- Optimal type and ratio of foaming agent, foaming stabilizer and fluxing agent of foam glass fabricated with red mud and coal gangue
Jina Wang, Kaidong Xu*, Zhixin Li, Yilong Yang, Qingxiao Li, Yun Bao, Huan Yang, Lingling Ding, Ruixin Zhang, Yuanyuan Wang and Lan Yao
School of Material and Chemical Engineering, Henan University of Urban Construction, Pingdingshan 467036, P. R. China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In order to effectively reuse red mud and coal gangue, foam glass was successfully prepared by sintering. The foaming of foam glass is ensured by adding foaming agent, foam stabilizer and fluxing agent to the raw materials including red mud, coal gangue and waste glass. Furthermore, the defects in the foam glass are also reduced. The obtained powder was dried at 105 ºC and characterized by XRF, DSC-TG analysis, etc. According to cellular structure, apparent density and water absorption, the optimal type and ratio of foaming agent, foaming stabilizer and fluxing agent were revealed. The results indicate that the optimized formula of foaming agent, foaming stabilizer and fluxing agent is 3% MnO2-2% Na3PO4-2% borax. The foam glass prepared under optimized conditions exhibits uniform porous distribution and suitable pore size with an apparent density of 0.575 g/cm3 and water absorption of 4.61%. The research in this work could establish the technical foundation of red mud-based porous material for industrial production
Keywords: Foam glass, Foaming agent, Foaming stabilizer, Fluxing agent, Optimal type and ratio
Red mud has been the major solid wastes of Bayer process of alumina extraction from bauxite, while the annual production of red mud reaches 50 to 80 million tons in the global world [1]. Researchers have made many efforts to recycle the red mud in different fields, such as cement, sintered brick, ceramsite, adsorbent materials and extraction of noble metal. However, the utilization of red mud owing to its relatively high alkali content is still a worldwide problem and thus largely restricts its potential application [2-7]. Coal gangue is one of the solid wastes in the process of coal mining and washing. The amount produced in China is reached above several million tonnes and increases by about one million tonnes per year [8]. Although it has been reused in the fields of cement and concrete, etc., the utilization ratio is still comparatively low compared to the high generation of 1.3 billion tons every year [9-11]. The disposal of waste glass has also become a global environmental problem. It amounts to about 2–8% in urban waste [12], and the amounts of generation reach up to 7.5 million tons every year in China. However, due to the low recovery rate of 25%–30%, most solid wastes can only be placed in the open air. Therefore, it is of great importance to find a suitable way to recycle red mud, coal gangue and waste glass.
