- Breakdown characteristics of geopolymers according to curing and aging conditions
Minjeong Kim and Yootaek Kim*
Department of Materials Engineering, Kyonggi University, Suwon 16227, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Lightweight geopolymers made of integrated gasification combined cycle (IGCC) slags and Si sludges exhibit an unusual breakdown, known as dentable characteristics. In this study, dentable characteristics of lightweight geopolymers were investigated under different curing and aging conditions. The relationship between these conditions and dentable characteristics was determined by measuring compressive strength, conducting XRD and FT-IR, and by optical microscopic observations of pore size, distribution, and shape. Dentable characteristics cannot be simply controlled by changing the curing and aging conditions. Homogeneous pore distribution, size, and shape by extra mixing did not enhance the dentable characteristics. Although the moisture and Si content of the geopolymer would control the dentable characteristic in some measure, aging conditions were found to be the most important effect on the dentable characteristics and stability of geopolymers in water.
Keywords: Geopolymer, Dentable characteristics, IGCC slag, Si sludge, Curing, Aging
All the nations worldwide have encountered severe environmental problems and have tried to reduce carbon dioxide exhaust. It is an urgent issue to minimize climate change due to global warming [1-3]. According to the Kyoto agreement signed in 1997, advanced coun- tries should reduce the production of carbon dioxide by 5.2% compared to that in 1990s. In 2015, the Paris agreement specified that an increase in the average temperature of Earth must be maintained well below +2.0 oC, whereas an increase in the average temperature of Earth should be limited to less than +1.5 oC [4, 5]. One of the largest carbon dioxide producing industries is the cement industry because considerable energy and resources are used for the production of cement. The amount of carbon dioxide produced in the cement industry is approximately 7% of the total carbon dioxide production; therefore, it is important to develop environ- mentally friendly materials instead of conventional Portland cement for sustainable development in the construction industry in the future [6-8].
Research has focused on the development of environ- mentally friendly techniques for a substantial decrease in carbon dioxide production and for the replacement of ordinary Portland cement by more ecological materials [9, 10]. The geopolymer was invented by Davidovits in 1978. Alumino-silicates were activated with an alkaline solution to develop a geopolymer with a structure similar to that of zeolite without the use of a sintering process [11]. Geopolymers have a three-dimensional structure similar to that of a polymer; therefore, it is called a geopolymer [12]. Geopolymer consists of an aluminosilicate gel network, including SiO4 connected to tetrahedral AlO4, and has cations such as Na+ in the framework cavities to strike a charge balance. Therefore, the quantities of SiO2 and Al2O, as well as the concentration of alkali activator, significantly affect the geopolymer properties [13-15]. Geopolymer is an environmentally friendly material because of its much low production of carbon dioxide (less than 20% com- pared with that of cement) during its manufacturing process [6, 16, 17].
It is a well-known that most ceramic materials, including geopolymers, exhibit fragile and abrupt failure (fractural) characteristics under external stress. Well-known machinable ceramics such as zirconia can be milled, drilled, and saw cut, with traditional metal- working machine tools without the need for diamond tooling [18]. However, machinable ceramics require high-temperature sintering process, which is as hard as a rock but not as soft as jelly. Hence, geopolymers under specific conditions exhibited dentable characteristics, which can be defined as a soft jelly or sponge-like material and a material that shows no abrupt breakdown characteristics under stress or pressure [19]. The dentable characteristics of geopolymers were first found in our laboratory several years ago during the bloating process when Si sludge was used as a bloating material. Since then, no systematic study has been conducted because of the reproducibility and repeatability problems. Therefore, in this study, the dentable characteristics of geopolymers were investigated by changing the curing and aging conditions because moisture content and aging conditions were considered as the most prospective factors at the first stage of determining the dentable characteristics of geopolymers [20, 21]. Thus, this study primarily aims to determine the relationship between dentable charac- teristics and curing and/or aging conditions, as well as to determine the cause of the presence of the dentable characteristics in geopolymers. The novelty of this work is to firstly report and investigate totally new concept ceramics showing dentable property which has not been previously reported before.
It is assumed that the application of dentable geo- polymers would be a breakthrough in ceramic materials, which will change the concept of traditional ceramics; it can be realized if the mechanism of dentable charac- teristics is explained and proved through further inves- tigations. We believe that this study is the first step to demonstrate the mechanism and conditions for the reproducibility of dentable geopolymers.
Materials
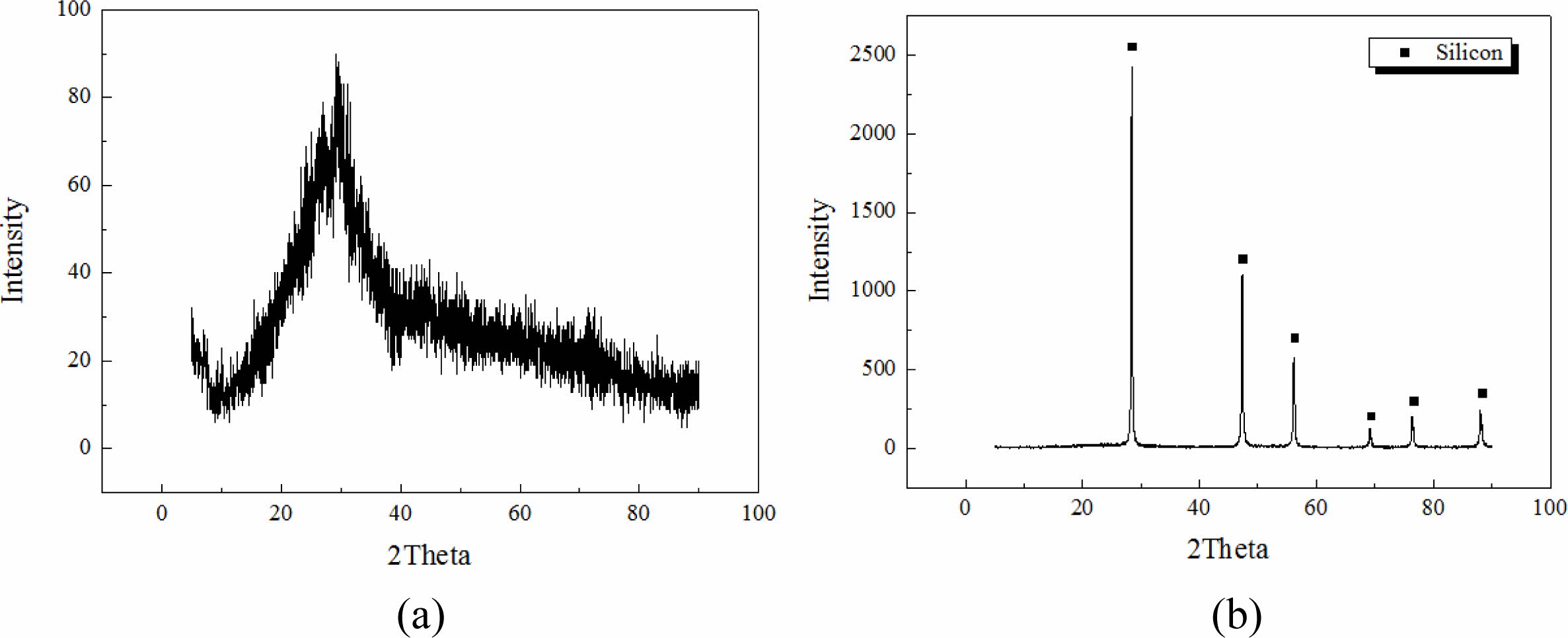
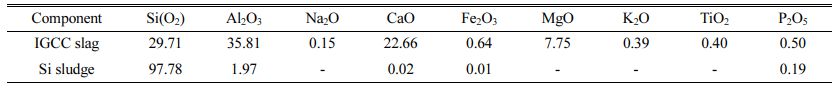
Integrated gasification combined cycle (IGCC) slags produced by the domestic power plant in Taean province were used as raw materials for geopolymers, and Si sludges produced during the polishing process used in the semiconductor wafer or solar cell industry were used as the bloating material. The chemical compositions of IGCC slag and Si sludge are listed in Table 1. As observed from the data presented in Table 1, IGCC slag has a Al/Si ratio that is essential for obtaining geopolymers. It was found to be amorphous by X-ray diffraction (XRD) analysis, proving them suitable for providing sufficient Si+4 and Al+3 ions by an alkaline activator during the geopolymerization reaction. XRD patterns of IGCC slags and Si sludges have been shown in Fig. 1. As shown in Fig. 1, IGCC slag is turned out to be total amorphous and Si sludge shows a typical polycrystalline pattern.
Hydrogen gas is produced during the bloating pro- cess, which creates pores in the geopolymer. Therefore, the hydrogen gas production must be controlled by adjusting the Si amount, alkaline activator concentration, geopolymer paste viscosity, and mixing method and time. Controlling the bloating process is particularly important for producing dentable geopolymers because it is assumed that the pore size, shape, and distribution are major factors for determining the dentable charac- teristics of geopolymers.
