- Mechanical properties of geopolymers after immersion
Yootaek Kim* and Hakmin Kim
Materials Engineering Department, Kyonggi University, Suwon, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Herein, the variation in the mechanical properties of lightweight geopolymers, which contain integrated gasification combined cycle slag and Si sludge, were investigated by varying the curing conditions and introducing an additional drying process after immersion. The changes in the compressive strength and dentable characteristics were monitored for various samples corresponding to different curing conditions, immersion periods, and an optional post-immersion drying process. The microscopic morphology of the geopolymers was observed before and after immersion to investigate the enhancement in the mechanical properties of the geopolymers. The oven-cured specimens exhibited dentable characteristics, whereas the autoclaved specimens exhibited abrupt breakdown characteristics without any dentable characteristics. Further, the dentable characteristics observed in the specimens immersed for 3 days disappeared when the specimens were immersed for 7 days. The autoclaved specimens showed little difference in the compressive strength profile after 3 and 7 days of immersion; this stability in water indicates that the geopolymerization reaction was complete in the autoclaved specimens. In addition, after an additional oven drying process was used, a substantial increase in the compressive strength (from 4.1 to 7.1 MPa) was observed. It is speculated that the additional drying process promotes geopolymerization, which completes the reaction during the 1 day process
Keywords: Geopolymer, IGCC slag, Si sludge, Immersion, Curing, Aging
The importance of environmental protection and the reduction in atmospheric carbon dioxide have attracted significant attention in recent years. Therefore, the sub- stitution of conventional cement with eco-friendly materials can have considerable environmental benefits.
The integrated gasification combined cycle (IGCC) process has attracted considerable attention not only for eco-friendly power generation but also for the production of synthetic natural gas [1]. IGCC is an innovative power generation method that is used in conventional coal thermal power plants, chemical plants, and combined cycle power plants. Although IGCC plants have higher efficiency and lower emissions than the conventional coal thermal power plants, they produce considerable amounts of solid waste. For example, 165,000 tons of slag are produced annually as the solid byproduct at the Taean power plant in Korea. This slag is recovered without any recycling because no viable recycling technology has been developed to date, in spite of the research effort in this field. Therefore, the development of a safe, efficient, and large-scale recycling technology for IGCC slag is crucial [2-4].
Si sludge is an industrial byproduct that is produced during the cutting and polishing of single-crystal or polycrystalline Si ingots and/or wafers. Si reacts with alkaline activators, mostly used in the manufacture of geopolymers, to produce hydrogen gas. Therefore, Si sludge can be used as a bloating material to form pores in geopolymers. In this study, IGCC slag and Si sludge were used as environmentally friendly raw materials for geopolymers with the aim of replacing Portland cement, which requires a large amount of energy and produces a large amount of carbon dioxide during its production [5, 6].
When more than 10 wt.% of Si sludge is used for obtaining lightweight geopolymers, dissolution takes place upon immersion, if the curing period after pro- duction is not sufficient [7]. Therefore, it is necessary to overcome the problem of dissolution during or after the immersion of geopolymers containing a higher fraction of Si sludge than normally used for the reliable application of geopolymers as construction materials.
An inorganic material was developed by reacting aluminosilicate with an alkaline activator for polymeri- zation [8]; this inorganic material is known as a geo- polymer because it has a three-dimensional structure similar to that of a polymer. Geopolymers consist of an aluminosilicate gel network including SiO4 connected to tetrahedral AlO4; therefore, the quantities of SiO2 and Al2O3 significantly affect the properties of the geopolymer [9-11].
In our previous study, a geopolymer specimen, which was not cured, dissolved within a few minutes upon immersion in water, and a large number of bubbles were observed because unreacted Si sludge reacted with water and produced hydrogen gas. In addition, geopolymers that were cured for less than 14 days dissolved in water when they were immersed for 1 day, and their compressive strength reached only 63% compared to that of the non-immersed specimens. The geopolymers that were cured for more than 21 days did not dissolve in water during the 1-day immersion period, and their compressive strength was approximately the same as that of the non-immersed specimens [12].
In this study, the effects of the curing and aging on the mechanical properties of lightweight geopolymers during and/or after immersion were studied. The com- pressive strength and dentable characteristics were monitored by changing the immersion period and intro- ducing an additional drying process after immersion. To investigate the enhancement in the mechanical pro- perties of the geopolymers after immersion, microscopic observations of the geopolymers’ morphologies before and after immersion, as well as X-ray diffraction (XRD) analyses, were carried out.
The as-received IGCC slag was dry milled to an average particle size of 128 µm using a planetary mill or ball mill operating at 117 rpm. In addition, the as-received IGCC slag and Si sludge were analyzed through XRD (Rigaku MiniFlex2 with Cu Kα radiation at 40 kV and 40 mA) to identify the phases.
The dissolution level and the degree of polymeriza- tion of the starting material are known to change during geopolymerization depending on the type and molar concentration of the alkali solution. Herein, the mixed alkaline activator was prepared using a sodium silicate solution (Na2SiO3nH2O; Daejung Co.) and 98.0% NaOH solution. The mixing ratio was 1:1, and this ratio was maintained throughout the study. The concentration of the mixed alkaline activator was maintained at 15 M.
Geopolymers were prepared using IGCC fused slag and Si sludge. When the bloating reaction of the Si sludge with the alkaline activator takes place, the hydrogen gas produced during the reaction forms pores in the geopolymer paste during the molding process [13]. The Gibbs free energy of the bloating reaction (858.23 kJ mol−1) is lower than that of the geopolymeri- zation reaction, and thus, pores are formed in the matrix of the geopolymer before the completion of the geopolymerization reaction [14].
