- Effect of MgO-CaO-Al2O3-SiO2 glass additive on densification of Al2O3 ceramics
Dagyeong Leea,b, Hyun Seon Hongb, Hyeondeok Jeonga,* and Sung-Soo Ryua,*
aEngineering Ceramic Center, Korea Institute of Ceramic Engineering and Technology, Icheon 17303, Republic of Korea
bDepartment of Environment & Energy Engineering, Sungshin Women’s University, Seoul 01133, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
We investigated the effect of the MgO-CaO-Al2O3-SiO2 (MCAS) additive on the densification of Al2O3 ceramics. MCAS was synthesized using a polymeric-complex method. We analyzed the densification behavior, using dilatometric analysis up to 1600 oC, and the results showed that sinterability increased with increasing MCAS content. Al2O3 samples were prepared by isothermal sintering at 1400, 1500, and 1600 oC. The microstructure, phase formation, and hardness of the samples were analyzed and discussed in relation to both the MCAS content and sintering temperature. The density of the MCAS-doped samples sintered at 1500 and 1600 oC was over 98%, and the maximum relative density was 99.7%. The highest hardness (18 GPa) was achieved for the sample prepared with 3 wt.% MCAS and sintered at 1500 oC for 1 h because further doping resulted in excessive grain growth. These results elucidate the conditions required for pressure-free sintering
Keywords: Al2O3, Sintering additive, MCAS glass, Dilatometry, Densification
Alumina (Al2O3) is a ceramic material that exhibits excellent thermal, mechanical, and chemical properties. It is widely used in electronics, heat-resistant equipment, and mobile technologies. Many studies have been con- ducted on the densification and grain growth of Al2O3 materials [1-8]. However, recent technological advances in related industries have increased the demand for Al2O3 materials exhibiting enhanced dielectric properties and plasma resistance. Therefore, research on advanced sintering methods and additives for Al2O3 materials has been attracting increasing attention [9-14].
