- Characterization of SiC powders synthesized from rice husks and fused silica powder by carbothermal reduction reaction
Young-Hoon Yun*
Dept. of New & Renewable Energy, Dongshin University, Naju, 58245, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Fine β-SiC powders were synthesized from rice husks and fused silica powder. In this SiC powder synthesis, carbon flakes were carbonized by heating rice husks with silica powder, and then the carbon flakes underwent a secondary reaction with the SiO vapor formed by the carbothermal reduction reaction. The SiC powders obtained from carbonized rice husks and fused silica powder were characterized by XRD, XPS, SEM and TEM. In the XRD patterns, the spectra clearly showed very strong peaks for the (111) plane near 35o and weak (220) and (311) peaks at approximately 60o and 72o, respectively. According to the XRD pattern, the powder synthesized from a 6:4 mixture of carbonized rice husks and silica under an Ar atmosphere contained mainly cubic crystalline SiC and a relatively small amount of a hexagonal phase without residual carbon. TEM images and FFT pattern showed that this specimen contained relatively fine particles (under 0.5 µm) and a crystalline 3C-SiC phase with a (100) diffraction pattern
Keywords: Silicon carbide (SiC), Rice husks, Silica, Carbothermal reduction
Silicon carbide (SiC) is the most widely used nonoxide ceramic for many industrial applications because of its attractive properties, such as high mechanical strength and hardness, high thermal conductivity, excellent corrosion and thermal shock resistance, etc. [1-7]. Rice husks (RHs) have a high ash content, varying from 13 to 29 wt% depending on the variety, climate, and geographic location. Ash is largely composed of silica (87-97%) with small amounts of alkalis and other trace elements [8-9]. Silica is naturally distributed in the backbone of the cellulose structure of rice husks. The effects of carbon content