- Comparison of the effect of different metal alloys on the esthetic appearance of dentin porcelain
Gonca Deste Gökaya*, Rukiye Durkanb, Perihan Oyarc and Gülsüm Gökçimend
aBursa Uludağ University, Faculty of Dentistry, Department of Prosthodontics, Bursa, Turkey
bIstanbul Okan University, Faculty of Dentistry, Department of Prosthodontics, Istanbul, Turkey
cHacettepe University, School of Health Services, Dental Prosthetics Technology, Ankara, Turkey
dAfyonkarahisar Health Sciences University, Faculty of Dentistry, Department of Prosthodontics, Afyonkarahisar, TurkeyThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The purpose of this study was to examine the effect of aurofilm masking agents applied to various metal alloys on the color of porcelain in metal-ceramic restorations (MCRs). The study was conducted with 2 different base-metal alloys (Ni-Cr, Co-Cr) and 2 different noble alloys (Pd-based, Au-Pd) used for MCRs, as well as 1 high noble alloy (Au-based) that served as a control group. Eight experimental groups (n=7) and 1 control group were used in this study. An aurofilm masking agent was applied to 4 groups (AuPdM, PdM, CoCr, NiCrM). Opaque porcelain and dentin body porcelain were applied to all groups. CIEL*a*b* color coordinates were measured. The Pd group had the highest mean a* value (-5.82); however, in comparison to the control group, the differences in a* values were statistically significant only for the Cr-Co alloy groups (CoCr and CoCrM). The Pd group had the highest mean b* value (7.89). The ΔE value (2.13) of the CoCr group was significantly higher than all other alloy groups. Metal alloy substrate and aurofilm masking agents significantly affected the color of porcelain MCRs. However, color differences between base-metal and noble alloys and the control group were within clinically acceptable limits (ΔE<3.5).
Keywords: Metal-ceramic restorations, Ceramic alloys, Color matching, Ceramic
In case of missing teeth, fixed partial dentures (FPD) is a preferred treatment in addition to implant option for restoring esthetic and function. Metal ceramic restorations (MCRs) have served the dental profession well for nearly 50 years and are still considered to be the “golden standard for the fabrication of multiple-unit implant-or tooth-supported FDPs [1-3]. However, there are many all-ceramic options, these options tend to have lower survival rates compared to MCR, as a meta-analysis has shown [2, 4, 5].
Color is an important factor in the esthetic of metal-ceramic restorations. In addition, color reproduction in restorations represents one of the most challenging aspects of esthetic dentistry [6]. The metal framework provides necessary strength to the restorations. However, it negatively affects the esthetic appearance of the restoration [7, 8]. An initial thin layer of opaque porcelain (OP) that is applied to mask the dark metal oxide and promote the adhesion of the dental porcelain (DP). DP provides the anatomical tooth form and plays an important role in the esthetic outcome of the MCRs [9]. Özçelik et al. (2011) [10] has shown that OP thickness and/or its susceptibility to diffusion of oxides might affect the final color of the OP layer after it has been veneered to its metal framework. A number of authors [11-13] have observed color changes in porcelain that they attributed to specific metal ions, such as Pd and Ni, in the dental alloys used for MCRs. In cases where the OP layer is unable to satisfactorily camouflage the metal framework [14], commercially available masking agents designed for base-metal or noble alloys can be applied directly on the metal framework for more esthetic outcomes [15].
The masking ability of restorative materials can be assessed using the CIE L*a*b* color system to measure color differences. A spectrometer is used to measure values for L*(light-dark) a* (red-green) and b* (yellow-blue) color parameters, and differences between colors (ΔE) is calculated using the formula ΔEL,a,b = [(L1 − L2)2 + (a1 − a2)2 + (b1 − b2)2] [16]. The system makes it possible to use a spectrometer to detect even slight differences in color between two objects [17, 18]. Color differences may not be perceived by the human eye and thus the ΔE has been used to define both perceptual and clinically acceptable thresholds. [16]. If the ΔE between two objects is greater than the perceptual threshold, a color mismatch will be detected by the human eye. However, due to optical conditions in the oral environment, the clinically acceptable threshold is higher than the perceptual threshold [19]. If a restorative material has an ideal masking ability, color measurements of the material performed on white and black substrates will have a ΔE of zero [20]. This indicates that the substrate has no effect on material color.