Experimental procedures
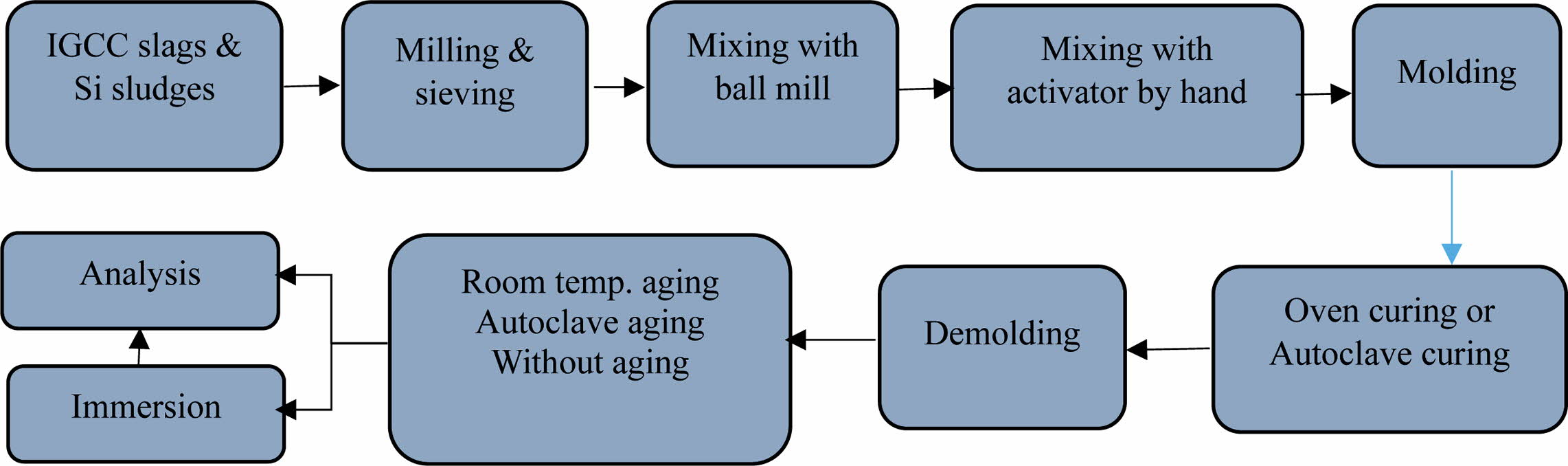
Fig. 2 shows the procedures used for the preparation of dentable geopolymer specimens. IGCC slags and Si sludges were sieved through a 106-µm sieve, and then, the IGCC slags that passed through the sieve were milled using an alumina ball mill at 117 rpm for 8 h. Bulk Si sludge was not milled because it was finely milled during the polishing and cutting process of Si ingots. Therefore, they were used directly in the bloating process without a further milling process after the sieving.
The mixed alkaline activator was used for the geo- polymerization and bloating reactions by mixing NaOH pellets (97.0% NaOH; Daejung Co., Korea) and sodium silicates (Na2SiO3∙nH2O; Daejung Co., Korea), known as water glass with a ratio of 1:1. The concen- tration of NaOH was 15 M, and the water solid ratio (W/S) was controlled at 0.2. The processed IGCC slags and Si sludge samples were mixed using a ball mill in a dry state for 1 h, and then, the mixed materials were mixed again with an alkaline activator by hand for more than 5 min prior to molding. The mixed pastes of the geo- polymer were molded with a stainless steel mold (5 × 5 × 5 cm3), and then, the entire mold was sealed with a polyethylene bag that can be used at temperatures above 150 oC during the curing process to prevent the early evaporation of water during the curing process.
The molded and sealed specimens were cured using either an autoclave (1.18–2.3 atm, 103-123 oC for 24 h) or dry oven (83-103 oC for 24 h) and then demolded. After the curing process, the specimens were aged in three different ways, as shown in Fig. 2: without aging at all, aging at room temperature (15-25 oC) for 72 h, and aging in an autoclave (1.18-2.3 atm, 103-123 oC) for 24 h. Dentable characteristics were compared according to the curing and aging conditions.
Some specimens were immersed in distilled water long-term, and then, the stability of the specimens in water was estimated. The specimen stability was estimated by measuring the pH after specimen immersion and by observing the microstructural differences on the surface and in the bulk after immersion. A previous study reported that the stability of the specimens is closely related to the degree of geopolymerization [22, 23].
Dentable characteristics were tested using a universal test machine (UTM) by measuring the compressive test and evaluated by fracture profiles. The crystalline phases and chemical bonding states were also analyzed by X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopy. Pore size, shape, and distribution were analyzed and observed using a CamscopeTM (digital optical microscope).
|
Fig. 1 XRD pattern. (a) IGCC slag and (b) Si sludge. |
|
Fig. 2 Schematic of the procedures for producing dentable geopolymer specimens by two curing processes, namely autoclave curing and oven curing, and three different aging methods, namely room-temperature aging, autoclave aging, and without aging. |
|
Table 1 Chemical composition of the IGCC slag and Si sludge as evaluated by XRF (wt.%) |
