- Effect of La2O3 upon optical properties of oxy-fluoride glasses
Taewan Ha and Seunggu Kang*
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this paper, the bonding states between composition ions and changes in the optical properties of glass according to the amount of substitution of La2O3 in La2O3-CaF2-Al2O3-SiO2 glass (hereinafter referred to as LCAS glass system) were studied. By analyzing the molar volume, density, and number of chemical bonds per unit volume of LCAS glass, it was confirmed that the added La2O3 acted as a glass network modifier. As the amount of substitution of La2O3 increased, the numbers of non-bridging oxygen and electron polarization increased, thereby reducing the optical band gap of the glass. As a result, it was confirmed that semiconducting behavior was exhibited in the glass. The La2O3-CaF2-Al2O3-SiO2 glass prepared in this study had a light transmittance of 75% or more, and the optical band gap energy could be changed as a function of amount of La2O3 substitution. It is concluded that LCAS glass can be used in various optical device fields such as optical sensors and lasers by controlling the amount of La2O3 substitution
Keywords: La2O3, Modifier, Optical band gap, Oxy-fluoride glasses
In general, glass is manufactured by melting an oxide powder at a high temperature and then exposing it to the room temperature atmosphere for rapid cooling. The properties of glass are mainly affected by the bond type and strength of constituent atoms. Therefore, many researches have been performed to control the structure and properties of glass by controlling the numbers of bridging oxygen and non-bridging oxygen by adding an intermediate and a modifier to the glass former oxides [1-4].
Among studies cited above, interest in optical glass with capability of controlling optical properties by changing the phonon energy of the structural lattices has recently been increasing. Examples of application of optical glass to high-tech industrial fields include displays, lasers, optical fiber, and biomedical devices [5-11].
Alumino-silicate glass has excellent chemical durability and mechanical stability due to the excellent glass-forming ability of Al3+ and Si4+ cations among constituent ions. In addition, owing to the advantage of high trans- mittance to visible light, it has been utilized in various fields [12-16]. However, due to the high phonon energy of the Si-O-Si bond, when ions of rare earth and transition metals are added to glass, the luminous efficiency is low, and thus there is a limitation in application to optical fibers and laser materials.
On the other hand, oxy-fluoride glass has the advantage that high luminous efficiency can be expected due to low phonon energy. However, the disadvantage of oxy-fluoride glass is that it has low chemical durability. Recently, as improved luminous efficiency as well as durability by precipitating fluoride-based crystals on an oxide glass matrix is known, research on oxy-fluoride glass is rapidly progressing [17]. When a rare-earth material is doped into an oxy-fluoride glass, a specific electron transition occurs depending on the type of ion of the rare-earth element, and the glass will thereby emit a specific wavelength [18].