Generally, only a small amount of Si sludge (usually less than 5 wt.%) is added to produce lightweight geo- polymers because the excess Si component does not participate in the geopolymerization reaction and remains in an unreacted state [15]. The unreacted Si component then dissolves in water when the geopolymer is immersed for long time periods. Consequently, the Si content in the geopolymer was fixed at 0.5 wt.% throughout this study, unless otherwise mentioned.
Herein, 15 moles per liter of mixed alkaline activator, 0.5 wt.% of Si, and water/solid ratio of 0.2 were used for the experiments. The IGCC fused slag, Si sludge, and mixed alkaline activator were added and mixed in a bowl. The mixture was then transferred into a brass cube mold (50 mm × 50 mm × 50 mm) to determine the compressive strength. A demolding aid (form oil) was applied to the interior of the mold before trans- ferring the mixture to ensure the smooth removal of form. Curing was performed in a dryer at 100 oC and 100% relative humidity for 1 day. To prevent the generation of cracks caused by temperature-induced rapid moisture evaporation, curing was conducted in a polyethylene zipper bag. After oven curing, the specimens were immersed in distilled water (20 oC) for 3 and 7 days to observe the dissolution phenomenon. The change in pH according to the immersion time was monitored to determine the degree of geopolymerization. The specimens were also analyzed by Fourier transform infrared (FT-IR) spectroscopy, optical microscopy (CamscopeTM), and universal testing machine (UTM). Herein, all the data points in the figures represent the average value of the measurements obtained from three specimens.
Compressive strength
Immersion for 3 days
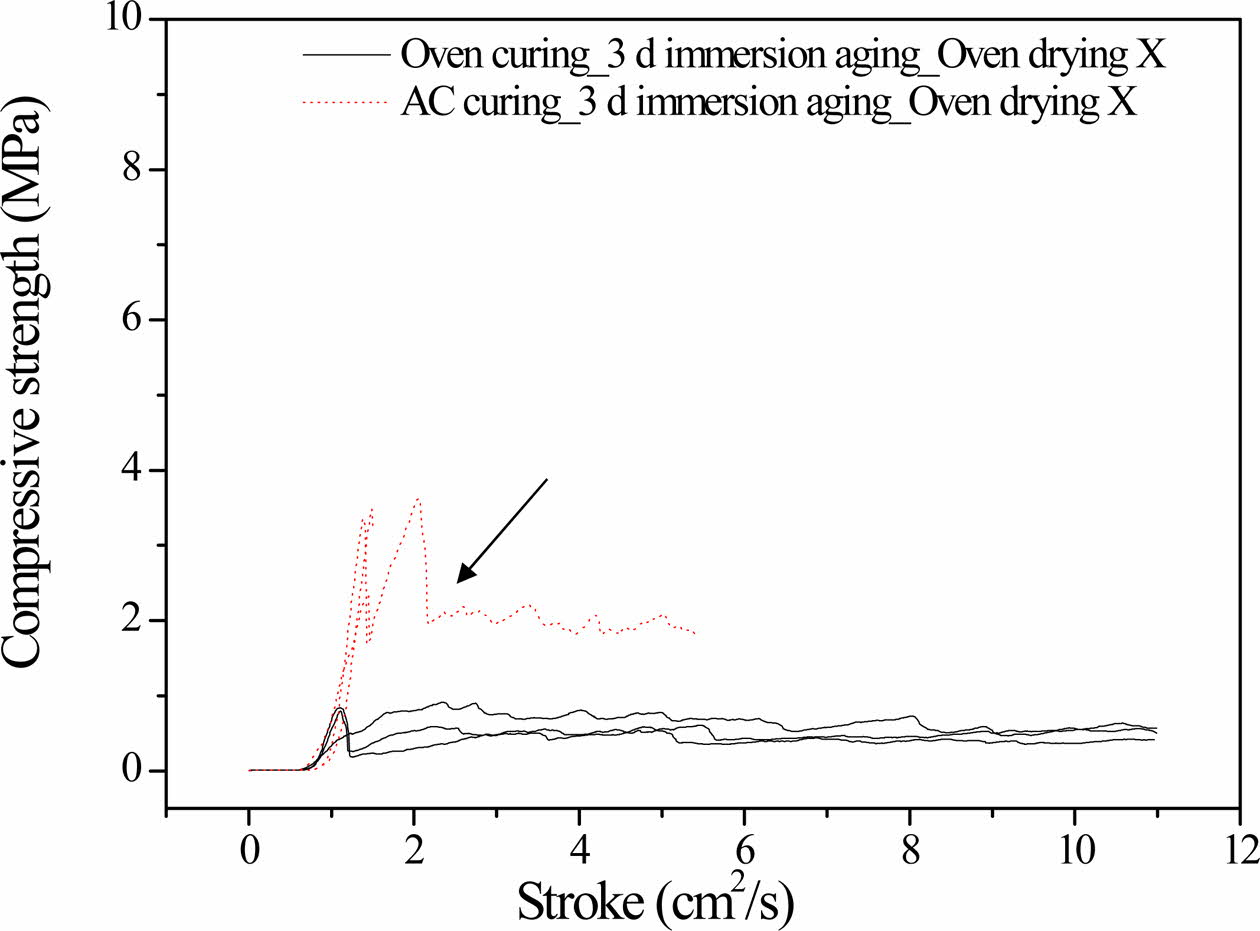
All the specimens used herein were either oven-cured at 100 oC for 1 day or autoclaved at 103 oC for 1 day. Thereafter, the cured specimens were immersed for 3 days in distilled water at 20 oC.