Room-temperature aging
All specimens in this section were aged for 3 d at room temperature (25 oC) if not mentioned separately.
Oven curing
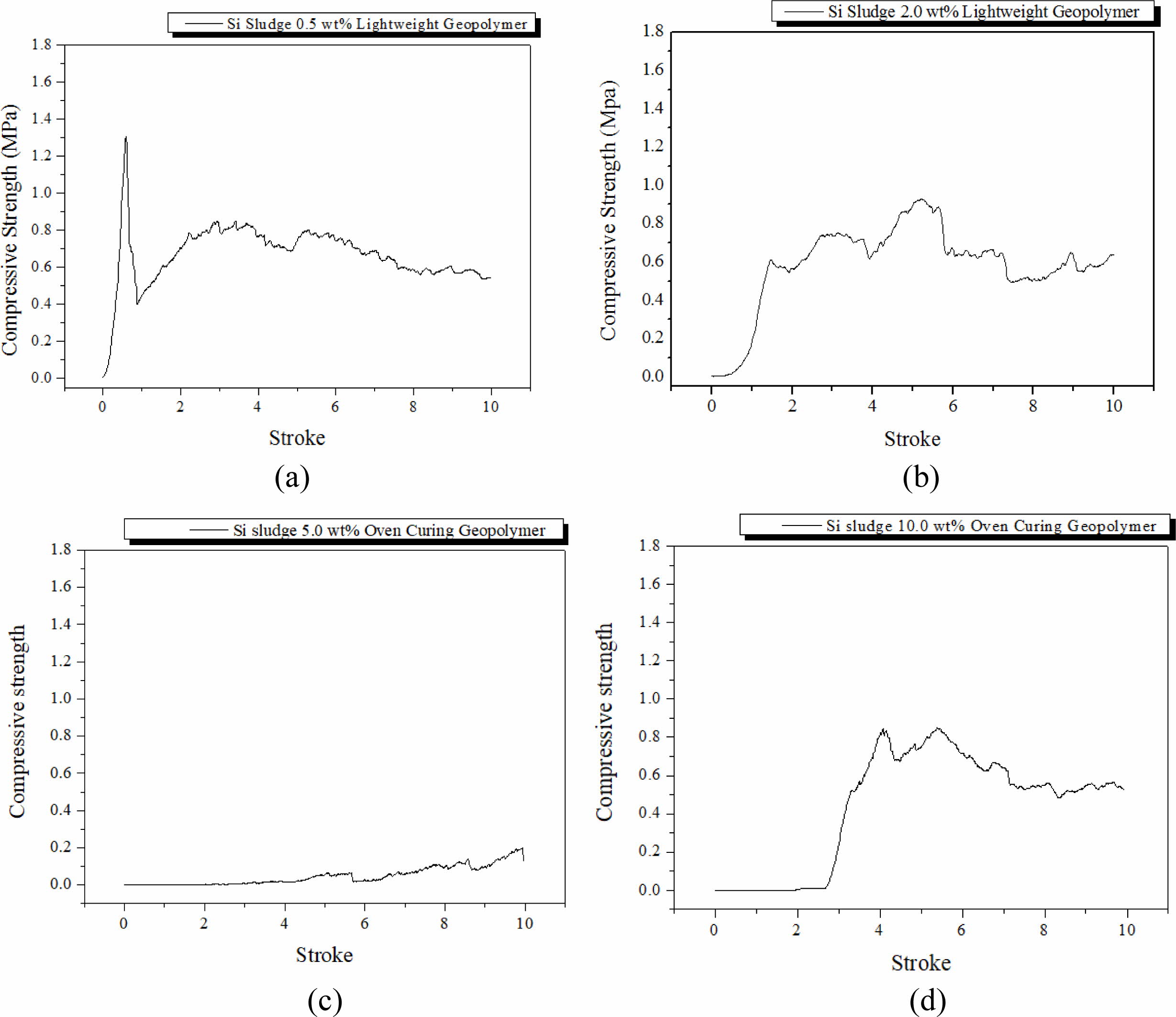
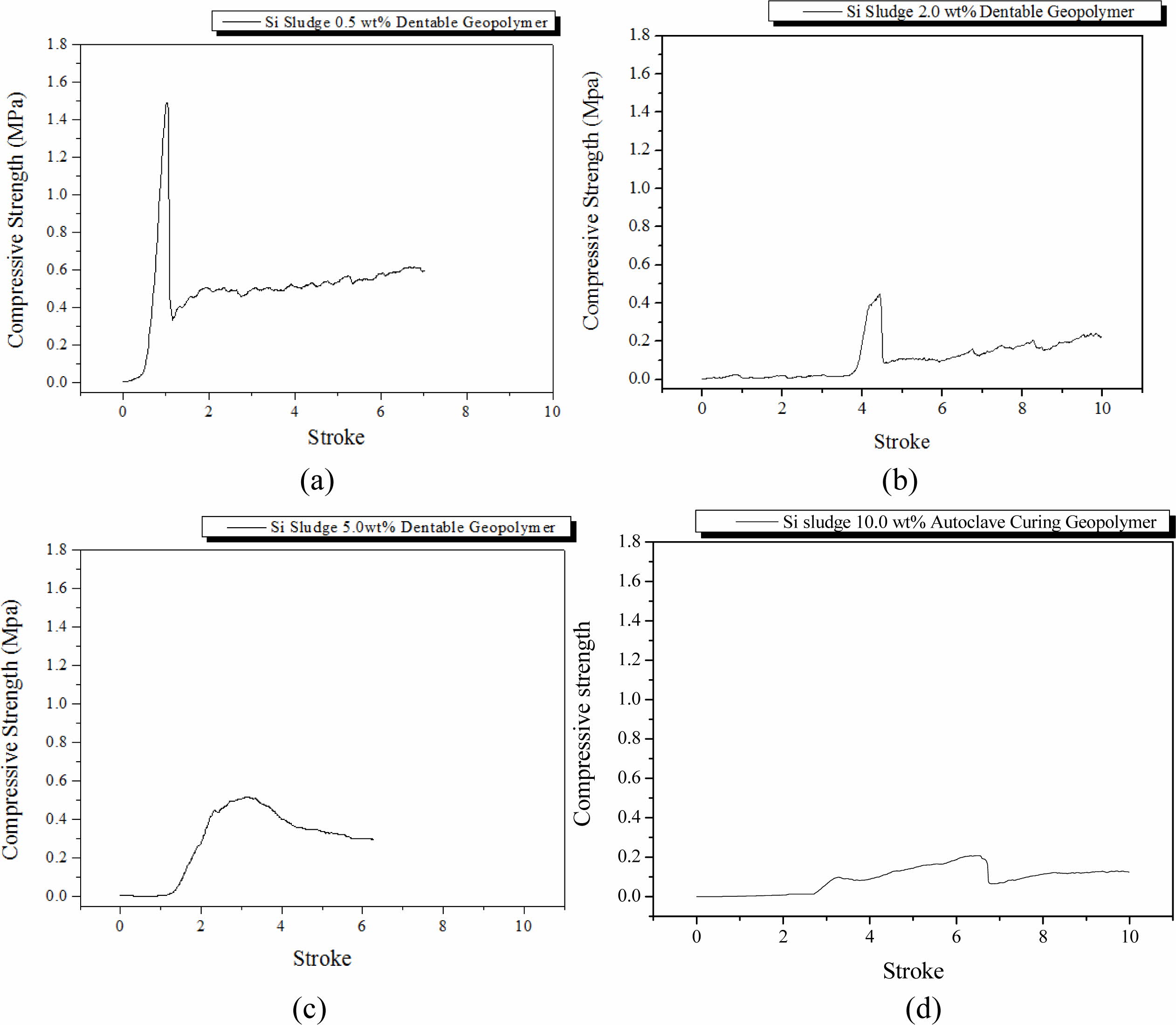
Specimens were mixed for 1 min and then cured in a dry oven at 103 oC for 1 d. After the dry oven curing, the specimens were aged for 3 d at room temperature (25 oC). The breakdown characteristics differed with the Si addition, as shown in Fig. 3. The maximum com- pressive strength during the compressive test tended to decrease with an increase in Si addition, and dentable characteristics were observed in the specimens contain- ing more than 2.0 wt.% of Si sludge. Herein, dentable means that there was no abrupt mechanical failure during the compressive strength test with UTM; more precisely, the specimen has dentable characteristics if it has no sharp drop in the compressive strength, as shown in Fig. 3(a).
It is well-known that most ceramics exhibit fragile or abrupt breakdown characteristics when exposed to ex- ternal stresses, such as strong impact or similar situations as compressive strength tests. However, some ceramics exhibit dentable characteristics, although they are rare in ceramics [15]. Geopolymers made mainly with IGCC slags with a small addition of Si sludge showed dentable characteristics, as shown in Figs. 3(b)-3(d). There was no sharp drop or change in compressive strength for the entire stroke period during the test, as shown in Figs. 3(b)-3(d). Therefore, it was concluded that geopolymers containing more than 2.0 wt.% of Si sludge exhibited dentable characteristics under the above-mentioned curing and aging conditions.
Furthermore, we observed a sudden decrease in com- pressive strength on the specimen containing 5.0 wt.% Si sludge. This phenomenon appeared in all specimens regardless of curing or aging conditions. Here is a brief explanation as follows: a proper pore structure for the good mechanical strength of the specimen was not created because the velocity of bloating is higher than that of geopolymerization in the geopolymer matrix. Therefore, large independent pores were formed, and the compressive strength of the specimen substantially decreased. A more detailed explanation will be given in Section Autoclave aging/Autoclave curing.
Autoclave curing
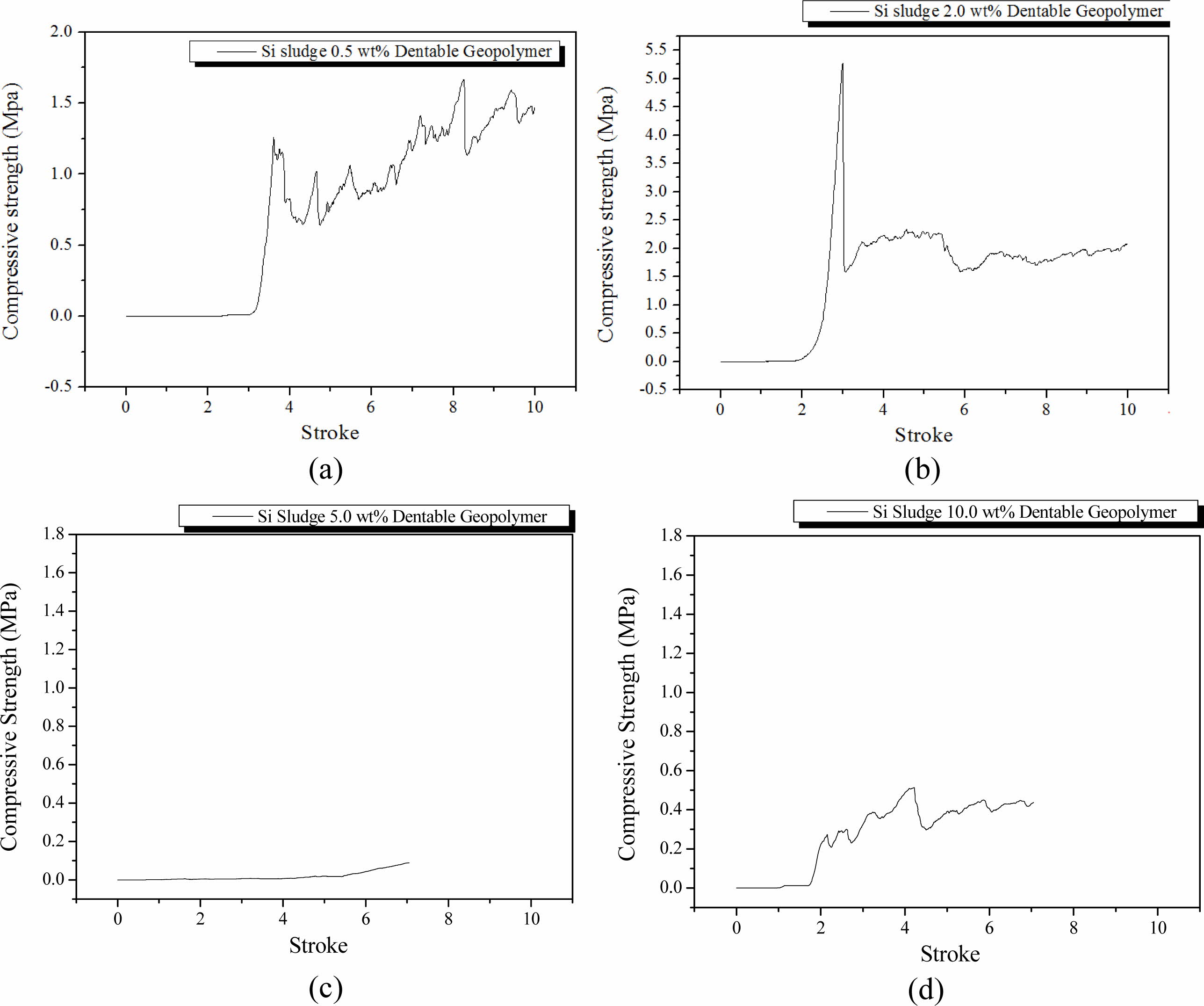
Specimens were mixed for 1 min as in the oven curing case and then cured in an autoclave at 103 oC for 1 d. After the autoclave curing, the specimens were aged for 3 d at room temperature (25 oC). The breakdown characteristics of the autoclave-cured specimens were not significantly different from those of the oven-cured specimens as shown in Fig. 4; however, the average compressive strength of the autoclave-cured specimens was lower than that of the oven-cured specimens. In the autoclave curing, the maximum compressive strength also tended to decrease with an increase in Si addition, and dentable characteristics were also observed in the specimens containing more than 2.0 wt.% of Si sludge, as in the dry oven curing case.