As the demand for energy saving and environmental-friendly products is increasing, foam glass has been applied in the fields of green construction, fire prevention and thermal insulation due to the dramatic feathers of anti-corrosion and thermostability. During the past decades, foam glass prepared by solid wastes, such as tailings, sludge, etc., have been widely studied [13-16]. Using solid waste in foam glass production is for the consideration of protecting environment and reducing the production cost of foam glass because it expands the range of choices for raw materials. It leads to a new trend in commercialized production of glass foams [17]. To our knowledge, coupling red mud with coal gangue to prepare foam glass has not yet largely studied. Furthermore, red mud and coal gangue is rich in SiO2, CaO and Al2O3, which is the basic composition of foam glass [18, 19]. In addition, it is beneficial to reduce the sintering temperature and cut the cost due to the high content of alkali metal elements in the red mud, which is consistent with the findings of D. S. Jung et al., who found the glass transition temperature was deceased from 517.3 oC to 468 oC when the amount of Na2O added to the product was changed from 0 to 5.7 wt.% [20]. Thus, it is a feasible way to prepare foam glass with the red mud, coal gangue and waste glass, and provides an important means to recycle red mud, coal gangue and waste glass scrap as well. However, considering the prepared foam glass with a low quality, it is necessary to investigate the proper type and ratio of foaming agent, foaming stabilizer and fluxing agent to accommodate with the material system and sintering temperature.
In this study, the red mud, coal gangue and waste glass were selected as the raw materials to prepare the foam glass. The types and contents of admixtures on cellular structure, apparent density and water absorption were investigated systematically. Meanwhile, the tech- nological parameters were well optimized. The research in this work could establish the foundation for preparing foam glass with solid wastes.
Materials
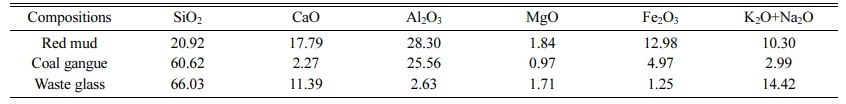
Red mud, coal gangue and waste glass were purchased from Henan shenhuo new materials Co. Ltd. Calcium carbonate (CaCO3), manganese dioxide (MnO2), sodium silicate (Na2SiO39H2O), trisodium phosphate (Na3PO4), sodium carbonate (Na2CO3), borax and boracic acid were purchased from Sinopharm Chemical Reagent Beijing Co. Ltd. All chemicals were analytical grade and used as received without further purification. The composition of red mud, coal gangue and waste glass were shown in Table 1.
Experiment design
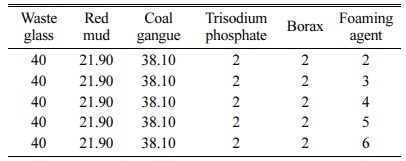
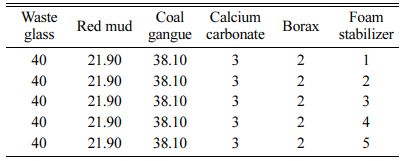
The amounts of foaming agent, foaming stabilizer and fluxing agent were shown in Table 2, 3 and 4, respectively.
Preparation of foam glass
The samples were prepared as follows. The red mud, coal gangue and waste glass are grinded into powder respectively and screened through a 180-mesh sieve. Then, the red mud, coal gangue, waste glass, foaming agent, foam stabilizing agent and flux are evenly mixed according to the design content of the formula. After aging process of 48 hours, the green body was obtained by pressed in mould and dried at 105 oC inside the drying oven. Finally, the dried green body was put into the resistance furnace for high temperature sintering, and the foam glass was prepared.
