- A comparative review on recovery of heavy metals from printed circuit boards (PCB’S) by chemical and bio-leaching
Murugesan Manikkampatti Palanisamya,*, Akilamudhan Palaniyappanb, Venkata Ratnam Mynenic, Kannan Kandasamyd, and Padmapriya Veerappane
aDepartment of Food Technology, Excel Engineering College, Namakkal, Tamil Nadu, India-637303
bDepartment of Chemical Engineering, Erode Sengunthar Engineering College, Erode, Tamil Nadu, India-638057
cDepartment of Chemical Engineering, Mettu University, Ethiopia
dDepartment of Chemical Engineering, Kongu Engineering College, Perundurai, Erode, Tamil Nadu, India -638060
eDepartment of Electronics Communication Engineering, Erode Sengunthar Engineering College, Erode, Tamil Nadu, India638057This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The electronics industry is the world's largest and fastest growing industry. This consumer-centric industry's combination of technology advancements and quick product obsolescence creates new environmental issues. There is an urgent need to address the volume and toxicity of electronic waste generated. Printed circuit boards (PCBs) are a significant component of electronic trash, containing mostly heavy metals such as copper (Cu), tin (Sn), zinc (Zn), and lead (Pb). Metal recovery and recycling from PCBs is an important step in pollution prevention. Researchers have devised many methods for recovering precious metals from PCBs, including gravity separation, magnetic separation, and electrostatic separation, as well as PCB separation using the organic solvent technique, leaching method, bioleaching method, or a combination of these methods. This research provides a brief summary of India's present e-waste status, environmental and health risks, continuing waste disposal and recycling activities, and emphasizes the recovery of heavy metals from PCBs by systematic leaching/bioleaching
Keywords: Printed Circuit Board (PCBs), Metal extraction, Chemical Leaching, Biological leaching, Adsorption
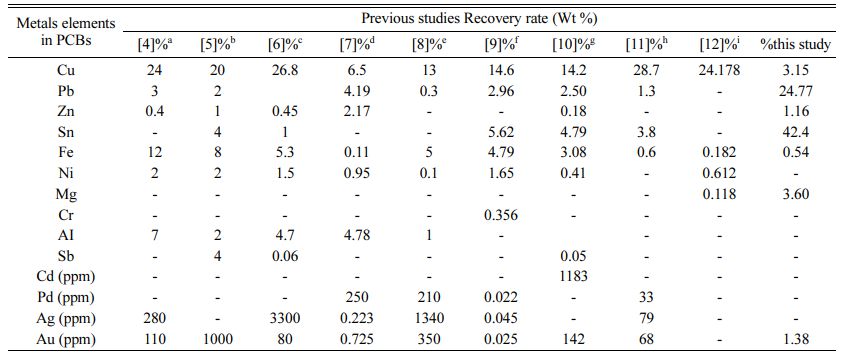
The discarded Printed circuit boards (PCBs) include a large number of heavy metals as well as non-metallic components. PCB scrap consists primarily of ferrous components (50%), plastics (21%), non-ferrous metals (13%), and miscellaneous substances (16%). Copper, tin, lead, mercury, cadmium, arsenic, nickel, and hexavalent chromium are found in excess of permitted levels [1]. PCBs may be removed from a variety of electronic devices, including television boards, CD players, and mobile phones, among others. According to researchers, the average rate of PCB manufacturing has increased by 8.7% each year, resulting in rising environmental concerns that need to be addressed in (Table 1) [2]. The typical metallic compositions of several PCBs are shown in (Fig. 1) [3]. Furthermore, ecologically friendly polymers and ceramic elements such as SiO2, Al2O3, polyethylene, polypropylene, PVC, and Nylon are present in electronic trash [4]. It is critical to evaluate alternative ways for dealing with these hazardous chemicals. A research [5] examined and proved that particle size reduction during milling operations boosted copper release to 100%. The metal concentrations were determined using hydro-metrological techniques, which yielded precious metal values of Ag 0.238 g kg-1, Au 0.725 g kg-1, Cu 6.5 g kg-1, and Ni 16.38 g kg-1 [6]. In a separate case, study on the composi- tion of desktop PCs revealed an average weight of 60lb of various metals. Switzerland generates 66,042 TPA of E-waste per year, Germany generates 1,100,000 TPA, the United Kingdom generates 915,000 TPA, the United States generates 2,124,400 TPA, Thailand, Denmark generates 118,000 TPA, Canada generates 67,000 TPA, and India generates 146,111 TPA [7]. Several investi- gations were also done on a variety of samples in various concentration ranges. In Japan in 2007, several different E-waste collecting facilities separated a sample of 20 personal computers (PCs). The chemical element analysis reveals metal concentrations ranging from 13.8% to 24.6%, Fe 0.2% to 4.79%, and Au 0.0076% to 0.02%, respectively [8].
E-waste is a huge rising concern throughout the world, with technological obsolescence accounting for around 80-90% of this trash. Several techniques of characterisation and therapy are described in the litera- ture [9]. Metal recovery was proven in the bioreactor followed by precipitation, and variations in treatment metal concentrations for metals such as copper, gold, platinum, and others were recorded [10]. In addition, the prior study looked at the continuous rise in E-waste creation rates as a result of the country's population and technological advancements [11]. The increased usage of contemporary electrical and electronic equipment results in the disposal of old equipment and the creation of a huge amount of e-waste for all types of equipment, such as personal computers, mobile phones, and so on. Because of the release of poisonous and hazardous components into the atmosphere, it will generate severe environmental issues [12]. Scanning electron microscopy will be used to characterize mobile scraps with diameters of 1 mm, 0.71 mm, 0.60 mm, 0.425 mm, 0.18 mm, and 0.075 mm. The research revealed that the tested materials included significant amounts of copper, carbon, and silicon [13].
India is one of the countries most affected by the e-waste problem, yet until 2012, there was no compre- hensive electronic waste law in existence.This might be because it was not seen as a potential threat that needed to be addressed appropriately. According to the Dangerous Wastes Rules (1989), e-waste is not deemed hazardous unless it is shown to have a higher concentration of specified substances. However, none of the above-mentioned environmental laws made a direct and specific reference to electronic waste processing as hazardous. In the Indian context, the study found that the yearly E-waste creation rate is predicted to be dangerously high [14], with Chennai (2 MT), Bangalore (21 MT), Mumbai (10.1 MT), and Delhi (9.1 MT) accounting for about 24% of total e-waste output. According to the research [15], the annual generation of E-waste has risen drama- tically every year, as seen by the graph below (Fig. 2). More than 40% of obsolete electrical goods in India are believed to be sitting idle in homes or warehouses because people are unsure what to do with them. Recycling and processing of discarded PCBs in e-waste are nearly exclusively handled by the informal sector and are totally driven by market forces. Because of insufficient base and metal recovery, the use of crude techniques creates occupational and environmental risks, as well as a loss of valuable resources.