Despite their widespread use, base metal alloys have rarely been the subject of colorimetric studies [8, 10, 21-23]. A literature review failed to identify any studies evaluating the effect of aurofilm masking agents (AMA) applied to noble and base-metal dental alloys on the color of dentin porcelain. Therefore, the current study aimed to assess the effect of AMAs and different alloys on the color of dentin porcelain. The null hypothesis was that the Au-rich aurofilm masking agents and different dental alloys would not affect the color of a 1-mm-thick layer of DP.
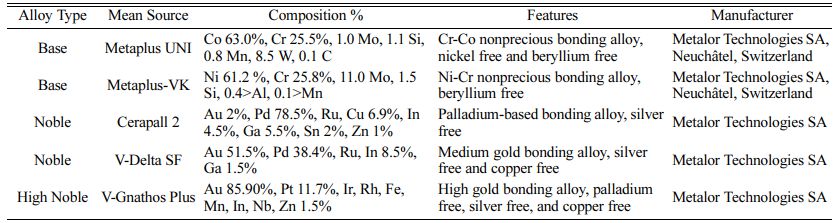
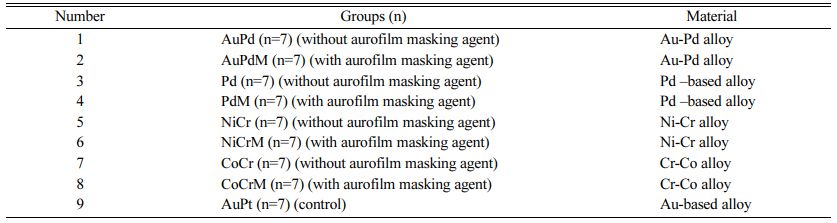
A total of 5 metal alloys were used in the study, including 2 noble alloys, the Au-Pd alloy (Group AuPd, AuPdM) and the Pd-based alloy (Group Pd, PdM); 2 base-metal alloys, the Co-Cr alloy (Group CoCr, CoCrM) and the Ni-Cr alloy (Group NiCr, NiCrM); and 1 high-noble alloy, the (Au-based) alloy (Group AuPt), which was designed as the control group. Material and group details are provided in Tables 1 and 2.
For each alloy, 14 wax disks 10x 1.00 0.1 mm (for control, 7 wax disks) were invested in phosphate bonded investment (AlphaCast Vario; Schütz Dental GmbH, Rosbach, Germany) and casted according to the manu- facturer’s recommendations. Alloys were casted in a centrifugal casting machine. Specimens were airborne-particle abraded with 250-μm aluminum oxide (Korox 250; BEGO, Bremen, Germany). Disk thickness was measured with a micrometer (Praecimeter S, 0.01 mm; Renfert GmbH) at four reference points that were marked on the back of each disk using a permanent marker. The same micrometer and reference points were used to measure the initial thickness of the metal disk as well as the thickness of the AMA, OP, and DP layers.
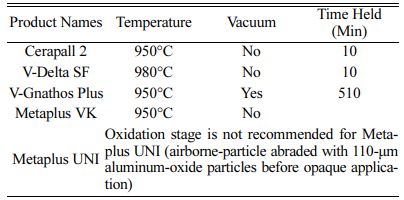
Specimens were steam-cleaned for 15 seconds and then oxidized (Programat X1; Ivoclar Vivadent, Schaan, Liechtenstein) according to the manufacturers’ instruc- tions (Table 3). Following oxidation, the Co-Cr alloy specimens (Group CoCr, CoCrM) were airborne-particle abraded with 110-μm aluminum oxide (Basic classic; Renfert GmbH).
A brush was used to apply a 0.1 mm (0.05 mm) coating of Metolar NP (Aurofilm NP, Metalor) aurofilm masking agent (AMA) to the specimens of Groups AuPdM, PdM, NiCrM, and CoCRM. Coated specimens were then placed in a ceramic furnace (Programat X1; Ivoclar Vivadent) set at 400 oC with the door open. After 6 min, the door was closed, and the furnace was heated at a rate of 80 oC per minute to a final temperature of 980 oC. Specimens were held at this temperature for 1-2 min, in accordance with the manufacturers’ instruc- tions, and then removed from the furnace [15].
Opaque porcelain (Shade A1, IPS d.SIGN Opaquer; Ivoclar Vivadent) was applied to all specimens. An initial layer was applied as a thin slurry, and after firing, a second, corrective layer was applied with a brush to compensate for shrinkage, and the specimens were fired again to obtain a uniformly thick 0.1 mm (0.05 mm) layer of OP.
Following OP application, a 1-mm thick coating of dentin body porcelain (Shade A1) was applied to all specimens. DP was also applied and fired according to the manufacturer’s instructions in two layers, with the second layer acting as a corrective layer to compensate for shrinkage [24]. OP and DP firing protocols are given in Table 4.
CIE L* (light-dark) a* (red-green) and b* (yellow-blue) [25] values were measured using a colorimeter (CR- 321; Konica Minolta, Tokyo, Japan; diameter of measurement area: 3 mm, with 45 circumferential illuminations by 30 optical fibers and 0-degree viewing geometry) [26, 27]. To ensure accurate results, a custom-fabricated teflon template was used to fix the position of the colorimeter so that the head was in contact with the specimen during measurement. L* a* b* values were measured at 3 different points on each specimen, for a total of 9 measurements per specimen.