and preheating treatment on the formation of SiC from mixtures of silica found in rice husks and carbon black have been reported [10, 11]. Rice husk ash contains the necessary carbon and silica, which are intimately dispersed, to provide nearly ideal source material for the production of SiC material [12].
The processes of SiC synthesis can be classified into two steps, (i) carbonizing at a relatively low temperature (400-800 oC) to remove volatile materials, and (ii) reacting the carbonized rice husks with silica at a higher temperature (>1300°C) to form SiC [13-15]. During this carbothermal reduction reaction of silica, crystalline SiC powder was formed by reactions between the gaseous phase SiO and carbon source. Several other researchers have attempted various routes to characterize the formation of SiC materials such as nanowires, whiskers and powders during carbothermal processes [8, 11, 16-20]. Using rice husks with a very high surface area and taking advantage of close contact between amorphous silica and carbon, they formed SiC powder at lower temperatures (1,200-1,550 oC), which resulted in the agglomeration and association of particles [21]. The synthesis of SiC was most often performed with an excess of carbon relative to silicon at high temperature [22-25]. First, SiO and CO gases are created through the SiO2+C→SiO+CO reaction; then, the carbon particles are converted to polycrystalline SiC phase through a spontaneous reaction between SiO and a carbon (SiO+2C→SiC+CO) [26-33]. After the nucleation reactions finish, the gas-phase molecules, SiO(g) and CO(g) would occur multiphase chemical reactions to generate new phases (SiC phase) [19].
In this research, SiC powders were synthesized from rice husks and silica powder by a carbothermal reduction reaction. This paper describes the results of experiments regarding SiC powder synthesis and characterization. The SiC powders obtained from carbonized rice husks and fused silica powder were characterized by XRD patterns, XPS results, and microstructural morphology according to FE-SEM and FE-TEM.