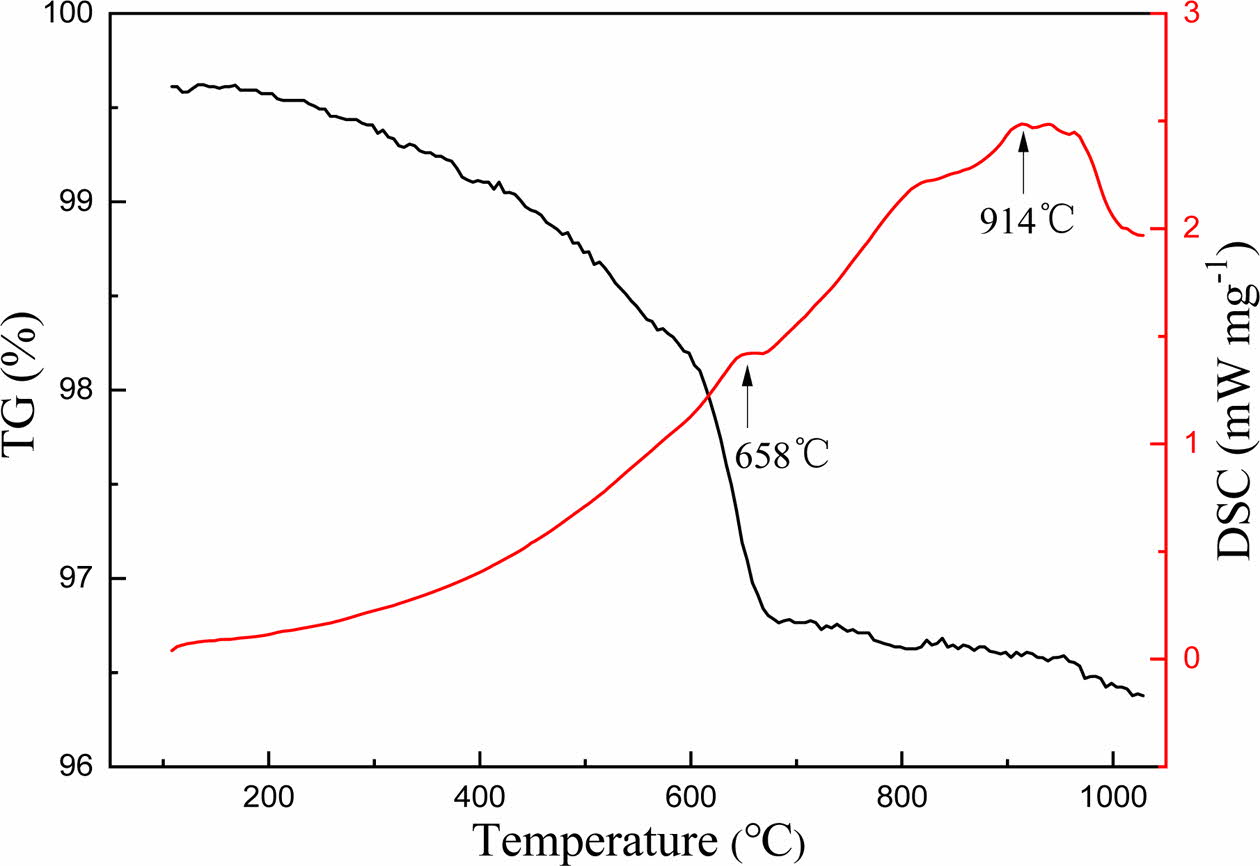
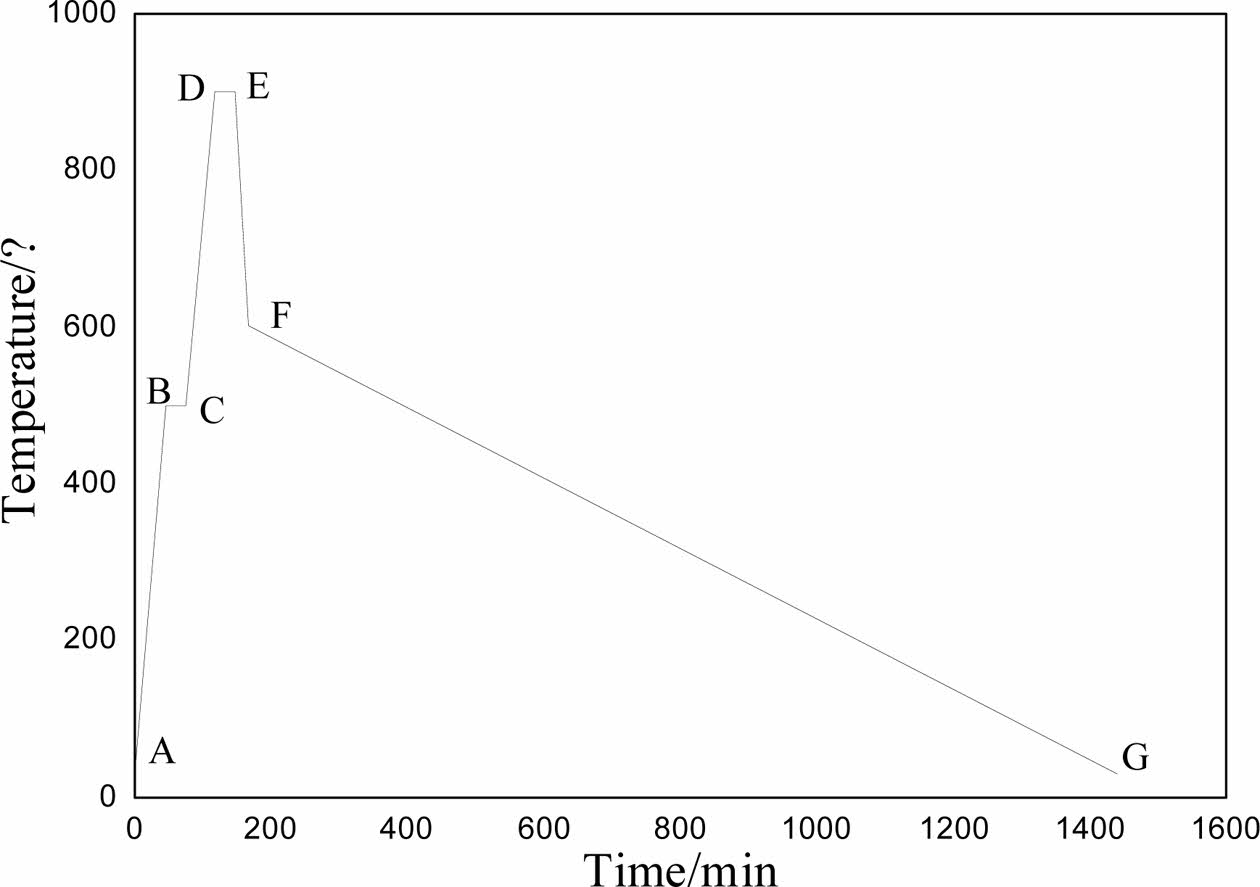
The DSC-TG spectra of the sample was shown in Fig. 1. According to the results of DSC-TG spectra, the preparation process of foam glass was illustrated in Fig. 2. The first stage (ABC, preheating), the green body was heated to 500 oC at a rate of 10 oC min−1 and maintained at 500 oC for 30 minutes. The second stage (CDE, foaming), the sample was heated to 950 oC and maintained at this temperature for 30 min. The third stage (EF, foam stabilization), the cellular structure was formed at this stage. The last stage (FG, annealing), cooling to room temperature within the furnace, the internal stress of foam glass was eliminated gradually to avoid the appearance of cracks.
Characterization of the foam glass
The chemical compositions of red mud, coal gangue and waste glass were measured by X-ray fluorescence spectroscopy (XRF), and the cellular structure of foamed glass was observed by using camera. The DTA-TG analysis of samples was carried out by the DSC/TG (Netzsch STA449F3, German). The apparent density was measured according to the Archimedes principle : F = ρgv, F: buoyance, ρ: density of H2O, g: acceleration of gravity, v: volume of drained water. Water absorption can be calculated based on the equation: Wm=(m2-m1)/m1 , Wm: water absorption, m1: the mass of material in dry condition, m2: the quality of material in saturated state of water absorption.
|
Fig. 1 DSC/TG curves of sample. |
|
Fig. 2 The sintering process of foam glass. |
|
Table 2 Raw materials formula and additive design of foaming agent for foam glass (wt/%) |
|
Table 3 Raw materials formula and additive design of foam stabilizer for foam glass (wt/%) |
|
Table 4 Raw materials formula and additive design of fluxing agent for foam glass (wt/%) |
The influence of foaming agent on cellular struc- ture and properties of foam glass
The influence of foaming agent on cellular structure
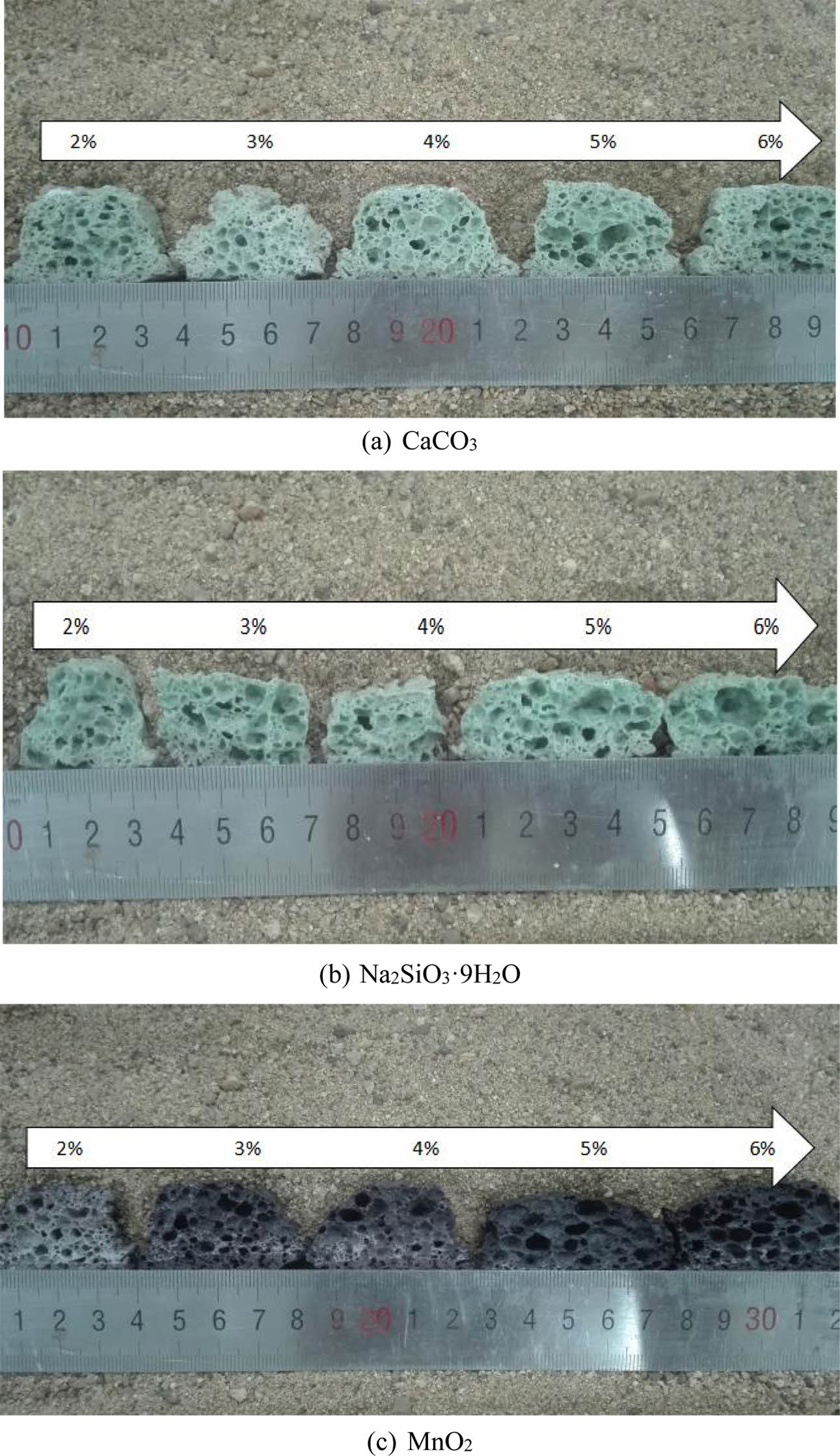
The digital images of foam glass with different foaming agent and the adding amount were illustrated in Fig. 3. It can be seen that the type and amount of foaming agent showed a significant impact on the cellular structure. For CaCO3, the pore size of honeycomb hole becomes larger gradually and the wall got thinner along with increasing the contents. The products showed a thick pore wall and low porosity with lower content of CaCO3. However, if the mass fraction of CaCO3 was increased by more than 4%, the pore coalescence emerged and cellular structure has been broken as depicted in Fig. 3(a). For Na2SiO39H2O, the generated cellular structure was irregular with serious pore coalescence (as shown in Fig. 3(b)). For MnO2, As shown in Fig. 3(c), the foaming process was fully achieved