- Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode with extraordinary electrochemical performances
Chea-Yun Kang and Seung-Hwan Lee*
Department of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
We report the synthesis of Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode via a co-precipitation method. The key factor to enhance the electrochemical performances of lithium ion batteries is to suppress the structural reconstruction because of the irreversibility of the H2-H3 phase transition, resulting in rapid performance decay. The as-prepared Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode delivers an initial discharge capacity of 221.4 mAh g-1 with a high coulombic efficiency of 88.5 %. Moreover, it shows better polarization of 0.33 V and superior cycle stability of 98.8% after 58 cycles. These values are obviously higher than those of pristine LiNi0.9Co0.05Mn0.05O2 cathode. Therefore, we can believe that Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode is one of the effective way for high-performance cathode material in lithium ion batteries
Keywords: Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode, structural reconstruction, H2-H3 phase transition, performance decay
Li-ion batteries (LIBs) is widely used for various applications such as electric vehicles (EVs), data centers, uninterruptible power supply (UPS) and hybrid electric vehicles (HEVs) [1]. As well as this, as interest in environment-friendliness increases worldwide, research and applications of sustainable renewable energy (solar, wind and biogas energy) are expanding. Accordingly, interest in energy storage devices (ESDs) that efficiently store and distribute such renewable energy is also explosively increasing. Among the various ESDs (fuel cell, supercapacitor, hybrid supercapacitor and LIBs), LIBs are the most used because of high energy density despite shortcomings in low power density and short lifetime [2].
The cathode is known to determine the electrochemical performance of LIBs. Recently, in order to realize high energy, Ni-rich LiNiCoMnO2 (NCM) has been extensively studied as a promising candidate. However, the cycle stability in Ni-rich NCM is inferior due to unwanted side reactions, H2-H3 phase transition, gas generation, and reduction of Ni4+ [3-5]. In order to overcome the shortcomings and maximize the performances of Ni-rich NCM cathode materials, doping, surface modification and morphology control have been reported [6, 7]. Among them, the appropriate amount cation doping is the simplest and easiest way to effectively increase Li ion intercalation, resulting from suppressed phase transformation and voltage decay [8].