In this work, SiC powders were synthesized through carbothermal reduction of carbonized matter in a mixture of rice husks and fused silica powder (mean particle size: 50 µm, covalent materials). The formation of silicon monoxide (SiO) gas by the carbon reduction reactions at 1,850 oC under an Ar atmosphere would have created crystalline silicon carbide powder in a furnace with graphite heating elements and a graphite reaction vessel. To control the emission of carbon monoxide (CO) gas generated during the synthesis of silicon carbide, the experiment in this study was performed by conducting the synthesis inside a graphite crucible that was inside a larger graphite crucible. The interiors of the graphite crucibles were coated with BN spray to prevent reactions with the generated silicon monoxide gas and covered with graphite foil to prevent additional reactions. When the partial pressure of carbon monoxide reached or exceeded a certain pressure in the inner crucible, it was naturally exhausted through a gap in the graphite cover of the outer crucible. Powders were synthesized from carbon-containing material and silica powder with mixing ratios of 6:4, 7:3 and 8:2 (weight ratio). Each mixture of silica powder and rice husks was placed in a graphite crucible and heated to 1,850 oC (2 hrs) at two heating rates (10 oC/min to 1,500 oC and 7 oC/min to 1,850 oC) under an Ar gas flow.
The SiC powders obtained from the carbothermal reduction reaction of silica and carbonized rice husks were investigated by XRD patterns, XPS, SEM and TEM. The SiC specimens were analyzed using an X-ray diffractometer (X-Pert Pro, PHILIPS) and observed via SEM (HITACHI S-4800, Japan) and 200 kV TEM (Field Emission Transmission Electron Microscope, Tecnai G2 F20). The XPS pattern of the synthesized powders was analyzed by X-ray photoelectron spectro- scopy (K-Alpha, Thermo).