|
Fig. 1 Metal compositions present in PCBs |
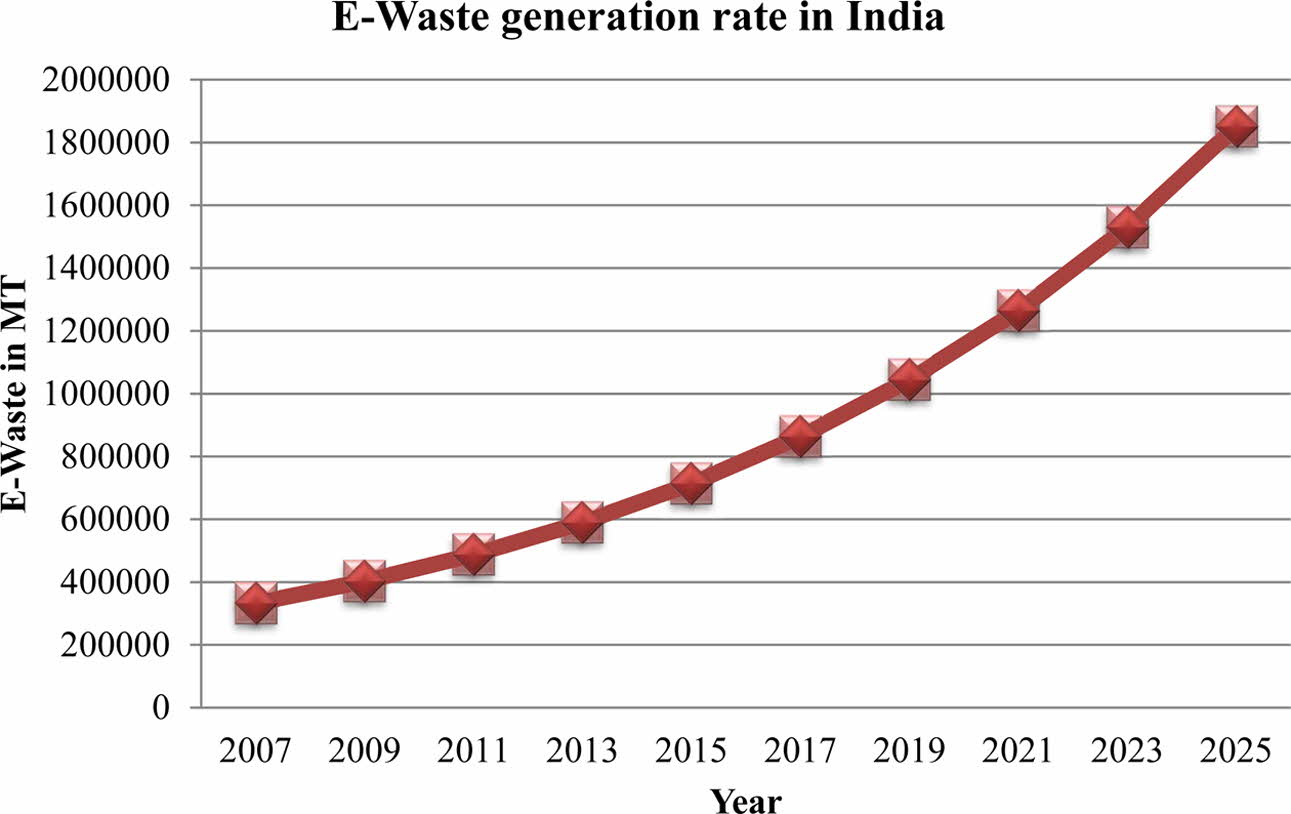
|
Fig. 2 Provides information about E-waste generation rate in India. |
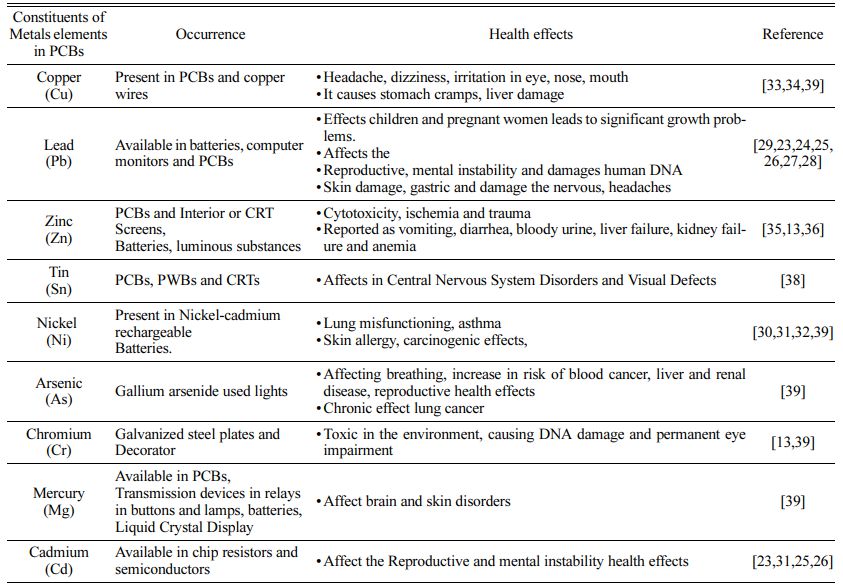
The chemical composition of PCBs is an essential characteristic that may be determined via inductive coupled plasma mass spectroscopy [16]. The metal composition study indicates metal concentrations in PCBs of 35.70 gm L-1 copper, 44.91 gm L-1 lead, and 21.77 gm L-1 iron [17]. PCBs include a variety of toxic and dangerous chemicals, many of which can cause significant issues if not properly recycled or handled [18, 19]. E-waste comprises not only home and com- mercial electrical equipment, but also parts such as batteries, capacitors, castings, and so on.Recycling of such garbage has occurred both formally and informally in a number of nations, including China, India, Ghana, Thailand, and Vietnam [20]. Formal recycling systems are well-developed methods for ensuring protection and successful separation, but they are quite expensive to build and run. Compromise on treatment stages can result in the release of numerous pollutants into the atmosphere, causing a variety of health problems [21]. Metals contained in PCBs are very hazardous to living creatures. These metals enter the human chain via media such as dust, air, water, and soil.Metals such as lead (Pb) and cadmium (Cd) have been linked to reproductive health; development, mental disease, and DNA damage [22-25]. Low amounts of lead (Pb) exposure also cause major development difficulties, skin damage, sickness, ulcers, blood, and reproductive system problems in children and pregnant people [26, 27]. It was also shown that staff pulmonary dysfunction and Skin Allergy are carcinogenic as a result of inhaling nickel-contaminated air [28-30]. Exposure to copper (Cu) at waste sites causes health consequences such as headaches, dizziness, eye irritation, nose and mouth irritation, and so on [31,32].
Nausea, vomiting, discomfort, cramps, diarrhea, renal failure, and cytotoxicity are all side effects of PCBs, batteries, and luminous chemicals [33, 34]. Its hazardous components, which are combined with soil and air and have severe consequences, include acid discharge, poisonous compounds such as heavy metals, carcinogenic chemicals, and heavy metal bio-magnification [35]. Tin exposure from PCBs, PWBs, and CRTs causes intellectual impairment in children, as well as harm to the blood or reproductive systems and visual defects [36, 37]. Table 2 provides information on the negative impact on human health and the environment of the presence of dangerous hazardous components in various heavy metals.Researchers at PCB disposal sites are exposed to dangerous toxic metal components (primarily copper, lead, arsenic, tin, zinc, and mercury) and other toxic substances discharged into it via water, air, or landfills and food chains, which may lead to micronucleus formation and chromosomal aberrations, resulting in genetic instability in the exposed individual [38]. Metals enter the human body and go to various organs such as the liver, kidney, bone, pancreas, and brain, where they are processed and can be engaged in a number of physiological processes. PCB trash may pollute soil and food systems with heavy metals in diverse forms that humans consume. Heavy metal concentrations are increased through the biomagnification process. Oc- cupational exposure to metals to the mother body leads to early pregnancy loss, genetic disorder, preterm bright, development of disabilities and behavior disorders, abnormal growth, and development [39].
Another study on 50 electrical gadgets reveals that the leachate is harmful to aquatic life. Acute toxicity assay, Selenastrum capricornutum chronic algal growth inhibition assay, Met plate acute test for heavy metal toxicity assay [40]. The impacts of hazardous components such as Pb, Cu, Sn, and Zn would create major human health and environmental concerns if PCBs were not properly disposed of and recycled. Previous research has included techniques for solidification and landfill- ing [43]. These techniques, however, contaminate the essence of the soil by decreasing the mass transfer rate of the interface and generating an atmosphere of pollution.
|
Table 2 Hazardous toxic metals present in printed circuit boards and their Health effects |
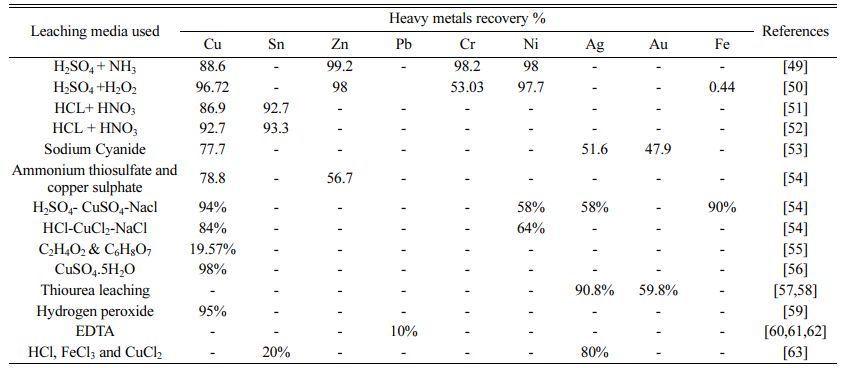