Herein, in the present study, we intend to synthesize the Sn-modified LiNi0.9Co0.05Mn0.05O2 cathode with extremely uniform particle size and investigate the electrochemical performances. We reported in a previous paper that Ni-rich NCM doped with 0.05 mol% V had excellent electrochemical performance [3]. Therefore, in this paper, 0.05 mol% Sn was selected as the doping amount. In addition, we discuss the role of the Sn modification in significantly improving electrochemical performances.
Spherical Sn-modified LiNi0.9Co0.05Mn0.05O2 powders were prepared by using co-precipitation method. Ni0.9Co0.05Mn0.05(OH)2 precursor was fabricated via appropriate amounts of NiSO4·6H2O, CoSO4·7H2O, MnSO4·H2O, Na2CO3 and NH3·H2O.Precipitating agentwas fabricated by NaOH and NH4OH. Spherical Ni0.8Co0.1Mn0.1(OH)2 was blendedwith LiOH·H2O (molar ratio 1.05 : 1) and 0.05 mol% SnO2. After that, the mixed powders were calcined at 500 oC for 6 h and then sintered at750 oC for 13 h in tube furnace under atmosphere.
The active materials (96 wt%), polyvinylidene fluoride (PVDF) (2 wt%) and carbon black (Super P, 2 wt%). Subsequently, N-methyl-pyrrolidinone (NMP) solvent was added to prepare slurry. The slurry was coated on Aluminum foil and dried at 120 oC for 12 h. The 2032 coin cells were assembled by Li foil (500 μm in thickness) as an anode in glove box filled with argon gas. 1 M LiPF6 in ethylene carbonate (EC), dimethyl carbonate (DMC) and ethyl methyl carbonate (EMC) (with a volumetric ratio 1 : 1: 1) were adopted as an electrolyte.
X-ray diffraction (XRD, X'pert MPD DY1219) and field emission scanning electron microscopy (FE-SEM, Hitachi S-4800) were measured to confirm the structural properties (crystallinity and morphology). The electro- chemical equipment (TOSCAT-3100, Toyo system) was adopted to measure the charge-discharge tests in the voltage range of 3.0 – 4.3 V. Multi potentiostat (VSP300, Bio-Logic) was used to measure the cyclic voltammetry (CV) in the frequency range of 1 MHz to 10 mHz with 5 mV amplitude.
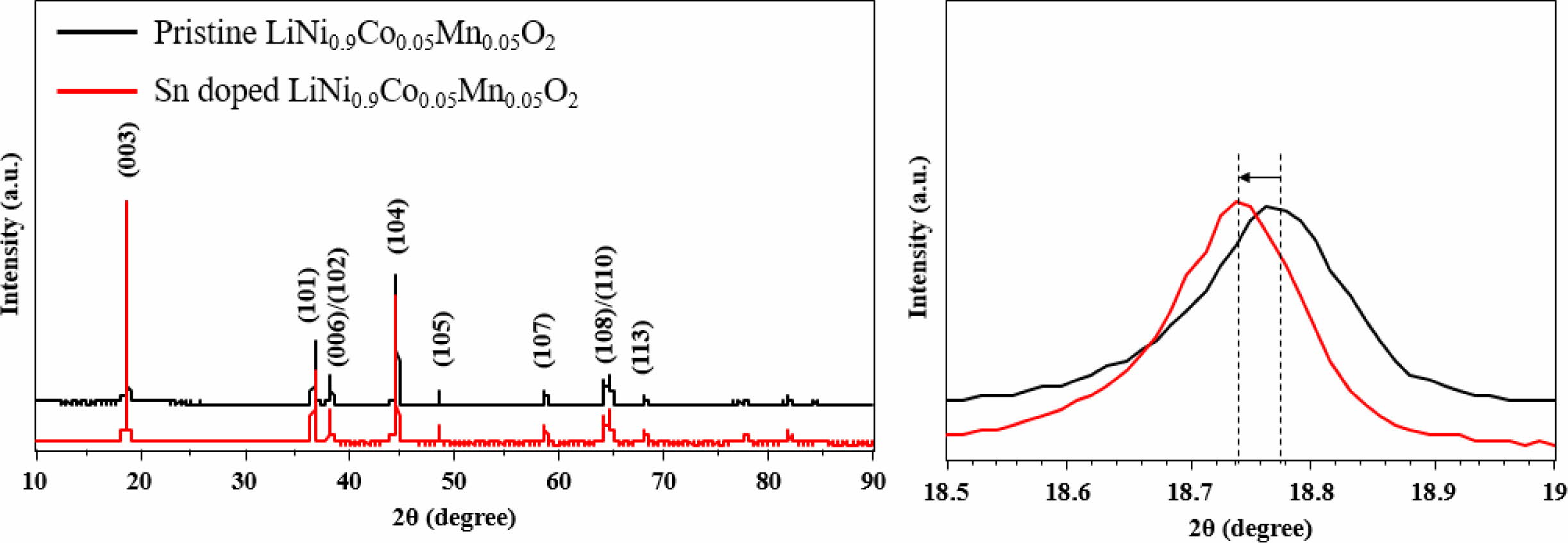