with a uniform pore size and thin pore wall. Similarly, if the mass fraction of MnO2 was enlarged more than 3%, the pore coalescence appeared. Based on the above analysis, the mechanism can be illustrated as below: with the increasing addition of foaming agent, gas was generated from the decomposition of foaming agent, leading to increased internal pressure and formation of connectivity pore. This agrees well with the findings of N. Chen et al., who found when the content of foaming agent was too small, it was difficult to mix glass powder and foaming agent evenly, resulting in uneven distribution of pores. And excessive content of foaming agent can cause the accumulation of small pores to form large holes, leading to non-uniformity of pore size and bad foaming quality [12].
The influence of foaming agent on apparent density
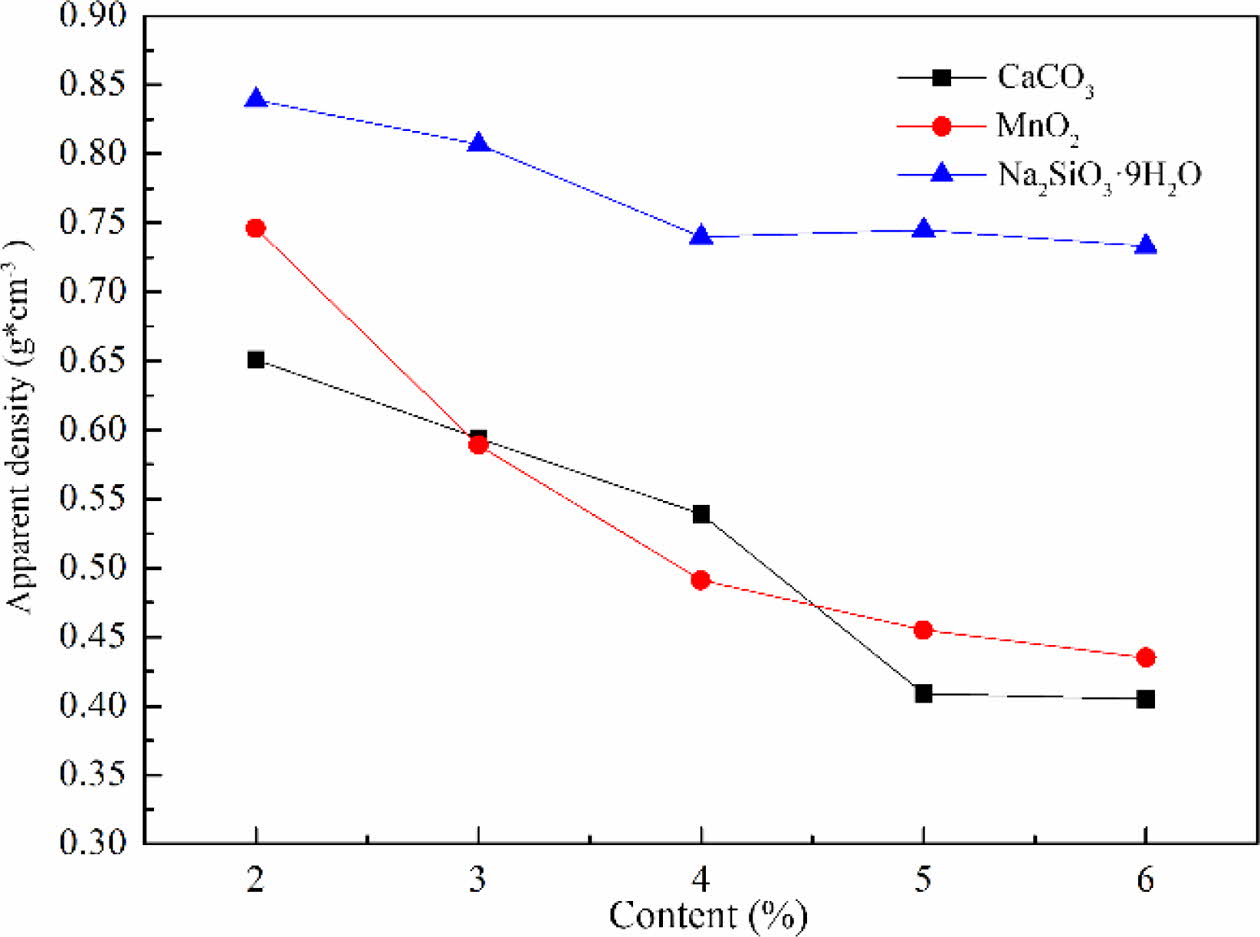
The apparent density of foam glass with different foaming agent and the adding amount were shown in Fig. 4. It can be noted that the apparent density of foam glass decreased along with increasing the contents of foaming agent, especially for the CaCO3 and MnO2. Nevertheless, for Na2SiO39H2O, the foam glass ex- hibited a higher apparent density with a much slower tendency of decline comparing to other two foaming agents. Moreover, the apparent density of sample obtained with Na2SiO39H2O was almost 1.6 times higher than that with the MnO2 when the addition was up to 5%. These results were quite agreed with the features of cellular structure shown in Fig. 3. In general, the foaming performance of samples with CaCO3 or MnO2 was better than that with Na2SiO39H2O due to the lower apparent density.
The influence of foaming agent on water absorption
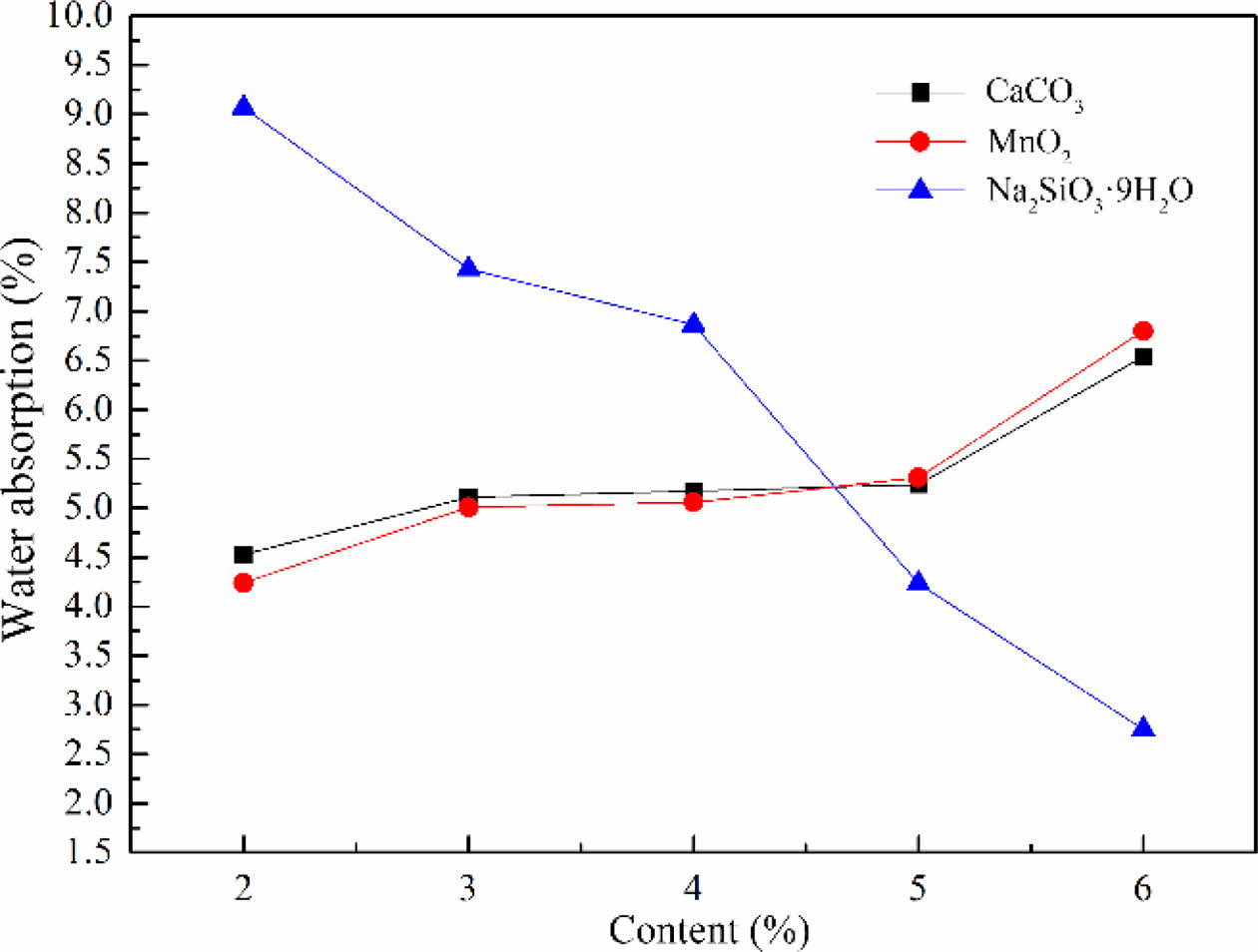
The water absorption of foam glass with different foaming agent and the adding amount were shown in Fig. 5. It should be noted that the water absorption of foam glass increased with increasing the contents of foaming agent, especially for the samples with CaCO3 or MnO2. When the CaCO3 or MnO2 was added no more than 5%, the water absorption increased slowly. Furthermore, the water absorption of the samples with CaCO3 or MnO2 increased significantly with an addition of more than 5%. This result indicated that the increased water absorption came from the microporous and interconnecting pores, resulting from the gas decomposed from the foaming agent and the risen internal pressure. While the Na2SiO39H2O was chosen as the foaming agent, it exhibited a reverse tendency that the water absorption declined with increasing the contents of Na2SiO39H2O. From these considerations we concluded that Na2SiO39H2O was not matched well with the material system, leading to the gas releasing from glass melt. This was agreed with foregoing influences of foaming agent on cellular structure and apparent density of foam glass. Based on the above results, MnO2 was selected as the foaming agent and the optimized mass fraction was about 2-3%.
