- The application of TiO2 and noble metal nanomaterials in tele materials
Ruihang Huang and Xiaoming Yang*
Donghua University, Shanghai, China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The use of TiO2 and noble metal nanoparticles in telematerials was examined. The application of several nano-materials on fibre textiles is investigated, including semiconductor materials such as TiO2 and ZnO, as well as other metal nanoparticle materials such as Au, Ag, Cu, and nano-clay, among others. TiO2 and its use in fibre textiles were thoroughly examined on this premise, including the photocatalytic principle of TiO2, the modification technique of TiO2, the fixation method of TiO2 nanoparticles, and the application of TiO2 in textile materials.Finally, the nano Ag antibacterial fiber fabric was investigated deeply, which containing the introduction of silver antibacterial agent, antibacterial mechanism of nano silver and loading method of nano silver
Keywords: TiO2, Nano material, Tele material
Nano technology is a new subject developed in the late 1990 s, which has good frontier and cross-cutting, because nano-materials generally have surface effects; Small size effect; Quantum size effect; Macroscopic tunneling effect; Advantages of dielectric effect [1-3]. Therefore, it is predicted that nanotechnology will become the “decisive technology” over network tech- nology and technology in the 21st century, and nano-materials will become the most promising materials. Countries all over the world have invested heavily in research, nanotechnology has been widely used in many fields, such as pharmacy, medical science, chemical and biological detection, manufacturing, optics and national defense. Especially in modern times, the textile industry has also introduced a large number of nanotechnology, new materials and functional textiles are constantly emerging, which is worthy of further research and discussion by scientists and technicians. Because of its small particle size, large specific surface area, strong magnetism, good photocatalytic perform- ance and strong ultraviolet absorption capacity, it has many advantages, such as good thermal conductivity, uniform dispersion, stable emulsion and so on. Therefore, it is a kind of nano-material with very broad application prospect at present [4, 5]. With the development of science and technology and the improvement of living standards, people are increasingly pursuing high-grade, comfortable and multifunctional fiber fabrics. People not only hope that the fiber fabric has the basic charac- teristics of heat preservation, breathability and elasticity, but also hope to endow the fiber fabric with more high value-added functions such as self-cleaning, antistatic, antibacterial, deodorization, ultraviolet protection, flame retardant, electromagnetic radiation protection and pollu- tion prevention. Applying nano-materials in textile is a method to prepare new textile materials. Nanomaterials refer to materials with at least one dimension in the three-dimensional space at the nanometer scale (1~100 nm). Based on the small size effect, quantum effect (macro quantum tunneling effect), surface effect and interface effect of nano materials, and their mechanical, magnetic, electrical, optical and chemical properties that macro materials do not have, by loading and com- pounding different nano-materials on fiber fabrics, and integrating multiple functions, nanotechnology has become one of the most active technical means for functional finishing of fiber fabrics, which has attracted great attention from the textile industry and consumers. The development trend of nanotechnology is irresistible and will bring huge economic benefits. This also brings a new economic growth point to the traditional tele industry-functional tele. People use nanotechnology to develop finishing fabrics with functions such as anti-ultraviolet, antibacterial and deodorization, which are being widely studied and applied. A variety of nano- materials, such as metals and metal oxide nanoparticles, including ag, Cu, au, MgO, ZnO, TiO2, and other nano- materials, such as carbon nanofibers and nanotubes, nano-silicon, chitosan, apatite, nano-clay, etc., have been used for functional finishing of fabrics, so that many aspects of the final products have better performance [6, 7]. Among them, some semiconductor nanomaterials and noble metal nanoparticles with photocatalytic pro- perties are particularly interesting.
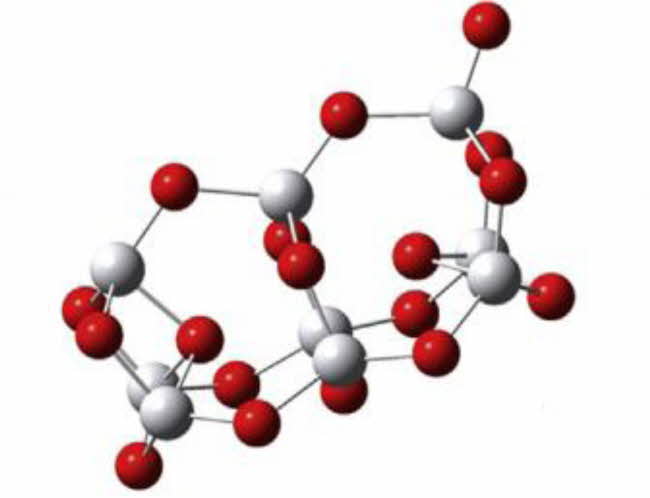
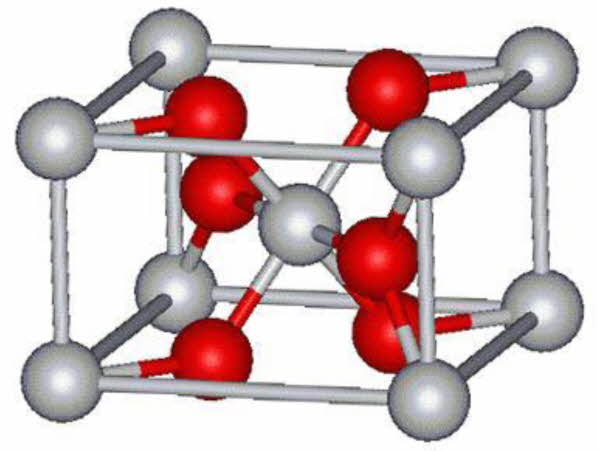
Nano-TiO2 is a kind of nano-material widely used at present. It has three crystal structures, namely anatase, rutile and brookite. These structures have a basic characteristic, that is, the basic unit of the structure is TiO6 octahedron, but the difference is that the skeleton is composed of TiO6 octahedron through common vertex or common edge. The anatase structure is composed of octahedron by common edges, while the anatase and rutile structures are composed of TiO6 octahedron by common vertices and common edges (as shown in Fig. 1 and Fig. 2). Both anatase and rutile are tetragonal, while brookite is orthorhombic. Although anatase and rutile are tetragonal, they have different space groups. Although all the three crystal configurations are based on TiO6 octahedra, the number of octahedra of each crystal form is different from that of other crystal forms. Anatase shares four edges, rutile is connected by two edges, and slate is connected by three edges [8-9].
Therefore, in this review, we will focus on the study of multifunctional fiber fabrics finished with TiO2 nano- materials. Firstly, this paper analyzes the application of different nano-materials on fiber fabrics, including TiO2, ZnO and other semiconductor materials. Au, Ag, Cu and other metal nanoparticles; Materials and nano-clay, etc. On this basis, in this paper, TiO2 and its appli- cation in fiber fabrics are analyzed in depth. Finally, this paper makes an in-depth investigation on nano Ag antibacterial fiber fabric.
