- A study on particle morphology and sintering behavior of yttria nano powder according to PVA mixing ratio in polymer solution synthesis route
Dae-Seon Leea, Heon Kongb and Sang-Jin Leea,b,*
aDepartment of Advanced Materials Science and Engineering, Mokpo National University, Muan 58554, Republic of Korea
bResearch Institute of Ceramic Industry and Technology, Mokpo National University, Muan 58554, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
A porous and single-phase yttria powder was synthesized using PVA polymer solution method and a dense sintered yttria body was fabricated without adding a sintering aid. The aggregation of nanoparticles in the synthetic powder and the effect of nano pores on sintering behavior were investigated under variation of the PVA mixing ratio. The yttria nano-powder showed excellent sintering behavior, but the micropores in the grain of sintered body, which are caused by the influence of the nano pores in the synthetic powder, resulted in intragranular fracture behavior with lower sintered density. As a result of sintering the yttria powder synthesized at a mixing ratio of PVA 4:1 at 1650 oC for 2 h, a dense yttria sintered body having a relative density of about 98.7% was obtained. Unlike other specimens with different PVA mixing ratios, it showed intergranular fracture behavior with no pore on the fracture surface
Keywords: Yttria, Nano powder, PVA, Sintering, Fracture behavior, Microstructure
Yttria (Y2O3) is a useful ceramic material that has excellent chemical stability and heat resistance up to a high temperature of 1800 oC or more, because it has a very high melting temperature of 2425 oC, a monoclinic structure up to 2325 oC and no phase transition [1]. Due to these characteristics, yttria is used in a wide range of fields such as high-temperature corrosion-resistant substrate materials, nozzle materials for jet casting of molten metals, and furnace materials for melting highly reactive metals [2]. In addition, yttria is used as the material for the internal walls of semicon- ductor manufacturing equipment, as it has excellent stability at high temperature and a plasma resistance higher than that of Al2O3 [3]. Accordingly, yttria sintered body having a dense microstructure is required, but it is not possible to achieve densification at a temperature around 1600C without a special sintering aid [4-6], and a high sintering temperature of about 1800 oC is required under normal pressure [7]. However, recently, yttria sintered bodies have been manufacture in a temperature range of about 1600~1700 oC under atmospheric pressure using high-purity yttria powder [8].
Methods for synthesizing yttria include the hydro- thermal reaction method [9], the co-precipitation method [10], the spray pyrolysis method [11], the discharge plasma method [12], the microwave stimulation method [13], the sol-gel method [14], and the combustion syn- thesis method [15]. Each method has advantages and disadvantages. In the case of mechanical alloying method, contamination by impurities can easily occur. With the use of a vapor phase condensation reaction, precipitation is difficult because the precursor of the vapor phase must proceed at a high temperature, and long heat treatment time and washing time are required. The sol-gel method can synthesize relatively high-purity, ultra-fine yttria powder, but has a disadvantage of low yields [16, 17].