The influence of foaming stabilizer on cellular struc- ture and properties of foam glass
The influence of foaming stabilizer on cellular structure
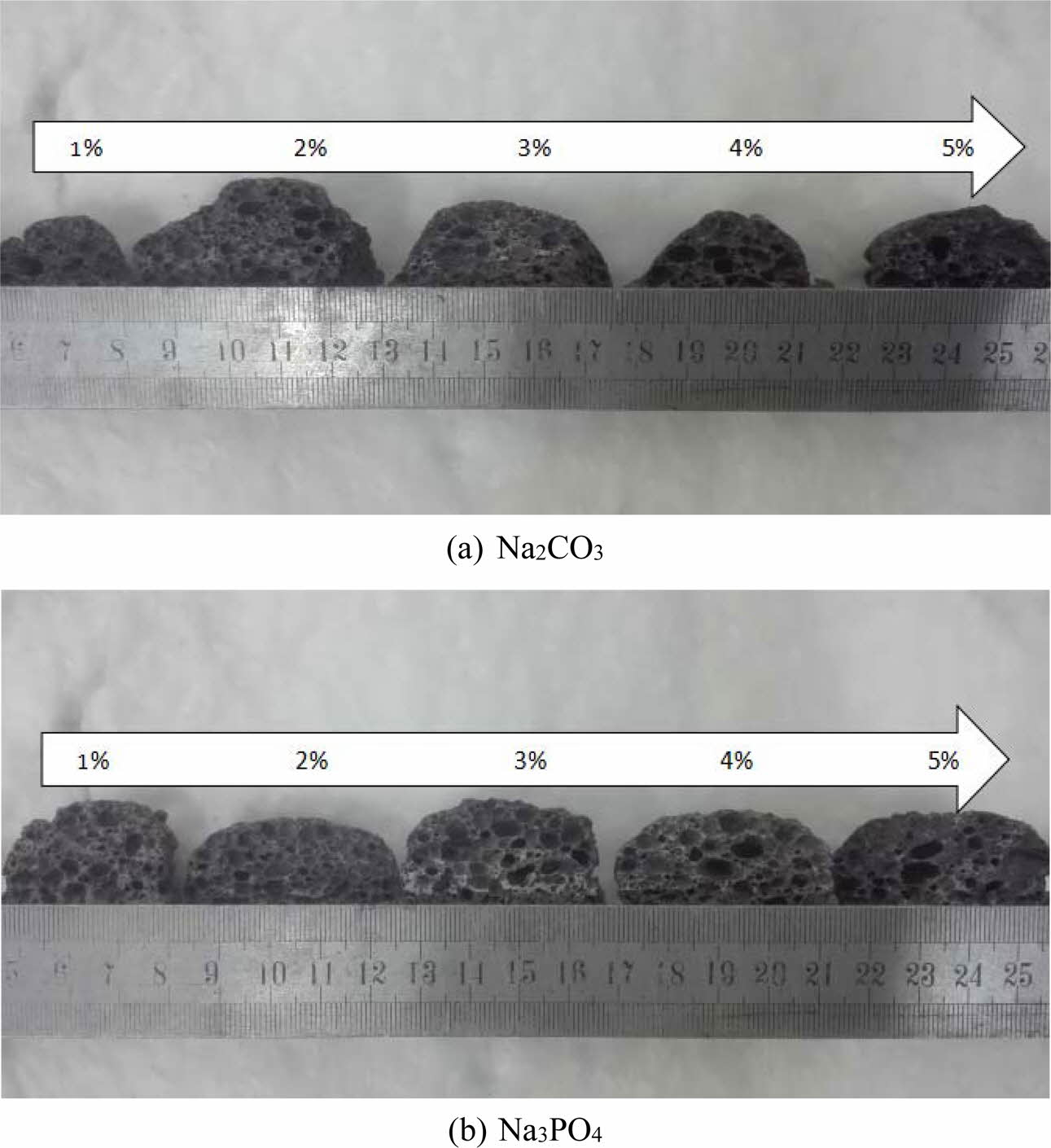
The digital images of foam glass with different foaming stabilizer and the adding amount were exhibited in Fig. 6. It can be seen that the addition of Na2CO3 caused the accumulation of small pores to form large or connected holes, while Na3PO4 led to an even distri- bution of pores when its content was appropriate. By increasing the contents of Na2CO3, the pore coalescence emerged seriously. Besides, peeling and blocking appeared on the surface of samples (as shown in Fig. 6(a)). For Na3PO4, if there was a low content in the mixture, it showed a quite good effect of foamstabilization. A typical 2 mm-hole was clearly identified, which was assigned to the mass fraction of 2% of Na3PO4. However, if the Na3PO4 was added more than 4%, pore coalescence emerged, resulting to the formation of connectivity pore (as shown in Fig. 6(b)). Based on the above analysis, the addition of Na3PO4 showed enhanced foamstabili- zation effect compared to that of the Na2CO3.
The influence of foaming stabilizer on apparent density
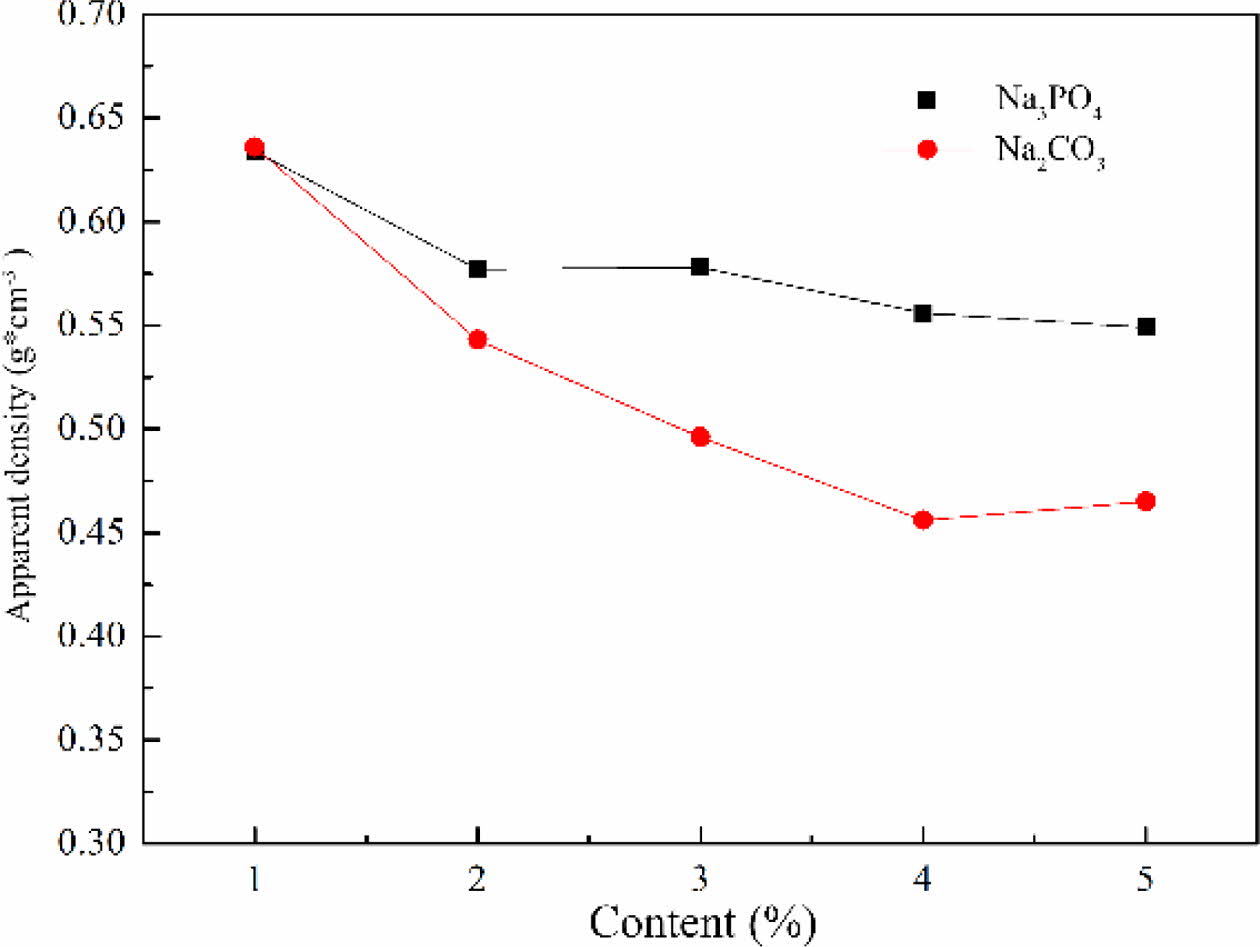
The apparent density of foam glass with different foaming stabilizer and the adding amount were shown in Fig. 7. It was found that the apparent density of foam glass decreased by increasing the contents of foaming stabilizer of Na2CO3 or Na3PO4. For a same addition of Na2CO3 or Na3PO4, the samples with Na2CO3 exhibited a lower apparent density than that with Na3PO4. It can be analyzed that when the Na3PO4 was selected as foaming stabilizer, the surface tension reduced and most of the gas run away, leading to a higher apparent density. For Na2CO3, based on the synergistic contri- bution of gas escaping and fluxing effect, the apparent density of the sample decreased remarkably with increas- ing the content of Na2CO3. This indicates that Na2CO3 as the foaming stabilizer caused the accumulation of small pores to form large or connected holes, thus decreasing the apparent density significantly.
The influence of foaming stabilizer on water absorption
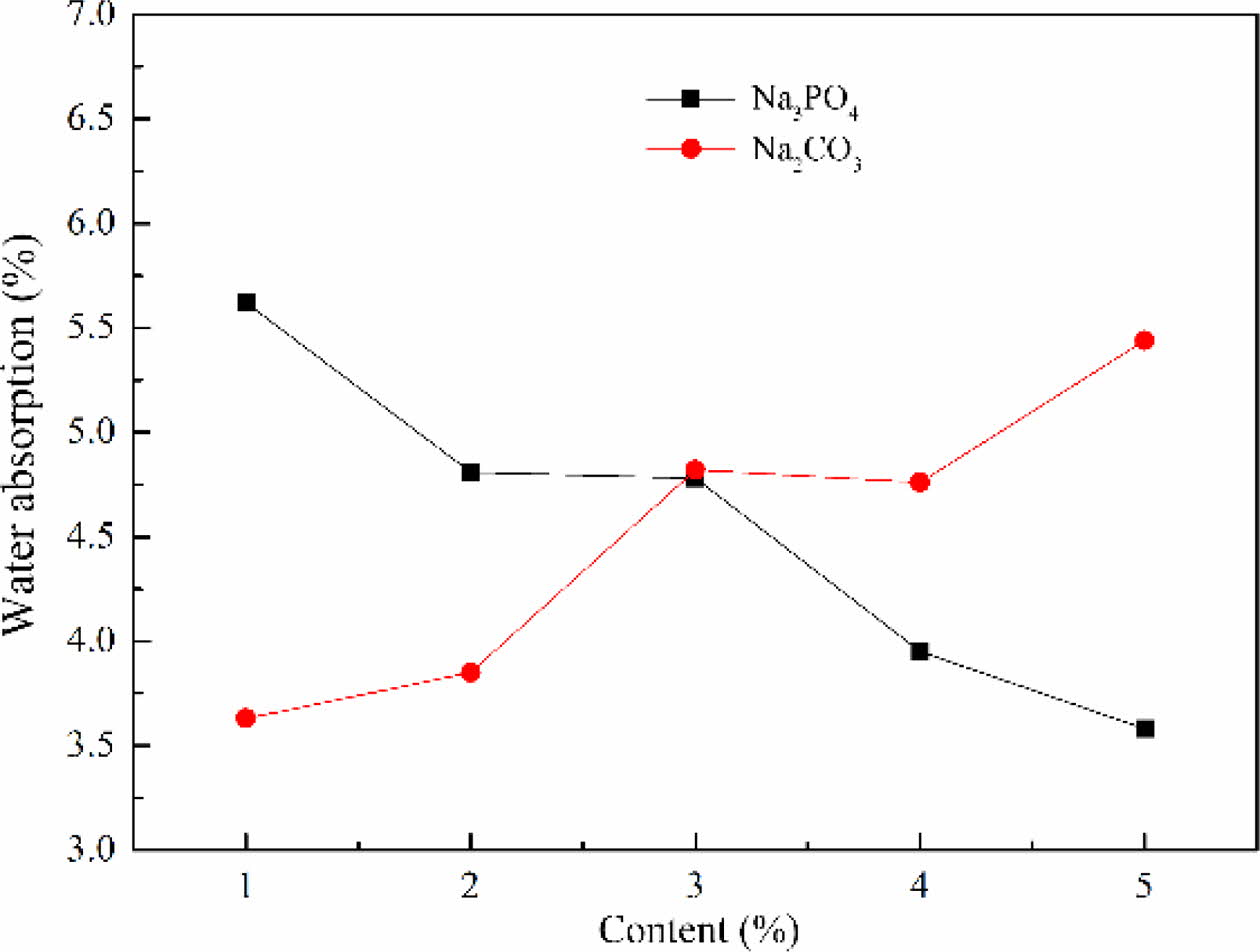
The water absorption of foam glass with different foaming stabilizer and the adding amount were shown in Fig. 8. This result implied that the water absorption of foam glass increased with increasing the content of Na2CO3. While the Na3PO4 was chosen as the foaming stabilizer, it exhibited a reverse tendency that the water absorption decreased with increasing content of Na3PO4. This result indicated that the increased water absorp- tion of sample with Na2CO3 obtained originating from the formation of pore coalescence and interconnecting pores, which was benefit for water to flow in the pores of foam glass. From these considerations we indicated that Na3PO4 showed well matching with the material system. Based on the above results, Na3PO4 was selected as the foaming stabilizer and the optimized mass fraction was about 2–3% with a low water absorption of 4.8%.