|
Fig. 1 Anatase octahedral structure |
|
Fig. 2 Rutile octahedral structure |
Semiconductor materials
Semiconductor materials such as TiO2, ZnO, Fe2O3 and SiO2 have been used in the research of finishing fiber fabrics, and a single or multifunctional fiber fabric with photocatalysis, antibacterial, self-cleaning, ultraviolet protection, nano-coloring, magnetic properties, antistatic, water treatment, flame retardant, super hydrophilic or super hydrophobic, sensing and energy storage has been developed. Zaleska, A conductive superparamagnetic fabric with microwave attenuation, antibacterial and UV protection was developed by using PEDOT/ magnetite [10]. The team also modified PET fiber and wool fabric with CuO nanoparticles, colored the fabric, and made the fabric have UV protection and antibacterial properties. However, due to the limitation of toxicity, light stability and photo-corrosion, among the above materials, only TiO2 and ZnO have been widely used, and people have made in-depth research on the fiber fabrics finished with TiO2 and ZnO. Because of the properties of this semiconductor, multi-functional fiber fabrics such as photocatalysis, antibiosis, self-cleaning, ultraviolet pro- tection and flame retardance can be obtained. Zhou, X prepared nano zinc oxide composite cotton fabric, and the finished fabric showed a good inhibitory effect on the growth of Lactobacillus johnsonii and Pseudomonas aeruginosa [11]. Junpeng, Wang Wang used microwave-assisted hydrothermal method to grow zinc oxide (ZnO) nanowires on cotton fabric, and prepared multifunctional cotton fabric with self-cleaning, superhydrophobic and UV blocking properties [12]. Wang J synthesized zinc oxide nanoparticles from corn silk, and introduced a new polyester fabric with antibacterial, high hydrophilicity, self-cleaning and low cytotoxicity [13]. Compared with ZnO, TiO2 has been widely studied in the modification and finishing of fiber fabrics because of its cheap, non-toxic, easy preparation, stable physical and chemical properties, broad-spectrum antibacterial property and good photocatalytic performance, and its application range is also wider, including antibacterial, photocatalytic, water treatment, UV protection, degradation of air pollutants, self-cleaning, flame retardance, etc. Lu, W. C.A cotton fabric coated with SiO2/TiO2 was prepared, and the degradation of red wine stains was achieved under sunlight [14]. Imai, H. prepared cellulose fibers coated with nano titanium dioxide by sol-gel technology at low temperature. The cellulose fiber coated with titanium dioxide has high photocatalytic decomposition efficiency for adsorbed methylene blue and asphalt fraction extracted by heptane under sun-like rays [15]. Hu, Y. treated polyester fiber with plasma to increase the adsorption of titanium dioxide on polyester fiber, and prepared a polyester fabric finished with titanium dioxide, which has good self-cleaning performance and stability [16].
Noble metal nanoparticles.
Most of the fiber fabrics finished with Au, Ag, Cu and other metal nanoparticles are used for antibacterial action and dyeing of fiber fabrics, or used to prepare conductive or electromagnetic shielding fabrics, or combined with semiconductor materials to play multiple roles. D Zare uses a one-step synthesis method to load nano-gold particles (AuNPs) on the viscose fiber, and through the LSPR action of AuN Ps, the treated fiber obtains a durable purple color. And dark colors were obtained at higher Au3+ concentration. In addition, the treated fiber has a good inhibitory effect on bacteria, fungi and other pathogenic bacteria [17]. In Li and D, Ginkgo biloba powder extract was used as reducing agent to synthesize gold nanoparticles on silk and cotton fabrics in a green way, which made the fabrics get firm coloring. Q.F.WEI deposited copper nano- structures with different thicknesses on the surface of polypropylene nonwovens by magnetron sputtering, which has a wide application prospect in antistatic, visible and ultraviolet shielding fields. Among them, nano-silver finished fiber fabrics are the most studied [18]. Ishchuk use lecithin to synthesize nano silver particles in situ, which improves the loading efficiency. The release rate of antibacterial agent in wool fiber was reduced [19]. Langlet, M used sonochemical method (ultrasonic treatment) and ethylene glycol as reducing agent to load nano silver coating on nylon and poly- propylene fabrics. After 20 washing cycles, the nano silver coating remained stable and showed excellent antibacterial performance [20]. AM Peiró uses aniso- tropic nano silver to dye wool fabric. The modified wool fabrics show brilliant colors due to local surface plasmon resonance. By controlling the shape and size of silver nanoparticles, blue, orange and yellow wool fabrics can be obtained, and the obtained wool fabrics also have obvious antibacterial effect on Escherichia coli. In addition, the fiber fabric coated with metal nanoparticles can also be used as flexible conductive fabric [21].
In addition, fiber fabrics finished with metal nano- particles have important applications in energy manage- ment and storage. Owieja et al. prepared fatty acid/nano-copper/poly (vinegar) composite materials for thermal management and improvement of thermal conductivity by in-situ synthesis of copper nanoparticles and direct adsorption of fatty acids into poly-cool fibers [22].
Carbon materials