The applied pressure on the geopolymer specimens and maintaining the moisture content during the curing process in the autoclave did not increase the compressive strength or dentable characteristics. Moreover, it seems that the conditions under the autoclave curing had an adverse effect on the compressive strength and dentable characteristics because maintaining the moisture level and extra pressure during the curing process did not increase the mechanical properties of the geopolymer nor the dentable characteristics after the completion of the geopolymerization process. In contrast, the conditions in the autoclave curing made the network structure of geopolymers looser because of extra moisture during the curing process, which caused lower compressive strength and formed inhomogeneous pore microstructures in the geopolymers.
Autoclave curing with extra mixing
The reports described the enhancement of compressive strength and homogeneous pore structure by extra mixing during the forming process [16, 17]. On the basis of the reports, the mixing time was increased from 1 to 5 min before molding to increase the compressive strength by making the pore structure more homo- geneous.
As expected from the previous report [17], the com- pressive strength of the specimens generally increased with an extra mixing, as shown in Fig. 5. In particular, the compressive strength of the specimen containing 2.0 wt.% Si sludge was substantially increased, as shown in Fig. 5(b), compared with those of the specimens without extra mixing, as shown in Fig. 4(b), and ex- hibited an abrupt fracture, which implies that dentable characteristics disappeared with extra mixing. This may be caused by the formation of a more homogeneous pore structure by extra mixing. To conclude, homogeneous pore distribution and structures did not contribute to increasing the dentable characteristics of the specimens; more precisely, various shapes of pores and distributions may be helpful for producing geopolymers with dentable characteristics. As previously mentioned, a sudden decrease in the compressive strength of the specimen containing 5.0 wt.% was observed under both the autoclave curing and extra mixing condition.
Autoclave aging
All the specimens in this section were aged at 83 oC for 3 d in an autoclave chamber if not mentioned separately.
Oven curing
Specimens were mixed for 1 min and then cured in a dry oven at 103 oC for 1 d under the same mixing conditions as the oven-cured and room-temperature aged specimens in Section Room temperature aging/Oven curing. After the dry oven curing, the specimens were aged for 3 d in an autoclave chamber at 83 oC. The breakdown characteristics were not significantly different from those of the specimens with room-temperature aging. Therefore, there was no effect of autoclave aging for the oven-cured specimens on the dentable charac- teristics.
Autoclave curing
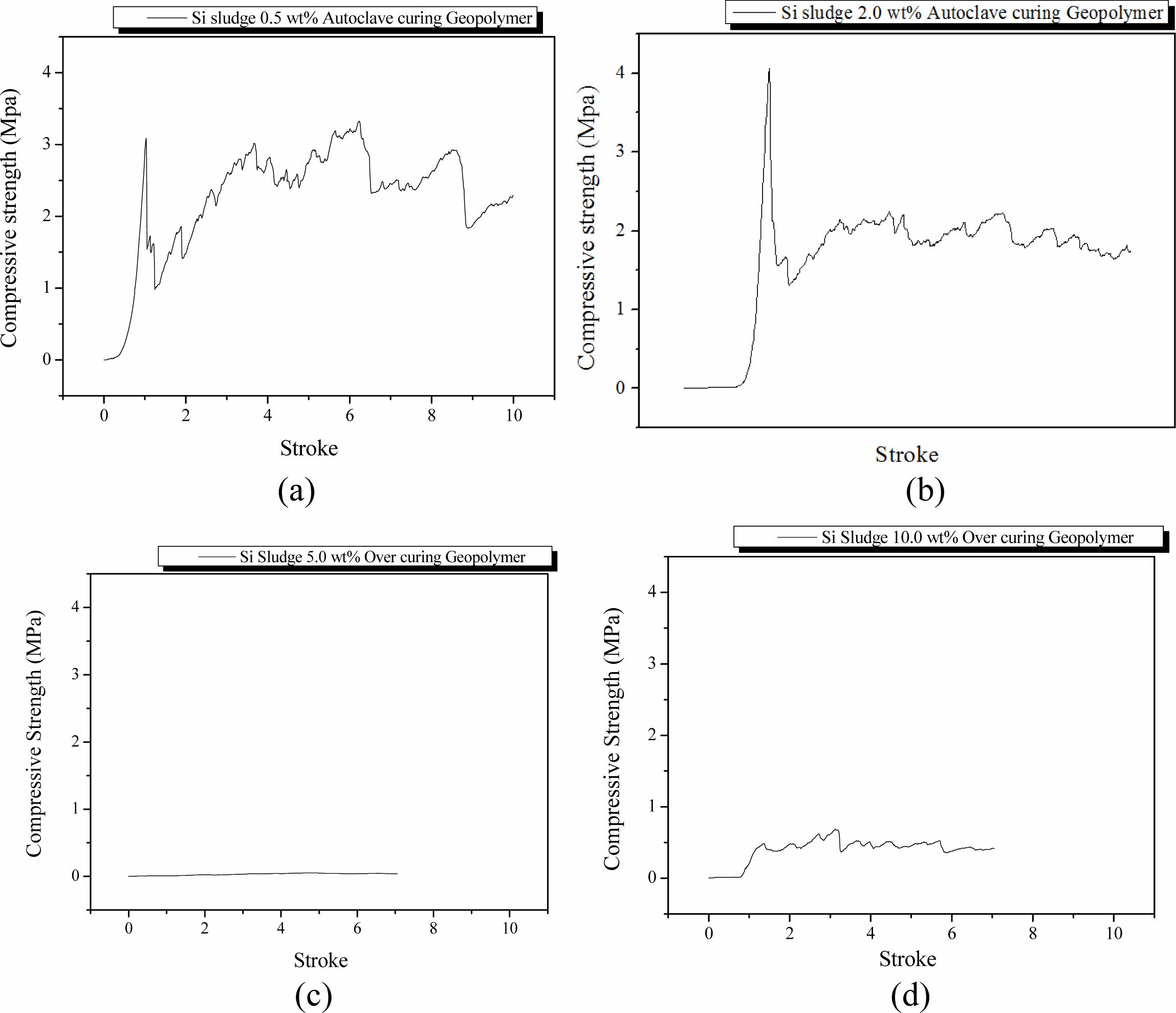
Specimens were mixed for 1 min and then cured in an autoclave chamber at 103 oC for 1 d under the same mixing conditions as the autoclave-cured and room-temperature aged specimens in Section Room tempera- ture aging/Autoclave curing. These specimens also exhibited dentable characteristics when the Si content was more than 0.5 wt.%; however, breakdown profiles of the specimens were similar to those of autoclave-cured and room-temperature aged ones. Because no particular difference was found in the specimens according to the aging method, it was necessary to investigate the effect of autoclave curing on the dentable characteristics of 5 min mixed geopolymers that did not show dentable characteristics with room-temperature aging.
The specimens were mixed for 5 min and then cured in an autoclave chamber at 103 oC for 1 d. After the autoclave curing, the specimens were aged at 83 oC for 3 d in the same autoclave chamber as curing. As shown in Fig. 6(a), the specimens containing 0.5 wt.% Si were fractured once and then exhibited dentable breakdown characteristics thereafter. The compressive strength was also slightly higher than that of the room-temperature aged one (Fig. 5(a)). This phenomenon was observed in the specimens with compressive strength in the range from 2.0 to 4.0 MPa, which is higher than that of dentable specimens but lower than that of high strength specimens exhibiting an abrupt fracture. The tendency of the breakdown characteristic and the values of compressive strength of other specimens except for the specimen containing 0.5 wt.% Si were comparable room-temperature aged ones (Fig. 5(b), 5(c), and 5(d)). Overall, there was little difference between room-tem- perature aging and autoclave aging for the autoclave curing specimens.
A sudden decrease in compressive strength on the specimen containing 5.0 wt.% was also observed in the autoclave curing and autoclave aging condition. Com- pressive strength of specimens containing more than 5.0 wt.% was lower than that of specimens mixed for 1 min under the autoclave curing and room-temperature aging conditions, as shown in Figs. 5(c) and 5(d). This decrease in compressive strength is caused by the difference in the reaction velocity of bloating. The pore size and distributions were artificially controlled by extra mixing up to 2.0 wt.%; however, they were not controllable because of the fast velocity of bloating reaction when the addition of Si was over 5.0 wt.%. An increase in Si addition caused a more exothermic bloating reaction; accordingly, the geopolymer paste became coagulated by the heat from the bloating reaction before the pores were united and formed large pores. When 5.0 wt.% of Si was added, the velocity of the bloating reaction was between 2.0 and 10.0 wt.%. Therefore, pores could be obtained continuously to form large pores after the pore size and distribution were controlled by extra mixing because the bloating reaction continued in the thick and sticky geopolymer paste before the geopolymer paste was completely solidified by the heat from the bloating reaction. Therefore, bloated geopolymer pastes overflowed from the mold and large pores were formed inside the specimens. Observing Fig. 6(c) and 6(d), the breakdown profiles of these specimens looked like having dentable characteristics; however, it is difficult to conclude whether these specimens have dentable characteristics because the compressive strength of these specimens was too low, and profiles could not be defined.