There are numerous approaches, including as inciner- ation, land filling, gasification, and pyrolysis processes that have advantages in terms of cost, environmental impacts, and metal recovery. To remove metals from PCBs, chemical leaching has been routinely employed. One such research [42] involved the removal of the unique metal ion copper by the leaching agent ammonia persulphate (Aqua regia) followed by electro winning for 14 hours, with recorded findings showing Cu, Zn, and Ni recovery rates of 99, 60, and 9%, respectively. Effectively, the recovery of leaching liquids by electro deposition method is also efficient.These techniques, however, have certain downsides since they employ hazardous chemicals in the process, which would have a negative impact on the quality of the environment [43]. Another typical technique combines mechanical grinding, density separation, and acid leaching [44]. The experimental approach [45], which involves an ultrasonic acid leaching procedure, resulted in the recovery of many heavy metals from electroplating sludge. During the leaching process, less valuable metals (Cu-96.72%, Ni-97.77%, Zn-98%, Cr-53.03%, and Fe-0.44%, respectively) were separated from waste sludge. The experimental techniques essentially offered selective metal separation, and the schematic process flow diagram of ultrasonic enhanced leaching was presented [45]. Some research, however, used pyrolysis and thermo-chemical procedures to recover polymers and bulk metals from PCBs. There are several disadvantages to these pyrolysis and thermo-chemical methods, such as high heat needs (thermal cracking temperatures employed range from 470 to 800 oC polluted gas formations, and expensive prices [46].
One researcher [1] thoroughly investigated E-waste treatment approaches used in chemical and biochemical leaching methods based on numerous previous studies with various types of E-waste materials such as PCBs, PWBs, DVD players, cell phone boards, calculator scraps, TV scraps, and personal computer scraps. The aforementioned analysis recovers other metals such as Pb, Cu, Ni, Sn, Al, Fe, Au, Ag, and Zn. Chemical extraction and biological leaching procedures, according to the study, have their own advantages and disadvan- tages, and there may be various scientific, economic, and environmental reasons for selecting one approach over the other. Table 3 summarizes the different com- bined leaching solvents used. Study using H2SO4 and H2O2resulted in recovery of Cu 96.72%, Zn 98%, Cr 53.03% and Ni 97.7% [47], using the leaching agent HCl+ HNO3, the removal of Cu 86.9% and Sn 98% [48, 49], using Sodium Cyanide, Cu 77.7%, Ag 51.6% and Au 47.9% recovered [50], Leaching solvents ammonium thiosulphate and copper sulfate are used to get Cu 78.8%, Zn 56.7% recovered [51] and H2SO4 + NH3 are Cu 88.6%, Zn 99.2%, Ni 98% recovered [46].
If hazardous chemicals are not properly disposed of, they can significantly contaminate the air, land, and natural environment [51]. With HCl-CuCl2-NaCl as the solvent solution and different parameters adjusted with the use of RSM, Cu recovered 94%, Ni recovered 58%, and Fe recovered 58%. In comparison to hydrogen peroxide, C2H4O2 and C6H8O7 treatments showed poor metal dissociation, whereas HNO3 increased metal solubility [52]. However, employing CuSO4.5H2O as an oxidizing medium, the purity of precious metals (Cu) in PCBs is reported to be 98% [53]. The results indicated that utilizing Thiourea leaching media with varied adjusted settings, Au and Ag recovery from PCBs was 90.87 and 59.8%, respectively [54, 55]. 95% of copper was recovered using hydrogen peroxide as a solvent [56]. Chemical leaching also necessitates the use of different chelating agents to recover heavy metals, with EDTA as a leaching agent resulting in enhanced lead recovery [57]. Heavy metals including chromium, copper, zinc, and nickel are also recovered using EDTA [58]. Etching is also a type of strong chemical leaching reagent that entails the recovery of metals from waste PCBs using chemicals like HCl, FeCl3, and CuCl2 [59].
Through this focused extraction of copper, only tiny amounts of other metals may be retrieved. The PCB sample size of 4 cm × 4 cm resulted in the separation of Cu, Zn, Sn, and Pb with compositions of 117.33 mg/g, 28.97 mg/g, 10.41 mg/g, and 9.34 mg/g, respectively, when employing HCL as a leaching agent under specified conditions [60]. When compared to the usual PCB metal composition, the quantity of Zn and Pb leached was negligible. The grades of Cu, Pb, Zn, and Sn are 16%, 2%, 1%, and 1%, respectively, when crushed PCBs (size between 0.43 mm-3.33 mm) are leached with sodium cyanide solution [61]. The Response Surface Methodology is also used to improve experimental settings (RSM).Au and Cu were stated to have been effectively leached with the aid of sulphuric acid and hydrogen peroxide and statistical optimization using RSM.