Novel sintering methods including isostatic pressing, hot pressing, and gas-pressurized sintering can be em- ployed to densify Al2O3 [1-3, 6]. These pressurization-based methods require expensive equipment, involve dangerous processes, and limit the size of the resulting ceramics. Therefore, researchers have used additives to attempt to develop pressure-free sintering methods. Several studies have used sintering aids such as NiO, SiO2, MnO, FeO, MgO, and TiO2 to improve sintering density and other properties [10, 15-17]. However, the additives usually degrade the mechanical and/or elec- trical properties of Al2O3 materials.
Glass-ceramic additives such as MgO-Al2O3-SiO2 (MAS), Li2O-Al2O3-SiO2 (LAS), CaO-Al2O3-SiO2 (CAS), and MgO-CaO-Al2O3-SiO2 (MCAS) are polycrystalline materials consisting of fine grains and effectively ac- celerate sintering and improve the properties of ceramic materials [11-13, 18-22]. Specifically, the addition of MCAS glass can increase the density and enable the control of the microstructure of ceramic materials without significantly altering their properties. Alumina materials prepared using glass-ceramic additives are expected to be applied to spark plugs, ceramic tiles/fibers, electrostatic chucks, and insulators.
C. Cheng et al. studied the addition of MCAS to BaTi4O9- and Ba2Ti9O20-based ceramics and reported that the densification temperature of the ceramic materials was lowered with the addition of the MCAS [19]. H. Lee et al. investigated the sintering behavior of AlN ceramics prepared with added MCAS and showed that the addition of 5 wt.% MCAS significantly densified the AlN ceramics [20, 21]. However, few studies have been conducted on the effect of MCAS on the densification properties of Al2O3 materials.
Therefore, to investigate sinterability, MCAS was added to Al2O3 in varying concentrations in this study. Isothermally sintered Al2O3 was also prepared at different temperatures; the density, phase, and microstructure were analyzed; and the relation between the MCAS content and the sintering temperature was investigated.
Commercial Al2O3 (AES-11, Sumitomo Chemical, Japan) and MCAS glass prepared using a polymeric-complex method were used as raw materials [23]. Fig. 1 shows SEM (scanning electron microscopy) images of the Al2O3 and MCAS used in this study. The Al2O3 (Fig. 1(a)) and MCAS (Fig. 1(b)) exhibited particle sizes of approximately 500 and 50 nm, respectively.