Fig. 1 shows the compressive strength profiles of the specimens as functions of time. The maximum com- pressive strength of the autoclaved specimen was four times higher than that of the oven-cured specimen. The oven-cured specimen exhibited dentable characteristics, as there was no abrupt drop in the compressive strength (breakdown in the solid lines). In contrast, the autoclaved specimen exhibited abrupt breakdown characteristics at the beginning of the compressive strength test; therefore, it did not exhibit dentable characteristics. However, the compressive strength was maintained at 2 MPa after the fractural breakdown point, as indicated by the arrow in Fig. 1.
Immersion for 7 days
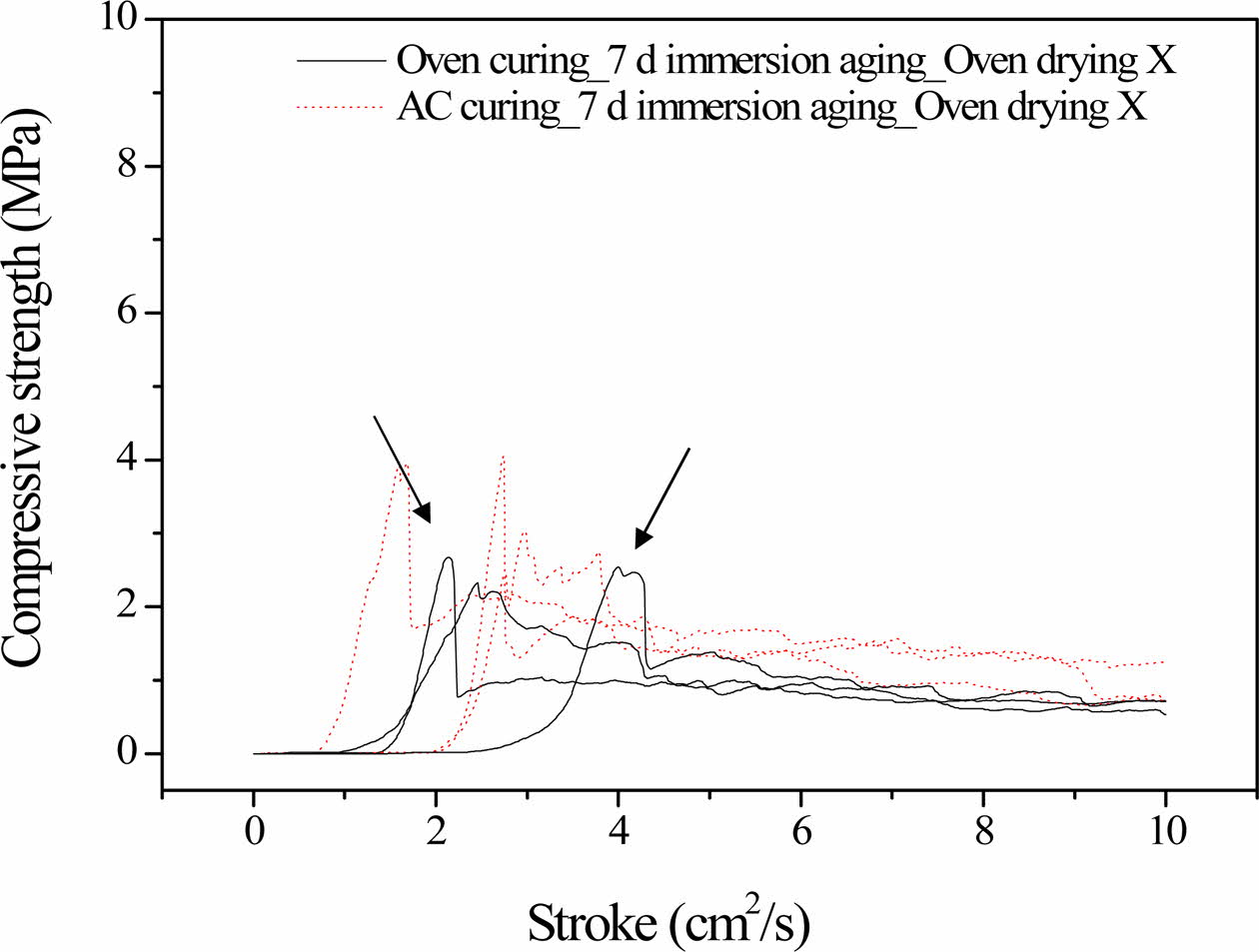
As shown in Fig. 2, there was a slight difference in the compressive strength profiles of the autoclaved specimens obtained after 3 and 7 days of immersion. However, there was a noticeable enhancement in the compressive strength of the 3-day-oven-cured specimens after they were immersed for 7 days, compared to im- mersion for 3 days. Furthermore, the dentable charac- teristics observed in the specimens immersed for 3 days disappeared after 7 days of immersion. Fractural breakdown (an abrupt drop in compressive strength) can be observed in the profiles, as indicated by solid lines in Fig. 2. The disappearance of the dentable characteristics can be attributed to the abrupt drop in the compressive strength.
The autoclaved specimens showed a slight difference in the compressive strength profile after 3 and 7 days of immersion, indicating that the geopolymerization reaction was almost complete, i.e., they were stable in water. In contrast, the geopolymerization reaction was not complete in the oven-cured specimens, and con- tinued during the additional 4 days of immersion. Therefore, the average compressive strength increased approximately two-fold and fractural breakdown was observed in the initial stage of the compressive strength test after 7 days of immersion, as indicated by the arrows in Fig. 2.