As one of the sol-gel methods, a PVA solution method applying Pechini process used in the past has been proposed. The Pechini process is one of the methods employing to manufacture fine ceramic powder, by which a chemically homogeneous and stable precursor can be obtained because the powder is produced from a sol containing the metal salt of the final oxide material [18]. Unlike the Pechini process, the PVA solution method has no chelation process, and the metal cations in the precursor sol are fixed only by the long polymer chain of PVA. This makes it possible to easily prepare a homogeneous and stable precursor with uniform dispersion. In addition, CO, CO2 and NOx gases derived from the PVA polymer and the nitrate salt causes many pores in the liquid precursor having a high viscosity, and thus a porous soft powder can be prepared. The pyrolysis of PVA during the calcination process enables powder synthesis at low temperature due to the charac- teristics of oxidation reactions accompanied by heat generation [19-22].
In this study, fine yttria powder with improved sinter- ability was synthesized using this PVA solution method, and the sintering behavior of the synthesized powder was investigated. Variations in particle shape according to the PVA mixing ratio were observed, and the effect of changes in the microstructure of particles on sintering behavior was investigated. Based on these results, we derived the optimal sintering conditions for densification of high-purity, single-phase, nano yttria powder prepared by the PVA solution method without an added sintering aid.
Synthesis and sintering of yttria powder
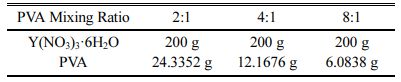
For the synthesis of fine yttria powder, yttrium nitrate (Y(NO3)3·6H2O, Sigma-Aldrich) and PVA (Polyvinyl alcohol, Sigma-Aldrich) having molecular weight of 146,000~186,000 were used and a 5 wt% PVA solution was prepared by dissolving 5 g of PVA polymer in 95 g of distilled water. Yttrium nitrate was completely dissolved in deionized water using a stirrer, and 5 wt% PVA solution was added in ratio of 2:1, 4:1 and 8:1 with respect to the total amount of metal cations, as shown in Table 1. Since 3+ yttrium ion has three times more electrons with respect to the -(OH) group obtained when PVA is dissolved, the actual ratio becomes 12:1 due to the difference in the amount of charge when added at a mol ratio of 4:1. Therefore, in order to match the ratio of 4:1, 5wt% PVA solution was added with a ratio of PVA : Y3+ = 4 : 3 considering that the mass of PVA monomer was 44 [4, 23]. For reference, in the case of 2:1, PVA : Y3+ = 2 : 3, where a larger amount of PVA is added. The reason why the valence ratio is important when PVA is added is that the amount of -(OH) of dissolved PVA affects the dispersion of the metal cations dissolved in the solution. The mixed solution of yttrium nitrate and 5wt% PVA was slowly stirred on a hot plate at 200 oC. When most of the moisture evaporated, yttrium nitrate was decomposed to generate NOx gas in