The synthesized MCAS (1, 3, or 5 wt.%) was added to Al2O3 and mixed using a planetary mill (PULVERISETTE 6, Fritsch, Germany). Subsequently, 20 g of the Al2O3-MCAS mixed powder, 0.2 g of dispersant (BYK-111, BYK, Germany), 100 g of Al2O3 balls (diameter: 5 mm), and 150 mL of isopropyl alcohol (Merck, Germany) were mixed at 300 rpm for 1 h. The mixture was dried in an oven at 80 oC for 20 h to evaporate the isopropyl alcohol. The 0, 1, 3, and 5 wt.% MCAS mixed powders were designated as M0, M1, M3, and M5, respectively.
The temperature-dependent sintering behavior was analyzed using a dilatometer (DIL-402C, Netzsch, Germany). The mixed powders were compacted into 12-mm-long cylinders, using a uniaxial press at 150 MPa. The thermal expansion of the pushrod was measured previously with nondoped Al2O3 samples. The linear shrinkage was measured while heating the prepared samples at 5 oC/min to 1600 oC, where the samples were held for 1 h. To determine the effect of the sintering tem- perature on the Al2O3 properties, additional specimens were heated at 5 oC/min and sintered at 1400 and 1500 oC for 1 h.
Sample density was measured using Archimedes’ principle. The microstructure was analyzed using a scanning electron microscope (SEM; JSM-6390, JSM-7500F, JEOL, Japan). The crystallinity was analyzed using an X-ray diffractometer (XRD; D/max-2500, RIGAKU, Japan) operating at 40-kV acceleration and 100 mA and with a scanning angle in the range 20-80o and a scanning rate of 10°/min. Sample hardness was measured under a load of 0.5 kg∙f, using a Vickers hardness tester (810-165K, Mitutoyo, Japan).
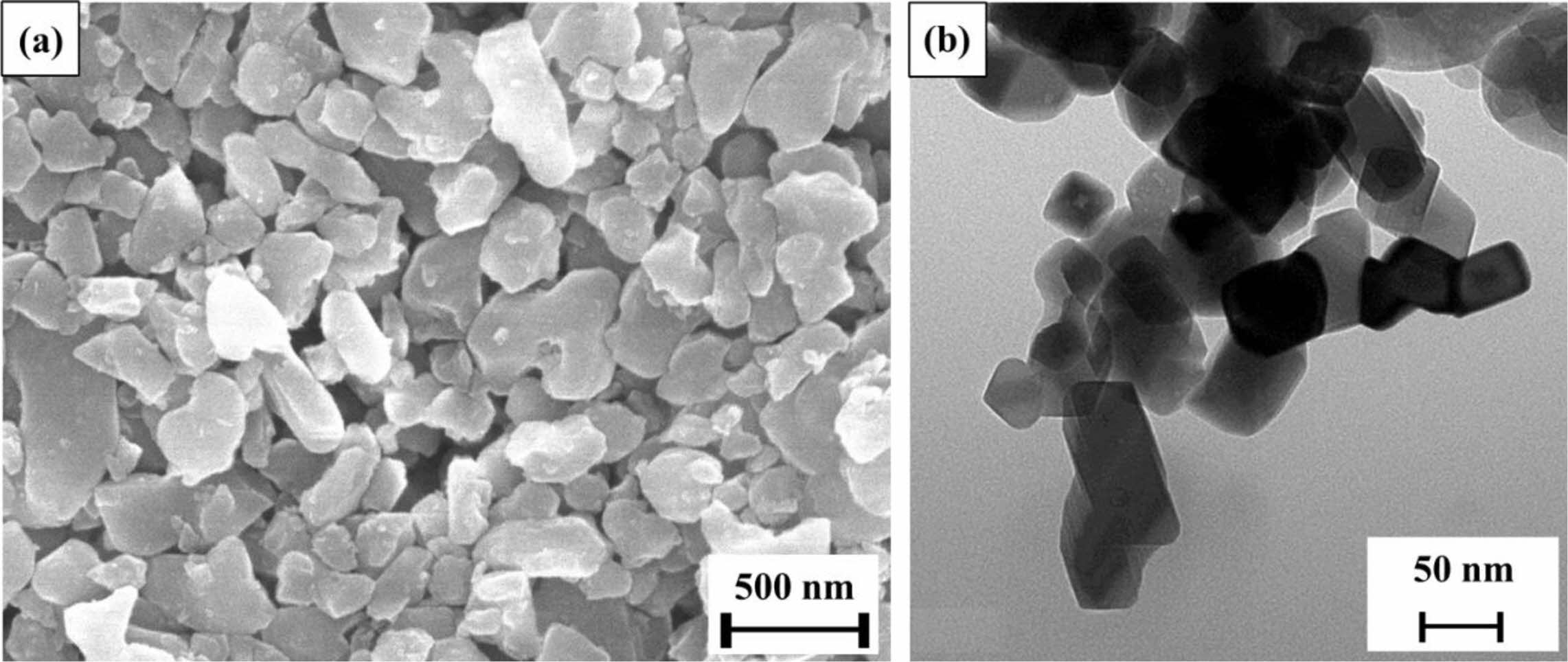
|
Fig. 1 FE-SEM image of (a) Al2O3 powder and (b) MCAS additive used in this study |
Dilatometer analysis
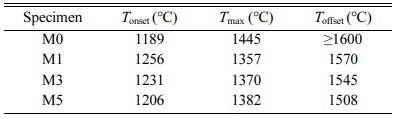
Fig. 2 presents the shrinkage behaviors of M0, M1, M3, and M5. Based on these results, the shrinkage onset and offset temperatures (Tonset and Toffset, respectively) and the maximum shrinkage temperature (Tmax), are summarized in Table 1. Fig. 2(a) shows that M0 ex- hibited a lower Tonset (1189 oC) than the other samples. However, M0 continued to shrink until 1600 oC, indi- cating slower densification over a wider temperature range. Additionally, as shown in the shrinkage-rate curves in Fig. 2(b), the maximum shrinkage rate of M0 was approximately 0.2 %/min, which is about half that of the MCAS-containing