Carbon nanotubes, graphene oxide and graphene are often used to prepare conductive fiber fabrics. The modified fiber fabrics can obtain good conductivity, UV protection and electromagnetic shielding performance, and can be used as wearable chemical and biological sensors, flexible conductive electrodes, etc. Paola, A.D. dyed conductive textiles with carbon nanotubes. Compared with undyed textiles, adding carbon nanotubes into tele can increase the conductivity by 4 orders of magnitude and double the capacitance [23]. Man-de Qiu reduced graphene oxide in situ on cotton fabric by simple heat treatment, and the modified reduced graphene oxide-cotton fabric showed good electrical properties, con- ductivity, surface hydrophobicity and UV protection [24]. Mustafa, Karaman uses conventional dyeing method to fix graphene oxide on the surface of acrylic yarn to manufacture conductive fabric. It is worth mentioning that finishing fiber fabrics with carbon nanotubes, graphene or graphene oxide may require high production cost and color (black) limitation [25].
Nano-clay
Nano-clay is a new environmental protection material developed in recent years. Nano-Shaotu is a kind of natural nano-material with high specific surface area, stable chemical and mechanical properties and various structures. It is widely used in polymer nanocomposites, coatings, ceramics and other fields, with large output and low price in China. Attapulgite (ATP), bentonite, montmorillonite, frog stone, mica, hydroxene, kaolin, montmorillonite (MMT) and other nano-clays have attracted wide attention because of their unique charac- teristics and cost-effectiveness. Nano-clay can be used to improve the flame retardancy, super-hydrophobicity, self-cleaning, antibacterial, anti-ultraviolet, thermal and mechanical properties of fabrics [26, 27]. Pant, HR, Park, prepared branched polyethylenimine and mont- morillonite (BPEI-MMT) coatings on cotton fabrics by layer-by-layer assembly, and obtained flame retardant cotton fabrics. In addition, since 2013, clay-based super- hydrophobic or superhydrophobic and oleophobic coatings have attracted more and more attention [28].
Photocatalytic principle of TiO2
The general process of photocatalysis is: when a semiconductor receives photon irradiation, it can absorb photon energy with energy larger than the forbidden band width (Eg) of the semiconductor, and some electrons on valence band are excited to transition to conduction band, at the same time, holes (h+) are generated. The photo-generated electron-hole pairs will trigger a series of redox reactions, which is the general process of photocatalytic reaction. Equations (1) and (2) show the above process.
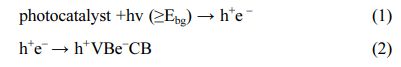
Under the irradiation of light with energy equal to or greater than the band gap energy of titanium dioxide (anatase is 3.2 eV), valence electrons transition from valence band to conduction band, leaving holes and negative electrons in valence band and conduction band respectively. The generated holes have strong oxidizability, while the negative electrons on the con- ductive band have reducibility. Holes (h+) react with water molecules adsorbed on the surface of titanium dioxide to form hydroxyl radicals (OH), while elec- trons react with oxygen molecules adsorbed on the surface of titanium dioxide to generate superoxide radical (Oi) and peroxy hydroxyl radical (OOH). These strong oxidizing groups can directly and thoroughly oxidize the target reactant into inorganic small molecules such as CO2and H2O [29].
The modification method of TiO2 Ion doping
Ion doping of semiconductor materials means that ions are introduced into the semiconductor lattice by physical and chemical means, and the introduced ions are in substitutional or interstitial state, which will cause distortion of the semiconductor lattice and formation of a large number of crystal defects. Furthermore, the energy band structure of the semiconductor is changed and the recombination rate of electron-hole pairs is reduced by introducing defects as electron capture points, so as to achieve the purpose of modifying the photoelectric properties of the semiconductor. According to the types of doped ions, ion doping can be divided into two types: nonmetal ion doping and metal ion doping. Generally, the metal ion Mx+ replaces Ti4+ in titanocene octahedron and enters the lattice to form M-O-Ti bond, while the doping of non-metal ions may exist in substitutional state or interstitial state or the coexistence of substitutional state and interstitial state. Elements used in nonmetallic impurity infiltration include N,C,F,B,S,P, etc., among which N is the most studied element [30]. The impurity NHaCI was charac- terised in an article published in 2005 by eilert, K. T. as moving the absorption threshold of TiO2 to the visible area during satin burning. However, this work has not received much attention in a long time. Relevant academics published the visible light photocatalytic response of nitrogen-doped titanium dioxide fifteen years after the publication of this study, which marked a breakthrough in visible light photocatalysis. Following that, a slew of papers on the approach of increasing the photocatalytic performance of titanium dioxide by nitrogen doping appeared.
Precious metal deposition
Precious metal deposition of TiO2 is based on the fact that the Fermi level of metal is lower than that of semiconductor in general. When the two are in contact, photogenerated electrons transfer to metal until the Fermi level of the two is equal. In this way, the metal surface is enriched with excessive electrons, while the semiconductor is enriched with excessive positively charged holes, forming a Schottky barrier, thus effectively inhibiting the recombination of electron-hole pairs. Promote the separation of electron-hole pairs and improve the photocatalytic activity. Common precious metal deposits include Pt, Au, Ag, Cu, Pd, Ru, Nb, etc. Precious metal deposition often has an optimal loading value, when it exceeds this value, metal deposition will have an adverse effect on photocatalytic activity. The existence of this optimal load value may have different reasons. For metal deposition exceeding the optimum value, due to the attraction of a large number of metal particles to electrons, the electron density will decrease. The resulting complex field structure adversely affects the charge separation and reduces the photocatalytic activity of the catalyst. In addition, the excessive coverage of TiO2 catalyst limits the amount of light reaching the surface and reduces the number of hole electrons generated by light, thus reducing the photocatalytic rate. Excessive loading will also cause new recombination centers, which is unfavorable to photocatalysis [31-32].