the highly viscous mixed solution, and the precursor solution explosively expanded. After stirring sufficiently until no more gas was generated, it was dried for 12 h in a drying furnace at 200 oC. In order to remove the PVA organics in the completely dried precursor, calcination was performed at 600 oC for 1 h with a temperature increase rate of 3 oC/min. In order to eliminate agglomeration of the as-synthesized yttria powder, the synthesized yttria powder was mixed with DI water and ball milling was performed for several hours at a speed of 120 rpm using a medium of zirconia balls having a diameter of 5 mm. After milling, the dried yttria powder was uniaxially pressed using a press. The uniaxial pressing was performed using a 15 mm cylindrical mold with pressure of 200 kgf/cm2. The obtained molded body was sintered at 1650 oC for 2 h at a heating rate of 2 oC/min in an electric box furnace under atmospheric condition.
Characterization
For thermal analysis of the precursor to which PVA was added, the precursor was analyzed using a thermo- gravimetric differential thermal analyzer (TG-DTA, STA6000, PerkinElmer, USA) up to 900 oC at a heating rate of 10 oC/min in an air atmosphere. For the crystal- line phase analysis of synthesized yttria powder with different PVA mixing ratios, the powder was analyzed using X-ray Diffractometry (XRD, X’ pert-pre MPD, PAN alytical, Netherlands) at 40 Kv, 30 mA with a scan speed of 4o/min. To examine the microstructure of the synthesized powder and sintered body, the powder and fractured surfaces of the sintered body were coated with Pt and then analyzed by a field emission scanning electron microscope (FE-SEM: JSM-7100F, JEOL, Japan) and a transmission electron microscope (TEM: JEM-1010, JEOL, Japan). In addition, the specific surface area and the pore size of the synthesized powder were measured using an Automated Surface & Pore Size Analyzer (Quadrasorb SI-Kr, Quantachrome Instruments, USA). The green density of powder compact and sintering density were measured using the Archimedes method, where each specimen was measured three times and the average value was used as the density value.
|
Table 1 Relative Amount of Yttria Nitrate and PVA Polymer according to PVA Mixing Ratio |
As the PVA polymer mixing ratio varied, there was a large difference in the final powder properties. With increasing content of PVA, the volume expanded dramatically during the stirring process with heat, and the dried precursor had very light weight and soft powder shape. This is ascribed to the NOx gas being continuously generated from the metal salts until com- pletely dried in a mixed solution having high viscosity due to the addition of high content polymer [14]. Accordingly, many micropores were created in the microstructure of the precursor, and soft and porous precursor could be manufactured.
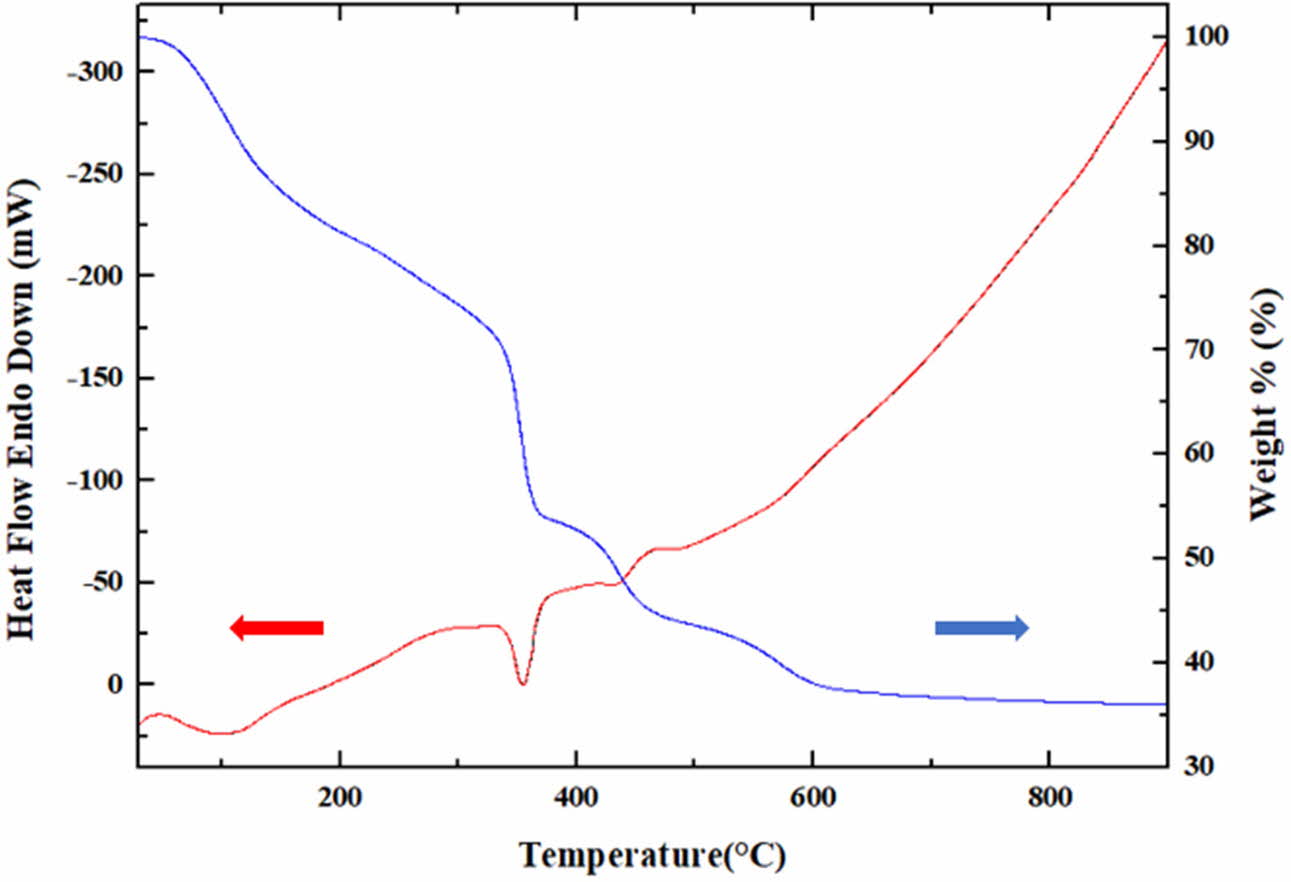