Oven drying after immersion
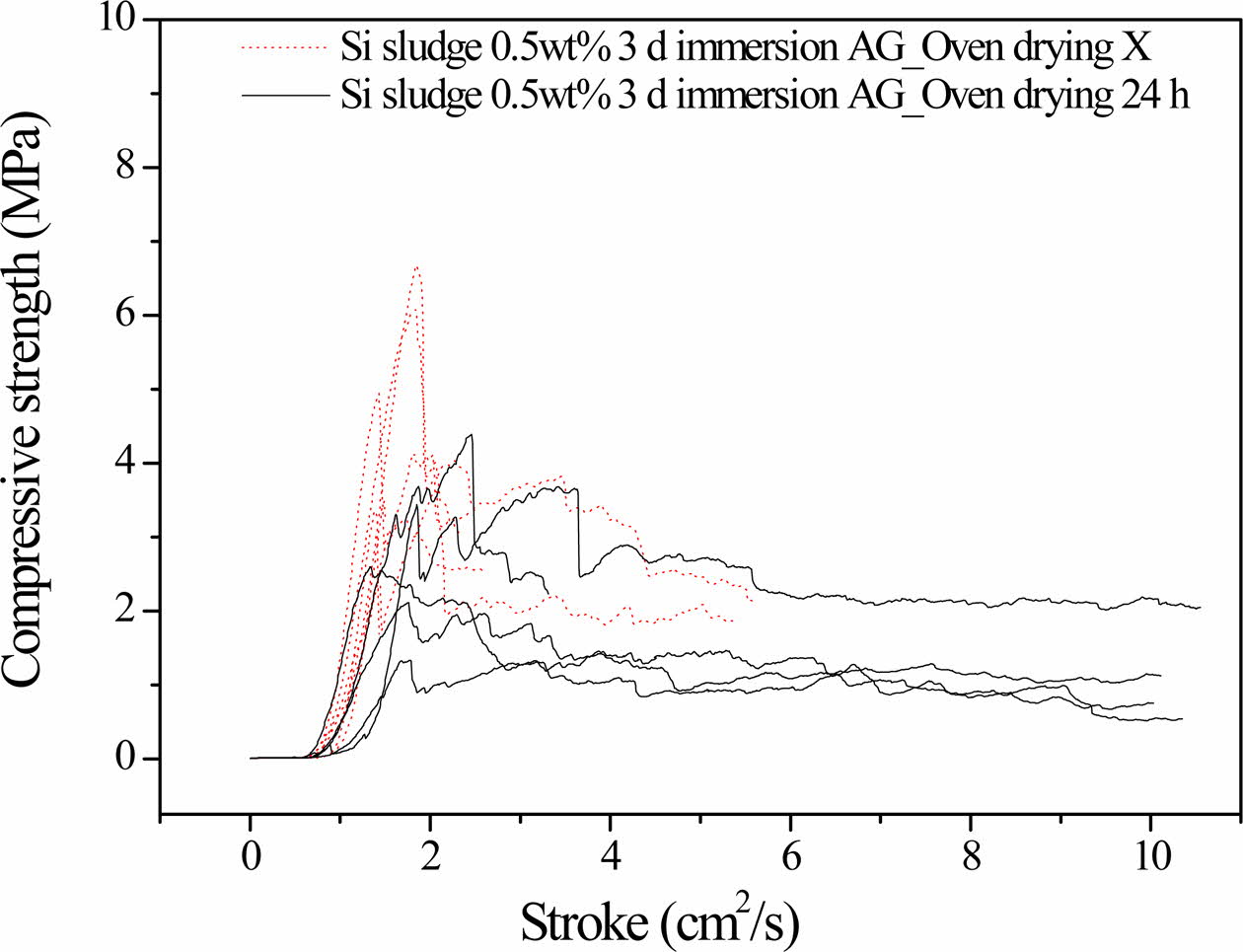
Figure 3 compares the compressive strength profiles of the 3-days-autoclaved specimens with and without the oven drying process immediately after 3 days of immersion, indicating that additional oven drying results in a substantial increase in compressive strength.
The specimens autoclaved for 3 days without the additional oven drying process exhibited a maximum compressive strength of 4.1 MPa. However, the maxi- mum compressive strength of the specimens prepared under the same conditions with the addition of oven drying at 100 oC for 1 day reached 7.0 MPa. This additional drying process after immersion is believed to promote the completion of the geopolymerization reaction by increasing the reaction temperature and removing residual water. Consequently, the specimens do not show any dentable characteristics after the additional drying process, as shown in Fig. 3.