Sensitization
Photosensitizing the surface of TiO2 with dye means fixing the dye on the surface of TiO2 by chemical adsorption or physical adsorption, and forming a complex with it, thus realizing the photosensitization of TiO2. After the photon absorbed by the composite is excited, as long as the excited state potential of the active material is more negative than the conduction band potential of the TiO2 semiconductor, electrons generated by excitation may be injected into the conduction band of the semiconductor, thus expanding the excitation wavelength range of TiO2and improving the photo- catalytic activity. Dyes and metal complexes used as sensitizers include lycopene B, Lloyd's violet, sub- stituted and unsubstituted bipyridine [33].
Method of loading and fixing TiO2nanoparticles on fiber fabric
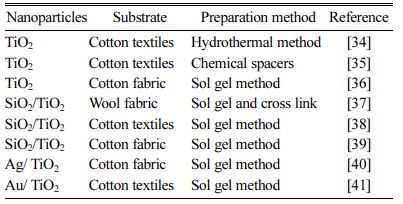
Scientists have tried various methods to fix titanium dioxide on various polymers and fiber fabrics, including sol-gel method, hydrothermal method, magnetron sput- tering method, electrochemical and chemical method, thermo-synthesizing method, Impregnation method, etc. Table 1 summarizes the methods of fixing titanium dioxide on different polymer and fabric substrates.
The application of TiO2 in tele materials Self-cleaning
It is a new concept proposed in recent years to obtain self-cleaning fibers by coating titanium dioxide nano- particles on fibers. Self-cleaning material refers to a kind of material that can keep its surface clean under natural conditions, automatically remove stains, without manual cleaning, and greatly reduce the cleaning cost. Developing self-cleaning materials can effectively reduce the washing times of clothes. Even realize the laundry-free, thus reducing the use of detergent and water, and reducing the sewage discharge, and finally achieving the effects of energy saving, water saving and environ- mental protection. The preparation of self-cleaning materials mainly has two principles: superhydrophobic self-cleaning and superhydrophilic self-cleaning. The photocatalytic self-cleaning fabric refers to the fabric loaded with nanoparticles with photocatalytic effect [42]. TiO2 can decompose organic pollutants under the action of ultraviolet light, so researchers often coat titanium dioxide on the surface of fiber fabrics to obtain self-cleaning fabrics. Titanium dioxide is illuminated by ultraviolet light.
Organic pollutants can be interpreted as carbon dioxide, water and inorganic acid, as shown in formula 3.
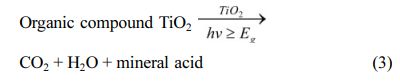
Tseng, C. H. coated the cotton fabric with a titanium dioxide film, and successfully degraded the stains of red wine and coffee under the irradiation of ultraviolet light. El-gabry, L.K. used hydrothermal method to load ultrafine titanium dioxide nanoparticles on cellulose fibers and effectively degrade methyl orange dye and formaldehyde under sunlight [44-45].
Improve the flame retardancy of fabrics
Most textiles are flammable, and many studies have proved that adding titanium dioxide nanoparticles can improve the flame retardancy and washability of fiber fabrics finished with flame retardant, and at the same time can reduce the side effects of flame retardant. Therefore, scientists have paid more and more attention to this research in recent years. A. El-Shafei adopted a “pad-dry-cure” method, in which TiO2 and flame re- tardant such as phytic acid (PA). Pyrovatex CP New were finished on cotton, wool and silk fabrics in the presence of crosslinking agent, which improved the flame retardancy of fabrics. the flame retardant and titanium dioxide often had combined flame retardancy [46, 47].
Ultraviolet protection
The wavelength range of ultraviolet rays is 200-400 nm, and the spectrum of 400-320 nm is called “ultraviolet UVA”. The spectrum of 320-280 nm is called “ultraviolet UVB”. The spectrum of 280-200 nm is called “ultraviolet UVC”. Excessive exposure to ultraviolet radiation, including UVA and UVB, can cause skin damage, such as sunburn, premature skin aging, allergy, and even skin cancer. The ultraviolet protection ability of ordinary textiles is not enough, therefore. It is necessary to use fabrics with anti-ultraviolet function to effectively resist ultraviolet radiation on the skin, and people's demand for anti-ultraviolet fabrics is increasing. There are two main ways to protect textiles from ultraviolet rays. One is ultraviolet absorption, which converts its energy into heat energy or electromagnetic waves with shorter wavelengths. One is the reflection and scattering of ultraviolet rays, which cannot convert light. Nano- materials, as an effective way to develop new functional teles and garments, have attracted extensive attention in recent years. One of the important properties of nano titanium dioxide is its anti-ultraviolet performance, so it is often used to develop anti-ultraviolet functional tele [48]. When TiO2 is excited by photons, electrons and holes are generated. Combines with electrons and holes, it releases energy in the form of heat or light, which is the theoretical basis of shielding ultraviolet rays. In recent years, there are a large number of fiber fabrics with UV protection properties finished with titanium dioxide.
Antibacterial performance