The dried precursor consists of residual NOx gas, PVA polymer and yttrium cations. Fig. 1 shows the results of thermal analysis of precursor with PVA added at a mixing ratio of 4:1. Upon increasing the temperature up to 300 oC, the weight gradually decreased due to the reduction of moisture and the evaporation of the residual NOx gas. A rapid weight loss is shown with endothermic reaction around 350 oC. It is estimated that this weight reduction is due to the pyrolysis of the PVA polymer. At this time, the color of the powder also turned to dark gray, and the PVA remained as carbon. Thereafter, the weight decreased with the exothermic reaction. This weight reduction occurs with the generation of CO2 by the oxidation of residual carbon. It was confirmed that the color of the powder became white again after 600 oC when the reaction was completed. Since the exothermic reaction is accompanied in the region where the oxidation reaction occurs, as described above, it is determined that the oxidation of the yttrium metal ions also occurs at the same time, and thus the yttria synthesis occurs.
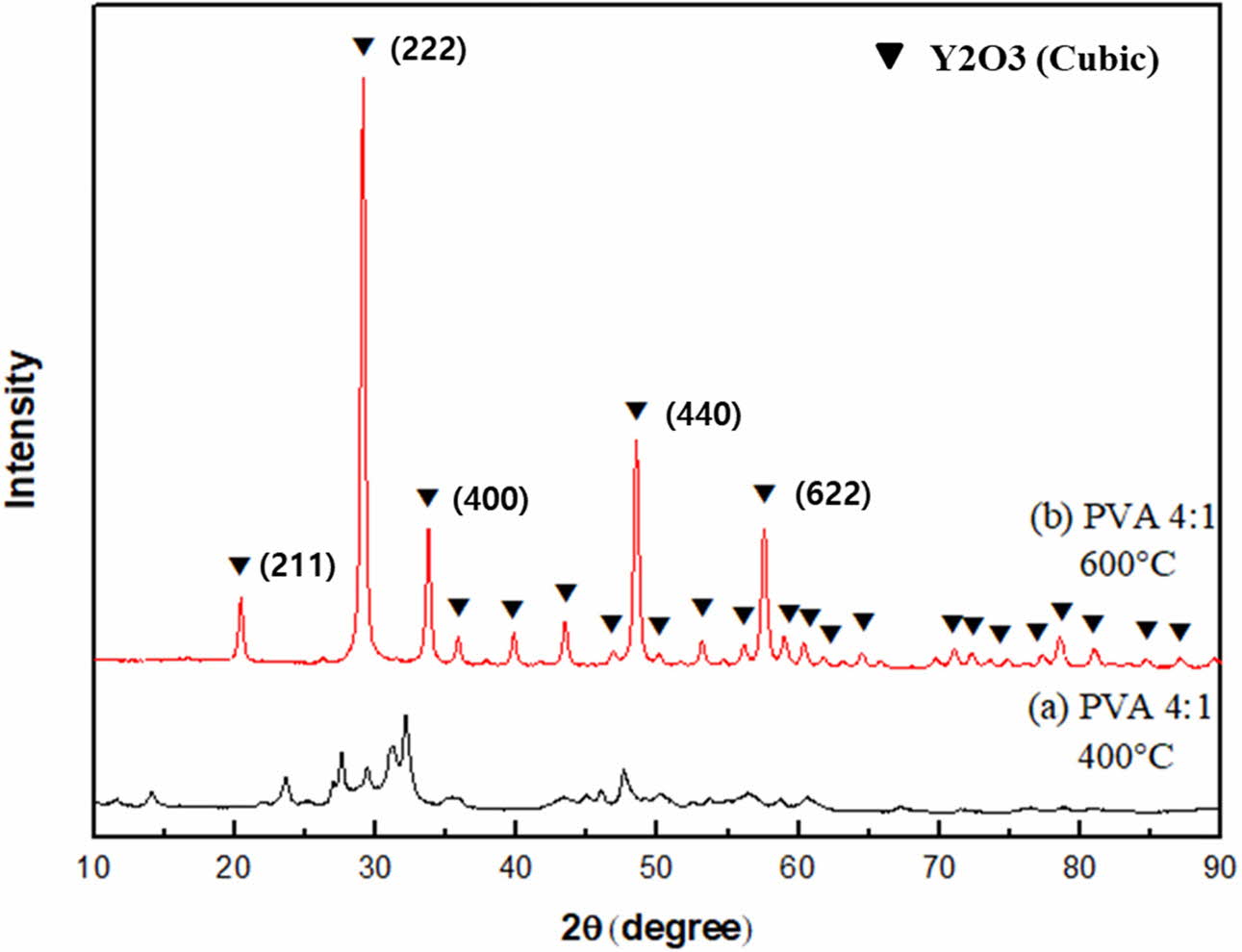
XRD analysis was performed to identify the crystalli- zation behavior according to the calcination temperature, as shown in Fig. 2. As a result of the heat treatment at 400 oC, a small amount of yttria crystal phase was observed, and it was also confirmed that other substances coexisted together. With heat treatment up to 600 oC, other substances disappeared and only yttria crystal phase remained. It was found that the yttria crystal phase developed rapidly after the exothermic reaction at around 500 oC.
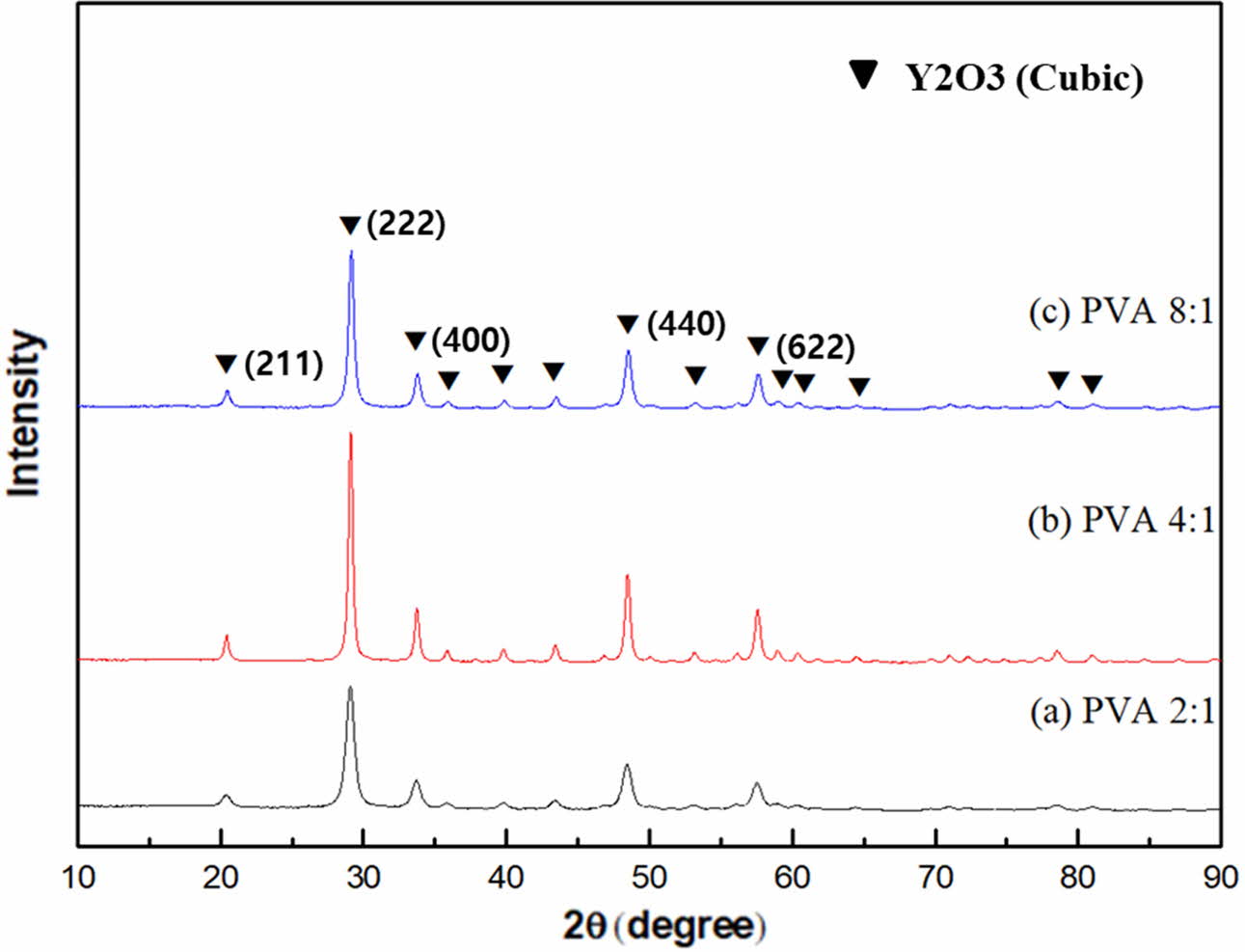
Fig. 3 shows the crystal phase of yttria powder according to the amount of PVA mixture. In the powders synthesized at 600 oC, only yttria peaks were observed. At the mixing ratio of PVA 4:1, the most well-developed yttria crystal phase peak was observed. It was thus found that the amount of polymer mixture influenced the oxidation of yttrium metal ions and the behavior of yttria crystal phase development [5]. It was estimated that the PVA mixing amount affected the dispersion of cations in the precursor sol and PVA polymer mixture: an appropriate amount optimized the dispersion of cations, and the growth of the crystalline phase then became more active during yttria synthesis. It was estimated that the amount of PVA mixed in the 4:1 ratio in this experiment was relatively optimized for cation dispersion among the three mixing ratios.
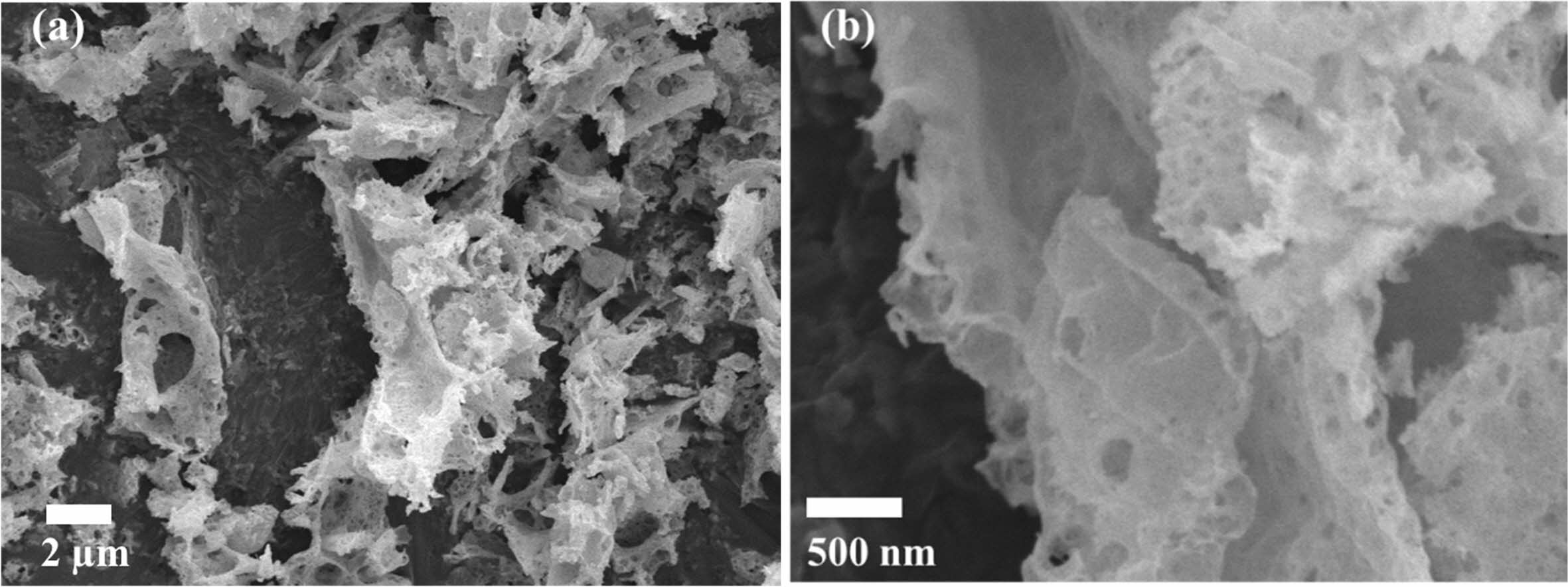
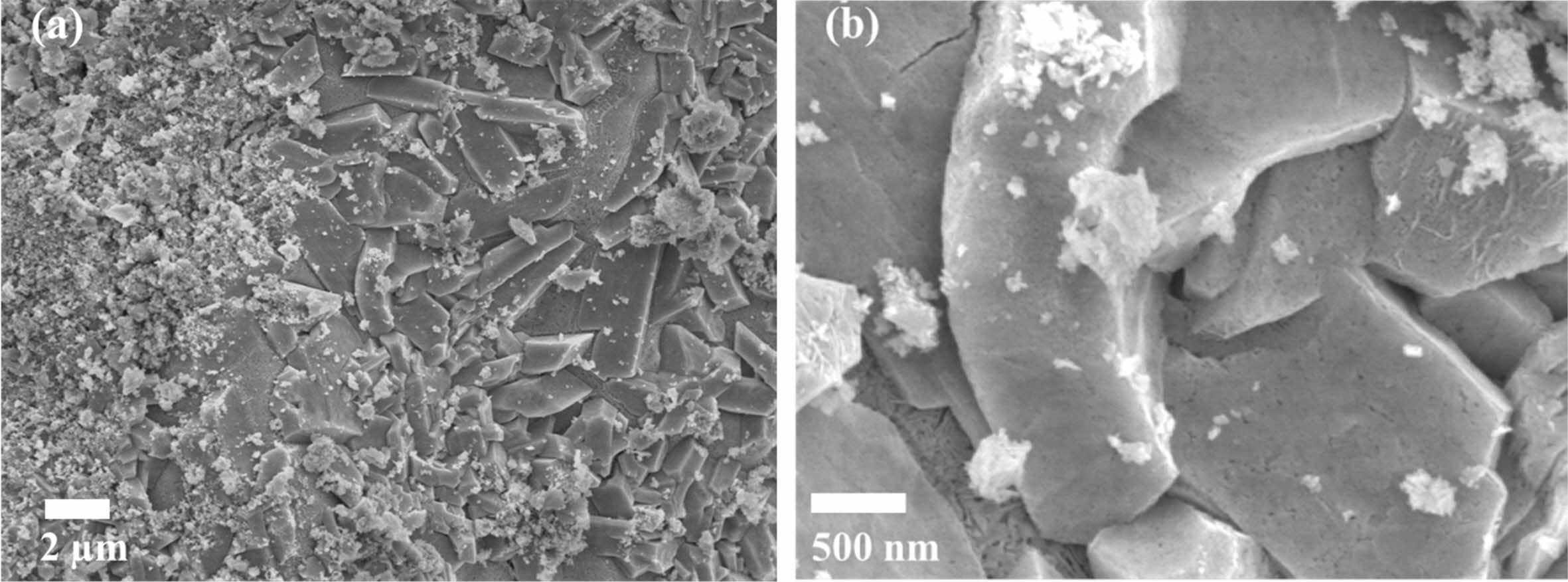
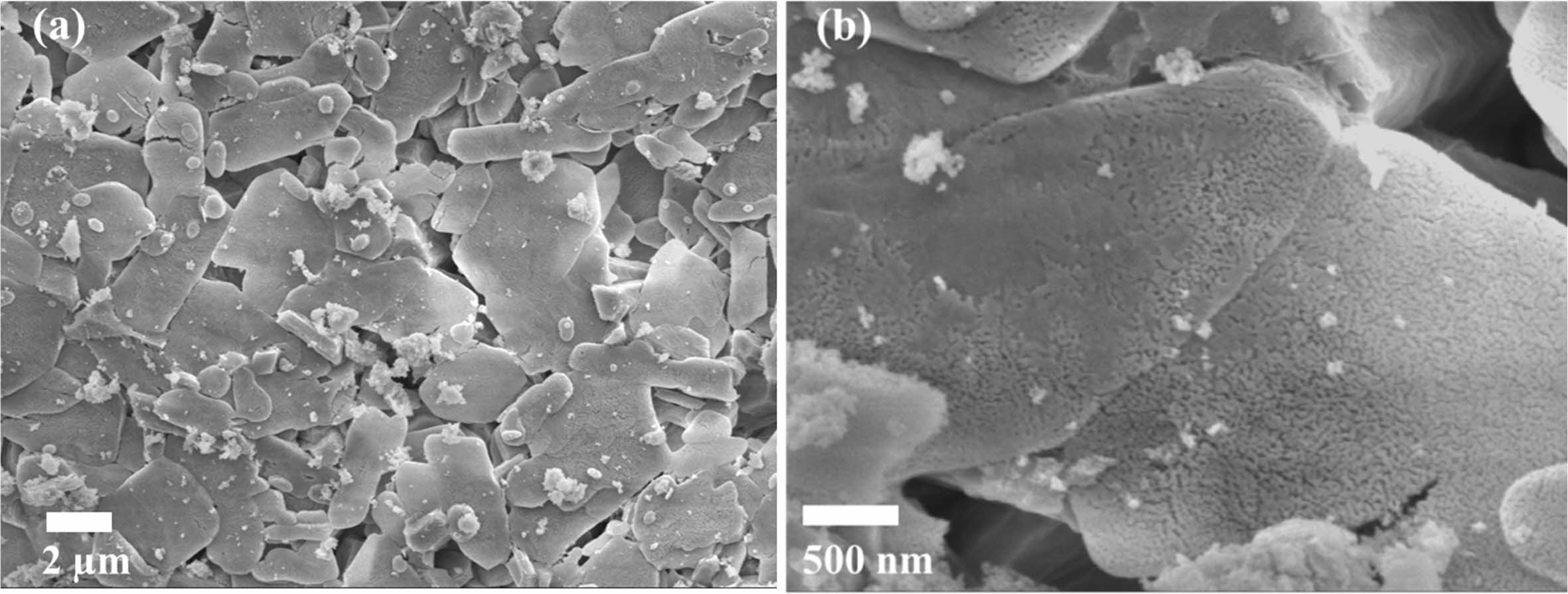
Fig. 4 ~ Fig. 6 show the microstructures of the yttria powder synthesized according to the various mixing amounts of PVA. The synthesized powders had a porous structure due to the generation of many voids by the NOx gas during drying process and the thermal decom- position and oxidation of PVA polymer during calcination process. It was found that there were differences in the size and the degree of aggregation of the particles, according to the PVA content. When PVA was added in a ratio of 2:1, a porous structure in which particles were connected in a mesh form was observed, and large and small pores existed between the particles. In the case of PVA 4:1 ratio, it was found that the particles agglomerated in the form of a plate together with the fine particles, and micropores existed inside the agglomerated particles, as seen in high magnifi- cation (Fig. 4(b)). At the ratio of 8:1 PVA, the smallest amount added, most particles had a more pronounced plate-like shape with strong aggregation. In the high magnification picture of the microstructure, many nano-sized pores were observed. In the case of the PVA 2:1 ratio, a porous microstructure due to the combustion of a large amount of PVA polymer was clearly observed, consistent with the results of Jung’s research [4].
