective ceramic tiles can be a novel ceramic-based cool roof product for building surface cooling and energy saving.
Energy-saving estimation
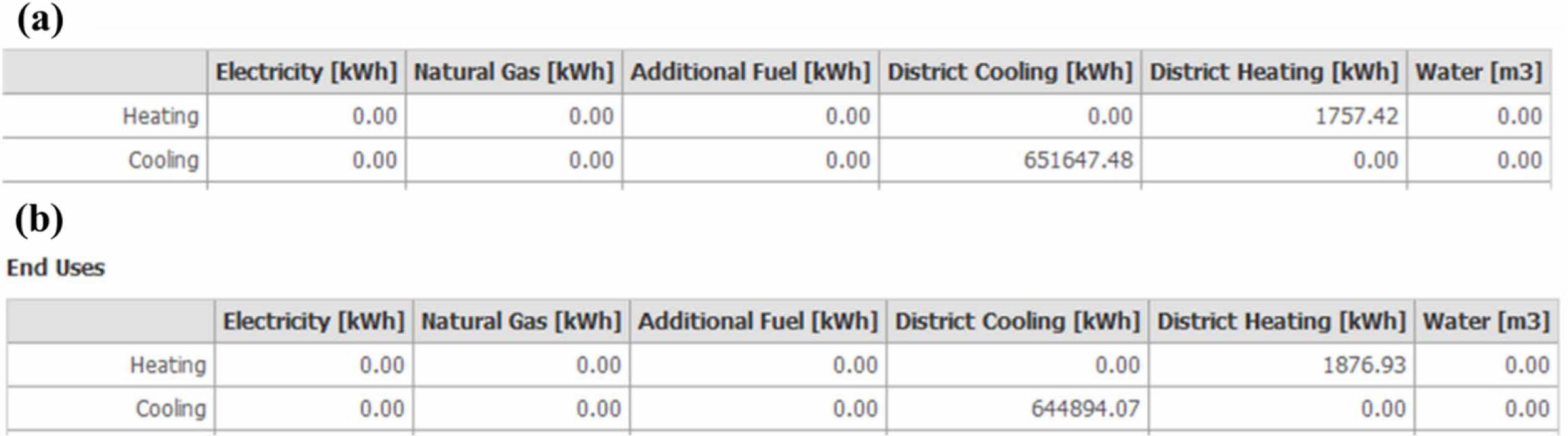
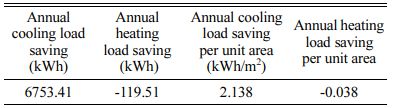
The energy saving of ZS@ZrSiO4 compositecool pigment on ceramic tiles were evaluated by Design Builder. Reducing solar radiation absorption coefficient to 0.3 (i.e., solar NIR reflective ceramic tiles) from 0.7 (i.e., cement wall) decreases the annual cooling load of the building from 651647.48 kWh to 644894.07 kWh, with a saving of 6753.41 kWh, while reducing its annual cooling load by 2.138 kWh/m2 (see Fig. 12). Meantime, the annual heating load increases from 1757.42 kWh to 1876.93 kWh, increasing its annual cooling load by 0.038 kWh/m2 (see Table 7).
According to the Electricity price list of Guangzhou, China (effective from July 1, 2018), energy cost savings in the industrial and commercial building is 1.63 RMB/m2 and in the residential building is 1.24 RMB/m2 (see Table 8), and the emission reductions of CO2, NOx and SO2 are 1.24 kg/m2, 7.77 g/m2and 148.48 g/m2, respectively.
|
Fig. 4 XRD patterns (a) and FTIR spectra (b) of ZS@ZrSiO4 composite cool pigment sintered at 700 oC. |
|
Fig. 5 TG-DSC curves of ZS and ZS@ZrSiO4 composite cool pigment. |
|
Fig. 6 Photographs of as-prepared solar NIR reflective ceramic tiles. |
|
Fig. 7 SEM micrographs of sample ZS@ZrSiO4 glaze-3: (a) cross-section image and (b) surface of ceramic glaze. |
|
Fig. 8 Solar reflectance spectra of solar NIR reflective ceramic glazes samples (NIR reflectance in the inset). |
|
Fig. 9 Schematic diagram of spraying of pigmented glaze on ceramic substrate surface (a) and structural diagram of solar NIR reflective glaze coating on ceramic substrate surfaces (b). |
|
Fig. 10 The cross section of solar NIR reflective ceramic tiles and optical and thermodynamic analysis. |
|
Fig. 11 Infrared irradiation images of ZS@ZrSiO4 glazes (a) and uncoated glaze-white with solar NIR reflective ceramic tiles (b). |
|
Fig. 12 Annual district cooling load and heating load calculated by Design Builder, (a) cement wall and (b) solar NIR reflective ceramic tiles. |
|
Table 3 The color coordinates of ZS@ZrSiO4 encapsulation pigment treated in different solutions |
|
Table 5 |
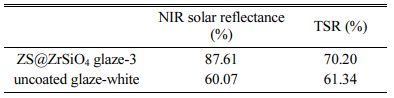
The NIR reflective properties of solar NIR reflective ceramic tiles, compared to those of uncoated glaze-white |
|
Table 6 The maximum surface temperature of solar NIR reflective ceramic glazes samples after exposing to the radiation for 10 min |
To improve the thermal stability of ZnSxSe1-x pigment, zircon-based in-situ encapsulated pigment (i.e., ZnSxSe1-x @ZrSiO4) were prepared with the assistance of soft mechano-chemical activation of ZrSiO4 precursors via high-shear homogeneous dispersing and subsequent sintering. The as-synthesized ZS@ZrSiO4 compositecool pigment possessed superior chromatic performance, high-temperature thermal stability and chemical stability. It is expected to be used as a thermal insulating coating material on ceramic tiles.
For thermal performance analysis, the as-prepared solar NIR reflective ceramic tiles were exposed to infrared lamps, and the surface temperature distribution of the coating was visually depicted by an infrared thermal camera. According to the temperature difference of 5.1-7.2 oC for enameled samples with ZS@ZrSiO4 compositecool pigment, solar NIR reflective ceramic tiles could be a novel ceramic-based cool roof product for building surface cooling and energy saving.
The application and energy saving of as-prepared solar NIR reflective ceramic tiles were evaluated. The annual building energy performance of applying solar NIR reflective ceramic tiles was simulated by Design Builder. The annual cooling load saving per unit area of building was 2.138 kWh/m2, the energy cost savings of building was 1.24-1.63 RMB/m2, and the emission reductions of CO2, NOx and SO2 are 1.24 kg/m2, 7.77 g/m2 and 148.48 g/m2, respectively. The simulations indicated that substituting solar NIR reflective ceramic tiles for cement walls could yield positive annual load, source energy, energy cost, CO2, NOx and SO2 savings in subtropical regions (e.g., Guangzhou, China).
The ‘cool roof’ solution for solar NIR reflective ceramic tiles had excellent energy-saving performance, promising popularization and application in public and residential buildings.
This work was supported by the China Postdoctoral Science Foundation (No. 2021M690722) and Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110487).
- 1. M. Ullah, H.J. Kim, J.G. Heo, D.K. Roh, and D.S. Kim, J. Ceram. Process. Res. 20 (2019) 86-91.
-