Fine SiC powders were synthesized from rice husks and fused silica powder through a carbothermal reduction process at 1,850 oC (2 hrs). During the synthesis of SiC, carbonized rice husks were contributed to being a reducing agent for fused silica powder; thus, in this study, there was an excess of carbon relative to the silica, i.e., a mixing ratio of 6:4 [34]. Then, the mixing ratios of the rice husks to the silica source was changed from 6:4 to 7:3 and 8:2.
The gaseous silicon monoxide (SiO) created from the mixture of rice husks and silica with a stoichiometric molar ratio (SiO2:C=1:3) would have caused nucleation of SiC and formation of CO gas, a byproduct [14]. This synthesis of the crystalline SiC would be easily accomplished with an excess of carbon through whole reaction, SiO2(s)+3C(s)→SiC(s) +2CO(v) [16, 19, 26]. The secondary reaction between the intermediate SiO gas and CO gas resulted in the formation of SiC on the carbon surface. The powder samples were investigated by XRD, SEM and TEM.
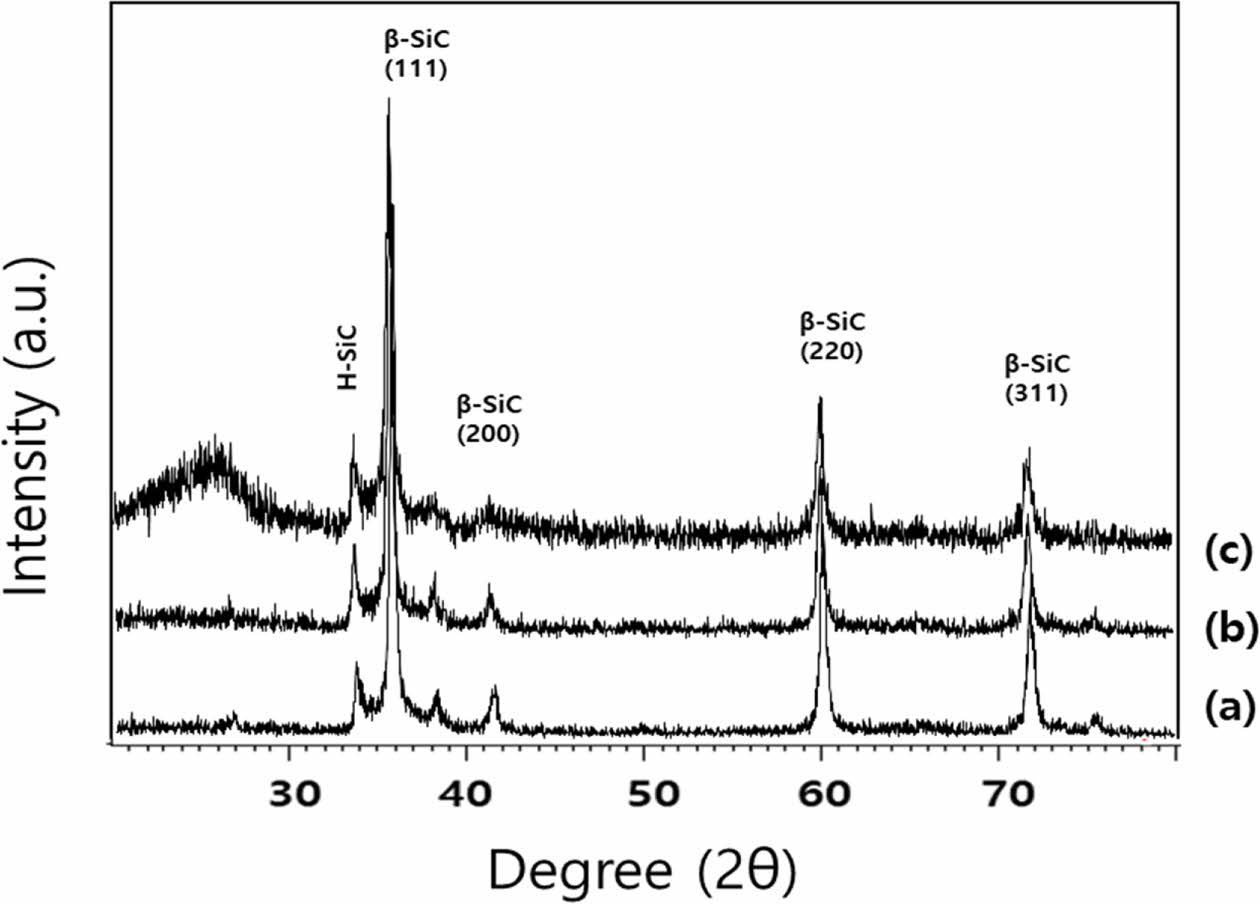
Fig. 1 shows the XRD patterns of SiC powders synthesized from rice husks and fused silica powder. The powders obtained from carbonized rice husks and silica, showed a very high peak (111) at 35o and relatively sharp (220) and (311) peaks at 60o and 72o (Fig. 1). The XRD pattern for a mixture of rice husks and fused silica powder with a mixing ratio of 8:2 showed a broad graphite peak at approximately 25o. For the sample prepared from a carbonized rice husk/silica ratio of 6:4 under an argon atmosphere, the XRD pattern did not show the presence of residual carbon, and there were major cubic SiC peaks with a relatively small hexagonal-structure ratio. The peak intensity of the hexagonal SiC peak at approximately 34o slightly increased with increasing mixing ratio of rice husks to fused silica powder from 7:3 to 8:2. The crystallization tendency of the SiC crystalline