pH change
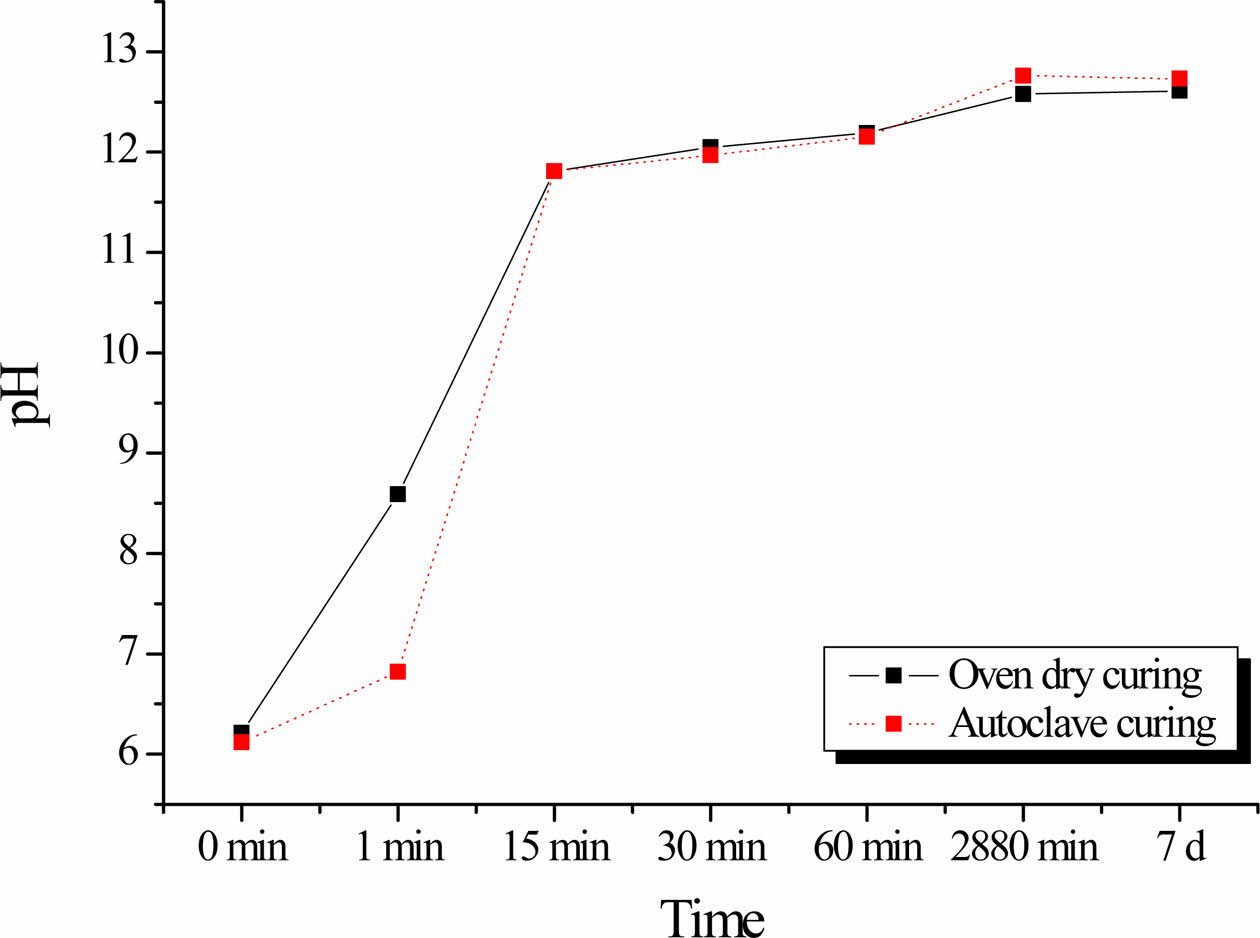
Fig. 4 shows the variation in the pH of the solutions as a function of the geopolymer immersion time. The pH of the solutions of both the oven-cured and auto- claved geopolymers sharply increase with increasing immersion time up to 15 min. Thereafter, the rate of increase decreases, even though the pH of the solutionsstill gradually increase up to 11.15 for both geopolymers.
As shown in Fig. 4, the pH values of the solutions after immersion of the autoclaved geopolymers was mostly lower than those of the oven-cured geopolymers. This suggests that the autoclaved geopolymers are more stable in water than the oven-cured specimens, as lower pH indicates a higher geopolymerization rate. Thus, the results of the pH studies corroborate the results obtained from compressive strength measurements (Fig. 3).
Morphology
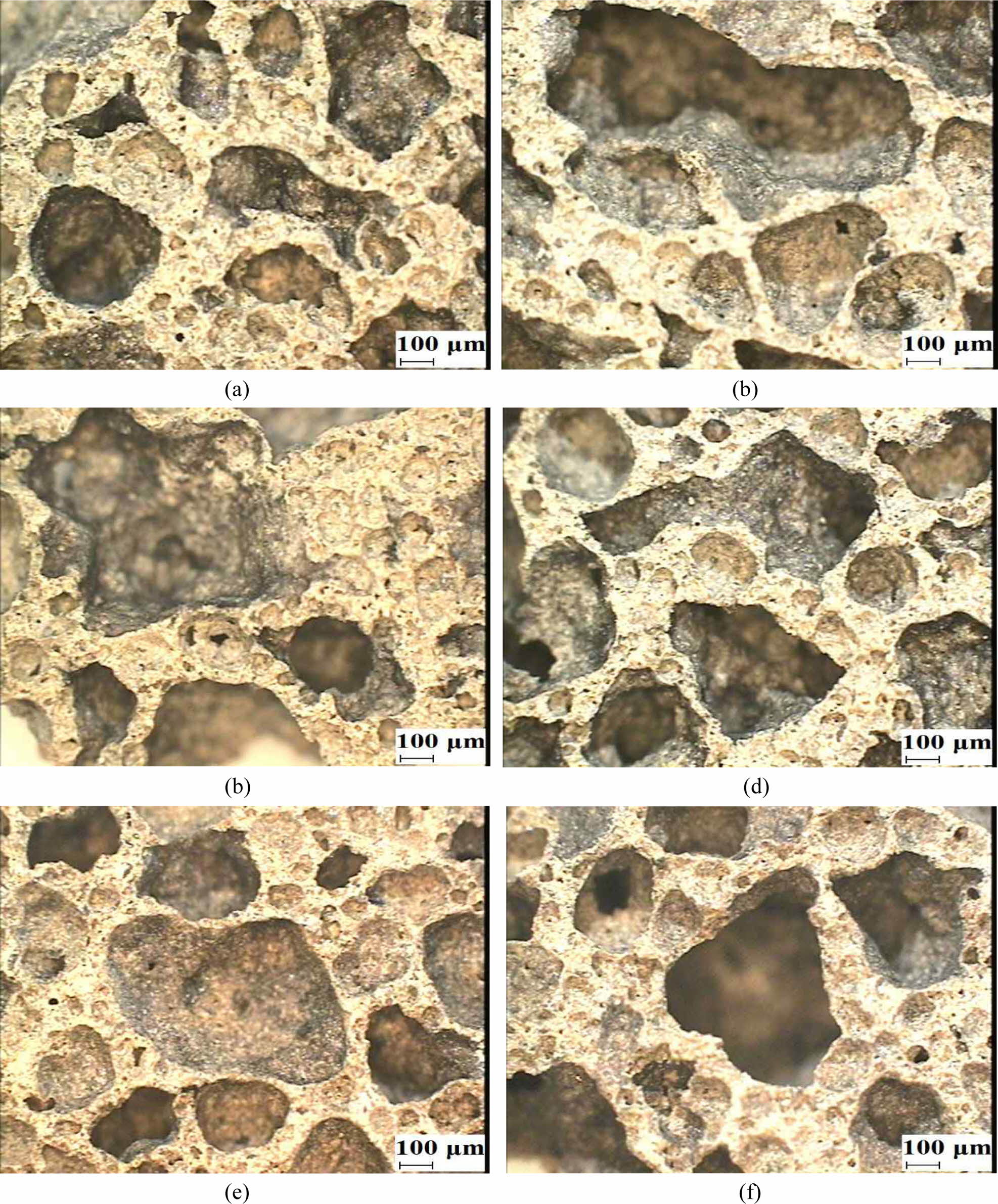
Figs. 5(a) to 5(d) show the morphologies of the oven-cured (left images) and autoclaved (right images) geo- polymer specimens with different immersion periods, and Figs. 5(e) and 5(f) show the morphologies of the autoclaved specimens after the additional 1-day oven drying process following immersion for 3 and 7 days, respectively.