Fig. 1 shows the XRD patterns of Sn-modified and pristine LiNi0.9Co0.05Mn0.05O2 powders. The XRD reflections indicate that both samples are indexed to layered hexagonal α-NaFeO2 structure with a space group R-3m. Both samples have similar XRD peaks, resulting from small amount of Sn introduction. The (003) peak of Sn-modified LiNi0.9Co0.05Mn0.05O2 shifts to lower angle compared to pristine sample via Bragg’s law (nλ = 2dsinθ) vis larger ionic radius of Sn (0.69 Å) than that of Ni2+ (0.56 Å), Co3+ (0.54 Å) and Mn4+ (0.53 Å). It can be inferred that Sn was effectively doped into the NCM bulk. The significant splitting of the (006)/(102) and (108)/(110) peaks of both samples, indicating excellent crystalline ordering in the hexagonal layered structure [1, 2]. Moreover, both samples have high (003)/(104) intensity ratios, higher than 1.2 (Sn-modified LiNi0.9Co0.05Mn0.05O2 : 1.41, pristine LiNi0.9Co0.05Mn0.05O2 : 1.29), revealing low cation disordering with lithium ions in 3a site, transition-metal ions in 3b site. This is because the (003)/(104) intensity ratios show the degree of cation mixing in the layered structure. More importantly, the bigger ionic radius of Sn, enabling smooth and rapid electrochemical kinetics [3].
The particle morphologies of both samples are shown in Fig. 2. As expected, the morphology and average particle size of Sn-modified LiNi0.9Co0.05Mn0.05O2 are almost identical to those of pristine LiNi0.9Co0.05Mn0.05O2. Both samples have spherical shape with approximately 11.0 μm, composed of numerous primary particles of about 150 nm diameter. As a result, we can infer that Sn-modification cannot change the microstructures.
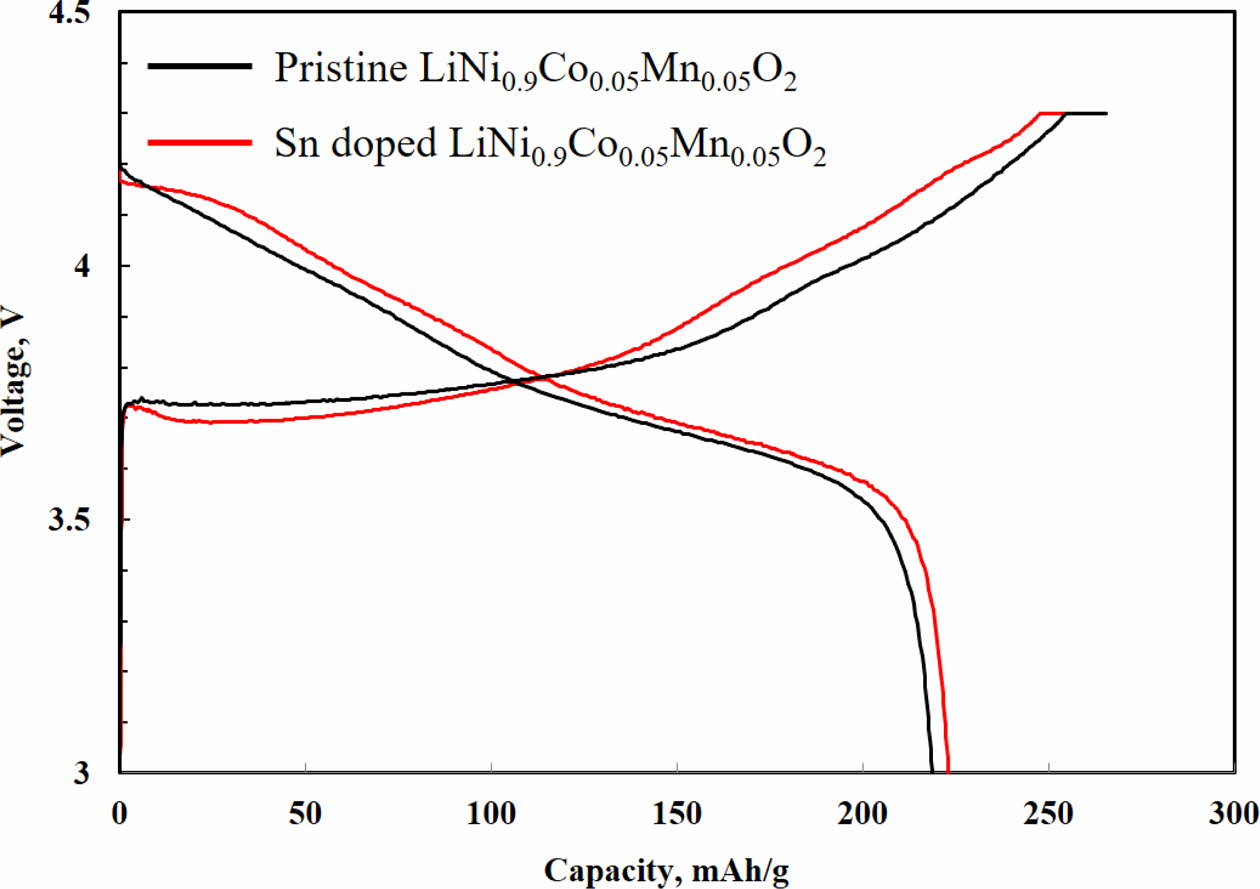
As shown in Fig. 3, the initial charge-discharge of both samples are measured in the voltage range of 3.0 – 4.3 V. There are no significant differences in charge-discharge behaviors, which is a usual curves of Ni-rich NCM cathode. The initial discharge capacities of Sn-modified LiNi0.9Co0.05Mn0.05O2 and pristine LiNi0.9Co0.05 Mn0.05O2 are 221.4 and 217.8 mAh g-1, respectively. Consequently, it can be confirmed that XX-modification has no significant effect on initial charge-discharge.
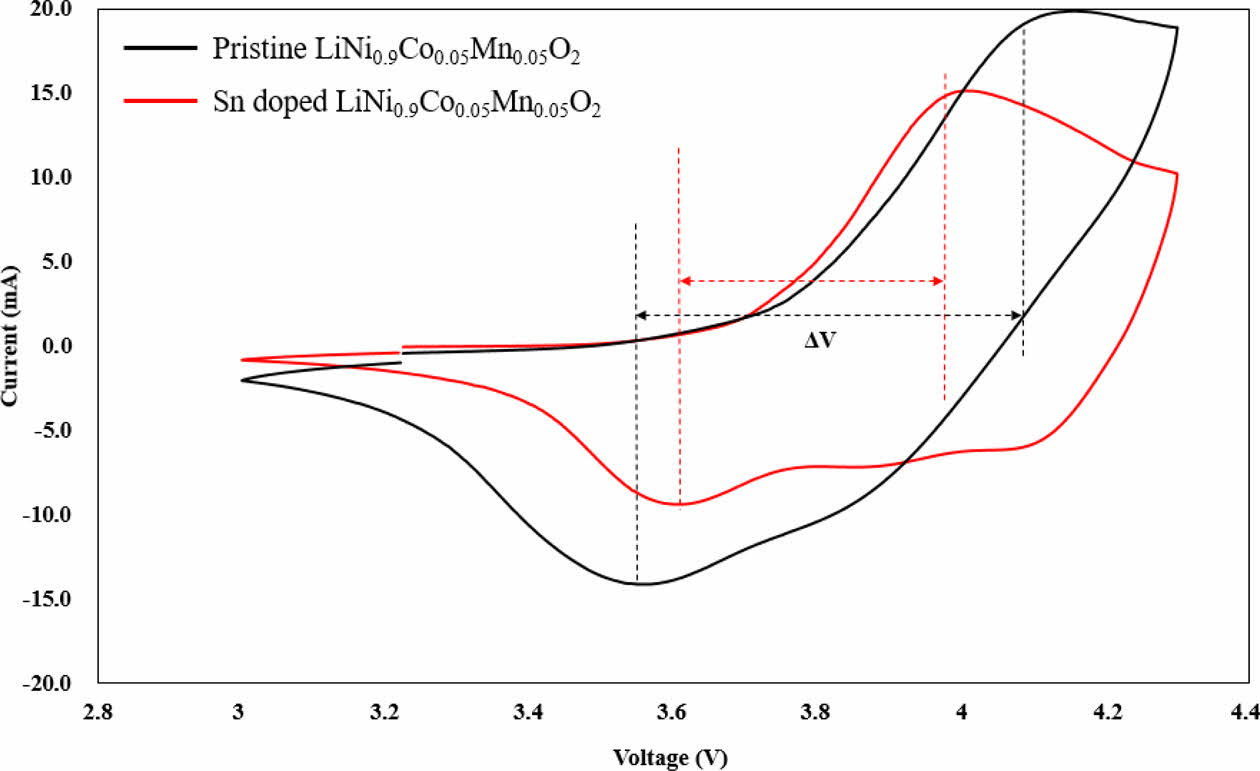