The photocatalytic sterilization mechanism of TiO2 includes direct and indirect effects. The photogenerated electrons and holes of titanium dioxide have very strong oxidation ability, and can directly oxidize cell walls, cell membranes or intracellular compositions, etc. Active oxygen (ROS), such as superoxide radical, radical radical, hydrogen peroxide radical and hydrogen peroxide, which are directly or indirectly generated by photogenerated electrons and holes respectively reacting with adsorbed oxygen and water molecules, can undergo biochemical reactions with cell walls, cell membranes or components in cells, destroying the cellular structure of bacteria and thus inactivating them. Mihailovi, D.A large number of antibacterial examples of catalysts were summarized, including Gram-positive bacteria, Gram-negative bacteria, and fungi [49-50].
|
Table 1 Methods of fixing titanium dioxide on different polymer and fiber fabric substrates |
Nanosilver is recognized as an efficient broad-spectrum antibacterial agent, which has the advantages of high safety, high stability, low toxicity, low pollution, long-lasting antibacterial performance, and is not easy to produce drug resistance. It has good application value in the antibacterial finishing of various fiber fabrics. As a non-antibiotic antibacterial agent, silver has not been found to be resistant to any bacteria at present. Therefore, nanosilver not only has long-term antibacterial effect, but also has the characteristics of wide antibacterial range and high safety. Because the antibacterial ability of silver ions is far stronger than other antibacterial metal ions and its safety is higher, nano silver inorganic antibacterial agents are widely used in antibacterial textiles. There are two main ways to apply nano silver in textiles: (1) preparing nano silver antibacterial fiber, and then processing into antibacterial textile; (2) A finishing agent containing silver antibacterial agent is prepared, and textiles are finished to obtain antibacterial properties.
Silver antibacterial agents
Antibacterial agents can be roughly divided into two categories according to their chemical composition: organic antibacterial agents and inorganic antibacterial agents. Organic antibacterial agents can be divided into natural antibacterial agents and synthetic antibacterial agents. Among them, the natural antibacterial agent is mainly extracted from animals, plants, and micro- organisms, which is safe and harmless to the human body and environment and is a green antibacterial agent. It is one of the main directions in the future development of antibacterial agents. At present, natural antibacterial agents mainly include chitosan, protamine, peppermint oil, Lohan tar, oxytetracycline, jinggang- mycin, etc. However, due to the limitation of resources and processing conditions, their short life, and poor heat resistance, they cannot be widely used. Represen- tatives of synthetic antibacterial agents mainly include quaternary salts, muscles, haloamine compounds, triclosan, and betaine, etc. This antibacterial agent has the advantages of strong initial lethality, fast sterilization speed, and wide antibacterial range, but it has some problems such as poor heat resistance, certain toxicity, easy seepage, easy ingestion by the human body, poor washing resistance, and short service life. Inorganic antibacterial agents mainly include metals, metal salts, and photocatalytic antibacterial agents. Many metals such as Ag, Hg, Cu, Cd, Cr, Ni, Pb, Co, Zn, and Fe have antibacterial effects, and it is reported that the antibacterial effects of these metal ions are from high to low in turn [51]. However, considering that some heavy metals such as mercury, pickaxe, nickel, chromium and lead are harmful to the human body, they are not suitable as ideal antibacterial agents. The commonly used metal antibacterial agents are Ag, Cu, and Zn, and their antibacterial properties decrease in turn, among which the antibacterial properties of Ag are about 1000 times that of Zn. Nanosilver antibacterial agents are widely used in antibacterial materials because of their combination of high efficiency and broad-spectrum, good heat resistance, low drug resistance, and high safety. Nanosilver can be used in medical burn treatment, dental materials, medical equipment coatings, tele fabrics, water treatment, sunscreen lotion, etc. It has low toxicity and high thermal stability to human cells at trace concentration. There are mainly n-type semi- conductor oxides such as TiO2, ZnO, CDs, w03. SnO2, Fe2o3, among which TiO2and ZnO are the most common ones. Photocatalytic antibacterial agents have spectral antibacterial properties, lasting antibacterial properties, and no secondary pollution. However, these kinds of antibacterial agents need to show excellent antibacterial properties under illumination, so their application is limited [52].
Antibacterial mechanism of nanosilver
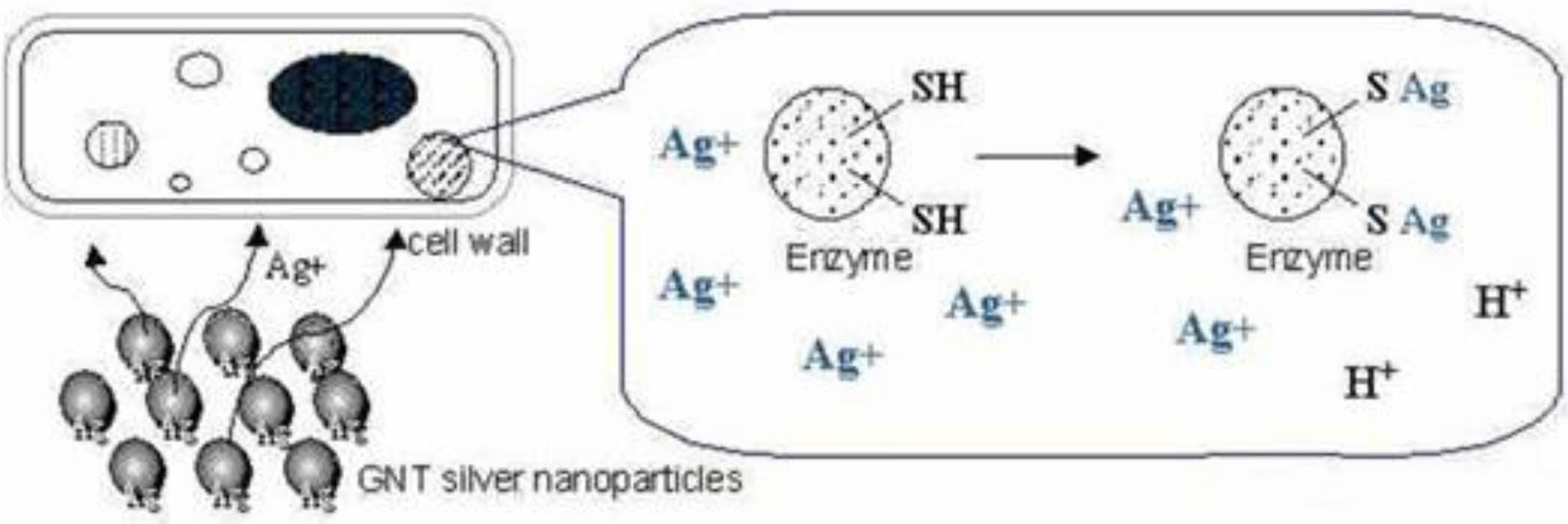
The antibacterial activity of nanoparticles is mainly affected by two aspects: (1) the physical and chemical properties of nanoparticles; (2) the type of bacteria. Up to now, the antibacterial mechanism of silver nano- particles has not been determined. Many studies have shown that the antibacterial effect of silver nanoparticles comes from silver nanoparticles themselves and is also related to the release of silver ions. Both silver nano- particles and silver ions can adsorb on the surface of the cell membrane, destroying the cell membrane, and penetrating into bacterial cells, thus affecting the infil- tration and respiratory function of cells. In addition, they can react with compounds containing sulfur and phosphorus groups, such as DNA. The reactive oxygen species (ROS) produced in this process can also kill bacteria, as shown in Fig. 3. The mechanism of toxicity of Ag nanoparticles to bacteria and other organisms has been discussed by researchers [53]. It is well known that silver nanoparticles can be oxidized in an aqueous solution exposed to air (Formula 4), thus releasing silver ions under acidic conditions (Formula 5).