Mean L* a* and b* values were calculated for each group both with and without the application of an AMA. Color measurements were performed twice on all specimens, once after the application of the OP (Ex) and again after the application of the DP (Ey). Differences between the two colour measurements (ΔE) were calculated as ΔE=Ey−Ex. Mean ΔE values for the groups in comparison to the control group were calculated using the mean L* a* b* values of the control group (Group AuPt) as a reference according to the equation ΔE(L,a,b) = [(L1 − L2)2 + (a1 − a2)2 + (b1 − b2)2] [16] where L1, a1 and b1 represent the values for the control group and L2, a2 and b2 represent the values for the groups.
Changes in L*, a*, and b* values and ΔE were evaluated using one-way analysis of variance (ANOVA) at a confidence level of 95% and the factors of alloy types, aurofilm masking agent, and the interaction between two factors. Mean values were compared using Bonferroni multiple comparison analysis (α = .05).
|
Table 4 Opaque porcelain, and dentin porcelain parameters according to manufacturers' instructions. |
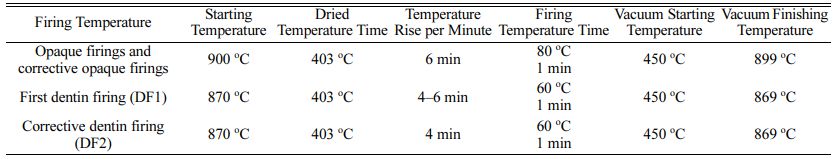
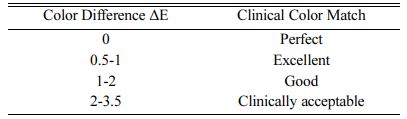
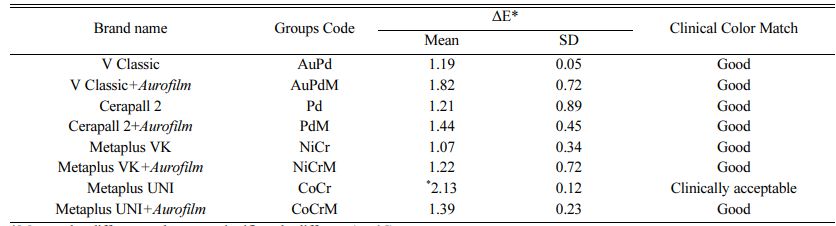
Clinical tolerance of color-matching according to ΔE values are given in Table 5 [28]. Means and standard deviations (SD) are given in Table 6, and ANOVA results are summarized in Table 7. ΔE values for all groups were found to be clinically acceptable.
The lowest L* values were obtained for Group PdM (92.450.76) (p>.05) and Group AuPd (92.450.45), and the highest L* value (L*=93.940.67) was obtained for Group CoCrM. Differences in L* values in comparison to the control group were not statistically significant (p>.05).
The lowest a* value was observed for Group NiCr (-4.480.12) and the highest for Group Pd (-5.820.54). In comparison to the control group, the differences in a* values were statistically significant for the Co-Cr alloy groups (CoCr and CoCrM), but not for any other groups.
The lowest b* value (5.950.29) was found in Group Pd and the highest b* value (7.890.19) in Group PdM. When an AMA was applied, the b* values of the Pd, NiCr, and CoCr groups did not vary significantly from those of the control group.
Statistically significant differences in color values were noted between the control group (Au-Pt) and the majority of the alloys tested (p<.001). The highest mean ΔE value (ΔE = 2.13) was measured in the CoCr group, and its color match was clinically acceptable (Table 7).
Translucency of all-ceramic restorations is an important factor in esthetic outcomes [29]. However, a high degree of translucency is not an advantage in all situations, for instance, in situations where restorations with metallic implant abutments, prefabricated cores, and cast metal post-and-cores are used [12, 30]. In these clinical conditions, in order to achieve acceptable esthetic results, a restorative material with optimal masking ability of the metallic frameworks is recommended [16]. This study investigated the effect of aurofilm masking agents on the color of porcelain used with various metal alloys. Based on the findings, the study’s null hypothesis that Au-rich aurofilm masking agents and different dental alloys would have no effect on the color of dentin porcelain was rejected.
The CIE L*A*B* color scale is an approximately uniform colour scale, i.e. a scale in which differences between points plotted in the colour space correspond to the visual differences between colours. The CIE L*A*B* color scale is organized in the form of a cube, with the vertical axis representing L*. The L* values range between zero, which corresponds to black, and 100, which corresponds to a perfect reflecting diffuser [31]. The horizontal axes a* and b* represent, respec- tively, red-green and yellow-blue continuums. The a* and b* values may be either positive (red and blue, respectively) or negative (green and yellow, respec- tively) and have no specific numerical limits [31].
Due to the close relationship between color per- ception and variations in ΔE [32], it is important to evaluate L* a* b* values separately. This provides an understanding of which color component has contri- buted the most to an observed color difference. Since the color of natural teeth tends towards yellow rather than red, porcelain restorations with positive L* and b* values and negative a* values are preferred. While differences in b* values (blue-yellow axis) between natural teeth and restorations can be tolerated, differences in a* values (red-green axis) are clinically unacceptable [33].