phase synthesized from SiO and CO gas created from the carbonized rice husks and fused silica powder is connected to their free energy and thermodynamic stability at the reaction temperature [16]. The whole reaction, SiO2(s)+3C(s)→ SiC(s) +2CO(v) is composed of two reactions as SiO2(s)+C(s)→SiO+CO(v) and 2C(s)+SiO(v)→SiC(s) +CO(v) [35]. In order to form the pure β-SiC phase, it was necessary to carry out the reaction under argon gas to maintain a stoichiometric ratio of approximately 1:3. The (111) peak intensity of the SiC powders increased with increasing mixing ratio of rice husks from 7:3 to 8:2.
Fig. 2 shows SEM images of SiC powders with mixing ratios of (a) 6:4 and (b) 7:3 in mixtures of rice husks and fused silica powder. It was presumed that during the synthesis process, the formation of SiC grains from carbonized rice husks and fused silica was accomplished by nucleation and growth around the boundary region of the constituent carbon solid phase and the created gaseous phase. To observe the surface morphologies, SiC specimens were ground with a zirconia agate mortar. Fig. 2 shows the SEM micro- structure under an Ar atmosphere. The (a, c) sample with a mixing ratio of 6:4 shows particles below 1 µm, and the (b, d) sample with a mixing ratio of 7:3. In the case of a mixing ratio of 7:3, the particles tended to be relatively large. As a carbon source, the rice husks mixed with silica would have induced changes in the partial pressures of silicon monoxide (SiO) gas and carbon monoxide (CO) gas generated in the process of synthesizing silicon carbide through carbon thermal reduction [22]. It was presumed that the microstructural features of the powder samples are related to the changes in partial pressures of SiO and CO gases and the effects of microstructural arrangements in the carbonized source.
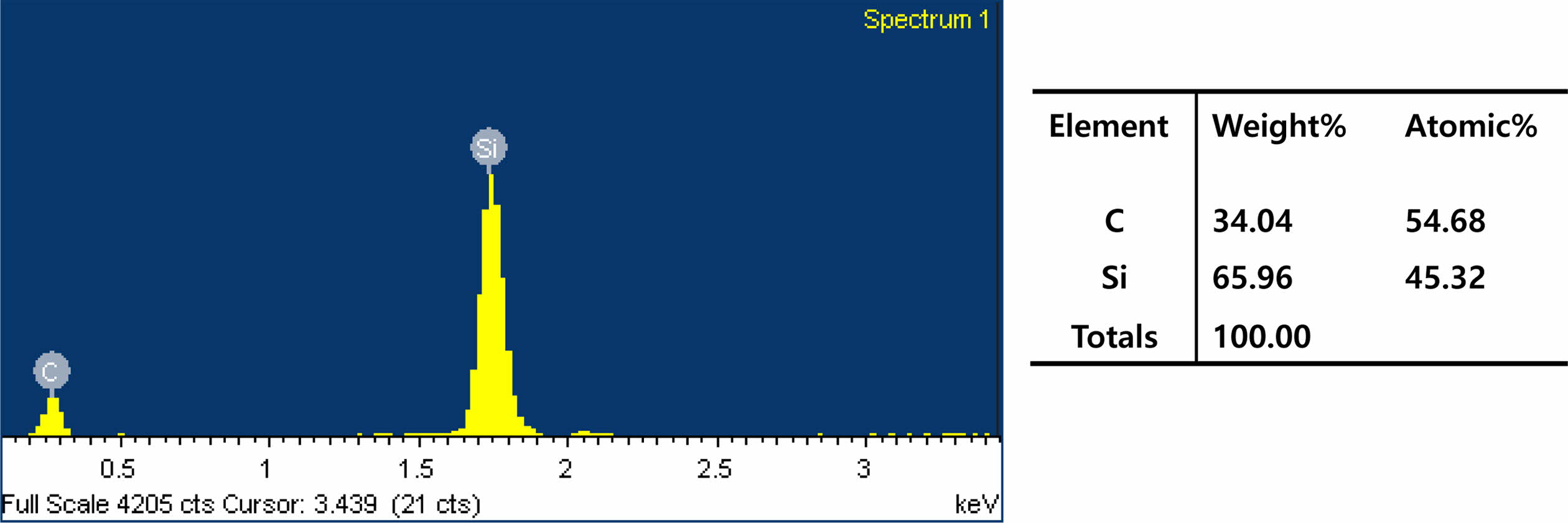