The recycling of PCBs is therefore a major issue, as not only the recycling, re-use, and waste disposal but also the separation of valuable metals from their respec- tive leaching media [60-62] is a an important aspect. Previous research findings have been recorded for the recovery of valuable metals from various PCB waste in various leaching media such as acid leaching, Aqua regia, NaOH, H2SO4+NH3, H2SO4+H2O2, HCl+HNO3, HCl+HNO3, Sodium Cyanide, Ammonium thiosulphate, and copper sulfate, H2SO4-CuSO4-NaCl, HCl-CuCl2-NaCl, C2H4O2&C6H8O7, CuSO4·5H2O, Thiourea leaching, Hydrogen peroxide, EDTA, HCl, FeCl3 and C6H8O7. These leaching media have individual dissolving and dissolving properties of metals and also have advantages and disadvantages for the particular metal recovery method. As a result, several researchers have successfully extracted heavy metals from printed circuit boards using hydrometallurgical techniques. However, these processes are associated with certain disadvantages that limit their application to the treatment sectors. Some common limitations of hydrometallurgical methods for recovering PCBs are listed here [63].
• Hydrometallurgical processes are sluggish and time-consuming in general, and they have an influence on the recycling economy. Concerns have been raised about the hydrometallurgical process's economics when compared to pyrometallurgical techniques for extracting heavy metals from PCBs.
• Because cyanide leakage causes pollution of aquatic water, which poses a serious health risk to the popula- tion, strict safety requirements are required.
• The effective recovery of heavy metals takes longer when size reduction procedures are used. These kinds of procedures result in a considerable drop in overall revenue.
• The use of Halide and thiourea leachates are very difficult to implement due to strong corrosive acids, oxidizing conditions, and high cost for leaching of heavy metals from PCBs.
The recovery of hazardous metals from PCBs is considerably more evident, and prior research has mostly focused on the metals Cu and Pb. The focus currently is on selective leaching to recover trace amounts of metals including Sn, Cr, Ni, Zn, Au, and Fe. Because of the various metallic components present, the concentration of the leached solution fluctuates depending on the leaching agent, making concentrated metal separations extremely challenging. Numerous researches in various nations have concluded that informal metal recovery techniques are dangerous owing to toxic contamination, non-technical processes, environmental impacts, and human health implications dependent on the type of metal recovery medium utilized for recovery. This analysis, therefore, overcomes these kinds of drawbacks. It initially deals with the recovery of heavy metals from PCBs using aqua regia as a two-stage leaching agent (the first stage is HCl and HNO3 and the second stage is HCl and H2SO4) and optimizes various operating parameters. Furthermore, experimental studies are carried out using the RSM to determine the recovery of heavy metal ions by central composite design (CCD). Currently, the electro-winning, electro-refining, and ion-exchange methods are used for the recovery of liquefied metals, but these methods have disadvantages. This study adsorption technique is therefore suggested to address environmental impacts and other disadvantages. Bentonite Clay (Bent) and Peanut Shell Carbon (PSC) are used in this study to be pristine. Thermally and chemically active types were used as adsorbents for the recovery of heavy metals from a leached solution.
Bioleaching of heavy metal ions, in particular, is regarded as one of the most promising technologies, with a cost-effective approach compared to chemical leaching and energy demands [64, 65]. The mainly acidophilic bacterial population plays an essential role in the bioleaching of heavy metals from PCB waste. Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptospirillum ferrooxidans [66] microorganisms, for example, are more actively involved in the break- down of organic and inorganic materials.The most significant heavy metal breakdown microorganisms [67] are iron and sulfur-oxidizing chemolythotrophs (effectively increasing and automatically fixing CO2 from the atmosphere). Bio-leaching is a cutting-edge technique for removing heavy metals from PCBs. Acidithiobacillus ferrooxidans [68], Thiobacillus thio- oxidans [1, 69-72], and Acidithiobacillus [73-75] were shown to be extremely effective in the leaching process. Because they are both ecologically friendly and cost-efficient, these treatments have shown to be highly effective. Bacteria and fungi [60] that are chemolytho- trophic [76], heterotrophic [77], and haemophilic [78] have been evaluated for the mobilization of basic metals such as Cu, Zn, Fe, Ni [1]. Scientists are concerned about this approach since it uses fewer reagents, uses less power, produces less pollution, and has other advantages. Rapid economic growth in Asia and the growing transboundary movement of secondary resources will progressively require both 3R endeavours (Reduce, Reuse, Recycle) in each country and private control of foreign material cycles, according to an excellently reviewed current status and research on e-waste issues in Asia [1]. In earlier research, critical analysis was performed by bacterial heavy metal leaching from different wastes, as shown in Table 4.