Autoclave curing and oven drying
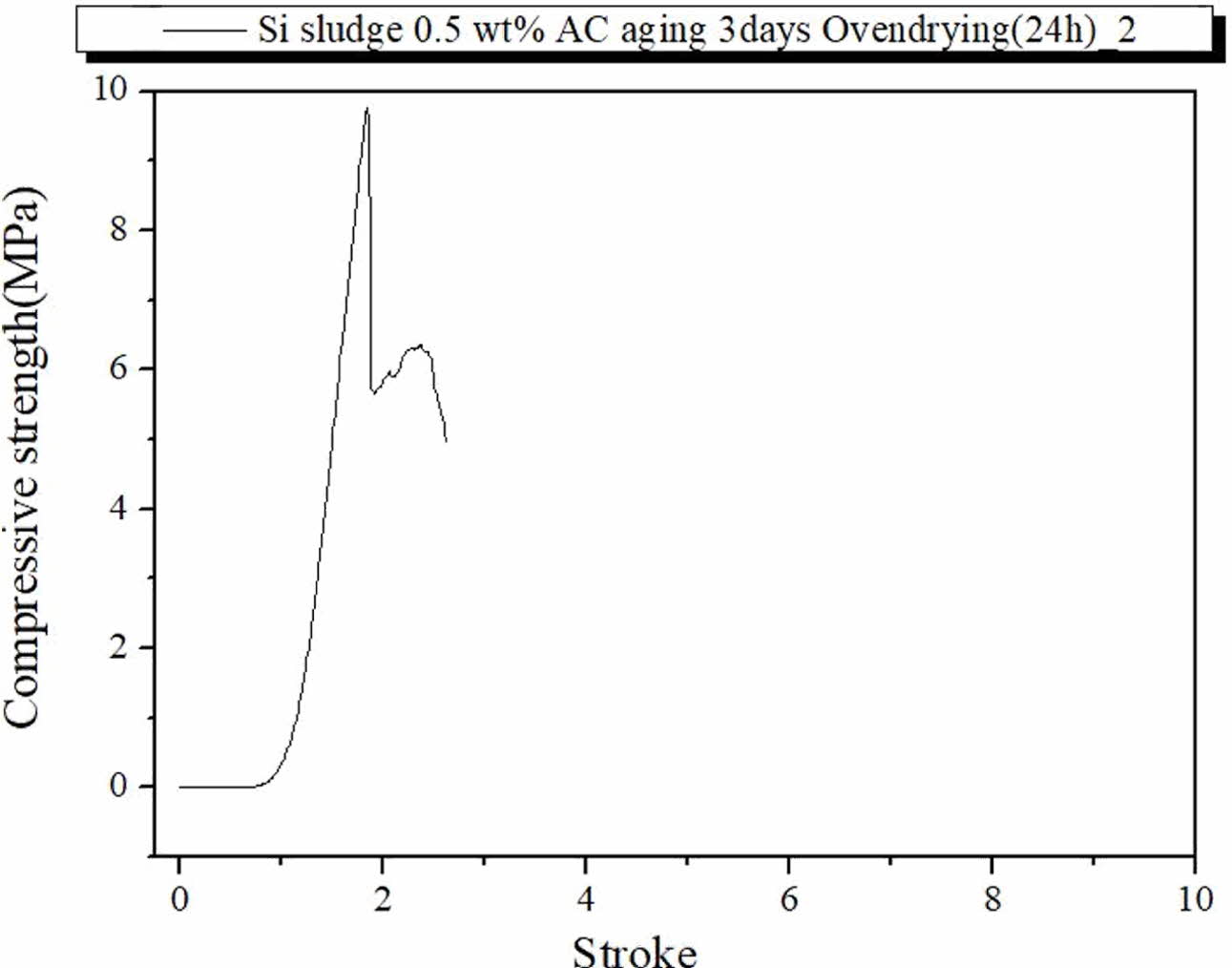
The specimens containing 0.5 wt.% Si sludge were mixed for 1 min and cured in the autoclave chamber at 103 oC for 1 d, and thereafter, specimens were additionally dried at 83 oC for 1 d in the dry oven. We chose the mixing time for 1 min because extra mixing time seemed to have an adverse effect on the dentable characteristics of the geopolymer specimens in all ranges of Si addition. Contrary to our expectation, none of these specimens exhibited dentable breakdown characteristics during the compressive test, as shown in Fig. 7. The compressive strength values of these specimens were more than three times higher than those of the other specimens containing the same amount of Si sludge. As shown in Fig. 7, all three data showed similar curves, and they were fractured abruptly and did not show any dentable characteristics. For comparison, a curve for the same specimen but only one different condition of immersion aging is shown in Fig. 8 in the next section.
Autoclave curing, autoclave aging, oven drying, and immersion
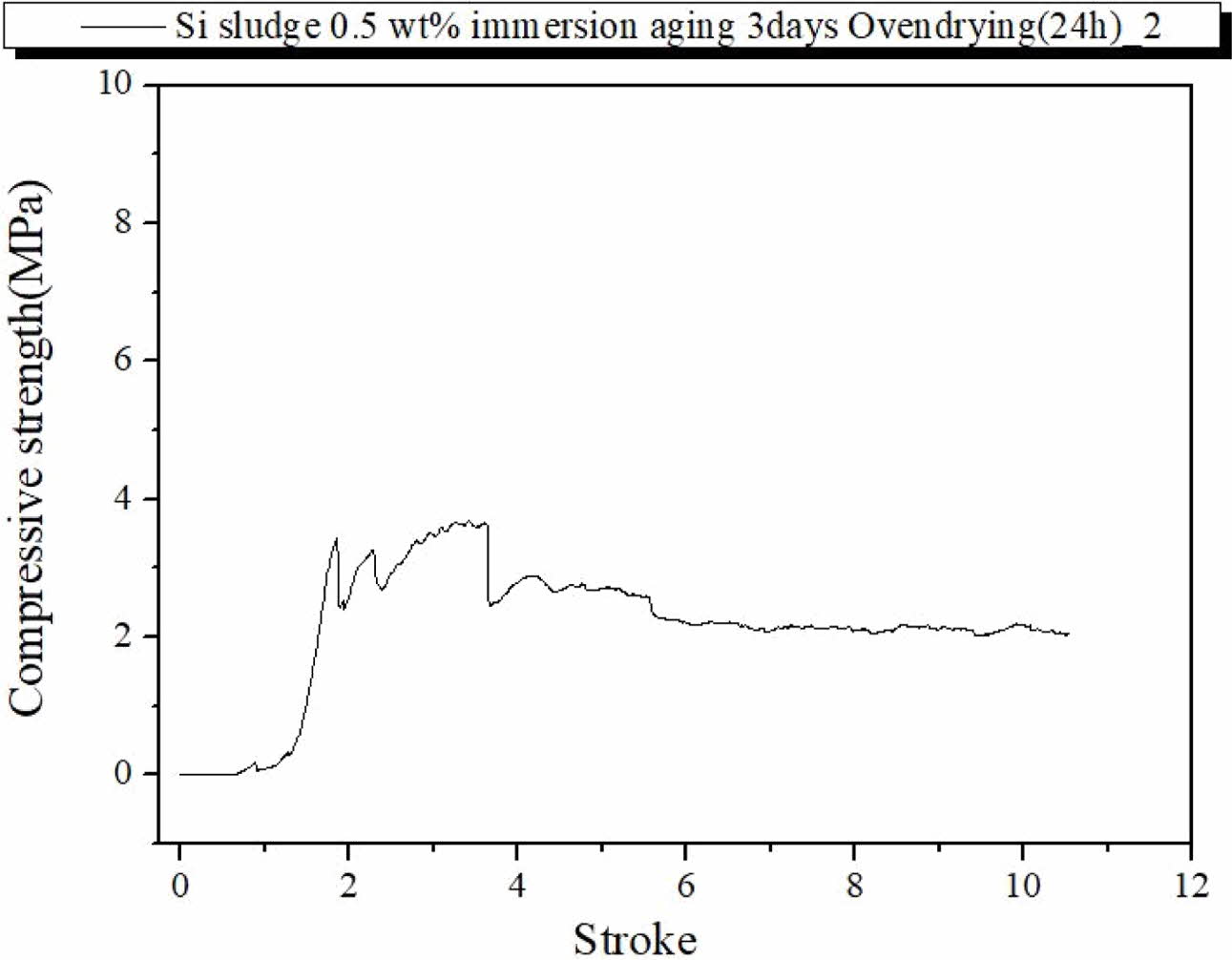
Fig. 8 show the fracture characteristics of the geo- polymer specimen prepared under the same curing and aging conditions but immersed for 3 d in distilled water after autoclave aging. As shown in Fig. 8, the compressive strength was less than half of those of the specimens prepared under the same conditions but immersed, as in Fig. 7. However, the immerged specimen exhibited typical dentable characteristics without any abrupt fracture during the test, as shown in Fig. 8. Therefore, an additional drying process before the compressive strength test may have contributed to enhancing the compressive strength of the specimens; however, it did contribute to enhancing the dentable breakdown char- acteristics of the specimens. From the above results, it was speculated that the moisture content may have an effect on the dentable characteristics and compressive strength of the specimen.