The influence of fluxing agent on cellular structure and properties of foam glass
The influence of fluxing agent on cellular structure
The digital images of foam glass with different fluxing agent and the adding amount were shown in Fig. 9. There was a big difference on fluxing effect between borax and boracic acid. The pore size of foam glass got bigger with increasing the content of borax. As shown in Fig. 3(a), a typical 2-3 mm honeycomb hole can be noted, which was assigned to the mass fraction of 2% of borax. However, if the borax was added more than 3%, pore coalescence emerged, leading to the irregular pore structure. Compared to borax, the pore coalescence emerged seriously along with the increasing content of boracic acid. This observation suggested that boracic acid was not matched well with the material system.
The influence of fluxing agent on apparent density
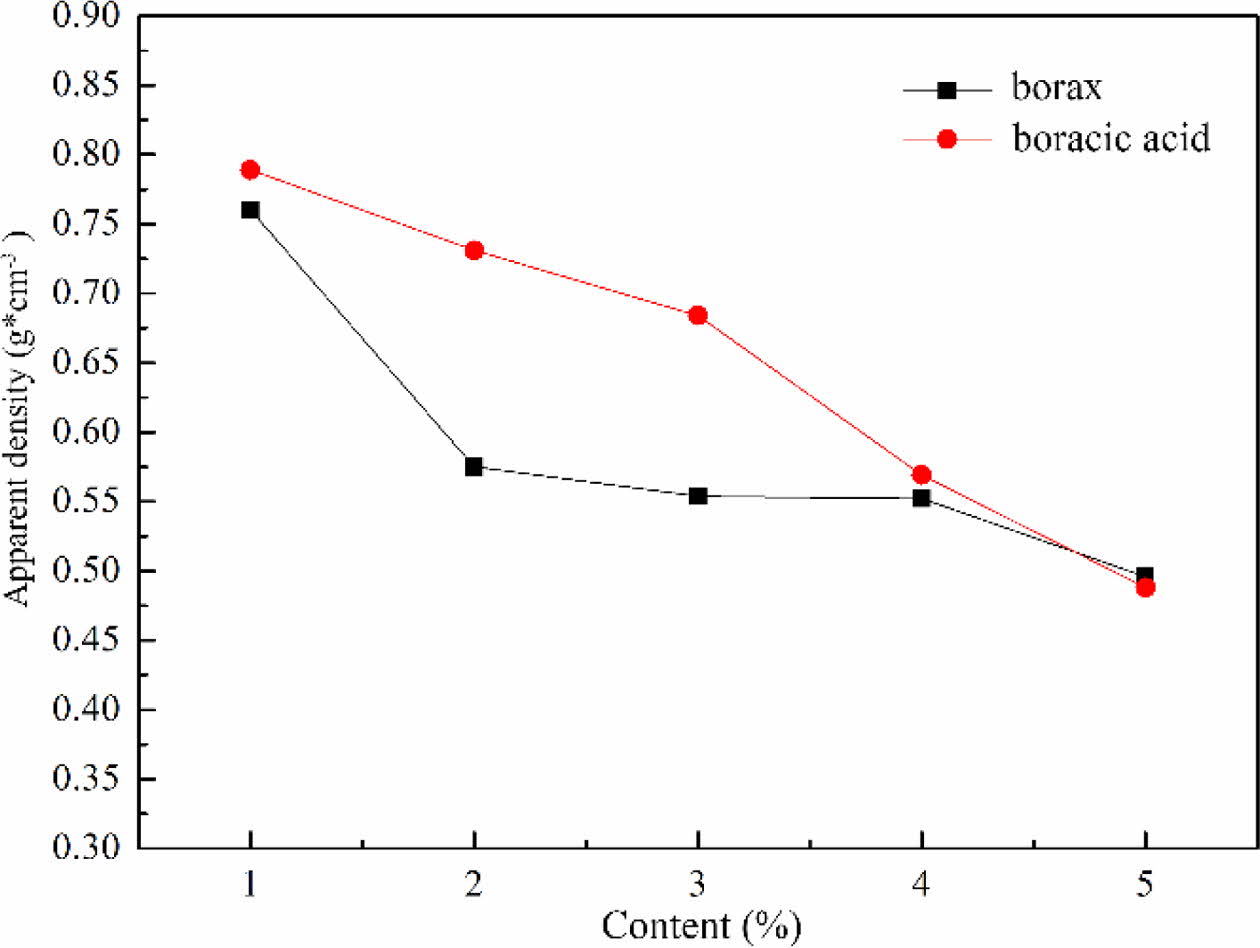
The apparent density of foam glass with different fluxing agent and the adding amount were shown in Fig. 10. It was obviously observed that the apparent density of foam glass dropped by increasing the content of fluxing agent. For a same addition of borax and boracic acid, the sample with borax exhibited a lower apparent density than that with boracic acid. These results were quite agreed with the features of cellular structure shown in Fig. 9. Based on the above results, borax was selected as the fluxing agent and the optimized mass fraction was about 2% with an apparent density of 0.575 g/cm3.
The influence of fluxing agent on water absorption
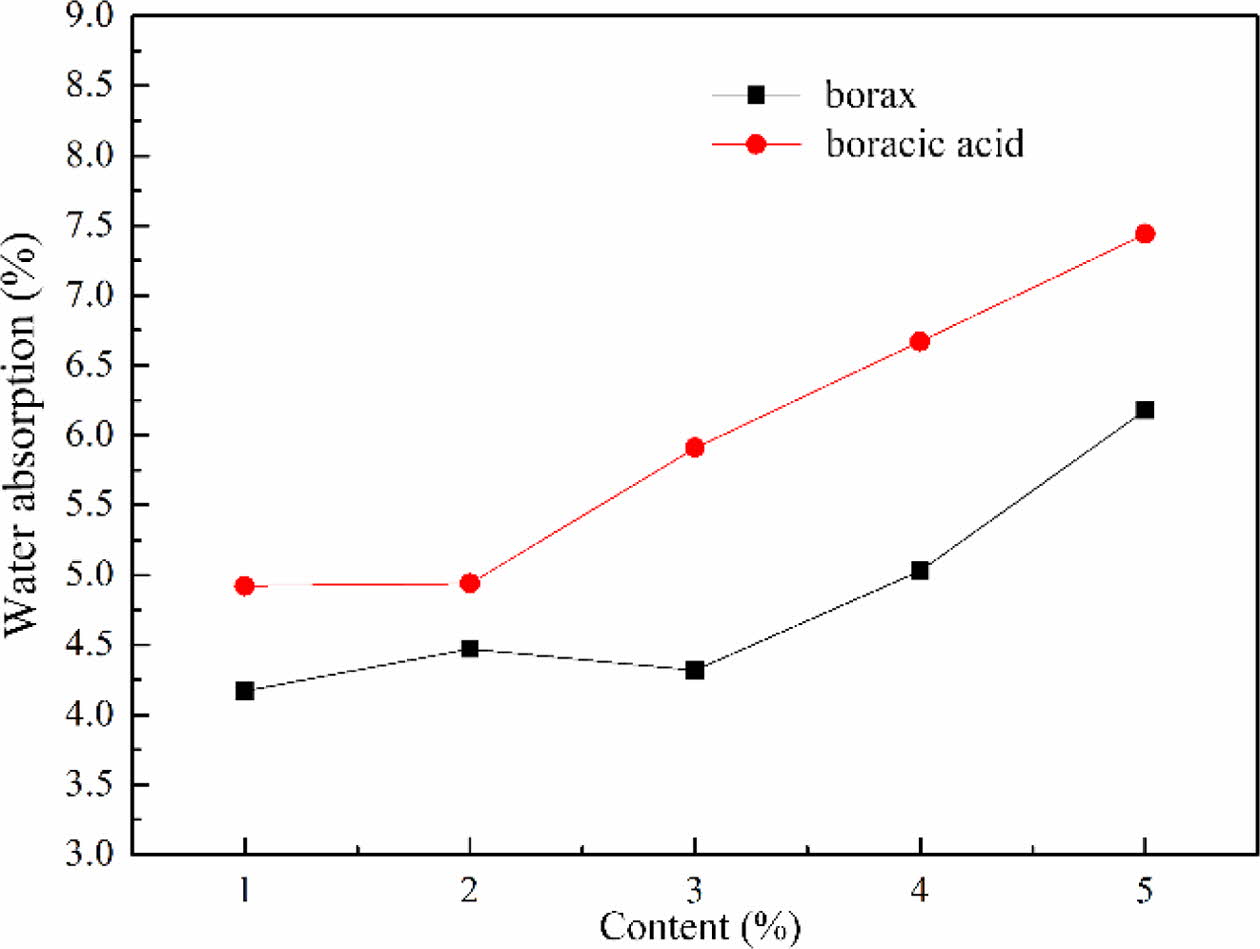
The water absorption of foam glass with different fluxing agent and the adding amount were shown in Fig. 11. The water absorption of foam glass increased with increasing the content of fluxing agent. For a same addition of borax and boracic acid, the sample with borax exhibited a lower water absorption than that with boracic acid. Under the same foaming tempera- ture, the viscosity of melting glass with fluxing agent can be significantly reduced compared to that without fluxing agent. This was favor for the gas to be generated, which induced to form the pore coalescence and increased the water absorption. From the analysis we considered that borax showed well matching with the material system and the optimized content was about 2% with a water absorption of 4.61%.
|
Fig. 3 The effect of the type and amount of foaming agent on the cellular structure of foam glass. |
|
Fig. 4 The effect of the type and amount of foaming agent on the apparent density of foam glass. |
|
Fig. 5 The effect of the type and amount of foaming agent on the water absorption of foam glass. |
|
Fig. 6 The effect of the type and amount of foaming stabilizer on the cellular structure of foam glass. |
|
Fig. 7 The effect of the type and amount of foaming stabilizer on the apparent density of foam glass. |
|
Fig. 8 The effect of the type and amount of foaming stabilizer on the water absorption of foam glass. |