The morphologies of the oven-cured specimens after the additional oven drying process are not shown because there was an insignificant difference before and after the drying process.
As shown in Fig. 5, all the oven-cured geopolymer specimens had large pores in the geopolymer matrices, regardless of the duration of immersion. Importantly, the size and number of the pores in the autoclaved specimens reduced significantly after the additional drying process, as shown in Figs. 5(e) and 5(f). These morphological differences resulted in a substantial increase in the compressive strength of the autoclaved specimens after the additional oven drying process, as shown in Fig. 3.
XRD
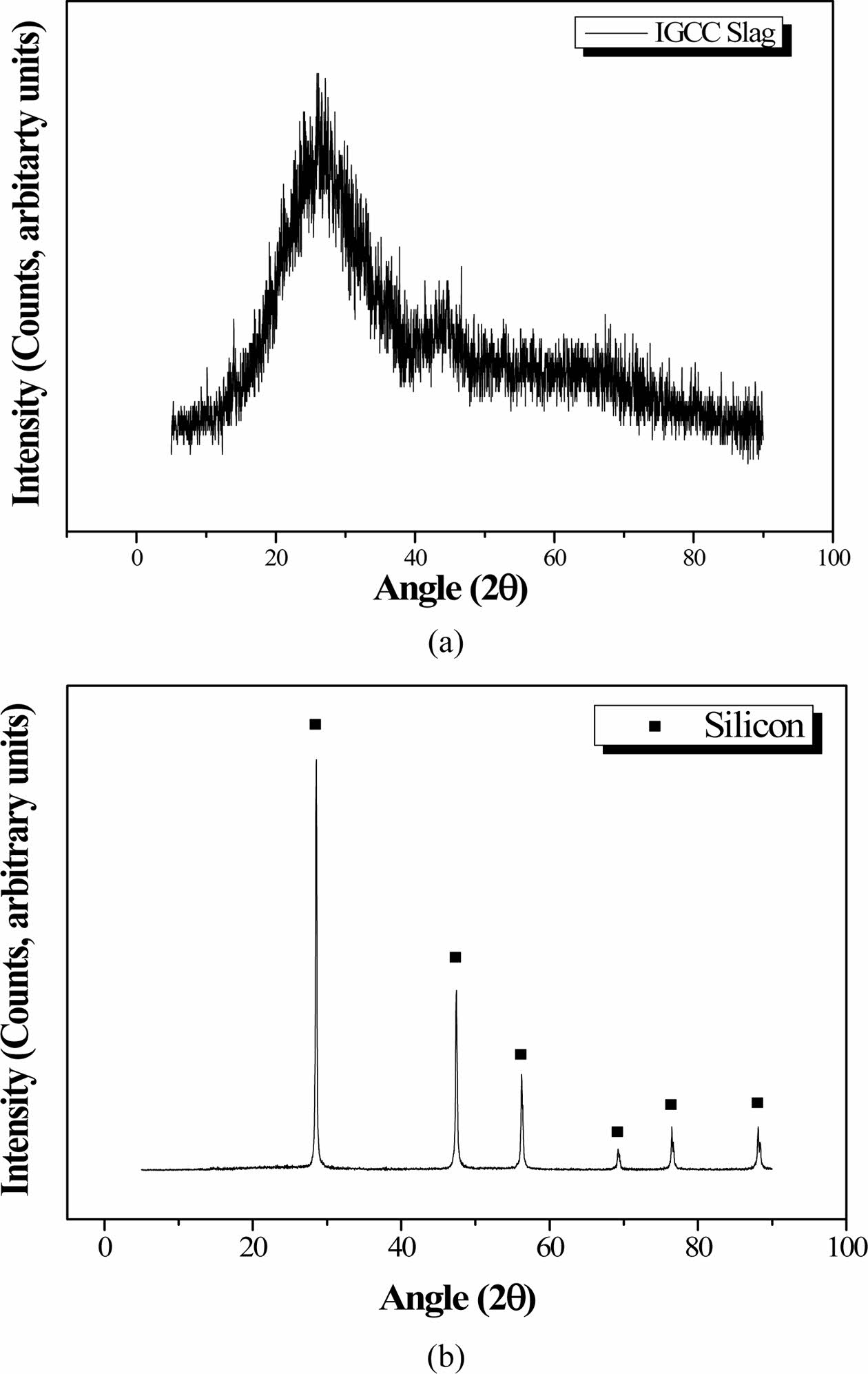
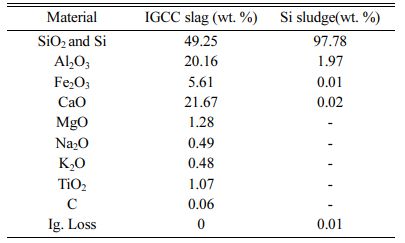
Figs. 6(a) and 6(b) show the XRD patterns of the IGCC slag after milling and the as-received Si sludge, respectively, indicating that the IGCC slag was com- pletely amorphous and the Si sludge was polycrystal- line. Table 1 lists the chemical compositions of the IGCC slag and the Si sludge based on X-ray fluorescence (XRF) measurements. The main components of IGCC slag are SiO2, Al2O3, and CaO. The Si sludge consists mainly of pure Si (97.8%).