The luminescence of rare earth ions is caused by electrons in the 4f orbital, but there are no electrons in the 4f orbital of the La3+ ion in La2O3. However, due to the high field strength of La3+ ion , there is a possibility of exhibiting high-quality luminescence intensity, and numerous studies on fluoride glass systems with added La2O3 thus have been conducted recently. In addition, since La2O3 has a high refractive index (2.0-2.3) and Abbe number, it lowers the optical aberration of the glass. Therefore, when used as an optical device lens, an image is formed accurately. Moreover, the band gap change can be controlled according to the composition, facilitating use in various optical materials.
Elkhoshkhany et al. [19] and Goel et al. [20] studied changes in the structure and the optical properties by adding La2O3 to zinc-tellurite glass and silicate glass, respectively. Although many researches have been conducted on the effects of La3+ ions on glass of various compositions, relatively few studies on oxy-fluoride glass have been reported. In this light, in this paper, the effect of La2O3 substitution on the structure and the optical properties of CaF2-Al2O3-SiO2 glass, which is an oxy-fluoride-based glass, was studied.
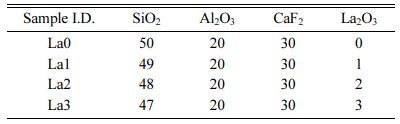
As starting materials for oxy-fluoride glass production, CaF2 (99.9%), α-Al2O3 (99.9%), SiO2 (99.9%), and La2O3 (99.9%) were used. The batches of La2O3-CaF2-Al2O3-SiO2 glass prepared in this experiment is shown in Table 1. The batch prepared as powder was mixed for 24 h, put in a platinum crucible, and melted at 1450 oC for 1 h. The obtained melt then was formed into a graphite mold of cylinder form and annealed at 400 oC for 1 h to prepare a glass from which stress was removed.
The density of the manufactured LCAS glass was measured using principle of Archimedes, and the molar volume of glass was also calculated through the measured density value. The formulas for density and molar volume are as follows:
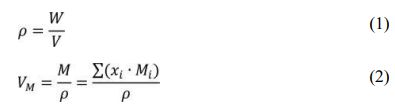
In Eq. (1), ρ is the specimen’s density (g/cm3), V is the volume of the glass specimen (cm3), and W is the weight of the specimen in air (g). In Eq. (2), VM is the molar volume (cm3/mol) of the glass specimen, M is the molar mass (g/mol) of the glass specimen, xi is the mole fraction of each molecule, and Mi is the molecular weight of each molecule. The crystalline phase generated in the prepared glass was confirmed using an x-ray diffraction analysis. For measurement of the light trans- mittance and absorbance, the glass was polished to a thickness of 3mm or less, and an ultraviolet-visible spectrophotometer was used. The refractive index of glass at a wavelength of 589 nm was obtained with an Abbe refractometer (ATAGO 2T, ATAGO CO., LTD. Japan).
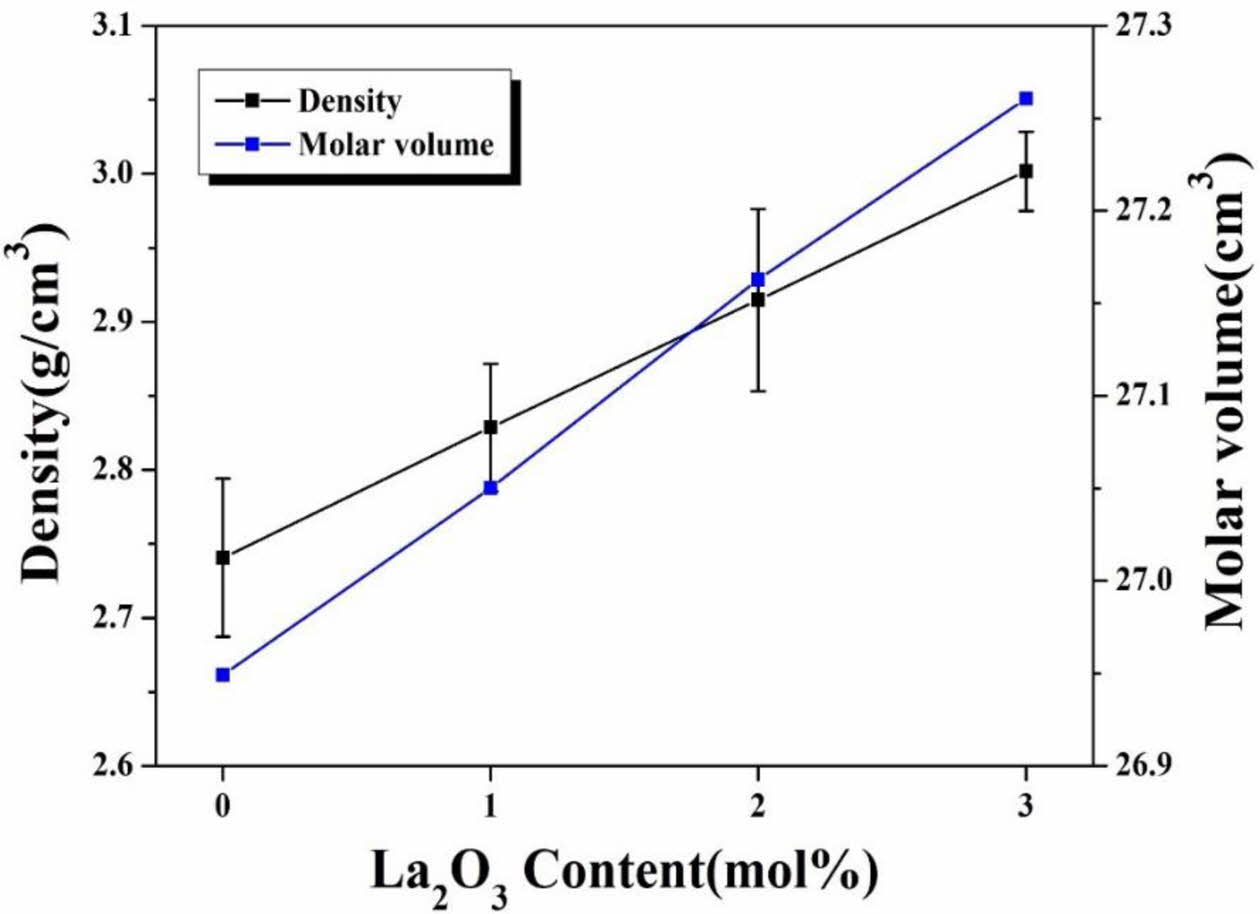
Among the properties of glass, density and molar volume are structural parameters and are closely related to factors indicating the degree of densification of the glass structure, the coordination number, and the presence or absence of internal pores [21-22]. The density of the glass increased linearly from 2.74 to 3.00 (g/cm3) as the amount of La2O3 substitution was increased, as shown in Fig. 1. This is because, as La2O3 (325.81 g/mol), having a heavier atomic weight than SiO2 (60.09 g/mol), was added, the density of the glass specimen was increased. In addition, the molar volume (VM) of the glass increased with the amount of La2O3 substitution. La2O3 is known to act as a modifier when added to oxide-silicate glass [23]. Therefore, it is thought that the numbers of non-bridging oxygen increased with the amount of La2O3 added, and VM also increased because the glass-network structure was opened accordingly [24].
The number of bonds per volume (nb) in glass is a factor showing the bonding properties of glass and can be obtained through the following equation [25]:

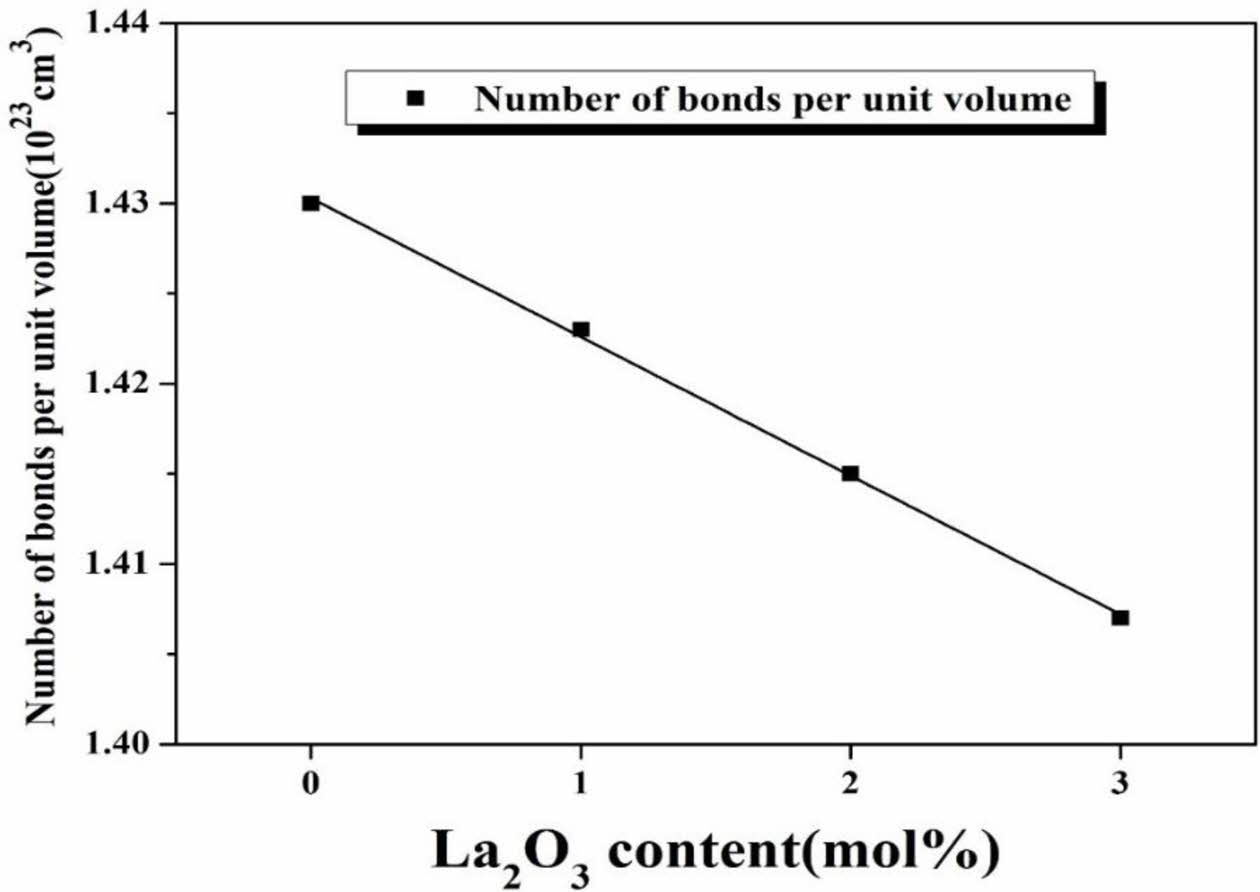
In Eq. (3), NA is number of Avogadro, nc is the coordination number of the cation, and xi is the mole fraction of each composition. Elkhoshkhany et al. studied the change of the ion radius, the numbers of oxygen displaced, and the glass network according to the NaF substitution amount in oxy-fluoride glass. As a result, it was found that Na+ ions act as modifiers and decrease the number of bond per unit volume [26].
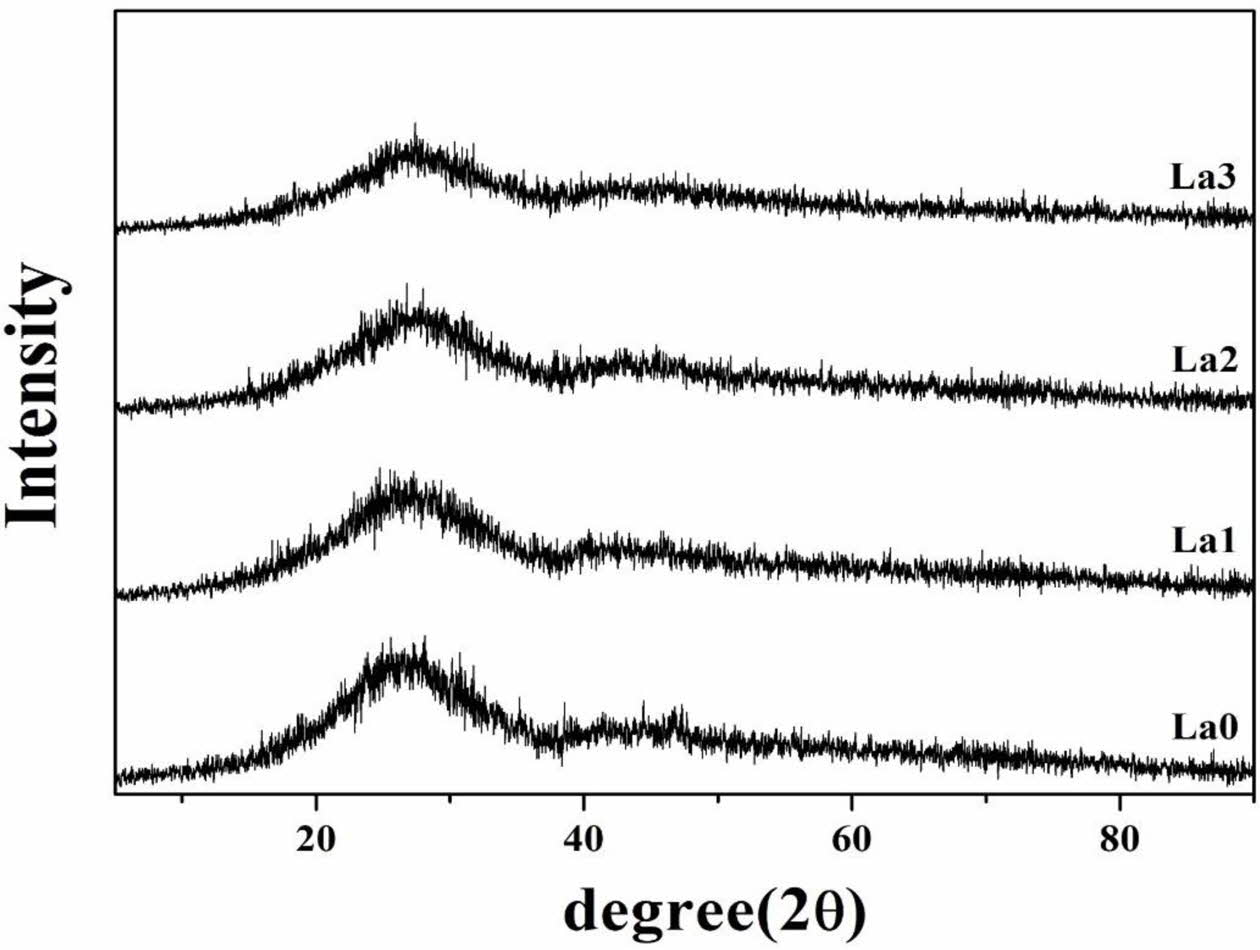
The number of bonds (nb) per unit volume of glass with the amount of La2O3 addition is shown in Fig. 2. nb decreased with amount of La2O3 substituted, which means that the network connectivity decreased and the glass network was loosened. Therefore, it was confirmed that La2O3 acted as a modifier for generating non-bridging oxygen in the network structure of glass. This was consistent with the analysis results of VM shown in Fig. 1. Fig. 3 shows the results of the XRD analysis for LCAS glass specimen. Sharp peaks representing specific crystals did not appear and only large hill-shaped peaks were noted. Therefore, all glass specimens have an amorphous structure, and it appears that only 0-3 mol% La2O3 substitution does not produce crystals in the glass.
In order to analyze the correlation between the bonding state and optical properties of the glass network, the polarizability, which has a close relationship with the electron transition, was calculated. The polarizability can be obtained using the Lorentz-Lorenz equation as follows [26]
:
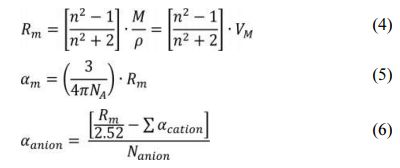
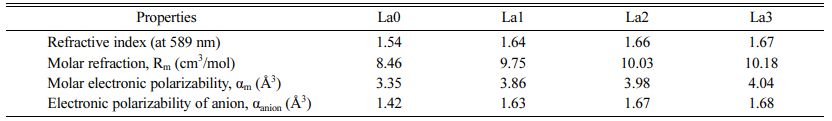