The CV curves of Sn-modified LiNi0.9Co0.05Mn0.05O2 and pristine LiNi0.9Co0.05Mn0.05O2 at a scan rate of 0.1 mVs-1 in the range of 3.0 – 4.3 V are shown in Fig. 4 to demonstrate the electrochemical phase transition and behavior. Both samples exhibit typical and similar shape of CV curves with several oxidation and reduction peaks. These is closely related to Ni2+/Ni4+ and Co3+/Co4+ redox couples [4]. These redox peaks are closely related to the phase transformation [5, 6]. There are three pairs of redox peaks, which corresponding to the 3-phase transformation from hexagonal phase to mono- clinic phase (H1→M), monoclinic phase to hexagonal phase (M→H2) and hexagonal phase to hexagonal phase (H2→H3) for both samples in the course of the charge/discharge. Among them, the H2-H3 phase transition resultingly causes quick lattice deformation along c-direction, results in electrochemical performance decay [7, 8]. The polarization between redox peaks for Sn-modified LiNi0.9Co0.05Mn0.05O2 is smaller (0.33 V) compared to that of pristine LiNi0.9Co0.05Mn0.05O2 (0.59 V), indicating superior reversible electrochemical performances [9, 10].
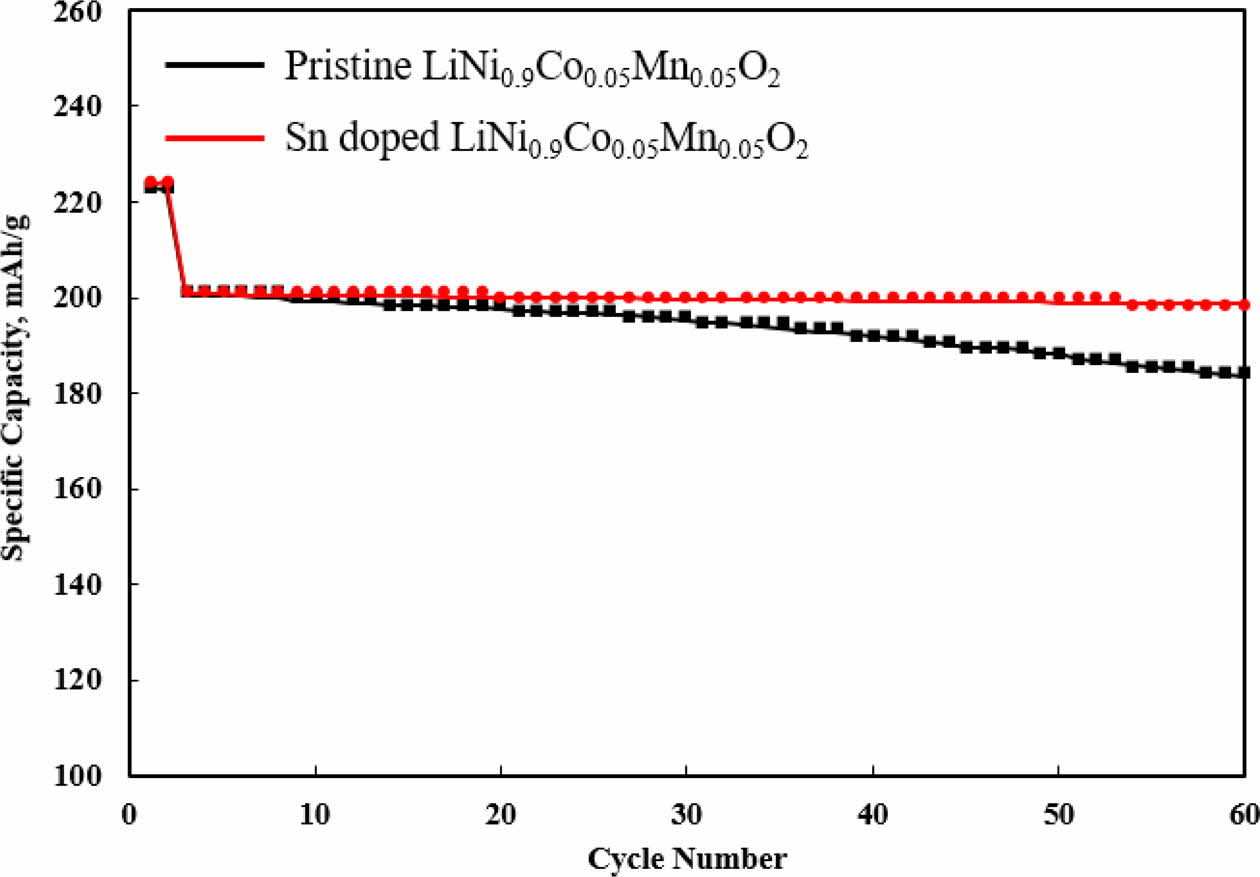
The cyclic performance of both samples is measured at 0.5 C between 3.0 V and 4.3 V, as illustrated in Fig. 5. As expected, Sn-modified LiNi0.9Co0.05Mn0.05O2 delivers higher retention than that of pristine LiNi0.9Co0.05Mn0.05O2 under the same conditions. The capacity retentions Sn-modified LiNi0.9Co0.05Mn0.05O2 and pristine LiNi0.9Co0.05 Mn0.05O2 are 98.8% and 92.1%, respectively after 58 cycles. These results are due to the superior interfacial stability on the surface of Sn-modified LiNi0.9Co0.05 Mn0.05O2 and, leading to the enhanced structural stability with less Ni dissolution [11, 12]. It can be assumed that the pristine LiNi0.9Co0.05Mn0.05O2 has a micro-cracking, resulting from the stress from volume expansion and shrinkage after cycling, which confirms our previous result [13, 14]. The secondary aggregate structure in pristine LiNi0.9Co0.05Mn0.05O2 practices highly anisotropic volume change in lattice dimension, which generate the cracking at the grain boundaries. It ultimately increase the contact area of pristine LiNi0.9Co0.05Mn0.05O2 and then cause more side reactions between cathode and electrolyte during long term cycling. Consequently, these phenomena disturb electronic and ionic transport thereby degrading electrochemical performances [15].
|
Fig. 1 XRD patterns of Sn doped and pristine LiNi0.9Co0.05Mn0.05O2. |