Without aging
Oven curing
Geopolymer specimens were mixed for 1 min and cured in a dry oven at 103 oC for 1 d. After the dry oven curing, specimens were tested for compressive strength without further aging to determine the aging effects. Compressive strength of all the oven curing specimens containing 0.5–10.0 wt.% of Si contents showed lower than 0.1 MPa, which was within the error range of the UTM. Therefore, it was concluded that the aging process was an essential step for oven curing geopolymers whether they exhibited dentable characteristics or not.
Autoclave curing
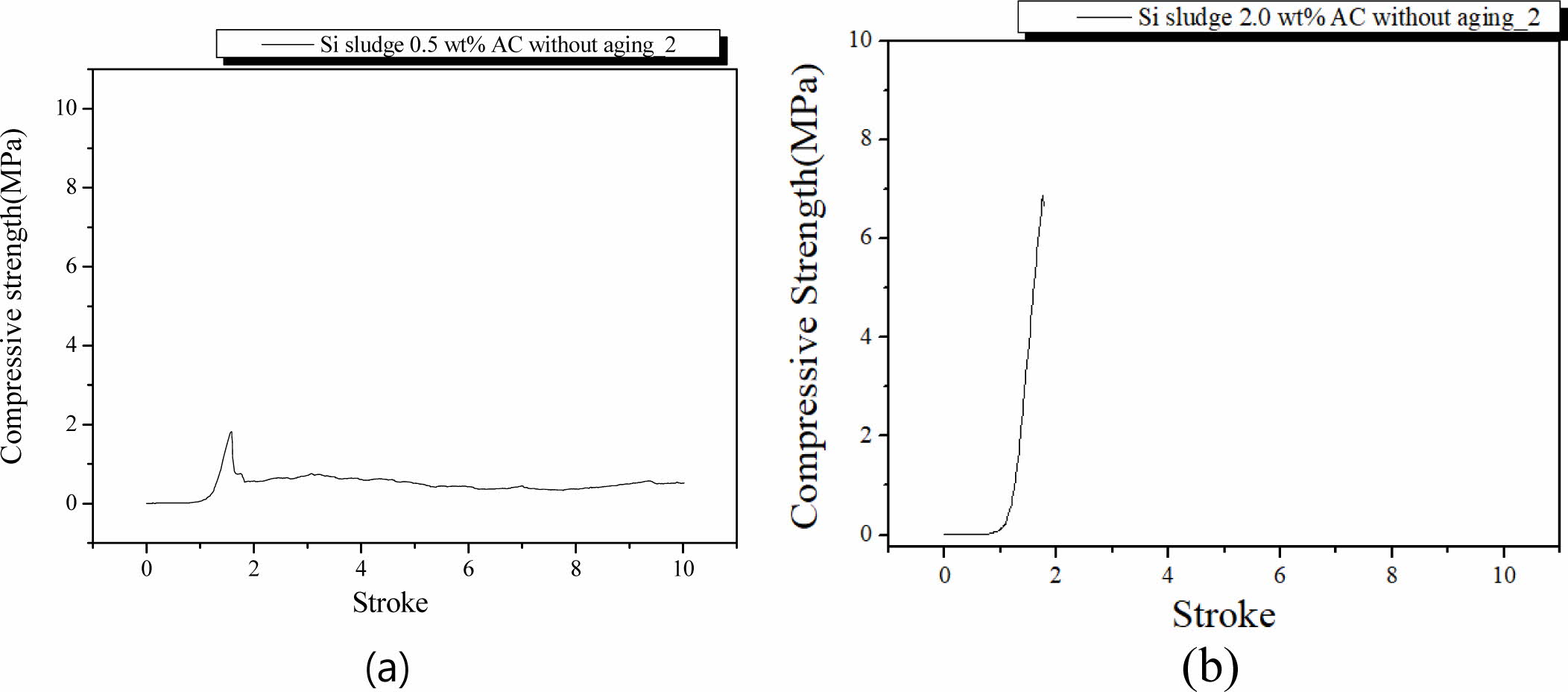
Specimens were mixed for 1 min and cured in an autoclave at 103 oC for 1 d. After the autoclave curing, specimens were tested for compressive strength without further aging. Fig. 9 shows the fracture characteristics of the specimens containing 0.5 and 2.0 wt.% Si with autoclave curing only. Unlike the oven-cured specimens without aging, the maximum compressive strength of the specimens containing 0.5 and 2.0 wt.% were 1.9 and 7.0 MPa, respectively. Compared to specimens that were only oven-cured, the compressive strength of the autoclave-cured specimens without aging was much higher and even comparable to those of the same specimens with extra mixing time and autoclave-aged, as shown in Figs. 5(a) and 5(b).
As shown in Fig. 9, no dentable characteristics were found in these specimens without aging. There was an abrupt fragile fracture on the specimen containing 2.0 wt.% Si, as shown in Fig. 9(b). It was concluded that the aging process is necessary for producing geopolymers with dentable characteristics.
XRD analysis
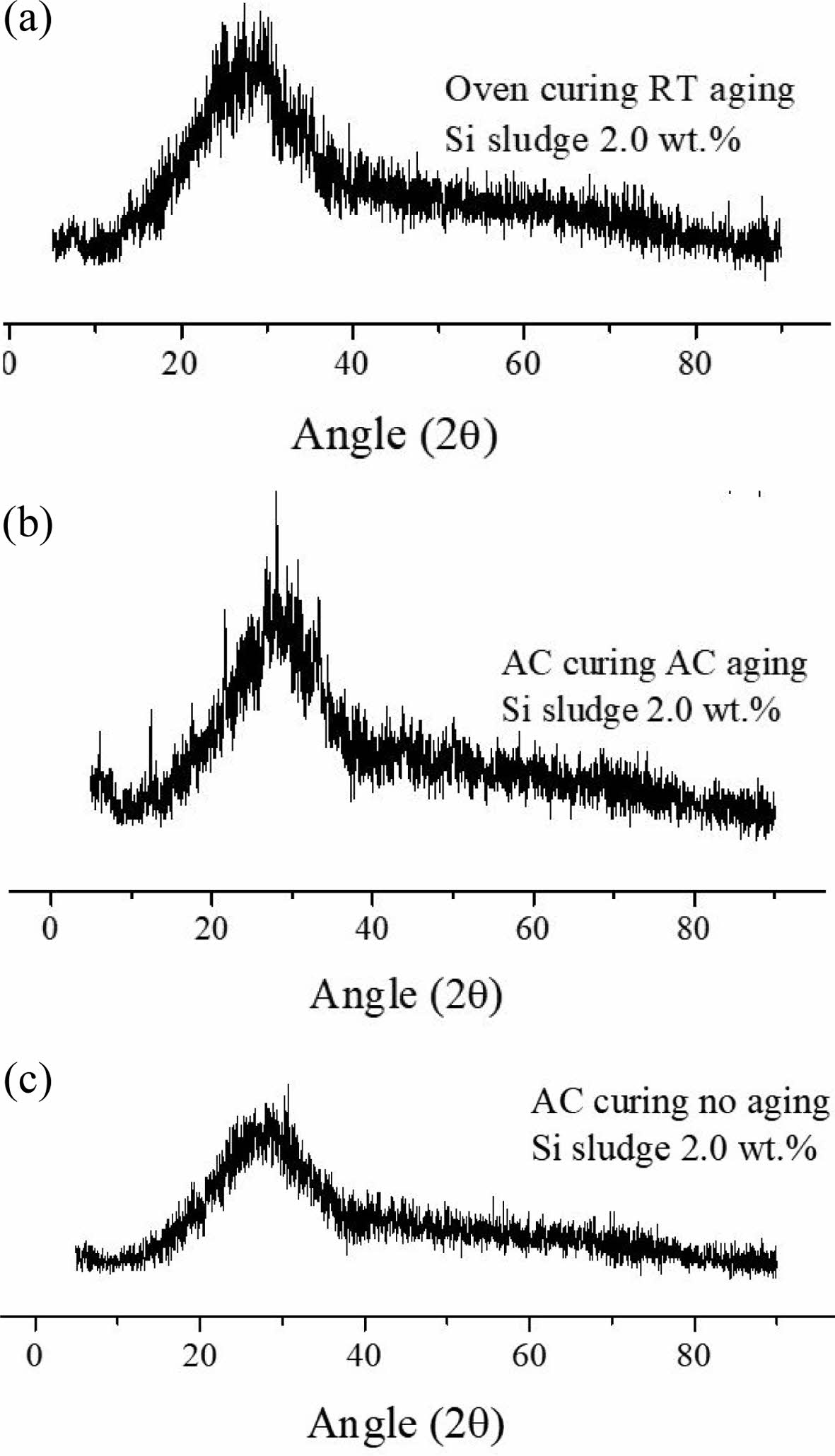
Fig. 10 shows the XRD patterns of three specimens containing 2.0 wt.% Si sludge aged via three aging methods, namely room-temperature aging, autoclave aging, and without aging. It was difficult to detect any difference among numerous peaks from the specimens prepared under various curing and aging conditions. Therefore, only selected peaks from the specimens prepared under the three different aging conditions are presented because most of the peaks exhibited a typical amorphous phase and no particular crystalline peak could be found.