In Eq. (4), Rm is the total molar refraction of the material and n is the refractive index. In Eq. (5), αm denote the molar polarizability of electron. In formula (6), αanion is the electronic polarizability of the anions (O2- and F-). Nanion means the total number of anions constituting the glass composition, and ∑αcation is the molar cation polarizability of the cations. In previously published studies, αSi = 0.033Å3, αAl = 0.054Å3, αLa = 1.052Å3, and αCa = 0.68Å3 were measured [27-29]. Table 2 shows the molar refraction, refractive index, electronic polarizability, and molar cation polarizability of the LCAS glass prepared in this study. As the amount of substitution of La2O3 increases, the refractive index increases. This can be interpreted as a simple rule of the mixture phenomenon that occurs because La2O3 is substituted for SiO2 with a low refractive index. The value of αm increases with the amount of La2O3 substituted, because the electron polarization of the La3+ cation is higher than that of Si4+. Therefore, it is considered that the substituted La3+ cations contributed in part to the glass refractive index increase. In addition, as the amount of substitution of La2O3 increased, the electron polarization of the anion increased, which is expected to facilitate electron transition [30-34].
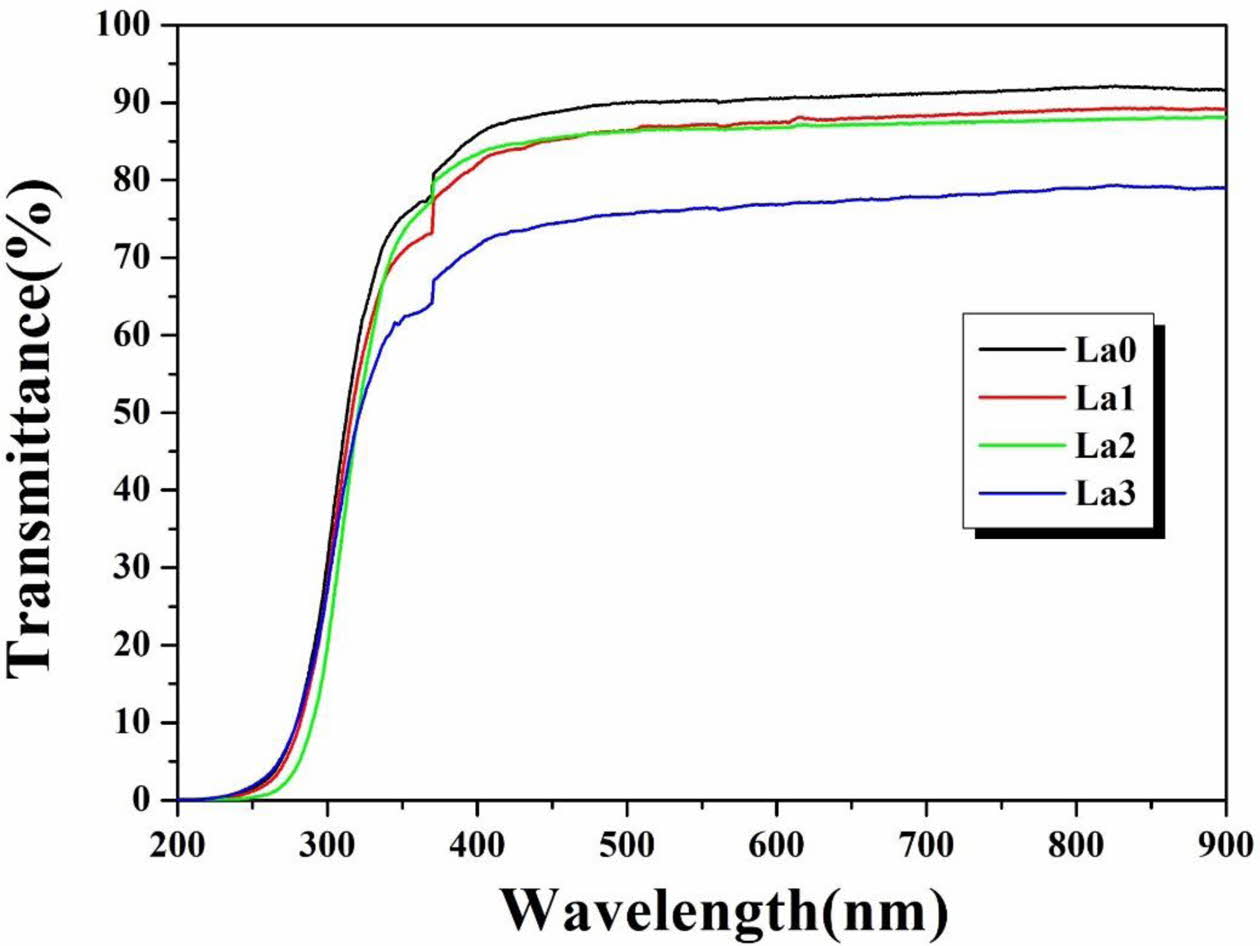
Fig. 4 shows the light transmittance of the glass with La3+ cation substitution. When 1 to 2 mol% of La3+ was substituted, the light transmittance in the range of 400 to 800 nm decreased slightly from 91% to 88%, but when 3 mol% was added, it decreased sharply to 75%. Cetinkaya et al. observed the change in light transmittance according to the amount of ZnO added, and explained that the light transmittance of glass decreased because ZnO acted as a modifier and the numbers of non-bridging oxygen increased [27]. Therefore, it is thought that the light transmittance of the glass prepared in this study also decreased as the numbers of non-bridging oxygen increased due to La3+.
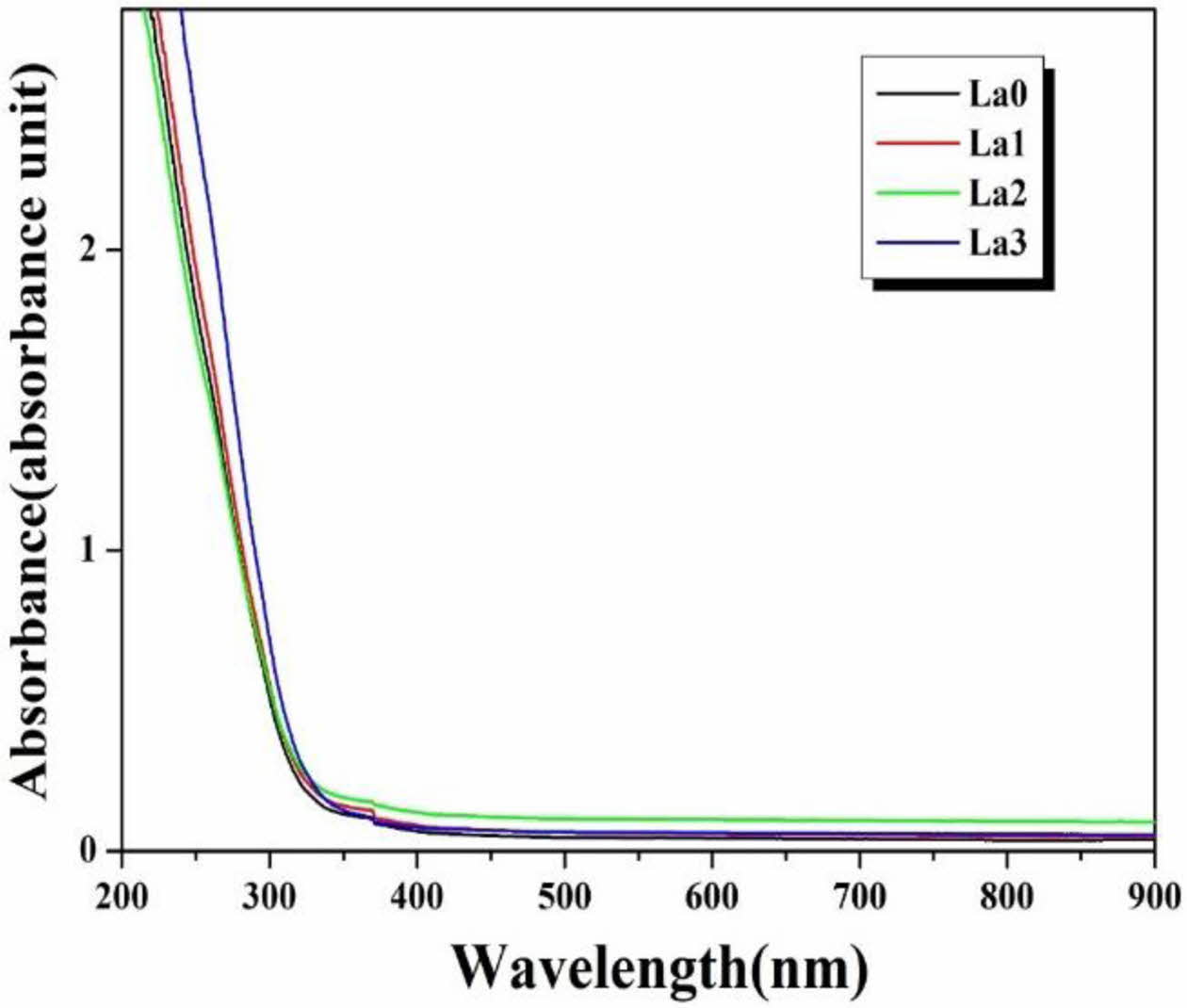
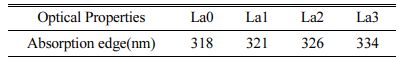