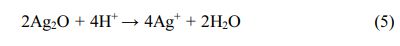
Method of loading nano silver on fiber fabric
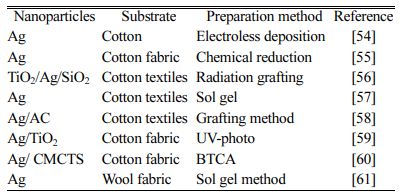
There are two main methods of loading nanoparticles on fiber fabrics, i.e., fibril processing and post-treatment processing. The preparation method of nano-silver finished fiber fabric is mainly post-processing, that is, finishing nano-silver antibacterial agent on fiber fabric by dipping, padding, deposition or coating. The method has simple process and relatively low cost.It is suitable for various textiles made of natural fibers or synthetic fibers, and is the most commonly used method for finishing textile fabrics with nano silver antibacterial agents at present. Table 2 lists some methods of loading nano silver on different fiber fabrics.
|
Fig. 3 Antibacterial mechanism of nanosilver. |
Scientists pay more and more attention to the research of nano-material finished fiber fabrics. With nanotechnology, scientists have developed more and more new and multifunctional textiles. However, by summarizing the current research status, we can find the main problems of nano-material finished fiber fabrics:
(1) Agglomeration of nanoparticles: Because nano- particles have a great specific surface area, huge surface energy, and are extremely unstable, they are easy to cluster together. No matter whether the nanoparticles are loaded by fiber processing or post-treatment, how to uniformly disperse nanoparticles on the fiber fabric is the key technology for the development and appli- cation of nano-functional fiber fabric. In order to solve the problem of aggregation of nanoparticles in solution, scientists usually adopt the methods of modifying nanoparticles and adopting appropriate dispersants.
(2) The loading of nanoparticles is not strong enough: for most inorganic nanoparticles, because the surface of fiber fabric lacks reactive groups, these nanoparticles are often not tightly combined with fabric fibers (matrix).
(3) Poor washing resistance or durability: Some finished nano-particles only exist in the fiber surface layer, and once they fall off, they cannot be replenished in time, so they have the disadvantages of poor washing re- sistance and durability, and may have safety problems due to the large initial dissolution amount.
The further development of nanotechnology provides a great possibility for the development of new multi- functional materials based on textile materials. The functionalization of textiles has also aroused the interest of many researchers in the world. We should treat the new multifunctional fiber fabric modified by nano- materials in the ascendant rationally, and we can neither deny it by one vote nor ignore the potential risks. While developing new products to meet the needs of human production and life, it is necessary to assess the biological and environmental risks simultaneously.
- 1. V. Brezová, M. Polovka, and A. StaKo, Spectrochim. Acta A Mol. Biomol. 58[6] (2002) 1279-1291.
-