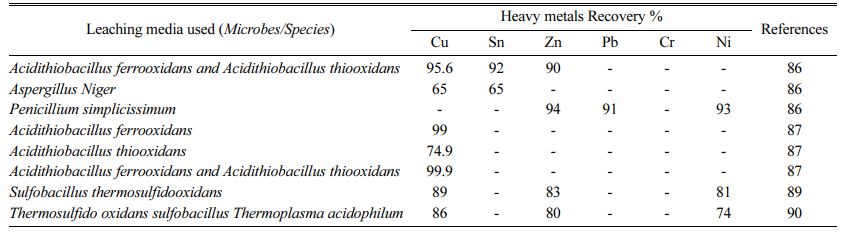
Previous research on the dissociation of heavy metals by microorganisms has used leaching operations under different controlled circumstances, such as temperature, duration, concentrations, and pH. Experiments are carried out with the appropriate variety of irons and the addition of a complexing agent, ensuring ideal circumstances for the microbe's development in the different para- meters listed above. For the recovery of heavy metals from PCBs by microbial leaching, these conditional parameters are used [79-82]. Acitithiobacillus ferrooxidans and Acitithiobacillus thiooxidans are Cu 95.6%, Sn 92%, Zn 90% recovered, Aspergillus Niger Cu 65% and Sn 65% recovered, Penicillium simplicissimus mare Zn 94%, Pb 91% recovered, Ni 93% recovered [78], Acidithiobacillus ferrooxidans 99% recovered, Acidithiobacillus thiooxidans 74.9% recovered, Acidi- thiobacillus ferrooxidans and Acidithiobacillus thiooxi- dans are Cu 99% recovered [83], Sulfobacillus thermo- sulfidooxidans are Cu 89%, Zn 83%, Ni 81% recovered [84], Sulfobacillus thermosulfidooxidans, Thermo plasma acidophilus bacteria are Cu 86%, Zn 80%, Ni 74% [85-86] separated from metal concentrates of waste PCBs.
As a result of the above research, it was determined that streaking bacteria were isolated species of bacterial colonies using Nutrient Broth and bacteria cultured on PCB waste. The suggested microbial strategy is the most powerful and capable of resolving the issues with chemical leaching approaches for PCB metal recovery. Bioleaching procedures are a great alternative to chemical leaching and developing technology that keeps the environment beautiful.
Every year, a large amount of PCB trash is rapidly increased across the world, causing significant human and environmental concerns. Inefficient, informal treat- ment and recycling methods such as soil filling, in- cineration, pyrolysis, and electrolysis have contributed to a wide range of environmental concerns in recent years, and may involve different methodologies, economic, and environmental factors for selecting successful techniques over others. Previous study, for example, identified a number of methods that were effective in terms of recovery rates, metal ions, and environmental advantages from the PCBs treatment process.However, based on the findings of leaching medium, this research suggests that some chemical reagents are given higher metal recovery rates, while some investigations have recorded a minimal recovery rate. In comparison to bioleaching, prior chemical leaching procedures required significant investments in leaching reagents, high tem- perature, pressure, and operations to operate. In this study, formal approaches such as leaching (two-stage chemical leaching and bioleaching) and adsorption were recommended for recovering heavy metals from PCBs.
Chemical leachinginvolves the recovery of heavy metals from PCBs by using aqua regia as a two-stage leaching agent (the first stage is HCl and HNO3 and the second stage is HCl and H2SO4) and optimizing various operating parameters. Leached metals are extracted by electro-winning, electro-refining, and ion exchange processes, but these processes have disadvantages. It is therefore proposed to resolve environmental impacts and other disadvantages. Chemical leaching, however, has some drawbacks due to the existence of toxic reagents that could have toxic effects on human health (human brain, central nervous system, issues with the kidneys and bones, skin allergies, cancers, and headaches) and environmental effects.
The Bioleaching technique is an important tool for reducing the metal content of PCBs. It's eco-friendly and easy to treat. PCBs will be treated with Acidithio- bacillus ferrooxidans and Acidithiobacillus thiooxidans for metal dissociation. Leaching would optimize the different parameters, such as contact time effects, pulp density, particle size, and temperature, to verify the optimal conditions and estimate the feasibility of bioleaching of PCB heavy metals. Hence, this review proposed technique has a big advantage in environ- mental protection as bioleaching does not result in the generation of any toxic wastes into the environment and leads to safer disposal of waste.
- 1. V.R. Jothi, R. Bose, H. Rajan, C. Jung, S.C. Yi, Adv. Energy Mater., 8 (2018) 1802615.
-