In the EDS analysis of the powders from a 6:4 mixing ratio of rice husks and fused silica (Fig. 3), silicon and carbon elements were detected. The ratio of silicon and carbon was approximately 1:1 in the atomic percent. The EDS spectra of the powders showed similar patterns according to the mixing ratios of the rice husks and the fused silica powder.
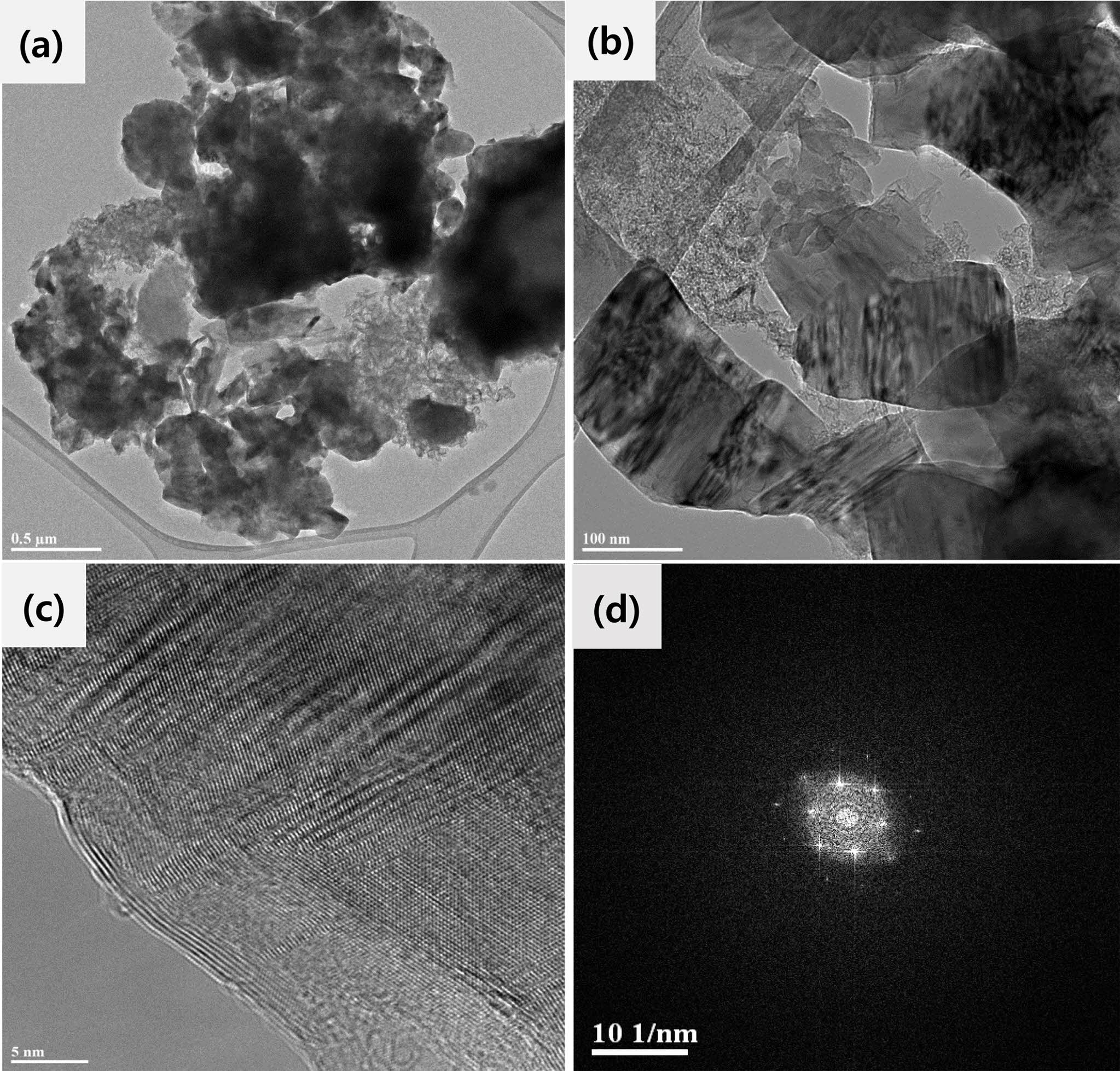
Fig. 4(a) and (b) show TEM BF images of the SiC particles interconnected. Fig. 4(c) and Fig. 4(d) show HRTEM image and fast fourier transform (FFT) pattern of SiC particle, respectively. In the TEM images, the SiC powder had morphologies of fine particles and a crystalline SiC phase with a (110) diffraction pattern [35]. When the mixing ratio of carbonized rice husks and silica was 6:4, the synthesized sample contained fine particles below 0.5 μm and revealed the diffraction pattern of 3C-SiC observed from one of the 110 zone axes. A (110) diffraction pattern of cubic crystalline silicon carbide was confirmed in the sample obtained by the carbothermal reduction reaction under an Ar atmosphere. In addition, SiC particles with a high degree of crystallinity were observed in the TEM microstructure analysis.
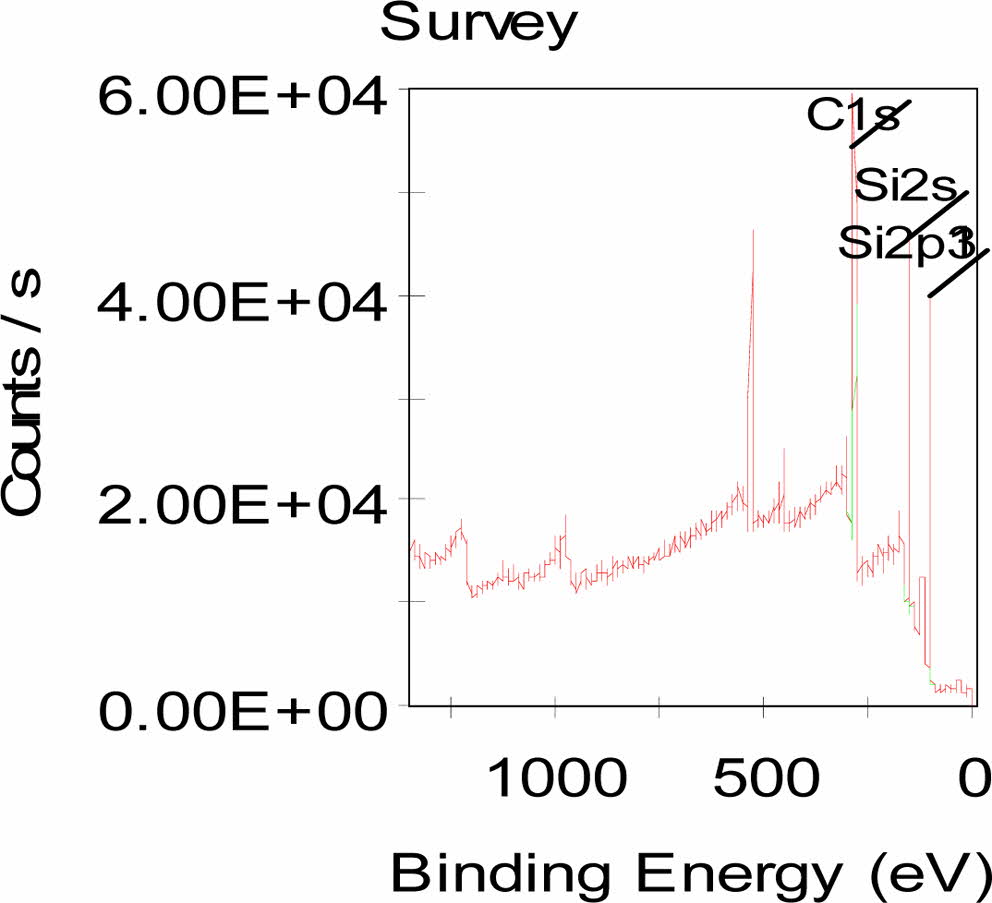
Fig. 5 shows XPS spectrum for the sample obtained through the carbothermal reduction reaction of the 6:4 mixing ratio of carbonized rice husks and silica under an Ar atmosphere. The XPS data containes a peak that corresponded to typically a crystalline SiC phase. The Si 2s at 150 ev and Si 2p3 peak at 99 eV corresponds to the silicon in b-SiC phase [36]. The C 1s peak at 283 ev is related to carbon in b-SiC phase.
From the XRD pattern, XPS spectrum and TEM analysis, it was certainly that the synthesis of the crystalline SiC powders is accomplished by carbothermal reduction reaction using rice husks and fused silica powder with graphite architecture.
|
Fig. 1 XRD patterns of SiC powders with mixing ratios of (a) 6:4, (b) 7:3 and (c) 8:2 for mixtures of rice husks and fused silica powder. |