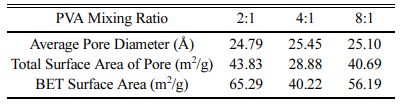
Table 2 shows the results of measuring the specific surface area of yttria agglomerated particles according to the various amount of PVA content, and the average pore size and total surface area of the pores in the yttria powder. For the BET specific surface area of the powder, the yttria powder prepared with a PVA 4:1 ratio showed the lowest value of 40.22 m2/g, that with a PVA 8:1 ratio showed 56.19 m2/g, and that with a PVA 2:1 ratio showed 65.29 m2/g. In general, a power synthesized from a precursor with a large amount of PVA mixture showed a more porous structure and had a higher specific surface area. It was reported that when the amount of PVA mixture is small or a PVA polymer is not used, the powder was seriously agglomerated and pores were not generated during the drying process or calcination process, and consequently the particle size was non-uniform and the specific surface area was small [25]. However, in this experiment, when the amount of PVA mixture was as small as 8:1, the powder showed a rather high specific surface area. It was estimated that this was related to the nano-sized micropores in the previously observed particles (Fig. 4~6), and hence the average pore size and the total surface area of the pores in the particles of each yttria powder were measured. The overall pore sizes varied from 24.79 Å to 25.45 Å, thus showing significant differences. As for the total surface areas of the pores, the case of PVA 2:1 ratio showed a value of 43.83 m2/g, the case of the PVA 4:1 ratio showed a value of 28.88 m2/g, and the PVA 8:1 ratio showed 40.69 m2/g. It was estimated that the nano-fine pores in the powder were greatly affected by the relative amount of metal cations and PVA polymer in the mixed solution. Accordingly, with better dispersion and homogeneity of yttrium cations by the appropriate amount of the polymer, a small amount of micropores is formed [4]. It was estimated that the nano-sized micropores were related to the aggregation between yttrium cations as well as to the aggregation of primary particles of yttria oxide during calcination. From these results, it was estimated that the case where the proper amount of PVA 4:1 ratio was effectively applied to the cation dispersion. Further, in the cases of 8:1 and 2:1 ratios, where insufficient or excessive amounts of PVA were added, the amount of nano-pores in the particle was relatively large.
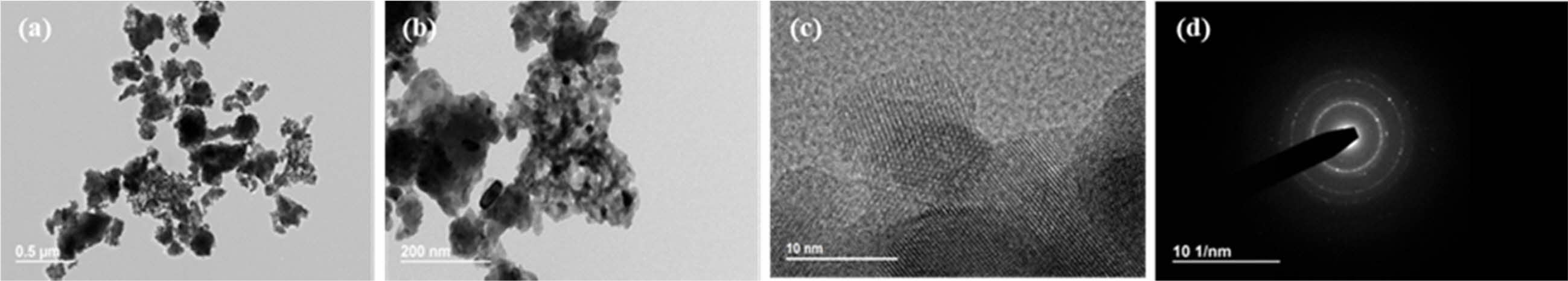
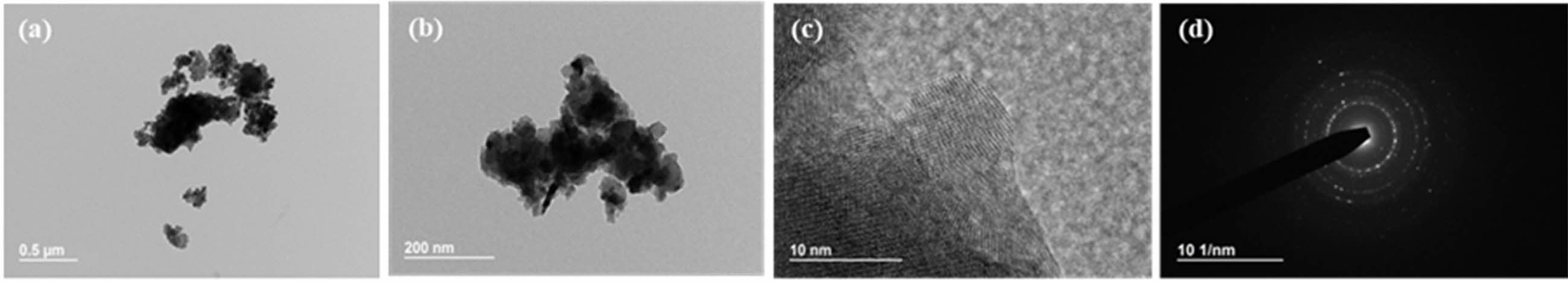
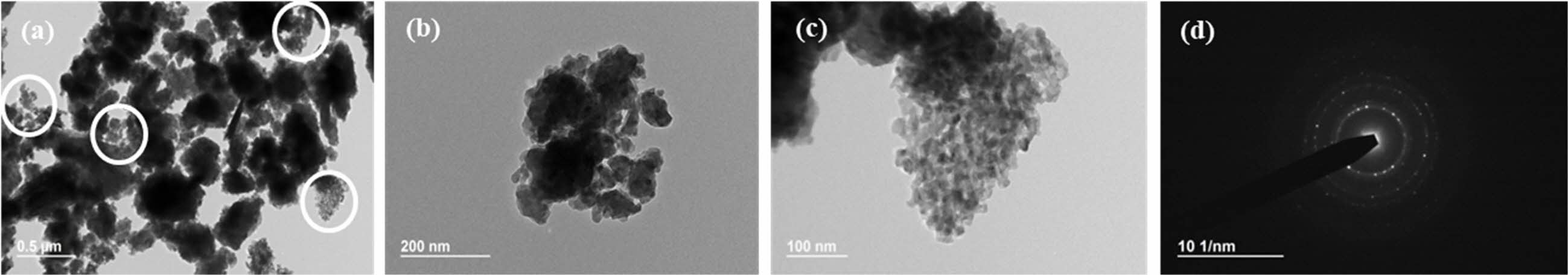