- 2. K. Huang, J. Guo, and Z. Xu, J. Hazard. Mater., 164 (2009) 399-408.
-

- 3. N. Menad, B. Björkman, and E.G. Allain, Resour. Conserv. Recycl., 24 (1998) 65-85.
-

- 4. S.-A. Shuey, E.-E. Vildal, and P.-R. Taylor, Pyro-metallurgical Processing of Electronic Waste. SME Annual Meeting, March St. Louis, MO. Preprint 06-037 (2006) 27-29.
- 5. Y. Zhao, X. Wen, B. and Li, D. Tao, Minerals and Metal Processing., 21 (2004) 99-102.
-

- 6. L. Flandinet, F. Tedjar, V. Ghetta, and J. Fouletier, J. Hazard. Mater., 214 (2012) 485-490.
-

- 7. A. Bandyopadhyay., Int. J. Env. Waste Manag., 2 (2008) 139-186.
-

- 8. J. Szałatkiewicz, Pol. J. Environ. Stud., 23, 6 (2014) 2365-2369.
- 9. W.-A. Bizzo., R.-A. Figueiredo, and V.F. de Andrade, Materials., 7 (2014) 4555-4566.
-

- 10. N.-J. Creamer, V. S. Baxter, J. Henderson, M. Potter, and L.E. Macaskie, Bio Technol. Lett., 28 (2006) 1475-1484.
-

- 11. D. Rimantho and S. R. Nasution, Int J. App. Environ. Sci., 6 (2016) 1451-1468.
- 12. I. Cherukuri, N. Sultana, and S. P. Podila., IOSR J. Environ. Sci. Toxicology and Food Technol., 12 (2018) 08-16.
-

- 13. L.-M. Terena, A.F. de Almeida Neto, M.L. Gimenes, and M.G.A. Vieira, Chem. Eng. Transactions., 56 (2017) 1945-1950
-

- 14. S. Lakshmi, A. Raj, and T. Jarin., Asian J. Appl. Sci. Technol., (AJAST), 9 (2017) 33-36.
- 15. R. Kumar, Karishma, Int. J. Scientific and Res. Publications., 6 (2016) 424-430.
- 16. I.-O. Ogunniyi, M.K.G. Vermaak, and D.R. Groot, Waste Manag., 29 (2009) 2140-2146.
-

- 17. S. Sobri and A.H.M. Ali, IOP Conf. Series: Mat. Sci. Eng., 17 (2011) 1-5.
-

- 18. P. Chatterjee. British medical Journal., 337 (2008) 376-377.
- 19. B. Ghosh, M.K. Ghosh, P. Parhi, P.S. Mukherjee, and B.K. Misshra, J. Clean. Prod., 94 (2015) 5-19.
-

- 20. S. Gupta, G. Modi, and R. Saini, Vijayaagarwala: Int. Refereed J. Eng. Sci., (IRJES), 3 (2014) 05-17.
- 21. D.-J. Jun, W.X. Feng, and Z.Y. Min. J. China Univ Mining Tech., 18 (2008) 0454-0458.
- 22. C. Frazzoli, O.E. Orisakwe, R. Dragone, and A. Mantovani, Environ. Impact Asses Rev., 30 (2010) 388-399.
-