- 2. M. Suwan, P. Premjit, P. Thavorniti, P. Kidkhunthod, and S. Supothina, J. Ceram. Process. Res. 18 (2017) 10-15.
- 3. C. Zhu, W. Lin, L. Chen, J. Lv, J. Zhang, and J. Feng, Sol. Energy Mater. Sol. Cells. 199 (2019) 129-135.
-

- 4. N. Xie, H. Li, W. Zhao, C. Zhang, B. Yang, H. Zhang, and Y. Zhang, Build. Environ. 163 (2019) 106334.
-

- 5. T. Kolås, A. Røyset, M. Grandcolas, M.T. Cate, and A. Lacau, Sol. Energy Mater. Sol. Cells 196 (2019) 94-104.
-

- 6. C. Piselli, V.L. Castaldo, and A.L. Pisello, Sol. Energy 192 (2019) 106-119.
-

- 7. A.L. Pisello, E. Fortunati, C. Fabiani, S. Mattioli, F. Dominici, L. Torre, L.F. Cabeza, and F. Cotana, Sol. Energy Mater. Sol. Cells 160 (2017) 34-42.
-

- 8. A.L. Pisello, E. Fortunati, S. Mattioli, L.F. Cabeza, C. Barreneche, J.M. Kenny, and F. Cotana, Energy Build. 112 (2016) 40-48.
-

- 9. L.M. Schabbach, D.L. Marinoski, S. Güths, A.M. Bernardin, and M.C. Fredel, Sol. Energy 159 (2018) 113-124.
-

- 10. Z. Li, M. Zhao, J. Zeng, C. Peng, and J. Wu, Sol. Energy 153 (2017) 623-627.
-

- 11. E. Enríquez, V. Fuertes, M.J. Cabrera, J. Seores, D. Muñoz, and J.F. Fernández, Sol. Energy 149 (2017) 114-124.
-

- 12. G.M. Revel, M. Martarelli, M. Emiliani, A. Gozalbo, M.J. Orts, M.Á. Bengochea, L. Guaita Delgado, A. Gaki, A. Katsiapi, M. Taxiarchou, I. Arabatzis, I. Fasaki, and S. Hermanns, Sol. Energy 105 (2014) 770-779.
-