|
Fig. 9 The effect of the type and amount of fluxing agent on the cellular structure of foam glass. |
|
Fig. 10 The effect of the type and amount of fluxing agent on the apparent density of foam glass. |
|
Fig. 11 The effect of the type and amount of fluxing agent on the water absorption of foam glass. |
In this study, optimal type and ratio of foaming agent, foaming stabilizer and fluxing agent of foam glass with red mud and coal gangue were investigated, the following conclusions were drawn:
(1) The type and amount of foaming agent show a significant impact on the cellular structure, and MnO2 is selected as the foaming agent and the optimized mass fraction is about 2-3%.
(2) The addition of Na2CO3 causes the accumulation of small pores to form large or connected holes, while Na3PO4 leads to an even distribution of pores when its content is appropriate at 2%. Thus, Na3PO4 as the foaming stabilizer is chosen.
(3) The borax shows well matching with the material system and the optimized content is about 2%, at this time, a typical 2-3 mm honeycomb hole can be noted.
(4) Under the optimized addition of 3% MnO2-2% Na3PO4–2% borax, the prepared foam glass exhibits good performance with an apparent density of 0.575 g/cm3 and water absorption of 4.61%. Meanwhile, the obtained products showed uniform macroporous struc- ture by introducing the admixtures in the precursor. Systematic studies clearly reveal that the dramatic foaming effect is ascribed to MnO2 and borax is matched well with the material system.
(5) According to the experimental and analyzed results, this study offers brand new idea to discover property and process parameters, which may conduce to a better grasp of sustainable applications for solid wastes.
This work was supported by the Special Fund for New Wall Materials of Henan Province and Natural Science Foundation of Henan Province [182300410135].
- 1. N.T. Trana, D.V. Q. Nguyena, V.M.H. Hoa, X.T. Danga and N.Q. Tran, J. Ceram. Process Res. 18 (2017) 366-372.
- 2. R.C.O. Romano, H.M. Bernardo, M.H. Maciel, R.G. Pileggi, and M.A. Cincotto, J. Therm. Anal. Calorim. 131 (2018) 2477-2490.
-