- 23. J. Zhang, Y. Jiang, J. Zhou, B. Wu, Y. Liang, Z. Peng, D. Fang, B. Liu, H. Huang, C. He, C. Wang, and F. Lu, Env. Sci. Technol., 44 (2010), 3956-3962.
-

- 24. X. Xu, H. Yang, A. Chen, Y. Zhou, K. Wu, J. Liu, Y. Zhang, and X. Huo, Reprod Toxicol., 33 (2012) 94-98.
-

- 25. X. Huo, L. Peng, X. Xu, L. Zheng, B. Qiu, Z. Qi, B. Zhang, D. Han, and Z. Piao, Environmental Health Perspectives, 115 (2007) 1113-1117.
-

- 26. J. Kishore, Monika., Indian J. Community Med., 35 (2010) 382-385.
-

- 27. C.-S. Poon, Waste Manag., 28 (2008) 1499.
-

- 28. G. Zheng, X. Xu, B. Li, K. Wu, T.A. Yekeen, and X. Huo, J. Expo. Sci. Environ. Epidemiol., 23 (2013) 67-72.
-

- 29. Y. Guo, X. Huo, Y. Li, K. Wu, J. Liu, J. Huang, G. Zheng, Q. Xiao, H. Yang, Y. Wang, A. Chen, and X. Xu, Sci. Total Environ., 408 (2010) 3113-3117.
-

- 30. N. Padiyar, P. Tandon, and S. Agarwal, Int. J. contemporary dentistary., 2 (2011) 80-83.
- 31. Q. Wang, H. Am, B. Gao, L. Chen, Q.Z. Yu, H. Guo, B.J. Shi, P. Jiang, Z.Y. Zhang, P.L. Li, Y.G. Sheng, M.J. Fu, C.T. Wu, M.X. Chen, and J. Yuan, J. Environ. Sci. Health A Tox. Hazard Subst. Environ Eng., 46 (2011) 669-676.
-

- 32. Y. Li, X. Huo, J. Liu, L. Peng, W. Li, and X. Xu, Environ. Monit. Assess., 177 (2011) 343-351.
-

- 33. L.-M. Plum, L. Rink, and H. Haase, Int. J. Environ. Res. Public Health., 7 (2010) 1342-1365.
-

- 34. R. Verma and P. Dwivedi, Recent Res. Sci. Technol., 5 (2013) 98-99.
- 35. M.-S. Sankhla, M. kumari, M. Nandan, S. Mohril, G.P. Singh, B. Chaturvedi, and R. Kumar, IOSR J. Enviro. Sci, Toxicology and Food Technol., 10 (2016) 98-104
- 36. M. Mahurpawar, Effects of Heavy metals on Human health, Int. J. Res., 1 (2015) 1-7.
- 37. M.-C. Vats and S.K. Singh, Int. J. Innovative Res. Sci. Engg. Technol., 3 (2014) 16917-16031.
- 38. Q. Liu, J. Cao, K.Q. Li, X. H. Miao, G. Li, F.Y. Fan, and Y.C. Zhao, Environ. Sci. Pollution Res., 16 (2008) 329-338.
-

- 39. S. Vimalraj, V. N. Sumantran, and S. Chatterjee, Reproductive Toxicology., 70 (2017) 30-48.
-

- 40. R. Dagan, B. Dubey, G. Bitton, and T. Townsend, Archives of Environ. Contaminations and Toxicology., 53 (2007) 168-173.
-

- 41. Y. Ikushima, K. Hatakeda, and N. Salto, J. Chem. Phys., 108 (1998) 5855-5860.
-

- 42. F. Bari, M. N. Begum, B. Jamaludin, and K. Hussin, University Malaysia Perlis., 1 (2007) 1-4.
- 43. I. Masavetas, A. Moutsatsou, E. Nikolaou, S. Spanou, A. Zoikis-Karathanasis, E.A. Pavlatou, and N. Spyrelis, Global Nest J., 11 (2009) 241-247.
-

- 44. H. Charvatova, D. Janacova, M. Fialka, and K. Kolomazník, Acta Montanistica Slovaca., 15 (2010) 58-61.
- 45. C. Li, F. Xie, Y. Ma, T. Cai, H. Li, Z. Huang, G. Yuan, and J. Hazard. Mater., 178 (2010) 823-833.
-

- 46. Y. Li, X. Huo, L. Junxiao, L. Peng, W. Li, and X. Xu, Environl. Monitoring and Assess., 177 (2011) 343-351.
-

- 47. R. Vijayaram, D. Nesakumar, and K. Chandramohan, Res. J. Eng. Sci., 2 (2013) 11-14.
- 48. R. Vijayaram and K. Chandramohan, Eng, J. Chem. Engg. Process Technol., 4 (2013) 1-3.
- 49. E. Kantarelis, W. Yang, W. Blasiak, C. Forsgren, and A. Zabaniotou, Appl. Energy., 88 (2011) 922-929.
-

- 50. A. Tripathi, M. Kumar, D.C. Sau, A. Archana, C. Sanchita, and T.R. Mankhand, Int. J. Metallurgical Engg., 1 (2012) 17-21.
-

- 51. E.-Y. Yazici and H. Deveci, Hydrometallurgy., 139 (2013) 30-38.
-

- 52. U. Jadhav and H. Hocheng, Nature Publishing Group., 5 (2015) 14574.
-

- 53. Z. Ping, F. Zeyun, L. Jie, L. Qiang, Q. Guangren, and Z. Ming, J. Hazard. Mater., 166 (2009) 746-750.
-

- 54. X.-L. Xu and J.Y. Li, J. Qingdao University (E&T)., 26 (2011) 69-73.
- 55. L. Jing-ying, X. Xiu-li, and L. Wen-quan, Waste Manage, Elsevier Ltd., 32 (2012) 1209-1212.
-