Fig. 5 shows the absorbance graph with La3+ cation substitution of the glass prepared in this study. When the La2O3 substituted specimen and the unsubstituted specimen were compared, a very similar pattern was observed over all wavelength bands. Also, the absorption edge can be obtained from the point where the tangent line drawn on the absorbance graph meets the wavelength axis. Table 3 shows the calculated absorption edge of the LACS glass. As the amount of substitution of La3+ cations increased, the absorption edge showed a tendency to shift to a longer wavelength band. This result is consistent with the results of research by Guo et al. showing that the absorption edge of mother glass shifts to a long wavelength band depending on the doping amount of Tb3+, a rare earth ion [32]. Guo et al. attributed this to Tb2O3 acting as a modifier to generate non-bridging oxygen. In other words, the absorption edge shifted toward longer wavelength because the non-bridging oxygen showed more polarization than the bridging oxygen. Therefore, it can be considered that the La3+ ions added to the glass prepared in this experiment also acted as modifiers.
Glassy materials do not show the long-range order that is a typical of crystalline solids with a periodic potential. The short range order in glassy materials, however, remains to some extent, causing to happen, thereby to a band-like structure of electron energy states comparable to that of crystalline solids. The localized electron levels occur near the band edges at somewhat high densities of state tailing into the band gap, called as diffuse band tail states. The direct and indirect optical transitions, which occurs at the absorption edge of non-crystalline materials. Glassy materials are often associated with a band tailing of density of states [33].
Mott and Davis suggested that the optical band gap according to the transition can be obtained from the following eq. (7): [33]