Fig. 7~Fig. 9 show TEM images of the yttria powder calcinated at 600 oC according to the amounts of PVA. It is seen that with lower PVA content, the grains of the particles were accordingly larger. The reason for this is that as the amount of PVA content increased, the agglomeration of particles by the polymer was minimized from the view-point of the macroscopic microstructure, and thus fine particles were formed [26]. These results may also be confirmed from the TEM diffraction pattern. It was found that when PVA was added in a ratio of 2:1, the particles were fine in nano size as a whole, and the diffraction results showed a ring pattern. As the amount of PVA mixture decreased, relatively large crystal grains were formed and spot patterns appeared in the ring pattern. Fig. 5 Fig. 8
For the shape and size analysis of the primary particles from TEM from a microscopic view-point, agglomeration of fine primary particles having size of about 10nm took place to a degree. Furthermore, many micropores were observed between these particles. In the PVA ratios of 2:1 and 8:1, considerable agglomeration of nano-particles was seen, and these micro-structures are shown in Fig. 7(b) and Fig. 9(c), respectively. In Fig. 9(a), the regions with nano-particles aggregation mixed with relatively large yttria grains are indicated by circular marks. The high specific surface area of the yttria powder at the 8:1 ratio of PVA amount was ascribed to the presence of a large number of these nanoparticle aggregations and thus the existence of nanopores. Such nano micropores are also shown in Fig. 6(b). In order to investigate the effect of the nano- pores on the sintering behavior of yttria, the micro- structure was observed after sintering in air at a temperature of 1650 oC for 2 h.
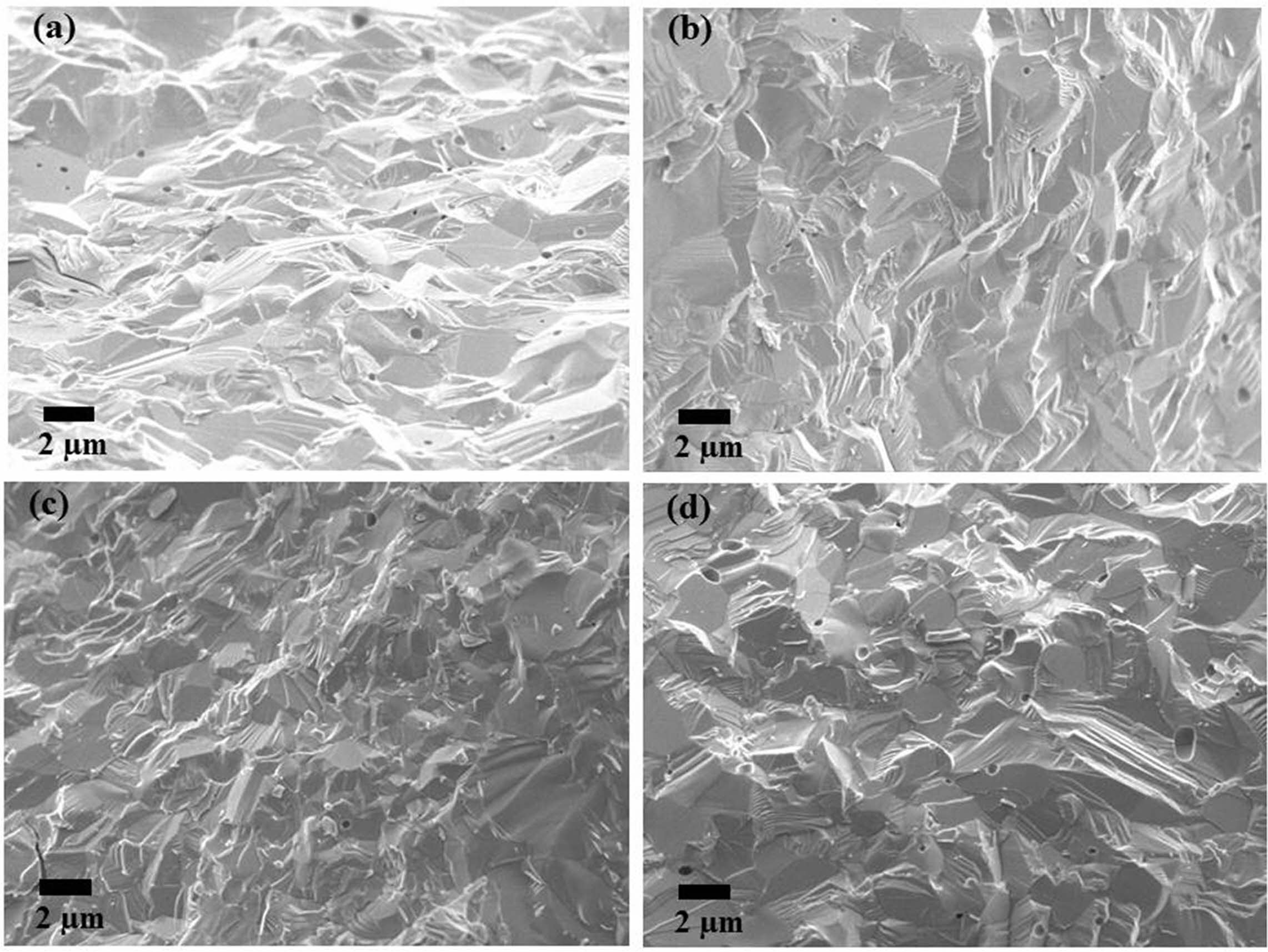
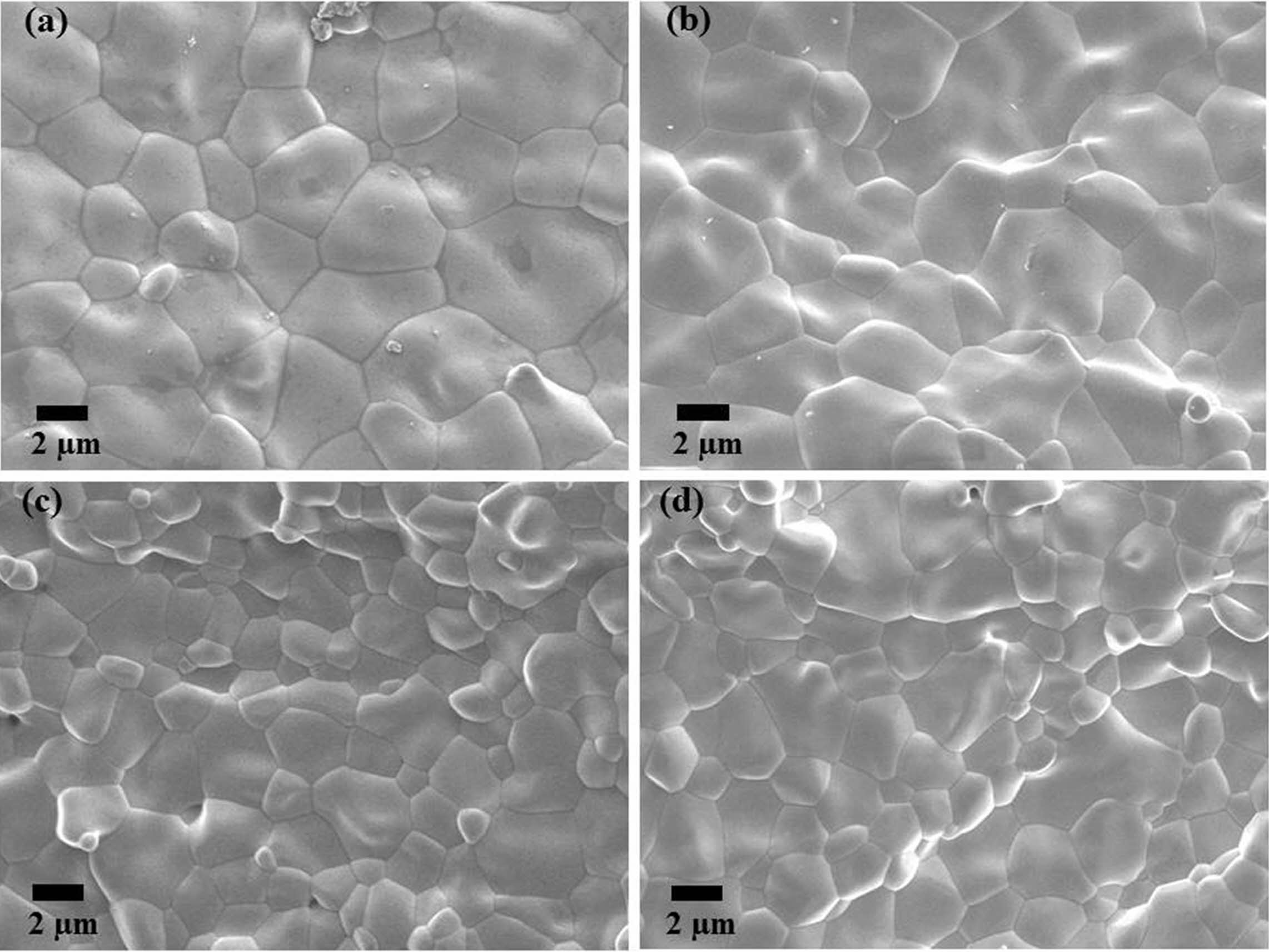
Fig. 10~Fig. 12 show the microstructures of the fracture surface of the sintered body of yttria powder synthesized according to the amount of PVA mixture. In each sample case, several areas of the fracture surface were indicated to ensure reliability of the fracture surface microstructure [27]. The most peculiar phenomenon is that intergranular fracture is only observed in a PVA ratio of 4:1, and relatively few pores were found (Fig. 11). On the contrary, in the microstructures of Fig. 10 and Fig. 12, it was found that intragranular fracture mainly occurred, and pores of about 0.5 mm in size were observed in the fracture surfaces. It was estimated that many pores existing between the nano-particles coalesced with each other during the sintering process and become large. They consequently could not be eliminated from the particles but existed as micropores inside the particles, which caused the intragranular fracture. On the contrary, in the case of PVA 4:1, it was shown that intergranular fracture mainly occurred, which means that there were few pores causing intra- granular fracture.
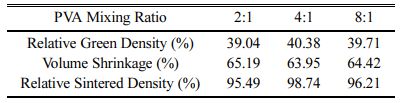
Table 3 shows the density of green body of each sample, as well as volume shrinkage and the relative density of the sintered body. The relative green densi- ties were similar. However, the sintered density was highest at the 4:1 ratio of PVA, followed by 8:1 and 2:1 ratios in order. These results are in good agreement with the microstructure analysis results of the sintered body. In all cases, since sintering between the nano-particles proceeded, the volumetric shrinkage rate showed a high rate of 60% or more.
