- Effects of binding agents in the production of SiC ceramic foam filters with replica method
Mehmet Bugdaycia,* and Gizem Barana,b
aChemical Engineering Department, Faculty of Engineering, Yalova University, 77200, Yalova, Turkey
bDepartment of Chemical Engineering (Msc), Institute of Sciences, Yalova University, 77200 Yalova, TurkeyThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
In this study, casting filter production parameters were investigated by coating SiC with superior mechanical and chemical properties on polyurethane sponge. Many methods in which SiC filters are produced have been researched and considering its simple applicability, the replication method has been chosen as the production method. In the experiments, polyurethane sponges with different porosities were coated with varying proportions of water, bentonite, gum arabic and SiC, and then subjected to pyrolysis and sintering. The characterization of the samples was carried out by weight loss, compression test, Archimedes scale and SEM methods. According to the test results, the highest weight loss was obtained with 46% by adding 30 mL of water into 30 ppi polyurethane sponge. In the sintering tests, the highest strength was obtained with the value of 472 kPa in the experiment performed at 1,450 ºC. The same sample gave the optimum result with a density of 3.101 g/cm3 and porosity of 1.24%. At the end of the study, the samples produced successfully were used in the real casting process and it was observed that they were effective in filtering
Keywords: Filter, SiC, Sintering, Refractories, Ceramic Foams
Silicon carbide (SiC) ceramics have gathered much attention in the area of porous materials in recent years. Silicon carbide's high mechanical strength, wear resist- ance, high thermal conductivity, low thermal expansion coefficient, high thermal shock resistance and high chemical resistance make it a preferred material in various applications. These properties of SiC make it superior when compared to its alternatives in many areas of application. SiC products are commonly used in a wide range of applications including grinding supplies, separation applications, catalyzer additives, sound insulation, high-temperature structural elements, heat insulation, strengthening of composite materials, refractory industry and casting filters [1-17]. There are many studies on SiC ceramics in the literature. In these studies, different properties of the material are emphasized. Ceramic materials have low toughness values compared to metals. In Seok Nam's study, it was observed that the addition of SiC increased the strength values by preventing crack-healing [18]. In the study of Joo et al., It was stated that SiC increased the thermal resistance in polymer-derived fibers [19]. Min So et al. examined the sintering conditions of SiC-TiC composites and showed that the material can be obtained with a hot press at 99.9% density. At this density, a hardness value of 25 GPa was reached, and the thermal con- ductivity value was found to be 80 Wm-1K-1. As can be seen, SiC significantly improves the properties of the structures to which it is added [20]. The work done by Hwan Ahn has shown that SiC's resistance to abrasion at high temperatures is increased and the material can be used with high performance in these applications [21].
SiC products are especially important in the area of filter production. These products are used in industry in the areas of water filtration, porous burners, honeycomb structured diesel particle filters, metal-matrix composites, polymer-matrix composites, membranes, hot gas filters and molten metal filters [22-26].
The main reason for using SiC as filter material is its low density, high selectivity and high surface area when it is produced as polymer composite. These properties are achieved by controlling the composition of the porous material. Amount of open and closed pores, distribution of pore size affect the properties of the material. All these properties depend on the production method of the material [27].
Many methods have been developed to produce porous ceramics for use in SiC filter production [28]. The main methods used in the production of cellular ceramics like foams, honeycomb structures, interconnected rods, fibers and hollow spheres were studied by Hye Eom recently [29]. In this study, the methods are divided into five categories as follows: (i) partial sintering, (ii) replica, (iii) sacrificial template, (iv) direct foaming and (v) bonding methods [29].
The partial sintering method is a basic method to produce SiC ceramics, which have porosity under 65% and pore dimensions ranging between 0.1 and 10 micrometers. In this process, the condensation is delayed by decreasing the sintering potential. A decreased sintering potential is achieved by lowering the sintering temperature, limiting the rough powder network, granulator sintering and recrystallization. The sacrificial template method is the most appropriate method to control the distribution of pore dimensions and the shape of pores in the production of porous SiC structures. The decisive parameters about these properties in this method are the dimensions, shape and composition of the template used in the process. In the sacrificial template method, the pore dimension is between 1 and 700 micrometers, and the porosity is between 15% and 95%. Pore dimension and porosity vary depending on the template and the process parameters. The direct foaming method is a simple and fast method for obtaining a wide variety of pore sizes, up to 95% porosity and both open and closed-cell structures. The bonding method is a cheap and low-temperature method to achieve a porosity value between 15% and 60% in the macro-porous SiC ceramics production. The process temperature varies between 800 and 1,550 oC depending on the composition of the binding agent. The replica method is used to produce open-cell SiC ceramics with the pore dimensions ranging between 10 micrometers to 5 millimeters [27].