In Eq. (7), when the value of n, the exponent, is 1/2, it means an allowed direct transition, and when it is 2, it means an allowed indirect transition. Here, α is the absorption coefficient, α0 is a constant, and EgOpt is the optical band gap. In addition, the absorption coefficient α can be obtained by substituting the absorbance A and the specimen thickness d in the following eq. (8) [31]:

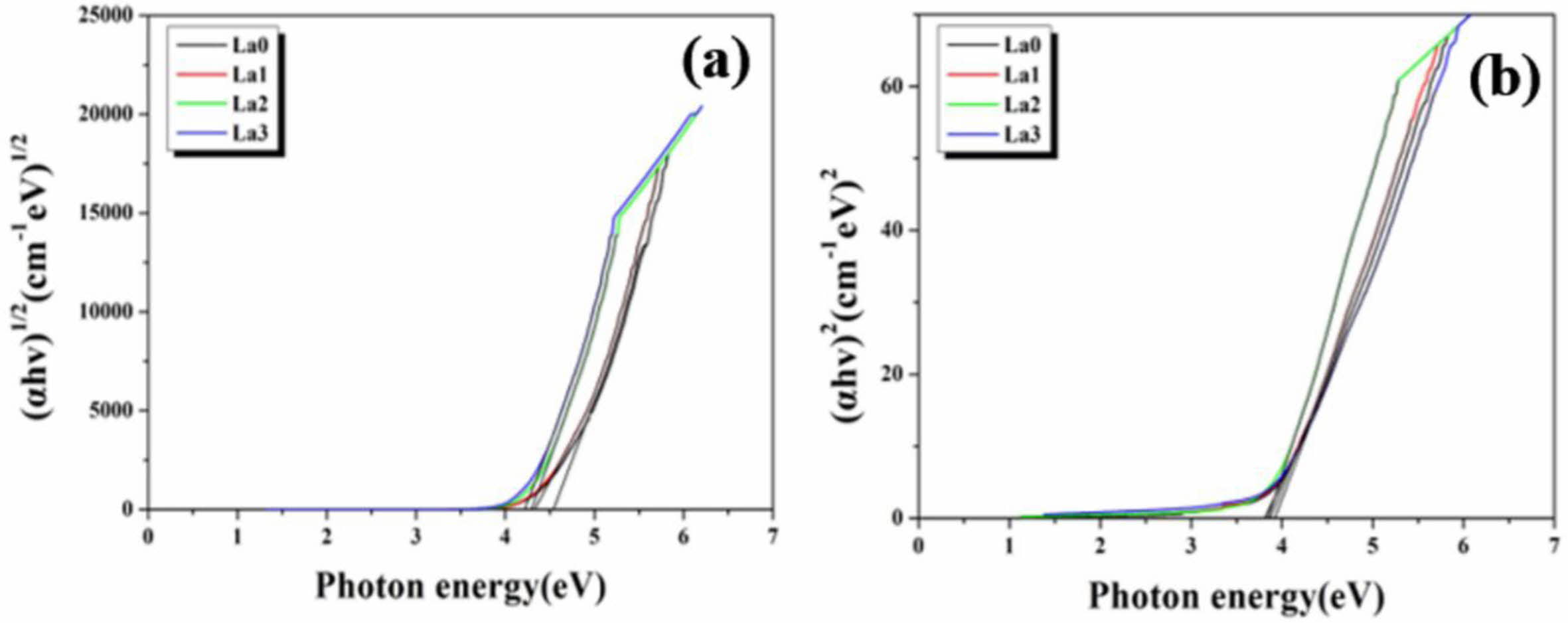
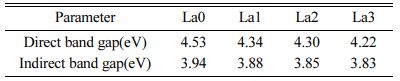
From the (αhv)n versus hv graph drawn using Eq. (7), the optical band gap (EgOpt) could be calculated when the (αhv)n (n=1/2 or 2) value is 0. [31] Fig. 6 shows a graph of (αhv)n (n=1/2 or 2) according to the energy of the wavelength, that is, the phonon energy (hv). The graphs in Figs. 6(a) and (b) are for n=1/2 and n=2, respectively. In each figure, when the (αhv)n value is 0 by extrapolation, EgOpt is obtained. In (a), the direct optical band gap is obtained, and in (b), the indirect optical band gap is obtained. The band gap values of LCAS glass obtained in this manner are shown in Table 4. Both the direct band gap and indirect band gap decreased as the amount of La2O3 substitution increased.
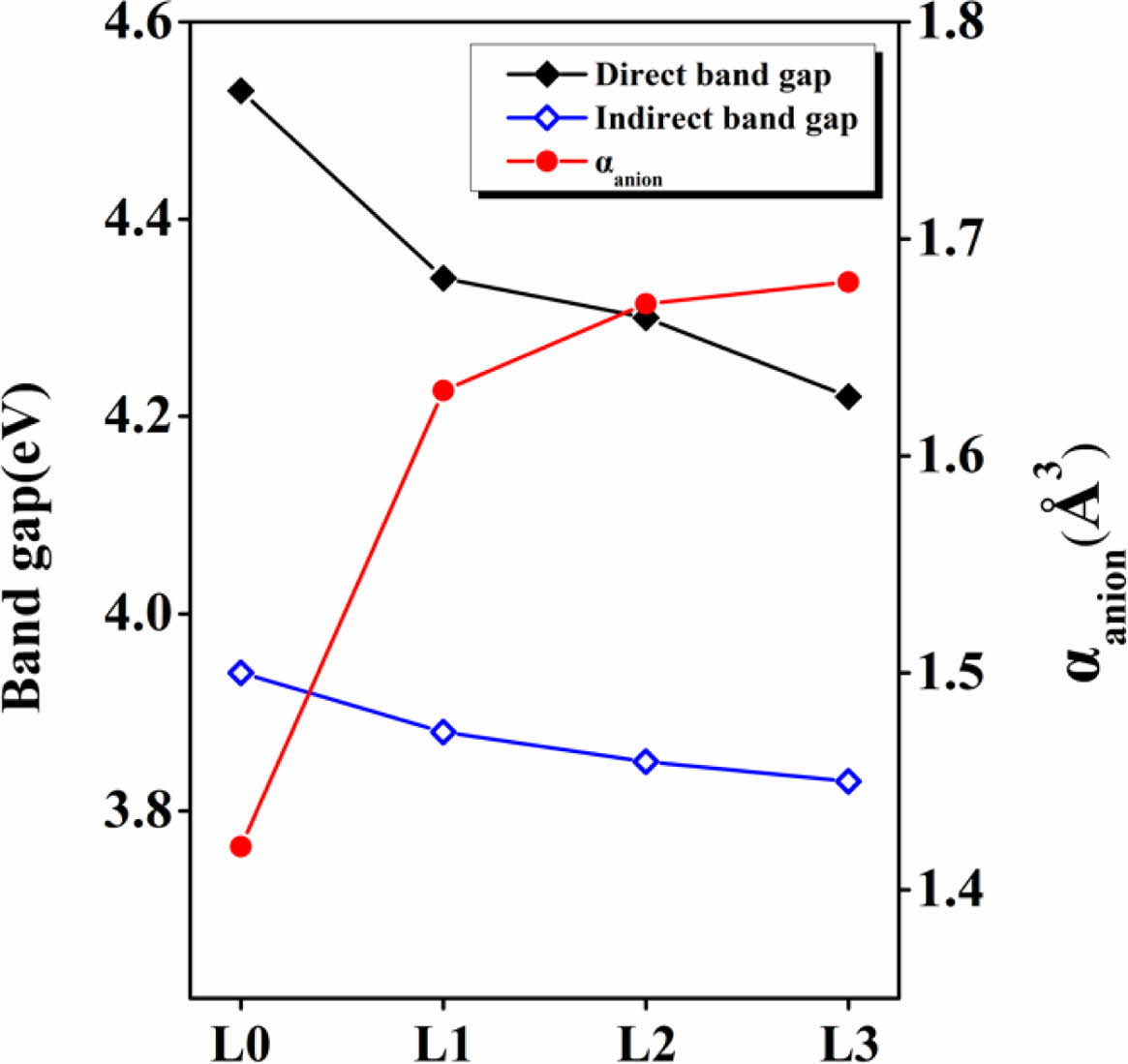
One of several factors that can indirectly determine the degree of electron transition is the electron polarization of an anion [34]. Fig. 7 shows the relationship between the electron polarization of anions and the band gap in LCAS glass. As the amount of substitution of La2O3 increased, the electron polarization of anions rapidly increased from 1.41 to 1.63. In addition, as the anion electron polarizability increased, both the direct band gap and the indirect band gap decreased, and the direct band gap was always higher than the indirect band gap in all specimens.
The reason for this is, first, the La3+ cation in the glass acts as a glass modifier, increasing the numbers of non-bridging oxygen that facilitate the optical electron transition. Second, as the amount of La3+ substitution increased, the electron polarization of anions increased. Therefore, it can be seen that the optical bandgap can be controlled by adjusting the amount of La3+ ions added to the LCAS mother glass prepared in this study [35-36]
|
Fig. 1 Density and volume per mole of LCAS glass system as a function of amount of La2O3 substituted. |
|
Fig. 2 Number of bonds in volume unit of LCAS glass system as a function of amount of La2O3 substituted. |
|
Fig. 3 XRD patterns of LCAS glass system according to amount of La2O3 substituted. |