|
Fig. 1 Simultaneous DTA/TG results of yttria precursor (PVA mixing ratio : 4:1). |
|
Fig. 2 XRD patterns of yttria precursor calcined at (a) 400 oC and (b) 600 oC (PVA mixing ratio of 4:1). |
|
Fig. 3 XRD patterns of yttria precursor calcined at 600 oC, prepared from different PVA mixing ratio of (a) 2:1, (b) 4:1, and (c) 8:1. |
|
Fig. 4 SEM micrographs of as-synthesized yttria powder calcined at 600 oC prepared from PVA mixing ratio of 2: 1. (a) low magnification, (b) high magnification. |
|
Fig. 5 SEM micrographs of as-synthesized yttria powder calcined at 600 oC prepared from PVA mixing ratio of 4: 1. (a) low magnification, (b) high magnification. |
|
Fig. 6 SEM micrographs of as-synthesized yttria powder calcined at 600 oC prepared from PVA mixing ratio of 8: 1. (a) low magnification, (b) high magnification. |
|
Fig. 7 TEM photographs of synthesized yttria powder calcined at 600 oC, prepared from PVA mixing ratio of 2:1. (a) low magnification, (b) high magnification, (c) high resolution, (d) SAD pattern image. |
|
Fig. 8 TEM photographs of synthesized yttria powder calcined at 600 oC, prepared from PVA mixing ratio of 4:1. (a) low magnification, (b) high magnification, (c) high resolution, (d) SAD pattern image. |
|
Fig. 9 TEM photographs of synthesized yttria powder calcined at 600 oC, prepared from PVA mixing ratio of 8:1. (a) low magnification, (b) high magnification, (c) high magnification of circle part of (a), (d) SAD pattern image. |
|
Fig. 10 SEM micrographs of sintered yttria fracture surfaces (PVA mixing ratio of 2:1). |
|
Fig. 11 SEM micrographs of sintered yttria fracture surfaces (PVA mixing ratio of 4:1). |
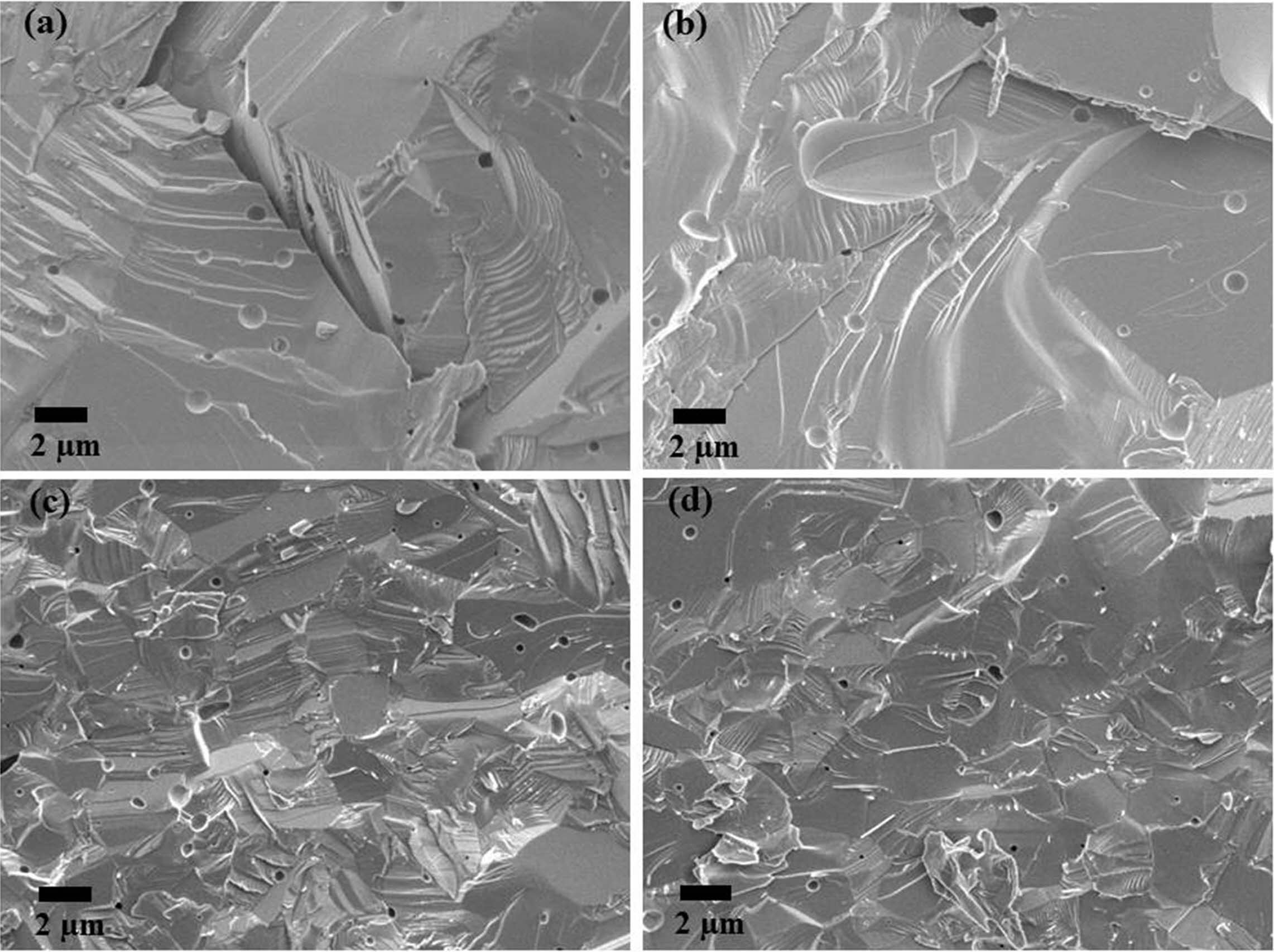
|
Fig. 12 SEM micrographs of sintered yttria fracture surfaces (PVA mixing ratio of 8:1). |
|
Table 2 Average Pore Diameter in Yttria Particles and BET Surface Area of Yttria Powder Synthesized with Different PVA Mixing Ratio |
|
Table 3 Relative Green Density of Yttria Powder Compacts and Volume Shrinkage and Relative Sintered Density of Sintered Yttria Samples at Different PVA Mixing Ratio |
The porous and nano-sized yttria powders, which have a specific surface area of 40~60 m2/g, was synthe- sized by PVA solution method. The nano-sized aggregated particles and the nano-pores in the green body affected the densification behavior. At the 4:1 mixing ratio of PVA, the yttria powder having a relatively smaller surface area of micropores was obtained due to a relatively homogeneous precursor and less aggregation of nano-particles. As a result, it showed the most developed yttria crystallinity and best sintering behavior. When a large number of nano-pores were included with the aggregated nano-particles in the 2:1 and 8:1 mixing ratio of PVA, the closed pores in the sintered grains were observed and the sintered sample showed intragranular fracture behavior. Finally, a fabrication method for denser yttria sintered body without a sintering aid is suggested in this research with a convenient process of sintering at below 1700 oC for short time under atmosphere condition.
- 1. J. S. Choi, T. Nakayama, and W. T. Bae, J. Korean Ceram. Soc. 50[5] (2013) 348-352.
-