samples. For M1, M3, and M5, shrinkage started at a relatively high temperature but occurred rapidly over a narrow temperature range. These results are presumably related to the thermal expansion and melting of the MCAS additive. The MCAS used in this study exhibited a higher coefficient of thermal expansion than Al2O3. Moreover, MCAS reportedly melts at approximately 1050 oC [24]. Thus, the heating- and melting-induced volumetric expansion appears to have offset the shrinkage caused by Al2O3 sintering. However, the MCAS melted and formed a neck between the Al2O3 particles and accelerated sinter- ing. M1, M3, and M5 were completely sintered below 1600 oC. In addition, the higher the MCAS content, the lower the value of Toffset.
Sintering density
Fig. 3 shows the relative densities measured as per Archimedes’ principle for the samples sintered at 1400, 1500, and 1600 oC for 1 h. The relative densities of M0 sintered at 1400 and 1600 oC were 81 and < 95%, respectively. The relative densities of M1, M3, and M5 were significantly higher than this. The M5, M3, and M1 specimens sintered at 1400, 1500, and 1600 oC, respectively, were the densest. Thus, at higher sintering temperatures, less MCAS was required for densification presumably because melting-induced loss occurred when excess MCAS was used [25, 26].
XRD analysis
The XRD spectra and corresponding full width at half maximum (FWHM) of the samples prepared with different MCAS contents and sintered at different temperatures are shown in Fig. 4. Fig. 4(a) shows the XRD spectra of the specimens sintered at 1500 oC. No MCAS peak was observed in the spectra of these samples because MCAS is an amorphous substance unsuitable for XRD analysis. Fig. 4(b) presents the FWHM for the main peak of the (116) plane for M0 sintered at various temperatures. According to the Scherrer equation, FWHM is related to crystallinity [27]. Clearly, FWHM decreased with increasing sintering temperature, indicating that grain growth had occurred. Fig. 4(c) shows the FWHM for the (116) peak at each sintering temperature for the samples prepared with different MCAS contents. Although increasing the MCAS content did not result in a clear FWHM trend for the Al2O3 samples sintered at 1400 oC, the FWHM of the samples sintered at 1500 and 1600 