|
Fig. 4 Transmittance graph of LCAS glass system as a function of an amount of La2O3 substituted. |
|
Fig. 5 Absorbance graph of LCAS glass system according to amount of La2O3 substituted. |
|
Fig. 6 Photon energy vs. (a) (αhv)1/2 and (b) (αhv)2 graph of LCAS glass system according to amount of La2O3 substituted. |
|
Fig. 7 Direct band gap, indirect band gap, and electronic polarizability of anions of LCAS glass system according to amount of La2O3 substituted. |
|
Table 2 Refraction–related properties of LCAS glass system according to amount of La2O3 substituted |
|
Table 3 Absorption edge of LCAS glass system as a function of amount of La2O3 substituted. |
|
Table 4 Band gaps of LCAS glass system as a function of amount of La2O3 substituted. |
In this study, CaF2-Al2O3-SiO2 based mother glass was prepared and its optical properties according to the change in the bonding states of the glass structure were analyzed as a function of amount of La2O3 substitution. The density increased with the amount of La2O3 sub- stitution, but the molar volume (VM) and the number of bonds in volume unit (nb) decreased, indicating that La3+ ions acted as modifiers. The light transmittance of the glass decreased with the amount of La2O3 substitution. From the absorbance results, it was confirmed that La3+ ions increased non-bridging oxygen, which caused a difference in the polarization of electrons, and that the absorption edge moved toward the long wavelength.
As the amount of La2O3 substitution increased, both the direct band gap and the indirect band gap decreased. This is thought to be attributable to the optical transition becoming easier due to the increased numbers of non-bridging oxygen and the increase in the electron polarization of anions. The La2O3-CaF2-Al2O3-SiO2 glass prepared in this study shows more than 75% transparency and the value of optical band gap energy can be controlled as a function of amount of La2O3 substitution. Therefore, it could be applicable to various devices for optics such as optical sensors and lasers. However, it is necessary to additionally conduct research on crystal growth control of LCAS-based glass, and changes in bonding structure and optical properties due to crystallization.
This work was supported by Kyonggi University Research Grant 2019.
- 1. A. Novatski, A. Steimacher, A.N. Medina, A.C. Bento, M.L. Baesso, L.H.C. Andrade, S.M. Lima, Y. Guyot, and G. Boulon, J. Appl. Phys. 104[9] (2008) 094910.
-