- 2. T.Y. Zhu, and J.F. Xia, Key Eng. Mater. 726 (2017) 179-183.
-

- 3. S.K. Rha, M.J. Lee, and Y.S. Lee, J. Korean Ceram. Soc. 57[3] (2020) 338-344.
-

- 4. C.H. Jung, J.S. Jang, and S.J. Lee, Met. Mater. Int. 17[3] (2011) 451-455.
-

- 5. S.J. Lee, and C.H. Jung, J. Nanosci. Nanotechnol. 12[1] (2012) 800-805.
-

- 6. J.H. Han, J. Korean Ceram. Soc. 34[12] (1997) 1247-1253.
- 7. Y.G. Yang, J.Y. Kwak, H. Kong, and S.J. Lee, J. Ceram. Process. Res. 21[4] (2020) 450-455
-

- 8. A.L. Micheli, D.F. Dungan, and J.V. Mantese, J. Am. Ceram. Soc. 75[3] (1992) 709-711.
-

- 9. Y. Liu, J. Song, L. Chen, H. Luo, G. Lin, P. Chen, C. Wei, and J. Liu, J. Ceram. Process. Res. 21[4] (2020) 488-494.
-

- 10. J. Hu, X. Tian, C. Hu, Y. Luo, H. Peng, and J. Luo, J. Cream. Process. Res. 17[1] (2016) 1181-1187.
- 11. H.J. Lee, S.K. Hong, D.S. Jung, Y.C. Kang, and K.Y. Jung, Korean Chem. Eng. Res. 43[3] (2005) 407-411.
- 12. M. Suarez, A. Fernandez, J.L. Menendez, and R. Torrecillas, J. Alloys Compd. 493[1-2] (2010) 391-395.
-

- 13. M.L. Saladino, G. Nasillo, D.C. Martino, and E. Caponetti, J. Alloys. Compd. 491[1-2] (2010) 737-741.
-

- 14. L. Gao, Y. Zhang, X. Yang, Y.B. He, and L.H. Song, J. Ceram. Process. Res. 21[6] (2020) 615-621.
-

- 15. S.K. Lee, and H.W. Choi, J. Korean Inst. Electr. Electron. Mater. Eng. 20[6] (2007) 536-540.
- 16. B.Y. Son and M.E. Jung, Korean J. Mater. Res. 21[8] (2011) 444-449.
-

- 17. F.M.B. Marques and G. P. Wirtz, J. Am. Ceram. Soc. 74[3] (1991) 598-605.
-

- 18. S.W. Yun, Y.J. Shim, and S.G. Cho, J. Korean. Ceram. Soc. 35[5] (1998) 498-504.
- 19. S.J. Lee and W.M. Kriven, J. Am. Ceram. Soc. 81[10] (1998) 2605-2612.
-

- 20. M.A. Gulgun, M.H. Nguyen, and W.M. Kriven, J. Am. Ceram. Soc. 82[3] (1999) 556-560.
-

- 21. S.J. Lee and C.H. Lee, J. Korean. Ceram. Soc. 39[4] (2002) 336-340.
-

- 22. S.J. Lee and W.M. Kriven, Ceram. Eng. Sci. Proc. 19[4] (1998) 469-476.
-

- 23. Y.H. Kim and S.J. Lee, J. Kor. Powder. Metall. Inst. 20[1] (2013) 37-42.
-

- 24. S.J. Lee, S.Y. Chun, C.H. Lee, and Y.S. Yoon, J. Nanosci. Nanotechnol. 6[11] (2006) 3633-3636.
-

- 25. D.C. Moon, K.H. Lee, C.S. Kim, and M.R. Kim, Anal. Sci. Technol. 13[1] (2000).
- 26. H.Y. Min and S.J. Lee, Korean J. Met. Mater. 52[5] (2012) 335-342.
-

- 27. Y. Liu, X. Yang, K. Peng, Q. Wang, J. Huang, Z. Zhang, J. Lua, H. Xu, J. Song, and L. Chen, J. Ceram. Process. Res. 20[4] (2019) 436-441.
 This Article
This Article
-
2022; 23(2): 137-144
Published on Apr 30, 2022
- 10.36410/jcpr.2022.23.2.137
- Received on May 5, 2021
- Revised on Aug 22, 2021
- Accepted on Aug 28, 2021
 Services
Services
Shared
 Correspondence to
Correspondence to
- Sang-Jin Lee
-
aDepartment of Advanced Materials Science and Engineering, Mokpo National University, Muan 58554, Republic of Korea
bResearch Institute of Ceramic Industry and Technology, Mokpo National University, Muan 58554, Republic of Korea
Tel : +82-61-450-2493 Fax: +82-61-450-2498 - E-mail: lee@mokpo.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.