oC decreased somewhat and considerably, respectively. Considering both the results shown in Fig. 4(c) and the shrinkage behavior shown in Fig. 2 suggests that grain growth did not occur at 1400 oC because shrinkage was incomplete. By contrast, at 1500 and 1600 oC, it appears that the addition of MCAS accelerated Al2O3-particle binding through liquid-phase formation and caused grain growth.
Microstructural analysis
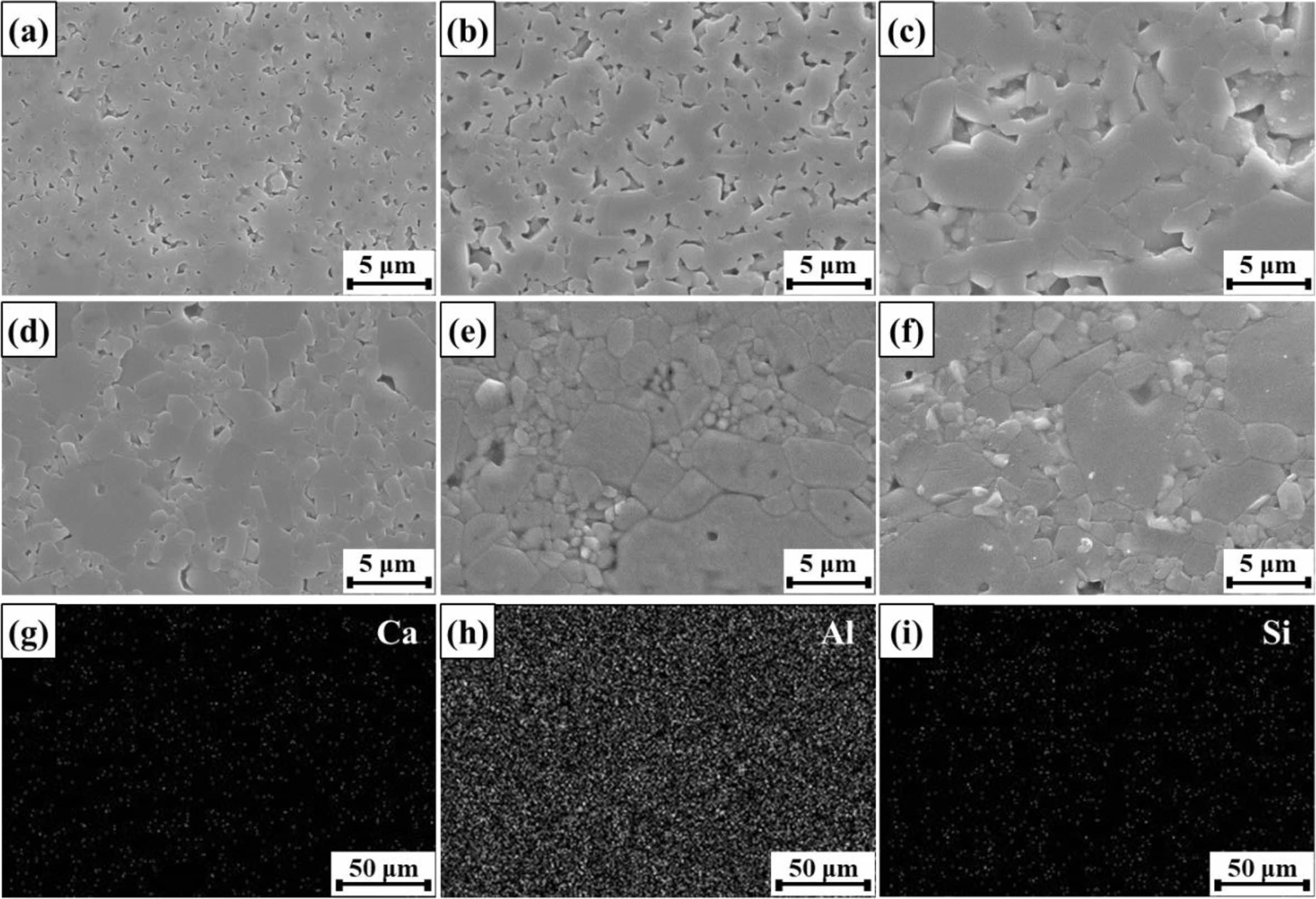
Fig. 5 shows polished cross-sectional images of sintered Al2O3. Fig. 5(a)-(c) presents microstructural images of M0 sintered at 1400, 1500, and 1600 oC, respectively. Like the raw material, the specimen sintered at 1400 oC exhibited particles several hundred nanometers in diameter. From the dilatometry results shown in Fig. 2, densification was incomplete at 1400 oC; thus, grain growth was not observed. In contrast, densification was complete above 1500 oC; thus, grain growth occurred, and grain size increased with increasing sintering temperature. The microstructures of M1, M3, and M5 sintered at 1500 oC are shown in Fig. 5(d), (e), and (f), respectively. Clearly, grain size increased with increasing MCAS content. MCAS has a low melting point (1050 oC), and its glass-transition temperature is approximately 670 oC [24]. Therefore, MCAS densified the Al2O3 particles below the M0 densification tem- perature and accelerated particle growth by facilitating the formation of necks between Al2O3 particles. The change in particle size observed through SEM was also consistent with the FWHM trend shown in Fig. 3. These results show that particle size can be controlled by adjusting both the sintering temperature and the MCAS content. Additionally, surface EDS analysis confirmed that individual MCAS elements were not segregated and were evenly distributed throughout the specimens, as shown in Fig. 5(g)-(i).
Hardness test
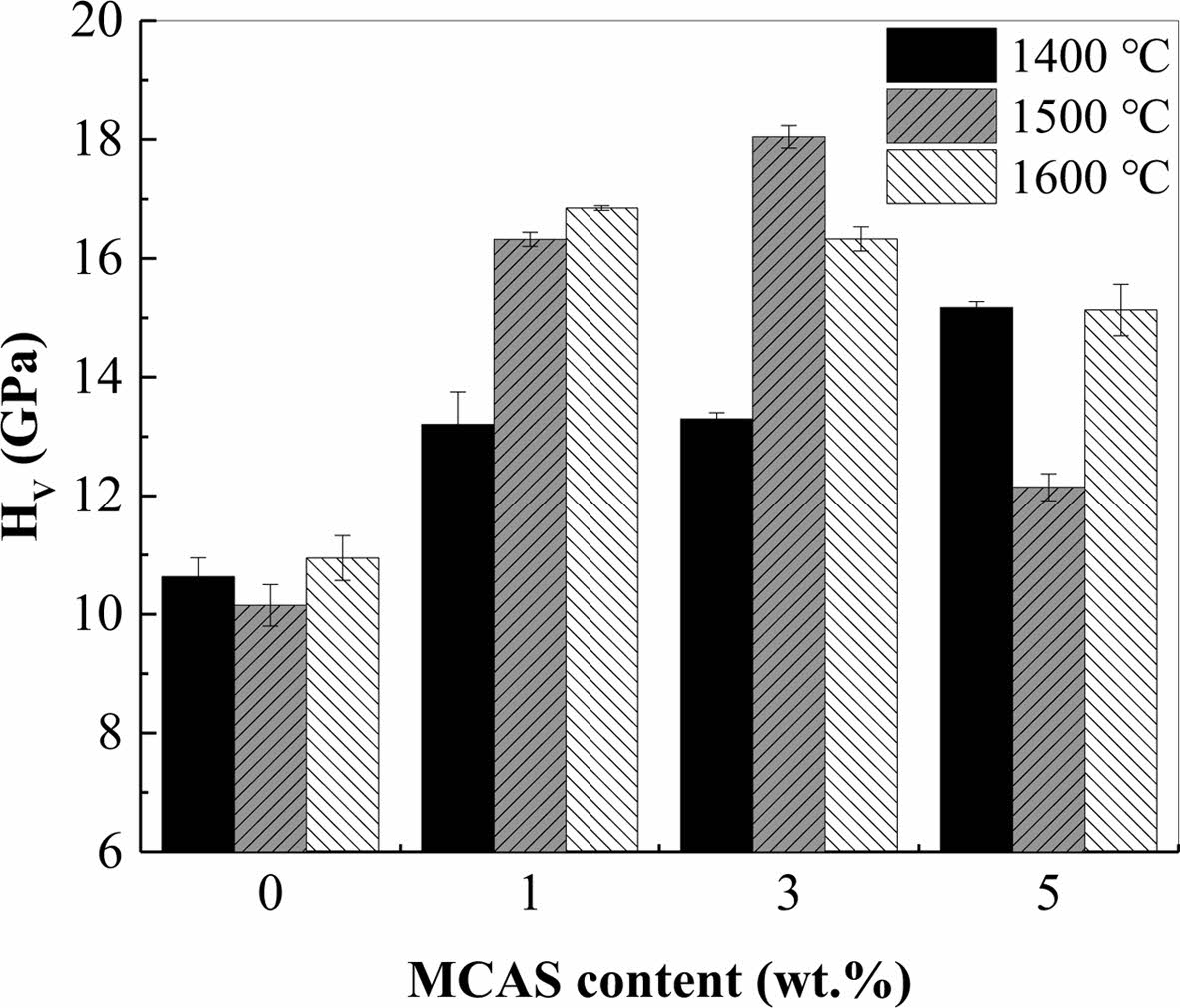
Fig. 6 shows the hardness of the Al2O3 bodies pre- pared with different MCAS contents and sintered at different temperatures. The measured hardness values showed a trend like that of the sintered-body densities, as shown in Fig. 4. For the specimen sintered at 1400 oC, the hardness was extremely low owing to the low sintering density, indicating low bonding