ΔE values of up to 3.5 have been reported to be clinically acceptable [34]. Considering that the highest ∆E value found in this study was 2.13, which was observed in the Cr-Co specimens (Group CoCr) without an aurofilm masking agent. All of the ∆E values in the present study are clinically acceptable. Whereas the Group NiCr had the best masking ability of any group when no additional AMA was applied, Group CoCr had the best masking ability when an AMA was applied. This is due to a 0.74 decrease in the ∆E value of the Co-Cr specimens (Group CoCr), from 2.13 to 1.39, that was observed when an AMA was used. In contrast, the ∆E values of all the other alloys tested increased in conjunction with AMA application.
In line with the findings of the present study, Özçelik et al. (2008) [8] reported significant color differences in OP applied to 1 Ni-Cr and 3 Co-Cr base-metal alloys in comparison to an Au-Pd control group, but in all cases the ΔE was reported to be below the clinically acceptable threshold of 3.5. According to the authors, most of the chromatic changes in base metal alloy finished porcelain exhibited higher b* values and lower a* values, resulting in a more yellow-green appearance. A study by Kourtis et al. (2015) [5] conducted with 4 different alloys used for MCRs, also found that the final color of the porcelain restorations was affected by the type of metallic framework alloy.
In the present study, L* values for the different groups ranged between 92.45 and 93.94, while the a* values for all groups were negative (green) and b* values for all groups were positive (yellow). The closest b* value (yellow) to that of the control group was observed in the Au-Pd specimens (Group AuPd). Regardless of whether or not the aurofilm masking agent was used, no significant differences in L* values were found for any of the groups in comparison to the control group. However, significant differences in a* values were found between all other groups and the control group, with the exception of the Co-Cr alloy specimens (Group CoCr, CoCRM).
Application of an AMA increased the brightness (L* values) of specimens in all groups with the exception of the Au-Pd alloy (Group PdM). AMA application also resulted in increased greenness (i.e. increases in a* values) in the Au-Pd specimens (Group AuPdM) and the Ni-Cr specimens (Group NiCrM) and increased yellowness (i.e. increases in b* values) in the Pd-based (Group PdM), Co-Cr (Group CoCrM), and Ni-Cr specimens (Group NiCrM). Because masking of the Co-Cr alloy samples improved with the increase in yellowness that occurred with the application of an AMA, a masking agent may be recommended in restorations constructed from Co-Cr alloys.
The authors did not identify any study in the literature that evaluated the effect of aurofilm masking agents on the color of metal-ceramic restorations, and differences in materials and techniques between the present study and earlier studies makes it difficult to draw clinically relevant conclusions. However, one previous study reporting on the effects of metal alloy on the color of dental porcelain stated that when compared to Ni-Cr and Co-Cr alloys, Au and Au-Pd alloys resulted in a greater shift towards yellow in the appearance of MCRs. The study also reported that noble alloys were easier to mask with an opaque layer than Ni-Cr alloys [35]. These findings are in line with the results of the present study.
This study had some limitations, namely, it had a small sample size and examined a limited number of materials. Future studies should examine the use of different porcelains or different shades on the same metal alloy, different surface treatments, and multiple firings, all of which may influence the final color of the metal ceramic restorations [36, 37].
Within the limits of the current study, the following conclusions can be drawn:
1. Type of alloy had a significant effect on the final color of metal ceramic specimens, with the CoCr’s ΔE value (2.13) was measured in statistically different. The color differences of base metal and noble alloys measured according to the control group are within clinically acceptable limits between 1.07 to 2.13.
2. The final color of metal ceramic specimens was influenced the overlying aurofilm masking agents. The masking agent was recommended in restorations with Co-Cr alloys.
- 1. M.S. Scurria, J.D. Bader and D.A. Shugars, J. Prosthet. Dent. 79 (1998) 459-464.
-