|
Fig. 2 SEM images of SiC powders synthesized from mixtures of rice husks and fused silica powder with mixing ratios of (a) 6:4 and (b) 7:3. |
|
Fig. 3 EDS spectrum of SiC powder with a 6:4 mixing ratio of rice husks and fused silica powder |
|
Fig. 4 (a), (b) TEM images and (c) HRTEM image and (d) FFT pattern of SiC powders prepared from a 6:4 mixing ratio of rice husks and fused silica powder. |
|
Fig. 5 XPS spectrum of SiC powders synthesized from a 6:4 mixing ratio of rice husks and fused silica powder. |
Silicon carbide powders were synthesized from a mixture of rice husks and fused silica powder by a carbothermal reduction process. According to the X-ray diffraction pattern in the case of a 6:4 mixing ratio of rice husks and fused silica reacted under an Ar atmosphere, it is estimated that the stoichiometric ratio of the carbothermal reduction reaction is close to silicon carbide, and it is assumed that the residual carbon was almost completely consumed. The (111) peak of the 3C cubic SiC crystal phase was detected at approximately 35o, and weak peaks for a hexagonal SiC crystal phase were detected at 33o, 37o, and 41o. As the stoichiometric ratio was approached at the 6:4 mixing ratio, the intensity of the (111) peak of cubic crystalline SiC gradually increased. An SEM image of a sample with a 6:4 mixing ratio showed particles smaller than 0.5 µm. In TEM analysis, a (110) diffraction pattern of cubic crystalline silicon carbide was confirmed in the sample obtained by the carbothermal reduction reaction of rice hulls and fused silica in an Ar atmosphere.
- 1. D.H. Lee, J.C. Kim and D.J. Kim, J. Ceram. Process. Res. 14 (2013) 322-326.
- 2. Y-T. Kim, C.S. Jang, J.W. Choi and E.S. Kim, J. Ceram. Process. Res. 15 (2014) 277-280.
- 3. E.J. Jung, Y.J. Lee, S.R. Kim, W.T. Kwon, J.K. Kim, D.J. Choi and Y.H. Kim, J. Ceram. Process. Res. 15 (2014) 447-450.
- 4. A. Okada, J. Eur. Ceram. Soc. 28 (2008) 1097-1104.
-