|
Fig. 2 FESEM images of Sn doped and pristine LiNi0.9Co0.05Mn0.05O2. |
|
Fig. 3 Initial charge-discharge profiles of Sn doped and pristine LiNi0.9Co0.05Mn0.05O2 at 0.5 C (1 C = 210 mAh g-1). |
|
Fig. 4 CV curves of Sn doped and pristine LiNi0.9Co0.05Mn0.05O2 in an EC–DMC-EMC solution at a scan rate of 0.1 mV s-1. |
|
Fig. 5 Cycle performances of Sn doped and pristine LiNi0.9Co0.05Mn0.05O2. |
In this study, we design and successfully synthesize the Sn-modified LiNi0.9Co0.05Mn0.05O2 for high-energy LIBs and investigate the electrochemical performances through phase transition. The Sn-modified LiNi0.9Co0.05 Mn0.05O2 enables smooth and rapid lithium ion transfer. Moreover, the Sn modification can maintain the original layered structure via stable surface chemistry. As a result, Sn-modified LiNi0.9Co0.05Mn0.05O2 can realize not only higher initial discharge capacity but also excellent long-term cycle performance by effectively suppressing H2-H3 phase transition. Therefore, we can conclude that Sn-modification can be one of the way for next-generation cathode materials.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1055979). Following are results of a study on the “Leaders in INdustry-university Cooperation +” Project, supported by the Ministry of Education and National Research Foundation of Korea.
- 1. Y. K. Li, W. X. Wang, C. Wu, and J. Yang, Trans. Electr. Electron. Mater. 23[1] (2022) 64-71.
-