- 56. A. Chaurasia, K.K. Singh, and T.R. Mankhand, Int. J. of Metallurgical Engg., 2 (2013) 243-248.
-

- 57. M. Cheikh, J.P. Magnin, N. Gondrexon, J. Willisn, and A. Hassen, Environmental Tech., 31 (2010) 1577-1585.
- 58. K.-J. Hong, S. Tokunaga, and T. Kajiuchi, J. Hazard. Mater., 75 (2000) 57-73.
-

- 59. L. Barbieri, R. Giovanardi, I. Lancellotti, and M. Michelazzi, Environmental Chemistry Letters., 8 (2010) 171-178
-

- 60. S.-G. Schmelzer and H. Wolf, Aufbereit,-Tech., 37 (1996) 149.
- 61. Z. Shunli, F. Eric, Res. Conserv. Recycl., 21 (1997) 247.
- 62. J. Cui, L. Zhang, and L.J. Hazard. Mater., 158 (2008) 228-256.
- 63. G. Hilson and A.J. Monhemius, J. Clean. Prod., 14 (2006) 1158-1167.
-

- 64. R. Montero, A. Guevara, and E. De La Torre, J. Earth Sci. and Eng., 2 (2012) 590-595.
- 65. A. Işıldar, E.R. Rene, E.D. Van Hullebusch, and P.N.L. Lens, Advances in Recycling and Waste Manag., 2 (2017) 1-9.
-

- 66. J.-R. Cui and L.F. Zhang, J. Hazard. Mater., 158 (2008) 228-256.
-

- 67. C.-L. Brierley, Trans. Nonferrous Met. Soc. China., 18 (2008) 1302-1310.
-

- 68. D. Mishra and Y.H. Rhee, Current Res. Technol. Edu. Appl Microbiology and Microbial Biotechnol., 2 (2010) 1289-1292.
- 69. N. Zhu, Y. Xiang, T. Zhang, P. Wu, Z. Dang, P. Li, and J. Wu, J. Hazard. Mater., 192 (2011) 614-619.
-

- 70. W.-J. Dai, J. Bai, J. Gu, W. Zhang, C. Wang, and P. Zhao, J. Spectroscopy., 1 (2014) 1-8.
- 71. N.-P. Prasanna and S.P. Kumar. J. Chemical and Pharma- ceutical Sci., 8 (2015) 268-72.
- 72. A. Mrazikova, R. Marcincaková, J. Kadukova, and O. Velgosova, J. Polish Mineral Eng. Soc., 2 (2013) 59-62.
- 73. J. Willner, J. Achivements in mat. And manufacturing Engg., 2 (2012) 860-863.
-

- 74. J. Willner and A. Fornalczyk, Silesian University of Technol., Department of Metallurgy, ul. Krasińskiego, 39 (2013) 198-208.
- 75. N.-N. Adhapure, S. S. Waghmare, V. S. Hamde, and A.M. Deshmukh, Appl. Biochemistry and Microbiology., 49 (2013) 279-284.
-

- 76. G. Weihua, B. Jianfeng, D. Jue, Z. Chenglong, Y. Wenyi, W. Jingwei, W. Pengcheng, and Z. Xin, J. Spectroscopy., 1 (2014) 1-8.
- 77. G. Liang, Y. Mo, and Q. Zhou, Enzyme and Microbial Technol., 47 (2010) 322-326.
-

- 78. N.-I. Chidi and O. Osibanjo. Waste Manag., 28 (2008) 1472-1479.
-

- 79. T.-D. Chi, J. C. Lee, B. D. Pandey, K. Yood, and J. Jeong, Minerals Engineering., 24 (2011) 1219-22.
-

- 80. M. Chen, J. Huang, O. A. Ogunseitan, N. Zhu, and Y.M. Wang, Waste Manag., 41 (2015) 142-147.
-

- 81. Y. Xiang, P. Wu, N. Zhu, T. Zhang, W. Liu, J. Wu, and P. Li, J. Hazard. Mater., 184 (2010) 812-818.
-

- 82. T. Yang, Z. Xu, J. Wen, and L. Yang, Hydro- metallurgy., 97 (2009) 29-32.
-

- 83. J. Wang, J. Bai, J. Xu, and B. Liang, J. Hazard. Mater., 172 (2009) 1100-1105.
-

- 84. S. Ilyas, M.A. Anwar, S.B. Niazi, and M.A. Ghauri, Hydrometallurgy., 88 (2007) 180-188.
-

- 85. S. Ilyas, C. Ruan, H.N. Bhatti, M. A. Ghauri, and M.A. Anwar, Hydrometallurgy, 101 (2010) 135-140.
-

- 86. P. Li, N. Zhu, T. Zhang, Y. Xiang, J. Wu, P. Wu, and Z. Dang, J. Hazard. Mater., 192 (2011) 614–619.
-

 This Article
This Article
-
2022; 23(1): 90-98
Published on Feb 28, 2022
- 10.36410/jcpr.2022.23.1.90
- Received on Sep 16, 2021
- Revised on Sep 25, 2021
- Accepted on Oct 4, 2021
 Services
Services
- Abstract
introduction about pcbs-environmental problem
toxic substance in pcbs and their harmful impacts on mankind
metal recovery from pcbs followed by chemical leaching
bioleaching of heavy metals from pcbs
conclusions
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Murugesan Manikkampatti Palanisamy
-
Department of Chemical and Electrochemical Engineering, Central Electrochemical Research Institute (CSIR˗CECRI), Karaikudi, Tamil Nadu, India-630003
Tel : +9566604416 - E-mail: murugesanmp@esec.ac.in






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.