- 5. G.M. Kim, G.S. Cho, D.S. Lim and S.W. Park, J. Ceram. Process. Res. 11 (2010) 377-383.
- 6. C. Brylinski, Diam. Relat. Mater. 6 (1997) 1405-1413.
-

- 7. B. Elyassi, M. Sahimi, T.T. Tsotsis, J. Membr. Sci. 288 (2007) 290-297.
-

- 8. R.V. Krishnarao and J. Subrahmanyam, Ceram. Int. 22 (1996) 489-492.
-

- 9. F.C. Lanning. J. Agric. Food Chem. 11 (1963) 435-437.
-

- 10. R.V. Krishnarao and M.M. Godkhindi, Ceram. Int. 18 (1992) 35-42.
-

- 11. R.V. Krishnarao, J. Mater. Sci. Lett, 12 (1993) 1268-1271.
-

- 12. J.C.C. Freitas, J.S. Moreira, F.G. Emmeric and T.J. Bonagamba, J. Non-Cryst. Solids 341 (2004) 77-85.
-

- 13. N.K. Sharma, W.S. Williams, A. Zangvil, J. Am. Ceram. Soc. 67 (1984) 715-720.
-

- 14. Y.L. Chiew and K.Y. Cheong, J. Ceram. Process. Res. 14 (2013) 117-124.
- 15. D.Q. Minh, T.V. Khai, H.N. Minh, N.V.U. Nhi and K.D.T. Kien, J. Ceram. Process. Res. 22 (2021) 246-251.
-

- 16. Y-T. Kim, C.S. Jang and E.S. Kim, J. Ceram. Process. Res. 15 (2014) 294-297.
- 17. W. Li, H. Guo, Mater. Lett. 215 (2018) 75-78.
-

- 18. Z. Chen, Z. Wu, J. Su, J. Li, B. Gao, J. Fu, X. Zhang, K. Huo and P.K. Chu, Surf. Coat. Technol. 406 (2021) 126641.
-

- 19. J-P. Chen, G. Song, Z. Liu, Q-Q. Kong, S-C. Zhang, C-M. Chen, J. Alloys Compd. 833 (2020) 155072.
-

- 20. Y-J. Lin and C-M. Chuang, Ceram. Inter. 33 (2007) 779-784.
-

- 21. M.F. Zawrah, M.A. Zayed, Moustafa R.K. Ali, J. Hazard. Mater. 227-228 (2012) 250-256.
-

- 22. A. Onojah, A.N. Amah and B.O. Ayomanor, Am. J. Sci. Ind. Res. 3 (2012) 146-149.
-

- 23. J.G. Lee and I.B. Cutler, Am. Ceram. So. Bull. 54 (1975) 195-198.
- 24. S.B. Hanna, N.A.L. Mansour, A.S. Taha and H.M.A. Abd-allah, Br. Ceram. Trans. J. 84 (1985) 18-21.
- 25. Y.H. Yun, S.C. Choi, J.C. Chang and J.C. Kim, J. Ceram. Process. Res. 2 (2001) 129-133.
- 26. M. Taghavi, E. Ghasemi and A. Monshi, J. Ceram. Process. Res. 15 (2014) 242-245.
- 27. J.S. Lee and S.C. Choi, J. Ceram. Process. Res. 3 (2002) 48-51.
- 28. Y.H. Yun, S.C. Choi, J. Ceram. Soc. Japan 108 (2000) 1045-1051.
-

- 29. Y.H. Yun, Y-H. Park, M-Y. Ahn, and S-Y. Cho, Ceram. Int. 40 (2014) 879-885.
-

- 30. Y.L. Chiew, K.Y. Cheong, Mater. Sci. Eng. B 176 (2011) 951-964.
-

- 31. M. Lodhe, A. Selvam, A. Udayakumar, M. Balasubramanian, Ceram. Int. 42 (2016) 2393-2401.
-

- 32. W. Li, Q. Huanga, H. Guo and Y. Hou, Ceram. Int. 44 (2018) 4500-4503.
-

- 33. S.T. Alweendo, O.T. Johnson, M.B. Shongwe, F.P.L. Kavishe and J.O. Borode, Procedia Manufacturing 35 (2019) 962-967.
-

- 34. H-D. Y, H-F. Yin, Y. Tang, Y. Xin, X. Pu and H. Zhang, J. Ceram. Process. Res. 21 (2020) 640-646.
-

- 35. Y-J. Joo, S-H. Joo, H-J. Lee, Y-J. Shim D-G. Shin, and H.Y. Cho, Nanomaterials 11 (2021) 2933.
-

- 36. J-H. Guo, K-Y. Song, B-B. Wu, X. Zhu, B. Zhang, and Y. Shi, RSC Adv. 7 (2017) 22875-22881.
-

 This Article
This Article
-
2022; 23(3): 247-251
Published on Jun 30, 2022
- 10.36410/jcpr.2022.23.3.247
- Received on Jul 20, 2021
- Revised on Feb 3, 2022
- Accepted on Mar 3, 2022
 Services
Services
Shared
 Correspondence to
Correspondence to
- Young-Hoon Yun
-
Dept. of New & Renewable Energy, Dongshin University, Naju, 58245, Korea
Tel : +82-61-330-7633 Fax: +82-61-330-2909 - E-mail: yunh2@dsu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.