- 2. T.R. Walton, Int. J. Oral Maxillofac. Implants 30 (2015) 851-861.
- 3. S.D. Heintze and V. Rousson, Int. J. Prosthodont. 23 (2010) 493-502.
- 4. T. Joda, F. Zarone and M. Ferrari, BMC Oral Health 17 (2017) 124.
-

- 5. B.E. Pjetursson, I. Sailer, N.A. Makarov, M. Zwahlen and D.S. Thoma, Dent. Mater. 31 (2015) 624-639.
-

- 6. R.D. Douglas and M. Przybylska, J. Prosthet. Dent. 82 (1999) 143-149.
-

- 7. L. Miller, J. Esthet. Dent. 5 (1993) 143-153.
-

- 8. T.B. Ozçelik, B. Yılmaz, I. Ozcan and C. Kircelli, J. Prosthet. Dent. 99 (2008) 193-202.
-

- 9. J.C. Wataha, J. Prosthet. Dent. 87 (2002) 351-363.
-

- 10. T.B. Ozçelik, B. Yilmaz, I. Ozcan and A.G. Wee, J. Prosthet. Dent. 106 (2011) 38-47.
-

- 11. S.G. Kourtis, A.P. Tripodakis and A.A. Doukoudakis, J. Prosthet. Dent. 92 (2004) 477-485.
-

- 12. M.M. Stavridakis, E. Papazoglou, R.R. Seghi, W.M. Johnston, and W.A. Brantley, J. Prosthodont. 9 (2000) 71-76.
-

- 13. M.M. Stavridakis, E. Papazoglou, R.R. Seghi, W.M. Johnston, and W.A. Brantley, J. Prosthet. Dent. 92 (2004) 170-178.
-