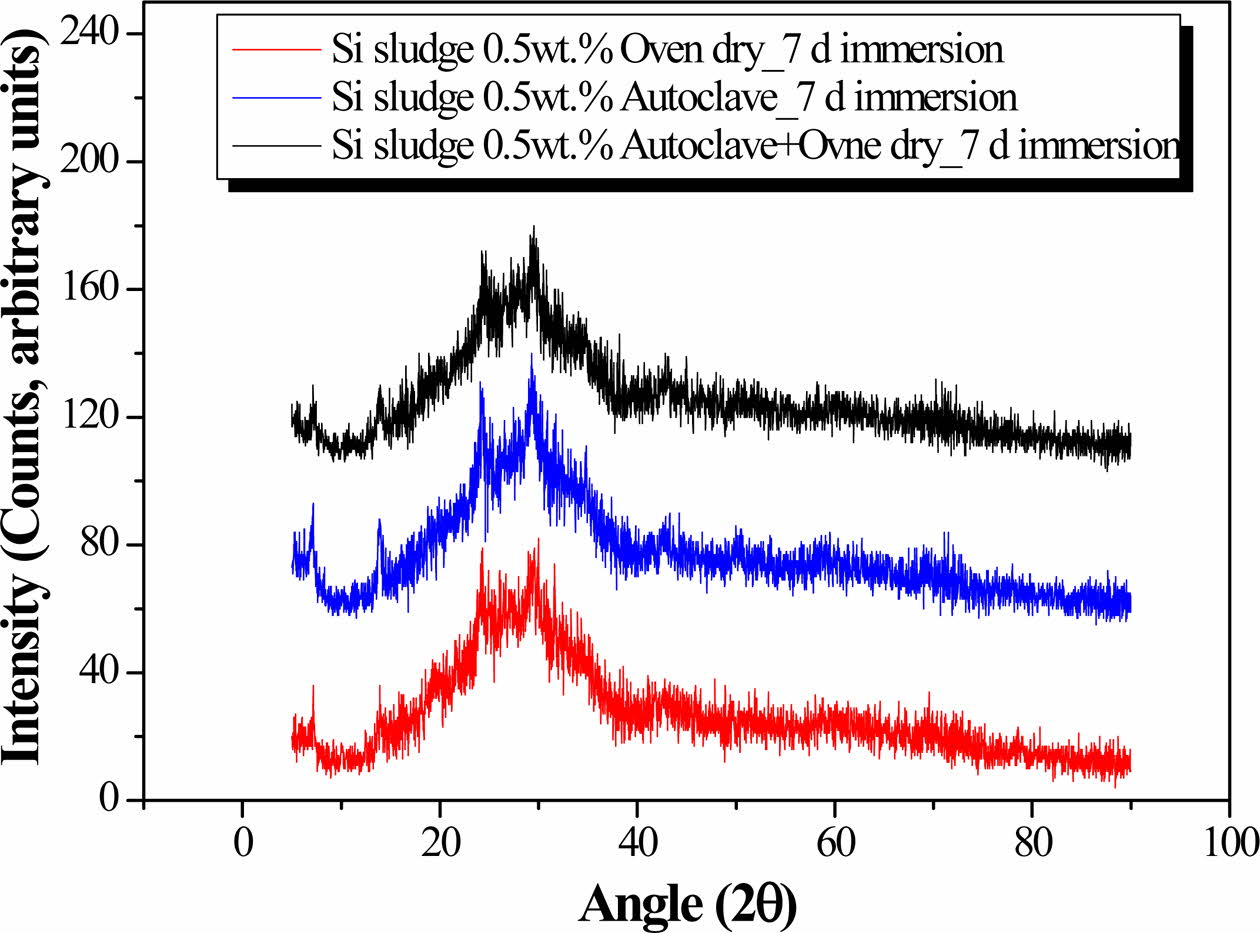
Fig. 7 shows the XRD patterns of the geopolymers that were oven-cured, autoclaved, and autoclaved with additional drying after 7 days of immersion. There is no significant difference in the XRD patterns, indicating that there was little difference in the phase or crystallinity of the geopolymers. In other words, the increase in the compressive strength of the geopolymers due to the additional drying process is not attributed to crystalliza- tion or the formation of different phases.