- 13. G.M. Revel, M. Martarelli, M. Emiliani, L. Celotti, R. Nadalini, A.D. Ferrari, S. Hermanns, and E. Beckers, Sol. Energy 105 (2014) 780-791.
-

- 14. I. Tatar, N. Ediz, and A. Aydin, J. Ceram. Process. Res. 16 (2015) 81-88.
- 15. N.T. Tran, D.V.Q. Nguyen, V.M.H. Ho, X.T. Dang, and N.Q. Tran, J. Ceram. Process. Res. 18 (2017) 366-372.
- 16. S.U. Bayca, T. Batar, E. Sayin, O. Solak, and B. Kahraman, J. Ceram. Process. Res. 9 (2008) 118-122.
- 17. J.G. Song, F. Wang, X.B. Bai, D.M. Du, Y.Y. Ju, M.H. Xu, and G.C. Ji, J. Ceram. Process. Res. 12 (2011) 357-360.
- 18. C. Ferrari, A. Muscio, and C. Siligardi, Procedia Eng. 169 (2016) 400-407.
-

- 19. N.M. Khalil, M.M.S. Wahsh, and A. Gaber, J. Ceram. Process. Res. 17 (2016) 478-484.
- 20. J. García-Ten, E. Monfort, P. Gomez, and S. Gomar, J. Ceram. Process. Res. 7 (2006) 75-82.
- 21. J.H. Lee, H.J. Hwang, J.W. Kwon, J.H. Kim, K.T. Hwang, and K.S. Han, J. Ceram. Process. Res. 20 (2019) 127-132.
- 22. F. Karimi, S. Baghshahi, M.N. Khezrabad, and N. Riahi-Noori, J. Ceram. Process. Res. 20 (2019) 357-362.
- 23. J. Zou and P. Zhang, Ceram. Int. 46 (2020) 3490-3497.
-

- 24. X. Huang, D. Liu, N. Li, J. Wang, Z. Zhang, and M. Zhong, Sol. Energy 202 (2020) 164-170.
-

- 25. J. Zou, T. Zhang, and X. He, Mater. Lett. 248 (2019) 173-176.
-

- 26. J. Zou and Z. Yu, Sol. Energy Mater. Sol. Cells 199 (2019) 99-107.
-

- 27. H.N. Deepak, K.S. Choudhari, S.A. Shivashankar, C. Santhosh, and S.D. Kulkarni, J. Alloys Compd. 785 (2019) 747-753.
-

- 28. C. Ferrari, A. Libbra, A. Muscio, and C. Siligardi, Ceram. Int. 39 (2013) 9583-9590.
-

- 29. R. Levinson, P. Berdahl, H. Akbari, W. Miller, I. Joedicke, J. Reilly, Y. Suzuki, and M. Vondran, Sol. Energy Mater. Sol. Cells 91 (2007) 304-314.
-

- 30. T. Zhang, Z. Pan, and Y. Wang, J. Sol-Gel Sci. Technol. 84 (2017) 118-128.
-

- 31. T. Zhang, Y. Wang, and Z. Pan, Ceram. Int. 44 (2018) 18851-18862.
-

- 32. C. Pecharromán, M. Ocaña, P. Tartaj, and C.J. Serna, Mater. Res. Bull. 29 (1994) 417-426.
-

- 33. M. Ahadi, M. Saber Tehrani, P. Aberoomand Azar, and S. Waqif Husain, Int. J. Environ. Sci. Te.13 (2016) 2797-2804.
-

- 34. K. Petzke, Physica B 273-274 (1999) 866-869.
-

- 35. T. Zhang, Y. Wang, and Z. Pan, Sol. Energy 184 (2019) 570-583.
-

- 36. P.N.S. PRC National Standard, GB/T 3810.13-2016 (2016) p.1-5.
- 37. A. Libbra, L. Tarozzi, A. Muscio, and M.A. Corticelli, Opt. Laser Technol. 43 (2011) 394-400.
-

 This Article
This Article
-
2022; 23(3): 326-334
Published on Jun 30, 2022
- 10.36410/jcpr.2022.23.3.326
- Received on Dec 3, 2021
- Revised on Feb 13, 2022
- Accepted on Feb 14, 2022
 Services
Services
- Abstract
introduction
experimental
result and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yanmin Wang a, Shanjun Ke b
-
aSouth China University of Technology, Guangzhou 510640, China
bFoshan Oceano Ceramics Co. Ltd., Foshan 528138, China
Tel : +86 20 87114883 Fax: +86 20 87110273 - E-mail: wangym@scut.edu.cn, sjkescut@163.com







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.