- 2. M. López-Vélez, F. Martínez-Martínez, and C.D. Valle-Ribes, CRC Crit. Rev. Food Technol. 43[3] (2003) 233-244.
-

- 3. Y. Cao, W. Yang, W. Zhang, G.Liu, and P. Yue, New J. Chem. 28[2] (2004) 218-222.
-

- 4. Y. Sakatani, J. Nunoshige, H. Ando, K. Okusako, H. Koike, T. Takata, J.N. Kondo, M. Hara, and K. Domen, Chem. Lett. 32[12] (2003) 1156-1157.
-

- 5. T. Iwasaki, M. Satoh, and S. Ichio, J. Mater. Process. Technol. 142[1] (2003) 131-138.
-

- 6. W. Choi, A. Termin, and M.R. Hoffmann, J. Phys. Chem. 98[51] (1994) 13669-13679.
-

- 7. M. Bradha, T. Vijayaraghavan, S.P. Suriyaraj, R. Selvakumar, and A. Ashok, J. Rare Earths 33[2] (2015) 160-167.
-

- 8. K.T Ranjit, I. Willner, S.H. Bossmann, and A.M. Braun, J. Catal. 204[2] (2001) 305-313.
-

- 9. X.L. Zhang, L.G. Ren, and W.Y Gao, Chinese Chem. Res. Appl. 23[9] (2011) 1195-1199.
- 10. A. Zaleska, E. Grabowska, J.W. Sobczak, M. Gazda, and J. Hupka, Appl. Catal. B. 89[3-4] (2009) 469-475.
-

- 11. X. Zhou, F. Peng, H. Wang, and H. Yu, J. Solid State Chem. 184[11] (2011) 3002-3007.
-

- 12. J. Wang, B. Huang, Z. Wang, X. Qin, and X. Zhang, Rare Met. 30[1] (2011) 161-165.
-

- 13. W.-C. Lu, H.D. Nguyen, C.Y. Wu, K.S. Chang, and M. Yoshimura, J. Appl. Phys. 115[14] (2014) 367.
-

- 14. H. Imai, and H. Hirashima, J. Am. Ceram. Soc. 82[9] (1999) 2301-2304.
-

- 15. Y. Hu, and C. Yuan, J. Cryst. Growth 274[3-4] (2005) 563-568.
-

- 16. D. Li, N. Gautier, B. Dey, S. Bulou, M. Richard-Plouet, W. Ravisy, A. Goullet, P. Choquet, and A. Granier, Appl. Surf. Sci. 491 (2019) 116-122).
-

- 17. S. Ishchuk, D.H. Taffa, O. Hazut, N. Kaynan, and R. Yerushalmi, ACS Nano. 6[8] (2012) 7263-7269.
-

- 18. M. Langlet, A. Kim, M. Audier, and J.M. Herrmann. J. Sol-Gel Sci. Technol. 25[3] (2002) 223-234.
-

- 19. A.M. Peiró, J. Peral, C. Domingo, X. Domènech, and J.A. Ayllón, Chem. Mater. 13[8] (2001) 2567-2573.
-

- 20. M. Oćwieja, Z. Adamczyk, M. Morga, and K. Kubiak. Adv. Colloid Interface Sci. 222 (2015) 530-563.
-

- 21. A.D. Paola, M. Addamo, M. Bellardita, E. García-López, G. Marcì, and L. Palmisano, Materials Science Forum. Trans Tech Publications. 587-588 (2008) 795-799.
-

- 22. M.-D. Qiu, G.-Y. Bai, X.-Y. Wang, Y.-Q. Zhai, and Z.-H. Yao, Asian J. Chem. 25[2] (2013) 1133-1136.
-

- 23. M. Karaman, F. Saripek, Ö. Köysüren, and H.B. Yıldız, Appl. Surf. Sci. 283[1] (2013) 993-998.
-

- 24. J. Sheng, L. Shivalingappa, J. Karasawa, and T. Fukami, J. Mater. Sci. 34[24] (1999) 6201-6206.
-

- 25. Y. Hui, Q. Shen, and J. Gao, Rare Metal Mat. Eng. 37[5] (2008) 8-11.
- 26. H.R. Pant, C.H. Park, B. Pant, L.D. Tijing, H.Y. Kim, and C.S. Kim, Ceram. Int. 38[4] (2012) 2943-2950.
-

- 27. T. Yuranova, R. Mosteo, J. Bandara, D. Laub, and J. Kiwi, J. Mol. Catal. A Chem. 244[1-2] (2006) 160-167.
-

- 28. K.T. Meilert, D. Laub, and J. Kiwi, J. Mol. Catal. A Chem. 237[1-2] (2005) 101-108.
-

- 29. M.J. Uddin, F. Cesano, F. Bonino, S.G. Bordiga, G. Spoto, D. Scarano, and A. Zecchina, J. Photochem. Photobiol. A. 189[2] (2007) 286-294.
-

- 30. W.S. Tung, and W.A. Daoud, Acta Biomater. 5[1] (2009) 50-56.
-

- 31. M.J. Uddin, F. Cesano, S. Bertarione, F. Bonino, S.G. Bordiga, D. Scarano, and A. Zecchina, J. Photochem. Photobiol. A. 199[1] (2008) 64-72.
-

- 32. W. Fang, L. Zhou, B. Shen, Y. Zhou, Q. Yi, M. Xing, and J. Zhang, Res. Chem. Intermed. 44 (2018) 4609-4618.
-