- 14. C.J. Goodacre, Dent. Clin. North. Am. 48 (2004) 359-385.
-

- 15. https://metalor.com/wp-content/uploads/2020/06/PreciousMetal-PlatingSolutions_MTEP06-EN2018-05.pdf (Accessed 6 December 2020).
- 16. A. Vichi, C. Louca, G. Corciolani and M. Ferrari, Dent. Mater. 27 (2011) 97-108.
-

- 17. V.L. Swain, I.J. Pesun and J.S. Hodges, J. Prosthet. Dent. 99 (2008) 468-476.
-

- 18. R.R. Seghi, E.R. Hewlett and J. Kim, J. Dent. Res. 68 (1989) 1760-1764.
-

- 19. R.D. Douglas, T.J. Steinhauer and A.G. Wee, J. Prosthet. Dent. 97 (2007) 200-208.
-

- 20. F.C. Chu, T.W. Chow and J. Chai, J. Prosthet. Dent. 98 (2007) 359-364.
-

- 21. I. Sailer, B.E. Pjetursson, M. Zwahlen and B.E. Pjetursson, Clin. Oral Implants Res. 18 (2007) 86-96.
-

- 22. B. Stevension and R. Ibbetson, J. Dent. 38 (2010) 361-368.
-

- 23. S.H. Jacobs, C.J. Goodacre, B.K. Moore and R.W. Dykema, J. Prosthet. Dent. 57 (1987) 138-145.
- 24. M.H. Atala, E.B.G. Aygün and A. Doğan, J. Ceram. Process. Res. 21 (2020) 407-415.
-

- 25. S. Imamura, H. Takahashi, I. Hayakawa, P.G. Loyaga-Rendon and S. Minakuchi, Dent. Mater. J. 27 (2008) 802-808.
-

- 26. B. Yılmaz, T.B. Özçelik, and A.G. Wee, J. Prosthet. Dent. 101 (2009) 395-404.
-

- 27. K.R. Pyon, K.S. Han and B.H. Lee, J. Ceram. Process. Res. 12 (2011) 279-288.
- 28. W.J. O’Brien, K.S. Kay, K.M. Boenke and C.L. Groh, Dent. Mater. 7 (1991) 170-173.
-

- 29. R. Ghinea, M.M. Perez, L.J. Herrera, M.J. Rivas, A. Yebra and R.D. Paravina, J. Dent. 38 (2010) e57-64.
-

- 30. J. Hasssija, V. Hegde and N. Sridhar, J. Indian Prosthodont. Soc. 14 (2014) S86-92.
-

- 31. S.G. Kourtis, A.P. Tripodakis and A.A. Doukoudakis, J. Prosthet. Dent. 92 (2004) 477-485.
-

- 32. B.T. Xu, B. Zhang, Y. Kang, Y.N. Wang and Q. Li, J. Dent. 40 (2012) e3-9.
-

- 33. R.D. Douglas and J.D. Brewer, J. Prosthet. Dent. 90 (2003) 339-346.
-

- 34. F.C. Chu, T.W. Chow and J. Chai, J. Prosthet. Dent. 98 (2007) 359-364.
-

- 35. S.H. Oh and S.G. Kim, J. Adv. Prosthodont. 7 (2015) 368-374.
-

- 36. B. Stevenson and R. Ibbetson, J. Dent. 38 (2010) 361-368.
-

- 37. R. Ceylantekin, V. Uz, G. Yanik, M. Sirin and H. Kadioglu, J. Ceram. Process. Res. 13 (2012) 409-412.
 This Article
This Article
-
2022; 23(1): 16-21
Published on Feb 28, 2022
- 10.36410/jcpr.2022.23.1.16
- Received on Aug 13, 2021
- Revised on Aug 25, 2021
- Accepted on Sep 11, 2021
 Services
Services
Shared
 Correspondence to
Correspondence to
- Gonca Deste Gökay
-
Bursa Uludağ University, Faculty of Dentistry, Department of Prosthodontics, Bursa, Turkey
Tel : +90 (553) 431 30 40 Fax: +90 (224) 294 00 41 - E-mail: goncadeste@uludag.edu.tr







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.