strength between the Al2O3 particles. However, with increasing MCAS content, the bonding strength between the Al2O3 particles increased; therefore, hardness increased. For the specimens sintered at 1500 and 1600 oC, the increase in hardness was substantial. However, M5 sintered at these temperatures exhibited excessive grain growth, which decreased hardness [28]; therefore, the highest hardness of 18 GPa was achieved by M3 sintered at 1500 oC.
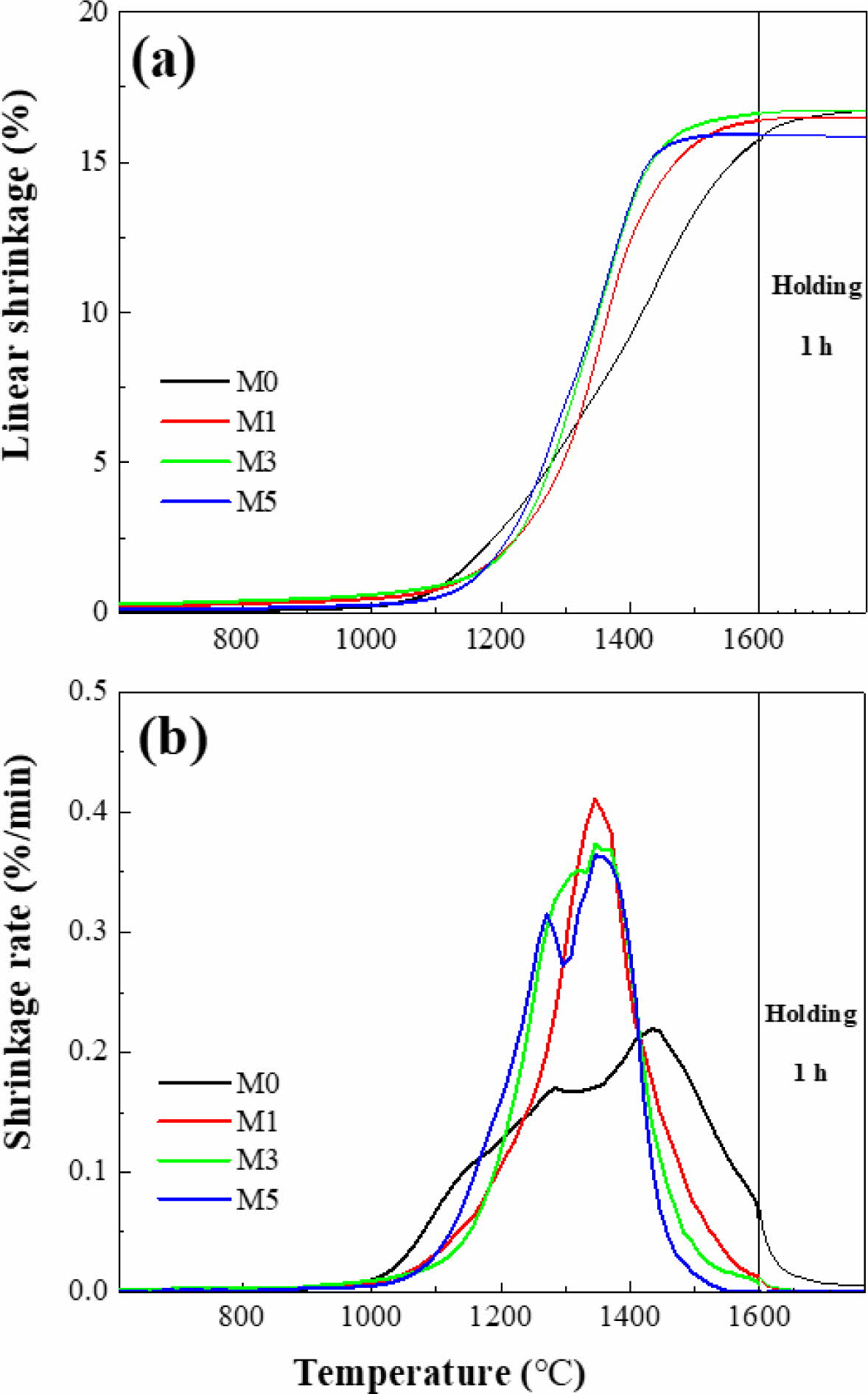
|
Fig. 2 Dilatometry analysis results for alumina with respect to MCAS content: (a) linear shrinkage and (b) shrinkage rate. |
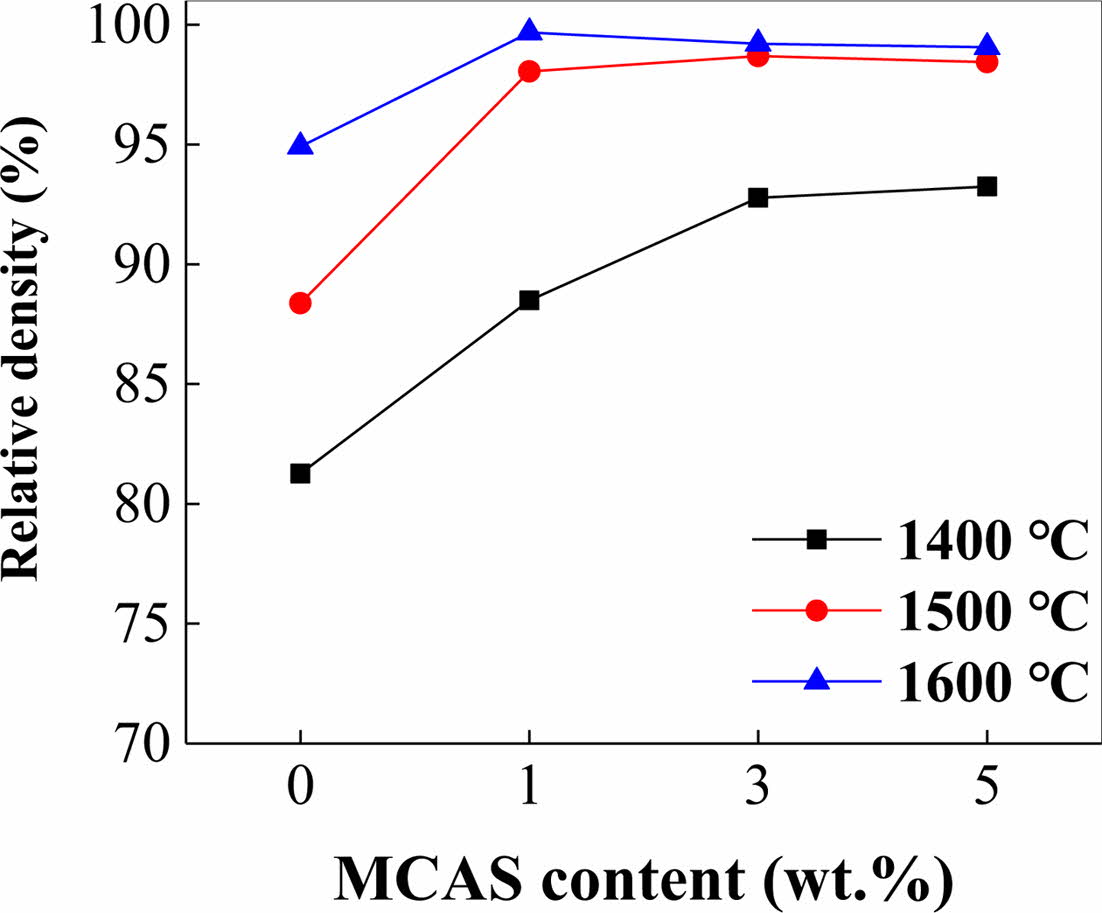
|
Fig. 3 Relative density of Al2O3 plotted as functions of MCAS content for different sintering temperatures. |
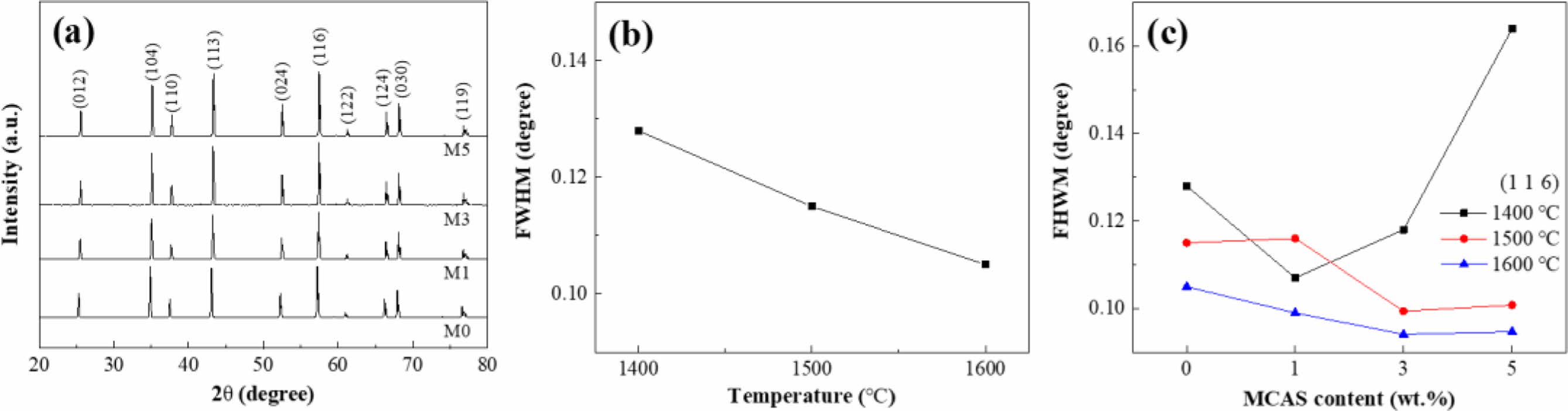
|
Fig. 4 (a) XRD spectra for M0, M1, M3, and M5 sintered at 1500 oC; FWHM for (116)-plane peak plotted as functions of (b) sintering temperature and (c) MCAS content. |
|
Fig. 5 Polished cross-sectional SEM images of M0 sintered at (a) 1400, (b) 1500, and (c) 1600 oC; polished cross-sectional SEM images of (d) M1, (e) M3, and (f) M5 sintered at 1500 oC; EDS maps of (g) Ca, (h) Al, and (i) Si for M5 sintered at 1500 oC. |
|
Fig. 6 Hardness of Al2O3 bodies prepared with different MCAS contents and sintered at different temperatures. |
This study investigated the effects of the sintering temperature and MCAS-additive content on Al2O3 sintering behavior. The main results are summarized as follows:
(1) Al2O3 sintering behavior was analyzed using a dilatometer, and the results showed that the addit- ion of MCAS accelerated densification, which was completed below 1600 oC.
(2) A high relative density of 98% was achieved by adding MCAS at a relatively low sintering tem- perature of 1500 oC.
(3) XRD and SEM analyses confirmed that the Al2O3 microstructure could be tailored by tuning both the sintering temperature and MCAS content.
These results will elucidate the conditions required to prepare high-density Al2O3 for practical applications.
This work was supported by the Korea Institute of Ceramics Engineering and Technology and the Ministry of Trade, Industry, and Energy (MOTIE), under Grant 20003891.
- 1. M.P. Harmer and R.J. Brook, J. Mater. Sci. 15 (1980) 3017-3024.
-