- 33. P.-C. Hsu, C. Liu, A.Y. Song, Z. Zhang, Y. Peng, J. Xie, K. Liu, C.-L. Wu, P.B. Catrysse, L. Cai, S. Zhai, A. Majumdar, S. Fan, and Y. Cui, Sci. Adv. 3[11] (2017) e1700895.
-

- 34. P.-C. Hsu, X. Liu, C. Liu, X. Xie, H. Lee, A. Welch, T. Zhao, and Y. Cui, Nano Lett. 15[1] (2015) 365-371.
-

- 35. L. Hua, and S. Wang, Fibers Polym. 13[10] (2012) 1272-1279.
-

- 36. D. Gao, R. Li, B. Lv, J. Ma, F. Tian, and J. Zhang, Compos. B. Eng. 77 (2015) 329 - 337.
-

- 37. Y.C. Li, J. Schulz, S. Mannen, C. Delhom, B. Condon, S. Chang, M. Zammarano, and J.C. Grunlan, ACS Nano 4[6] (2010) 3325-3337.
-

- 38. C. Liu, M. Li, J. Wang, X. Zhou, Q. Guo, J. Yan, and Y. Li, Chinese J. Catal. 37[3] (2016) 340-348.
-

- 39. F. Carosio, J. Alongi, and A. Frache, Eur. Polym. 47[5] (2011) 893-902.
-

- 40. D. Zhang, C. Jiao, J. Xiong, H. Lin, and Y. Chen, Jpn. J. Appl. Phys. 54[6S1] (2015) 06FH01.
-

- 41. C.H. Tseng, C.C. Wang, and C.Y. Chen. J. Phys. Chem. B. 110[9] (2006) 4020.
-

- 42. L.K. El-Gabry, A.A. El-Kheir, M. Salama, S. Mowafi, and H. El-Sayed. J. Appl. Sci. Res. 10[3] (2014) 218-229.
- 43. S. Amiri, L. Duroux, J.L. Nielsen, A.H. Nielsen, D. Yu, and K.L. Larsen, J. Text. Inst. 105[3] (2014) 327-336.
-

- 44. M. Montazer and S. Seifollahzadeh, Photochem. Photobiol. 87[4] (2011) 877-883.
-

- 45. M. Montazer, A. Shamei, and F. Alimohammadi, Prog. Org. Coat. 74[1] (2012) 270-276.
-

- 46. D. Mihailović, Z. Šaponjic, M. Radoičić, T. Radetić, P. Jovančić, J. Nedeljković, and M. Radetić, Carbohydr. Polym. 79[3] (2010) 526-532.
-

- 47. K. Hashimoto, H. Irie, and A. Fujishima, Jpn. J. Appl. Phys. 44[12] (2005) 8269-8285.
-

- 48. M. Rai, A. Yadav, and A. Gade, Biotechnol. Adv. 27[1] (2009) 76-83.
-

- 49. V.K. Sharma, R.A. Yngard, and Y. Lin, Adv. Colloid Interface Sci. 145[1-2] (2009) 83-96.
-

- 50. J.G. Mcevoy, and Z. Zhang, J. Photochem. Photobiol. C. 19 (2014) 62-75.
-

- 51. J. Ye, H. Cheng, H. Li, Y. Yang, S. Zhang, A. Rauf, Q. Zhao, and G. Ning, J. Colloid Interface Sci. 504 (2017) 448-456.
-

- 52. X. Luo, Y. Liu, Z. Qin, Z. Jin, L. Xu, Y. Liu, L. Cao, F. He, X. Gu, and X. Ouyang, J. Biomed. Nanotechnol. 14[3] (2018) 601-608.
-

- 53. Q.L. Feng, J. Wu, G.Q. Chen, F.Z. Cui, T.N. Kim, and J.O. Kim, J. Biomed. Mater. Res. 52[4] (2015) 662-668.
-

- 54. R.S. André, C.A. Zamperini, E.G. Mima, V.M. Longo, A.R. Albuquerque, J.R. Sambrano, A.L. Machado, C.E. Vergani, A.C. Hernandes, J.A. Varela, and E. Longo, Chem. Phys. 459 (2015) 87-95.
-

- 55. C. Khurana, A.K. Vala, N. Andhariya, O.P. Pandey, and B. Chudasama, Environ. Sci. Process Impacts. 16[9] (2014) 2191-2198.
-

- 56. J. R. Koduru, S.K. Kailasa, J.R. Bhamore, K.H. Kim, T. Dutta, and K. Vellingiri, Adv. Colloid Interface Sci. 256 (2018) 326-339.
-

- 57. K. Logaranjan, A.J. Raiza, S. Gopinath, Y. Chen, and K. Pandian, Nanoscale Res. Lett. 11[1] (2016) 520.
-

- 58. A. Ivask, A. Elbadawy, C. Kaweeteerawat, D. Boren, H. Fischer, Z. Ji, C.H. Chang, R. Liu, T. Tolaymat, D. Telesca, J.I. Zink, Y. Cohen, P. A. Holden, and H.A. Godwin, ACS Nano. 8[1] (2014) 374-386.
-

 This Article
This Article
-
2022; 23(2): 213-220
Published on Apr 30, 2022
- 10.36410/jcpr.2022.23.2.213
- Received on Nov 23, 2021
- Revised on Dec 4, 2021
- Accepted on Dec 4, 2021
 Services
Services
- Abstract
introduction
application of different nanomaterials in textile materials
tio2and its application in tele fabrics
noble metal and its application in textile materials
conclusion
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Xiaoming Yang
-
Donghua University, Shanghai, China
Tel : +021-67792259 Fax: +021-67792259 - E-mail: 1037234058@qq.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.