- 2. M.L.F. Nascimento and N.O. Dantas, Mater. Lett. 61 (2007) 912-916.
-

- 3. M.M. Hivrekar, D.B, Sable, M.B. Solunke, and K.M. Jadhav, J. Non-Cryst. Solids. 474 (2017) 58-65.
-

- 4. Z. Luo, J. Zhang, J. Liu, J. Song, and A. Lu, Solid State Ionics, 317 (2018) 76-82.
-

- 5. G. Hou, C. Zhang, W. Fu, G. Li, J. Xia, and Y. Ping, Ceram. Int. 45 (2019) 11850-11855.
-

- 6. Q. Zhang, L. Han, W. Liu, W. You, A. Lu, and Z. Luo, J. Ceram. Process. Res. 22[1] (2021) 8-15.
-

- 7. Y. Zhang, Z. Zhang, X. Liu, G. Shao, L. Shen, J. Liu, W. Xiang, and X. Liang, Chem. Eng. J. 401 (2020) 125983.
-

- 8. J. Lei, Q. Zhang, Y. Song, J. Tang, J. Tong, F. Peng, and H. Xiao, Opt. Laser Technol. 128 (2020) 106223
-

- 9. S. Kaewjaeng, S. Kothan, W. Chaiphaksa, N. Chanthima, R. Rajaramakrishna, H.J. Kim, and J. Kaewkhao, Radiat. Phys. Chem. 160 (2019) 41-47.
-

- 10. D. Shiratori, Y. Isokawa, H. Samizo, M. Koshimizu, N. Kawaguchi, and T. Yanagida, J. Ceram. Process. Res. 20[4] (2019) 301-306.
- 11. G. Okada, H. Masai, A. Torimoto, S. Kasap, and T. Yanagida, J. Ceram. Process. Res. 17[3] (2016) 148-151.
- 12. P.R. Watekar, S.M. Ju, S.J. Boo, and W.T. Han, J. Non-Cryst. Solids. 351 (2005) 2446-2452.
-

- 13. P.R. Watekar, S.M. Ju, and W.T. Han, Colloids Surf. A Physicochem. Eng. Asp. 313-314 (2008) 492-496.
-

- 14. A. Halder, M.C. Paul, S.W. Harun, S.K. Bhadra, S. Bysakh, and S. Das, M. Pal, J. Lumin. 143 (2013) 393-401.
-

- 15. A. Ashjari, B.E. Yekta, and H.R. Rezaie, J. Eur. Ceram. Soc. 40 (2020) 1368-1375.
-

- 16. N. Suebsing, C. Bootjomchai, V. Promarak, and R. Laopaiboon, J. Non-Cryst. Solids. 523 (2019) 119598.
-

- 17. M. Li, J. Zou, G. Guo, J. Liu, and G. Wang, J. Ceram. Process. Res. 22[5] (2021) 504-509.
-

- 18. D. Nakauchi, M. Koshimizu, N. Kawaguchi, and T. Yanagida, J. Ceram. Process. Res. 20[4] (2019) 307-313.
- 19. N. Elkhoshkhany, R. Essam, and E.S. Yousef, J. Non-Cryst. Solids. 536 (2020) 119994,
-

- 20. A. Goel, D.U. Tulyaganov, V.V. Kharton, A.A. Yaremchenko, and J.M.F. Ferreira, Acta Mater. 56 (2008) 3065-3076.
-

- 21. Z. Shen, Y. Zhao, Z. Tian, W. Huang, J. Wu, and H. Lin, J. Non-Cryst. Solids. 499 (2018) 17-24.
-

- 22. I. Kashif, A.A. El-Maboud, and A. Ratep, Results Phys. 4 (2014) 1-5.
-

- 23. A.K. Varshneya and J.C. Mauro, “Fundamentals of Inorganic Glasses” (Elsevier, 2019) p.1.
-

- 24. A. Gaddam, H.R. Fernandes, D.U. Tulyaganov, and J.M.F. Ferreira, J. Non-Cryst. Solids. 505 (2019) 18-27.
-

- 25. M. Abdel-Baki, F.A. Abdel-Wahab, and F. El-Diasty, J. Appl. Phys. 111 (2012) 073506.
-

- 26. N. Elkhoshkhany, M. Mahmoud, and E. Sayed-Yousef, Chalcogenide Lett. 16[6] (2019) 265-282.
- 27. Y.B. Saddeek, K.A. Aly, and S.A. Bashier, Physica B 405 (2010) 2407-2412.
-

- 28. M.K. Halmah, M.F. Faznny, M.N. Azlan, and H.A.A. Sidek, Results Phys. 7 (2017) 581-589.
-

- 29. R.D. Shannon, and R.X. Fischer, Am. Mineral. 101[10] (2016) 2288-2300.
-

- 30. S.C. Colak, I. Akyuz, and F. Atay, J. Non-Cryst. Solids. 432 (2016) 406-412.
-

- 31. A.H. Hammad, M.A. Marzouk, and H.A. El-Batal, Silicon 8 (2016) 123-131.
-

- 32. H. Guo, Y. Wang, Y. Gong, H. Yin, Z. Mo, Y. Tang, and L. Chi, J. Alloys Compd. 686 (2016) 635-640.
-

- 33. E.A. Davis, and N.F. Mott, Philos. Mag. 22[179] (1970) 903-922.
-

- 34. Z.A.S. Mahraz, M.R. Sahar, and S.K. Ghoshal, J. Mol. Struct. 1072 (2014) 238-241.
-

- 35. A. Herrmann, M. Tewelde, S. Kuhn, M. Tiegel, and C. Rüssel, J. Non-Cryst. Solids. 502 (2018) 190-197.
-

- 36. R.S. Gedam, and D.D. Ramteke, J. Phys. Chem. Solids. 74 (2013) 1039-1044.
-

 This Article
This Article
-
2022; 23(2): 237-242
Published on Apr 30, 2022
- 10.36410/jcpr.2022.23.2.237
- Received on Feb 23, 2022
- Revised on Apr 13, 2022
- Accepted on Apr 13, 2022
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seunggu Kang
-
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon, Korea
Tel : +82-31-249-9767 Fax: +82-31-249-9774 - E-mail: sgkang@kyonggi.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.