- 2. Z. Chen, J. Wang, D. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T.C. Sum, J. Lin, Z. Shen, Scientific Reports 6 (2016) 25771.
-

- 3. S.J. Sim, S.H. Lee, B.S. Jin, H.S. Kim, Scientific Reports 9 (2019) 8952.
-

- 4. S.J. Sim, S.H. Lee, B.S. Jin, H.S. Kim, Scientific reports 10 (2020) 11114.
-

- 5. S.H. Lee, B.S. Jin, H.S. Kim, Scientific reports 9 (2019) 17541.
-

- 6. T. Sattar, S.H. Lee, B.S. Jin, H.S. Kim, Scientific reports 10 (2020) 8562.
-

- 7. S.J. Jo, D.Y. Hwang, S.H. Lee, ACS Appl. Energy Mater. 4 (2021) 3693-3700.
-

- 8. D.Y. Hwang, S.J. Sim, B.S. Jin, H.S. Kim, S.H. Lee, ACS Appl. Energy Mater. 4 (2021) 1743-1751.
-

- 9. Y. Xia, J. Zheng, C. Wang, M. Gu, Nano Energy 49 (2018) 434-452.
-

- 10. S.H. Lee, K.Y. Kim, J.R. Yoon, NPG Asia Materials, 12 (2020) 28.
-

- 11. J. Zahnow, T. Bernges, A. Wagner, N. Bohn, J.R. Binder, W.G. Zeier, M.T. Elm, J. Janek, ACS Appl. Energy Mater. 4 (2021) 1335-1345.
-

- 12. Y. Zhou, Z. Hu, Y. Huang, Y. Wu, Z. Hong, J. Alloys. Comp. 888 (2021) 161584.
-

- 13. A. Habibi, M. Jalaly, R. Rahmanifard, M. Ghorbanzadeh, J. Alloys. Comp. 834 (2020) 155014
-

- 14. Y. Zhend, N. Xu, S. Chen, Y. Liao, G. Zhong, Z. Zhang, Y. Yang, ACS Appl. Energy Mater. 3 (2020) 2837-2845.
-

- 15. P. Vanaphuti, S. Bong, L. Ma, S. Ehrlich, Y. Wang, ACS Appl. Energy Mater. 3 (2020) 4852-4859.
-

 This Article
This Article
-
2022; 23(3): 243-246
Published on Jun 30, 2022
- 10.36410/jcpr.2022.23.3.243
- Received on Jun 3, 2021
- Revised on Sep 1, 2021
- Accepted on Sep 25, 2021
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seung-Hwan Lee
-
Department of Materials Science and Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea
Tel : ++82-42-280-2414 - E-mail: shlee@dju.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.