- 2. M.P. Harmer and R.J. Brook, J. Br. Ceram. Soc. 5 (1981) 147-148.
- 3. C. Nivot and F. Valdivieso, Ceram. Int. 34 (2008) 1595-1602.
-

- 4. K. Liu, Y. Shi, W. He, C. Li, Q. Wei, and J. Liu, Int. J. Adv. Manuf. Technol. 67 (2013) 2511-2519.
-

- 5. G. Maizza, S. Grasso, and Y. Sakka, J. Asian Ceram. Soc. 2[3] (2014) 215-222.
-

- 6. P. Guyot, G. Antou, N. Pradeilles, A. Weibel, M. Vandenhende, G. Chevallier, A. Peigney, C. Estournès, and A. Maître, Scr. Mater. 84-85 (2014) 35-38.
-

- 7. A. Moradkhani, H. Baharvandi, and A. Naserifar, J. Korean Ceram. Soc. 56 (2019) 256-268.
-

- 8. J. Song, Y. Liu, C. Pang, J. Zhang, L. Chen, X. Zhang, S. Guo, X. Wang, R. Wang, and A. Chen, J. Ceram. Process. Res. 19 (2018) 142-145.
- 9. K. Shigeno, H. Katsumura, H. Kagata, H. Asano, and O. Inoue, Ferroelectrics, 356 (2007) 189-196.
-