- 3. P. Castaldi, M. Silvetti, S. Enzo, and S. Deiana, Clay Clay Miner. 59 (2011) 189-199.
- 4. J. Somlai, V. Jobbagy, J. Kovacs, S. Tarjan, and T. Kovacs, J. Hazard. Mater. 150 (2008) 541-545.
-

- 5. X. M. Liu, N. Zhang, H.H. Sun, J.X. Zhang, and L.T. Li, Cement Concrete Res 41 (2011) 847-853.
-

- 6. V.M. Sglavo, R. Campostrini, S. Maurina, G. Carturan, M. Monagheddu, G. Budroni, and G. Cocco, J. Eur. Ceram. Soc. 20 (2000) 235-244.
-

- 7. C. Venkatesh, R. Nerella, and M.S.R. Chand, J. Korean Ceram. Soc. 57 (2020) 167-174.
-

- 8. W. Danga and H.-Y. He, J. Ceram. Process Res. 21 (2020) 69-74.
-

- 9. Z.C. Wang, Z.C. Wang, and W.T. Zhao, J. Wuhan Univ Technol, 33 (2018) 427-430.
-

- 10. S.J. Zhao, F.H. Muhammad, L. Yu, M. Xia, X. Huang, B.Q. Jiao, N. Lu, and D.W. Li, Environ. Sci. Pollut. Res. 26 (2019) 25609-25620.
-

- 11. D. Wu, R.K. Zhao, W.T. Hou, and S. Wang, Arab. J. Sci. Eng. 45 (2020) 3469-3478.
-

- 12. N. Chen, S. Zhang, X. Pan, S. Zhou and M. Zhao, J. Porous Mater. 27 (2020) 621-626.
-

- 13. O.V. Suvorova, and D.V. Makarov, Glass Ceram. 76 (2019) 188-193.
-

- 14. K.S. Ivanov, Soil Mech. Found. Eng. 57 (2020) 92-96.
- 15. Y. Vaisman, A. Ketov, and P. Ketov, Glass Phys. Chem. 41 (2015) 157-162.
-

- 16. K.S. Ivanov, Refract. Ind. Ceram. 56 (2016) 621-625.
-

- 17. Y. Chia, J. Lina, and B. Xu, Glass Phys. Chem. 45 (2019) 104-110.
-

- 18. S.K. Rout, T. Sahoo, and S.K. Das, Cent. Eur. J. Eng. 3 (2013) 316-328.
-

- 19. X.M. Hou, C.S. Yue, M. Zhang, and K.C. Chou, Int. J. Min. Met. Mater. 18 (2011) 77-82.
-

- 20. D.S. Jung, S.K. Hong, H.Y. Koo, Y.D. Eo, M.J. Lee and Y.C. Kang, J. Ceram. Process Res. 10 (2009) 152-157.
 This Article
This Article
-
2022; 23(1): 79-85
Published on Feb 28, 2022
- 10.36410/jcpr.2022.23.1.79
- Received on Jan 19, 2021
- Revised on Jul 28, 2021
- Accepted on Aug 28, 2021
 Services
Services
- Abstract
introduction
experimental materials and methodology
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kaidong Xu
-
School of Material and Chemical Engineering, Henan University of Urban Construction, Pingdingshan 467036, P. R. China
Tel : +86 18537505973 Fax: +86 18537505973 - E-mail: kdxu@hncj.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.