As shown in Fig. 10, there was little difference among XRD patterns; however, more small peaks that appeared probably from the crystallites grown in the geopolymer matrix are shown in Fig. 10(b), compared to the other two patterns. This implies that autoclave aging may enhance the geopolymerization and result in more peaks, which means that the crystallinity of the geopolymer increases. Another supporting evidence of this scientific inference is the stability of the geopolymer after immersion. As mentioned in a previous report [20] and in Section 3.2.4, the autoclave-cured and aged specimens exhibited good stability in water as well as dentable characteristics. This stability may be attributed to the increased crystallization of the geopolymer by autoclave aging.
FT-IR analysis
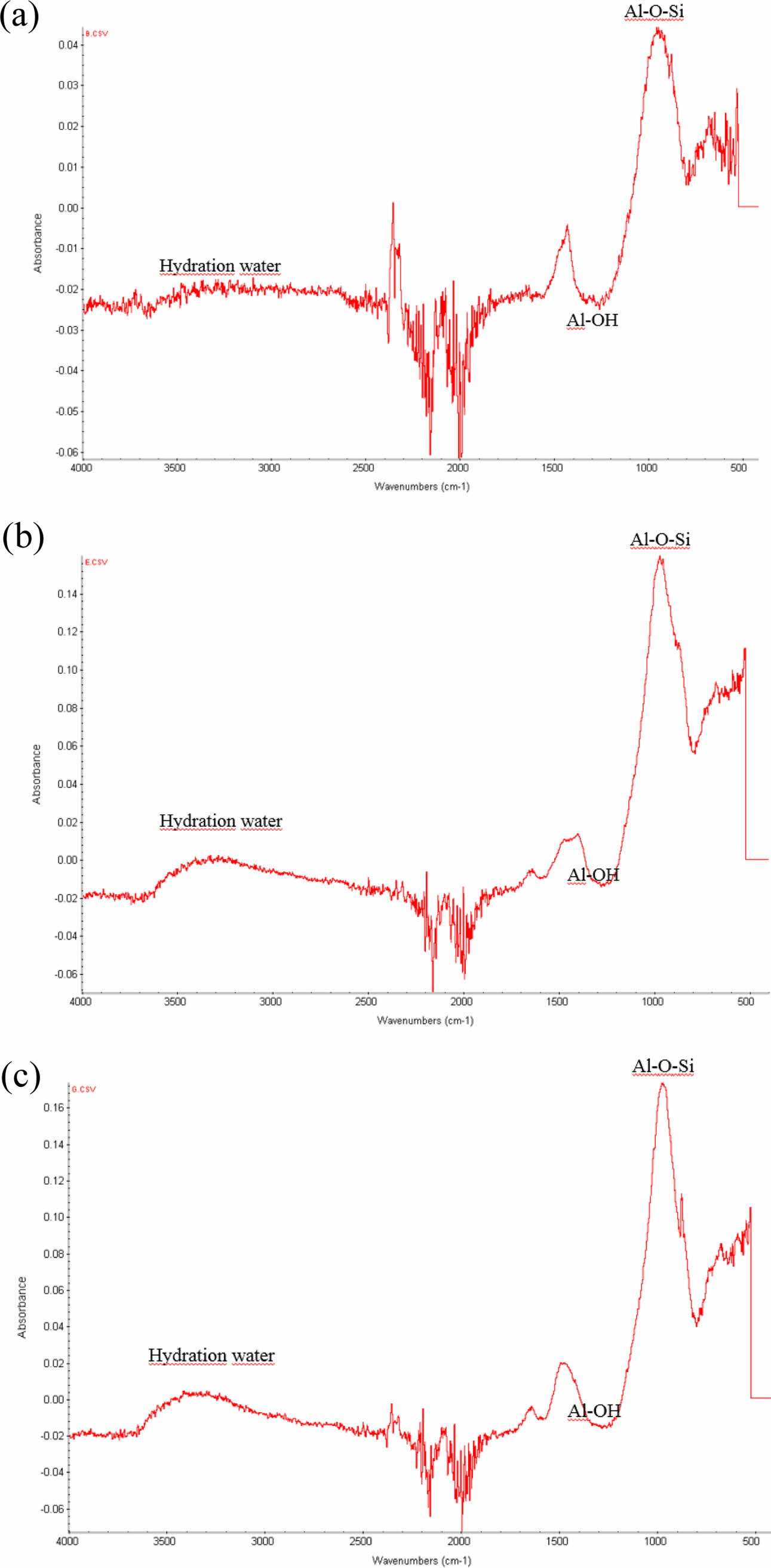
Fig. 11 shows FT-IR peaks from three specimens containing 2.0 wt.% Si sludge aged via three aging methods, namely room-temperature aging, autoclave aging, and without aging, as same sequence as in Fig. 11. Similar to the XRD results, it was also difficult to determine any difference among numerous FT-IR peaks from the specimens prepared under various curing and aging conditions. Therefore, only the selected FT-IR peaks, as shown in Fig. 11, from the specimens prepared under the three different aging conditions, are presented. Small differences among the given FT-IR peaks in Fig. 11 can be found according to the aging conditions.
Note that the intensity of each graph has different scales. The highest peak intensity of Al–O–Si bonding is shown in Fig. 11(b), observed in the autoclave-cured and aged specimens. The intensity of the Al–O–Si bonding peak was four times higher than that of the oven-cured and room-temperature specimens. This implies that more Al–O–Si bonds were created in the autoclave-cured and aged geopolymer, and it is con- cluded that more geopolymerization reaction progressed on the autoclaved and aged specimens, resulted in more stability in water because more Al–O–Si bonding means more networking in the geopolymer.
The Al–OH bonding peaks also have the same tendency as the Al–O–Si bonging peaks, that is, the intensity of the Al–OH peak in Fig. 11(b) is approximately four times higher than that of Fig. 11(a). It can be speculated that dentable characteristics can be explained by Al–OH bonding peaks because Al–OH bonding is closely related to the presence of water (H2O), and this bonding is not related to the strength of the geo- polymer, that is, it is not related to the formation of the network structure of the geopolymer. Therefore, it can be explained that Al–O–Si bonding peaks contribute to the stability of water, whereas the Al–OH bonding peaks contribute to the dentable characteristics of the autoclaved-cured and aged species.
It is difficult to draw a conclusion from the limited data, as shown in Fig. 10 and Fig. 11; therefore, it is necessary to investigate XRD and FT-IR data more extensively and systematically in the future to obtain solid conclusions. Nevertheless, the data in Fig. 10 and Fig. 11 are still worthwhile for the first report on dentable geopolymers.
Optical microscopic observation
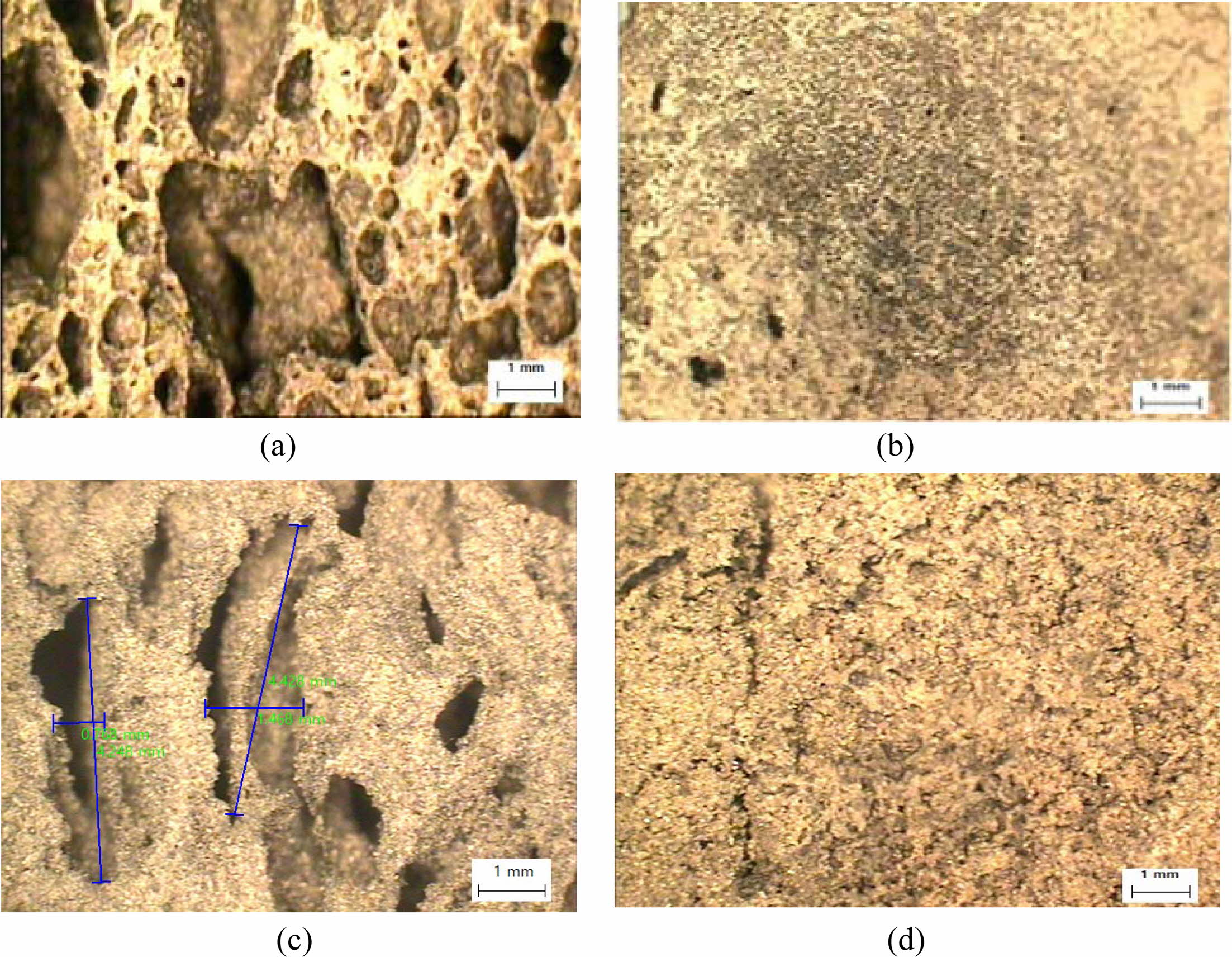
The shape, size, and distribution of pores were observed by an optical microscope, as shown in Fig. 12. Fig. 12 shows comparative optical micrographs of pore shape, size, and distribution before the compressive test without and with extra mixing, and after the compressive test without extra mixing and with extra mixing.