- 10. H. Unno, Y. Sato, S. Toh, N. Yoshinaga, and S. Matsumura, J. Electron Microsc. 59 (2010) S107-S115.
-

- 11. T.H. Byeon, H.S. Park, H. H. Shin, S. O. Yoon, and C.Y. Oh,, J. Korean Ceram. Soc. 47 (2010) 325-328.
-

- 12. I.J. Induja, P. Abhilash, S. Arun, K.P. Surendran, and M.T. Sebastian, Ceram. Int. 41 (2015) 13572-13581.
-

- 13. X. Luo, L. Ren, Y. Hu, Y. Xia, M. Xin, C. Zhang, and H. Zhou, Ceram. Int. 44 (2018) 6354-6361.
-

- 14. B.R. Cho, J.J. Lee, and S.K. Kang, J. Ceram. Process. Res. 10 (2009) 121-123.
- 15. W.R. Rao and I.B. Cutler, J. Am. Ceram. Soc. 56 (1973) 588-593.
-

- 16. J. Zhao and M.P. Harmer, J. Am. Ceram. Soc. 70 (1987) 860-866.
-

- 17. Z. Harun, N.F. Ismail, and N.A. Badarulzaman, Adv. Mat. Res. 488-489 (2012) 335-339.
-

- 18. S. Lei, H. Fan, and W. Chen, J. Alloys. Compd. 632 (2015) 78-86.
-

- 19. C.M. Cheng, C.F. Yang, S. H. Lo, and T.Y. Tseng, J. Eur. Ceram. Soc. 20 (2000) 1061-1067.
-

- 20. H.J. Lee, S.W. Kim, and S.S. Ryu, Int. J. Refract. Met. Hard Mater. 53 (2015) 46-50.
-

- 21. H.J. Lee, W.S. Cho, H.J, Kim, H.T. Kim, and S.S. Ryu, J. Korean Ceram. Soc. 54 (2017) 43-48.
-

- 22. O. Kaygili, F. Yakuphanoglu, and C. Tatar, J. Ceram. Process. Res. 17 (2016) 881-884.
- 23. H.J. Lee, W.S. Cho, H.J. Kim, W. Pan, M. Shahid, and S.S. Ryu, Electron. Mater. Lett. 12 (2016) 732-737.
-

- 24. K. Agersted and T. Balic-Zunic, Int. J. Appl. Ceram. Technol. 15 (2018) 255-266.
-

- 25. K. Chen, Y. Pu, N. Xu, and X. Luo, Electron. Mater. Lett. 23 (2012) 1599-1603.
-

- 26. S. Qi, H. Cheng, K. Yang, B. Song, S. Sun, and Y. Zhang, J. Mater. Sci.: Mater. Electron. 30 (2019) 6411-6418.
-

- 27. G.K. Williamson and W.H. Hall, Acta Metall. 1 (1953) 22-31.
-

- 28. G. Mata-Osoro, J.S. Moya, and C. Pecharroman, J. Eur. Ceram. Soc. 32 (2012) 2925-2933.
-

 This Article
This Article
-
2022; 23(2): 149-153
Published on Apr 30, 2022
- 10.36410/jcpr.2022.23.2.149
- Received on May 18, 2021
- Revised on Oct 5, 2021
- Accepted on Oct 9, 2021
 Services
Services
- Abstract
introduction
experimental method
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hyeondeok Jeong and Sung-Soo Ryu
-
Engineering Ceramic Center, Korea Institute of Ceramic Engineering and Technology, Icheon 17303, Republic of Korea
Tel : +82 31 645 1482 Fax: +82 31 645 1485 - E-mail: jhd526@kicet.re.kr (H. Jeong), ssryu@kicet.re.kr (






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.