Therefore, even though the enhancement in the com- pressive strength after the additional drying process indicates increased geopolymerization, it does not necessarily result in the formation of new phases or crystalline materials in the geopolymer matrix.
|
Fig. 1 Compressive strength profiles of oven-cured (solid lines) and autoclaved specimens (dotted lines) after 3 days of immersion. Oven dry X in the legend means there was no oven dry process after immersion. |
|
Fig. 2 Compressive strength profiles of oven-cured (solid lines) and autoclaved specimens (dotted lines) after 7 days of immersion. Oven dry X in the legend means there was no oven dry process after immersion. |
|
Fig. 3 Compressive strength profiles of autoclaved specimens without oven drying (solid lines) and those with oven drying after 3 days of immersion (dotted lines). Oven dry X in the legend means there was no oven dry process after immersion. |
|
Fig. 4 Variation in the pH values of the solutions of the oven-cured and autoclaved geopolymers as a function of immersion time. |
|
Fig. 5 Morphologies of the geopolymer specimens: (a) Oven-cured, immersed for 3 days; (b) autoclaved, immersed for 3 days; (c) ovencured, immersed for 7 days; (d) autoclaved, immersed for 7 days; (e) autoclaved, immersed for 3 days with 1-day additional oven drying; and (f) autoclaved, immersed for 7 days with 1-day additional oven drying |
|
Fig. 6 XRD patterns of (a) IGCC slag and (b) Si sludge |
|
Fig. 7 XRD patterns of the geopolymers after immersion for 7 days. The XRD patterns of oven-cured, autoclaved, and autoclaved with additional oven drying specimens are overlapped for the comparison. |
|
Table 1 Chemical composition of the IGCC slag and the Si sludge obtained from XRF measurements. |
IGCC slag and Si sludge were used as raw materials because both are spent materials, which can be recycled to reduce waste and protect the environment. Moreover, the compressive strength of geopolymers made of IGCC and Si sludge is higher than that of Portland cement.
The oven-cured specimens exhibited dentable charac- teristics, whereas the autoclaved specimens exhibited abrupt breakdown characteristics with no dentable characteristics. There was a noticeable enhancement in the compressive strength of the 1-day-oven-cured specimens that had been immersed for 7 days than those that were immersed for 3 days. Furthermore, the dentable characteristics observed in the 3-day-immersed specimens disappeared after 7 days of immersion. However, the autoclaved specimens showed little difference in the compressive strength profile after 3 and 7 days of immersion. This indicated that the geo- polymerization reaction had almost completed in the autoclaved specimens, such that they were stable in water.
A substantial increase in the compressive strength of the specimens from 4.1 to 7.1 MPa was noted following an additional oven drying process. This was attributed to the 1 day post-immersion drying process promoting the completion of geopolymerization reaction by raising the reaction temperature and removing the residual water.
This work was supported by a Kyonggi University Research Grant (2020).
- 1. K.P. Lee, S.S. Lee, and H.Y. Song, J. Kor. Arch. Inst. 27[11] (2011) 111-118.
- 2. L. Vickers, W.D.A. Rickard, and A. van Riessen, Thermochim. Acta. 580[20] (2014) 20-27.
-

- 3. J. Sim, C. Park, S.J. Park, and Y.J. Kim, J. Korean Soc. Civ. Eng. A. 26[1A] (2006) 213-218.
- 4. L. Duan, S. Sun, L. Yue, W. Qu, and Y. Yang, Energy 87 (2015) 490-503.
-

- 5. Y. Kim and M. Kim, J. Ceram. Process. Res. 22[1] (2021) 39-47.
-

- 6. D. Kioupis, C. Kavakakis, S. Tsivilis, and G. Kakali, Adv. Mat. Sci. Eng. 2018 (2018) 1-11.
-

- 7. M. Szechyn´ska-Hebda, J. Marczyk, C. Ziejewska, N. Hordyn´ska, J. Mikuła, and M. Hebda, Materials 12 (2019) 2999-3015.
-

- 8. J. Davidovits, J. Thermal Analysis 37[8] (1991) 1633-1656.
-

- 9. F.G.M. Aredes, T.M.B. Campos, J.P.B. Machado, K.K. Sakane, G.P. Thim, and D.D. Brunelli, Ceram. Int. 41[6] (2015) 7302-7311.
-

- 10. J.G.S. van Jaarsveld and J.S.J. van Deventer, Ind. Eng. Chem. Res. 38[10] (1999) 3932-3941.
-

- 11. A. Autef, E. Joussein, G. Gasgnier, and S. Rossignol, J. Non-Cryst. Solids 358[21] (2012) 2886-2893.
-

- 12. Y. Kim and M. Kim, Int’l J. Mech. Production Eng. 8[5] (2020) 47-51.
- 13. Y. Kim, S. Kim, and C. Jang, J. Ceram. Process. Res. 17[11] (2016) 1202-1207.
- 14. M. Szechyn´ska-Hebda, J. Marczyk, C. Ziejewska, N. Hordyn´ska, J. Mikuła, and M. Hebda, Materials 12 (2019) 2999-3015.
-

- 15. S. Kim and Y. Kim, J. Ceram. Process. Res. 18[1] (2017) 59-63.
 This Article
This Article
-
2022; 23(2): 126-131
Published on Apr 30, 2022
- 10.36410/jcpr.2022.23.2.126
- Received on Apr 29, 2021
- Revised on Aug 23, 2021
- Accepted on Aug 28, 2021
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yootaek Kim
-
Materials Engineering Department, Kyonggi University, Suwon, Republic of Korea
Tel : +82-31-249-9765 Fax: +82-31-249-9774 - E-mail: ytkim@kgu.ac.kr, gkrals9496@naver.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.