As shown in Fig. 12, pore shape, size, and distribu- tion significantly differ before and after extra mixing, as reported previously [5]. Pore shapes were also changed after the compressive test. As shown in Fig. 12, the average aspect ratio of the pores increased from near 1.0, as shown in Fig. 12(a), to near 5.0, as shown in Fig. 12(c), after the compressive stress test. It is assumed that this change in aspect ratio is closely related to the dentable characteristics. However, specimens with extra mixing of small size, homogeneous shape, and distribution did not exhibit dentable characteristics. Therefore, it can be speculated that the small size, homogeneous (circular) shape, and distribution do not contribute to enhancing the dentable characteristics of geopolymers.
This study is preliminary results of dentable geo- polymer which has not been reported yet; therefore, it is difficult to find out previous report or similar works to be compared with. Hopefully, this study could give a motive to other researchers for further study of dendable materials.
|
Fig. 3 Profile of compressive strength of specimens that were oven-cured and room-temperature aged with various Si sludge addition vs. stroke. Additions of Si sludges are: (a) 0.5, (b) 2.0, (c) 5.0, and (d) 10.0 wt.%. |
|
Fig. 4 Profile of compressive strength of specimens that were oven-cured and autoclave-aged with various Si sludge addition vs. stroke. Additions of Si sludges are: (a) 0.5, (b) 2.0, (c) 5.0, and (d) 10.0 wt.%. |
|
Fig. 5 Profile of compressive strength of specimens that were oven-cured, room-temperature aged, and extra mixed for 5 min with various Si sludge addition vs. stroke. Additions of Si sludges are: (a) 0.5, (b) 2.0, (c) 5.0, and (d) 10.0 wt.%. |
|
Fig. 6 Profile of compressive strength of specimens that were mixed for 5 min, autoclaved-cured, and autoclave-aged with various Si sludge addition vs. stroke. Additions of Si sludges are: (a) 0.5, (b) 2.0, (c) 5.0, and (d) 10.0 wt.%. |
|
Fig. 7 Profile of compressive strength of specimen containing 0.5 wt.% Si, autoclave-cured, autoclave-aged, and oven-dried at 83 o C for 1 d in the dry oven. |
|
Fig. 8 Profile of compressive strength of the specimen containing 0.5 wt.% Si, autoclave-cured, autoclave-aged, oven-dried, and then immersed for 3 d. |
|
Fig. 9 Profile of compressive strength of the specimens with two Si sludge addition vs. stroke. Additions of Si sludges are: (a) 0.5 and (b) 2.0 wt.%. |
|
Fig. 10 XRD patterns from three specimens containing 2.0 wt.% Si sludge aged via three aging methods, namely room-temperature aging, autoclave aging, and without aging. (a) Oven curing and room-temperature aging; (b) autoclave curing and autoclave aging; and (c) autoclave curing and no aging |
|
Fig. 11 FT-IR peaks from three specimens containing 2.0 wt.% Si sludge aged via three aging methods, namely room-temperature aging, autoclave aging, and without aging. (a) Oven curing and room-temperature aging; (b) autoclave curing and autoclave aging; and (c) autoclave curing and no aging. |
|
Fig. 12 Cross-sectional optical micrographs of geopolymers show the changes in pore shape, size, and distribution according to the before and after compressive test, and without extra mixing and with extra mixing conditions. All specimens were made with 2.0 wt.% Si sludge, autoclave-cured for 1 d, and aged for 3 d. (a) Without extra mixing before the compressive test; (b) with extra mixing before the compressive test; (c) with extra mixing after the compressive test; and (d) with extra mixing after the compressive test. |
Geopolymers made of IGCC slag and Si sludge showed dentable characteristics when they are prepared under the certain curing and aging conditions. It was assumed that small pore size and homogeneous shape and distribution did not enhance the dentable charac- teristics. The moisture content of the geopolymer is also one of the factors controlling the dentable charac- teristics. Aging conditions and mixing were determined to be among the most important factors for dentable characteristics and stability of geopolymers in water. Autoclave-cured and aged specimens exhibited stability in water and dentable characteristics.
The results of this study are preliminary and prema- ture because the studies on such a kind of dentable ceramics have hardly been found. Although the experi- mental conditions and research parameters for this study are not well organized and sophisticated, it is believed that this study is worthwhile as the first step in determining the mechanism and production conditions of dentable geopolymers and hopefully would be a basis for future studies on dentable geopolymers.
This work was supported by a Kyonggi University Research Grant 2020.
- 1. Brundtland Commission, in “Our Common Future” (Oxford University Press, 1987) p.27.
- 2. V. Jadhao and P. Pajgade, Int. J. Eng. Adv. Tec. 2[2] (2013) 148−153.
- 3. M.J. Kim, and Y.T. Kim, J. Korean Cryst. Growth Cryst. Technol. 29[6] (2019) 257-263.
-

- 4. https://unfccc.int/process-and-meetings/the-paris-agreement /the-paris-agreement. (Accessed 12 Dec. 2015)
- 5. Y.T. Kim and M.J. Kim, J. Ceram. Process. Res. 20[5] (2019) 522−529.
-

- 6. K.T. Koh, G.S. Ryu and J.H. Lee, J. Kor. Rec. Consrt. Res. Inst. 16 (2011) 119−126.
- 7. P. Purnell, Environ. Sci. Technol. 46[1] (2012) 454-461.
-

- 8. L.K. Turner, and F.G. Collins, Constr. Build. Mater. 43[6] (2013) 125-130.
-

- 9. Y.T. Kim, S.Y. Kim and T.S. Chae, J. Ceram. Process. Res. 18[3] (2017) 214−219.
- 10. G.H. Lim, J.B. Lee, H.K. Jeong and S.S. Kim, J. Rec. Const. Res. 4[4] (2016) 418-424.
-

- 11. J. Davidovits, in Proceeding of the 95th Annual Meeting of The American Ceramic Society, November 1993, edited by Moukwa M. (Amer Ceramic Society, 1993) p.1654.
- 12. J. Davidovits, J. Thermal Analysis 37[8] (1991) 1633-1656.
-

- 13. F.G.M. Aredes, T.M.B. Campos, J.P.B. Machado, K.K. Sakane, G.P. Thim, and D.D. Brunelli, Ceram. Int. 41[6] (2015) 7302–7311.
-

- 14. J.G.S. van Jaarsveld and J.S.J. van Deventer, Ind. Eng. Chem. Res. 38[10] (1999) 3932-3941.
-

- 15. A. Autef, E. Joussein, G. Gasgnier, and S. Rossignol, J. Non-Cryst. Solids 358[21] (2012) 2886-2893.
-

- 16. G. Habert, J.B Lacaillerie, and N. Roussel, J. Clean. Prod. 19[11] (2011) 1229-1238.
-

- 17. R. Kajaste and M. Hurme, J. Cleaner Prod. 112[5] (2016) 4041-4051.
-

- 18. https://precision-ceramics.com/what-are-machinable-ceramics.
- 19. Y. Kim, Annual research report to NRF (National Research Foundation of Korea), No. NRF-2018R1D1A1B07044819 (2019).
- 20. Y. Kim and M. Kim, J. Ceram. Process. Res. 22[2] (2021) 158-168.
-

- 21. Y. Kim and M. Kim, Int'l J. Mech. Product'n Eng. 8[5] (2020) 47-51.
- 22. W.K.W. Lee, and J.S.J. Deventer, Langmuir. 19[21] (2003) 8726-8734.
-

- 23. Y.T. Kim, S.Y. Kim and C.S. Jang, J. Ceram. Process. Res. 17[11] (2016) 1202-1207.
 This Article
This Article
-
2022; 23(1): 1-11
Published on Feb 28, 2022
- 10.36410/jcpr.2022.23.1.1
- Received on Feb 1, 2021
- Revised on Aug 8, 2021
- Accepted on Aug 28, 2021
 Services
Services
- Abstract
introduction
experimental procedures
results and discussions
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yootaek Kim
-
Department of Materials Engineering, Kyonggi University, Suwon 16227, Republic of Korea
Tel : +82-70-4024-9765 Fax: +82-31-249-9774 - E-mail: ytkim@kgu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.