The aim of this study is to produce open-cell SiC filters to be used in metallurgical casting applications. Metal casting technology is growing rapidly with 110.7 million tons of global shipment in 2019. The biggest problem of foundries is to produce parts according to the design and the quality specifications without the defects that occur during the casting process [29-32]. To produce quality products with the casting process, the design of the mold is one of the most important parameters. Using low-quality sand in the mold causes mold failure and insufficient mold strength during the casting process and thus lead to the production of lower-than-desired quality products. Parts produced by casting process are subjected to processes like sandblasting, welding and machining to obtain final products. In addition to these defects, the impurities and the inclusions in the material lead to undesired material compositions and to the need for additional processes mentioned above to finalize the product after the casting process. The impurities must be eliminated from the structure to ensure proper interaction between the molten metal and the mold surface during the grain formation from the molten metal on the inner surface of the mold during the solidification process. For this reason, the molten metal must be filtered before interacting with the mold surface [32]. The liquid metal filtration process is a widely used purification process in iron and non-iron alloy casting applications. The filtration process eliminates the residue that occurs in the melting (high temperature) process and thus increases the mechanical properties of the final product machinability. This process also increases the surface quality and density of the final product and thus decreases the cost of the machining process after the casting process [33, 34]. It can be used in all metal casting applications since SiC maintains its shape at temperatures above 1,500 ºC [35]. The permeability, the selectivity and the thermal resistance are the most important properties of a filter which will be used in the casting process. In addition to the superior selectivity and high-temperature resistance properties of SiC, the replica method, which has simple applicability, was chosen as the production process in this study. The aim of this study is to determine the optimum mixing and sintering parameters of SiC filter production with the replication method and to investigate the effects of using the produced filters in casting applications.
Material
SiC, bentonite, gum arabic and porous polyurethane sponge materials were used in the studies for the production of SiC-based ceramic foam filters with the replica method. Bentonite supplied by Smart Chemical was used to adjust the viscosity of the slurry and take advantage of its binding effect. XRD result of this sample is presented in Fig. 1. XRD analysis was performed with CuKα (k = 1.5406 Å) radiation in the range of 0-90 for 2 hours using 0.02º step size and 4 ve / min speed was used for the investigation of SiC samples. Sample structures were determined with the X Pert High Score program. When Figure 1 is examined, it is seen that SiC gives the highest intensity (10000) peak at 36 degrees. Apart from that, there are 13 more SiC peaks in the graph and each peak matches the pattern number 01-074-1302 pdf. According to Fig. 1 it is clearly seen that the sample does not contain any impurity.
Gum arabic was added to bentonite as a binder and it was supplied from Shaanxi Undersun Biomedtech. Gum arabic characteristic properties is shown in Table 1.
After adding binders to the sponge, SiC was added as the material to be formed on the foam, this material was supplied from Nanokar Chemical Materials Manufacturing and Trade Limited Company. Experiments were carried out with SiC’s in two different grain sizes of 100 and 32 μm. Polyurethane sponge, which was supplied from Sunger Dokum, was used as skeleton material in 5, 10, 30 pore/inch dimensions.
Method
The first stage of the experimental studies is the preparation of the sludge. At this stage, gum arabic and bentonite were dissolved by mixing with Thermomac magnetic stirring device at 350 rpm for 30 min, after SiC powder was added to the solution for 30 min, and a homogeneous sludge was obtained.
In the second stage of the experimental studies, the process of absorbing the sludge into the sponge and removing the excess sludge from the sponge was carried out. Polyurethane sponge was cut as 3 ´ 3 cm for 10 and 30 ppi pore size and 3x5 cm for 5 ppi pore size. The prepared ceramic mud was absorbed into the polyurethane sponge and the excess mud was squeezed away from the sponge.
The third stage is the drying process. The drying process was carried out in ETUV at 105 ºC for 1 h. Ceramic particles are deposited on the sponge as the result of the drying process.
The fourth stage of the experimental studies was pyrolysis. Nevo QTZ ash oven was used for this process. Nevo–QTZ Series furnaces have an operating temperature of 1,100 oC; 4 lt. and 7 lt. They are ash furnaces with internal volumes. Pyrolysis was applied for 30 min at 600 ºC at 10 ºC/min heating rate. Poly- urethane sponge and gum arabic were removed from the structure by the pyrolysis process.
The final stage of the experimental studies was the sintering process. The mixture, which was first subjected to pre-sintering at 1,050 ºC, was then subjected to final sintering at various temperatures. With the sintering process, the structure was provided to be concentrated. The process was carried out in MSE VF_1700 furnace. This furnace can heat from 450 ºC to 1,700 ºC under 10-5 mbar vacuum.
Characterization Techniques
In the first stage of characterization studies, weight loss amounts were determined with the SHIMADZU TX-423L scales. X-ray diffraction (XRD) analysis was done with BrukerTM D8 Advanced Series powder diffractometer (operated at 35 kV and 40 mA) with CuKα (k = 1.5406 Å) radiation at 2 h range of 0-90º using a step size of 0.02º and a rate of 4º/min was used for investigations of the SiC samples. Microstructure images of the products were taken with Carl Zeiss/Gemini 300 brand-model SEM-EDS device. A compression test was performed to measure the strength of SiC filters. Compression experiments were carried out by using a Zwick/Roell device according to ASTM D695 standart. TGA analysis (Seiko, TG/DTA 6300) was performed with the device. Chemical analysis of casting samples was performed with a Hitachi High-Tech Analytical Science Foundry Master series optical emission spectrometer. Finally, density measurements were made by Archimedes method using the ASTM C 373 standard.
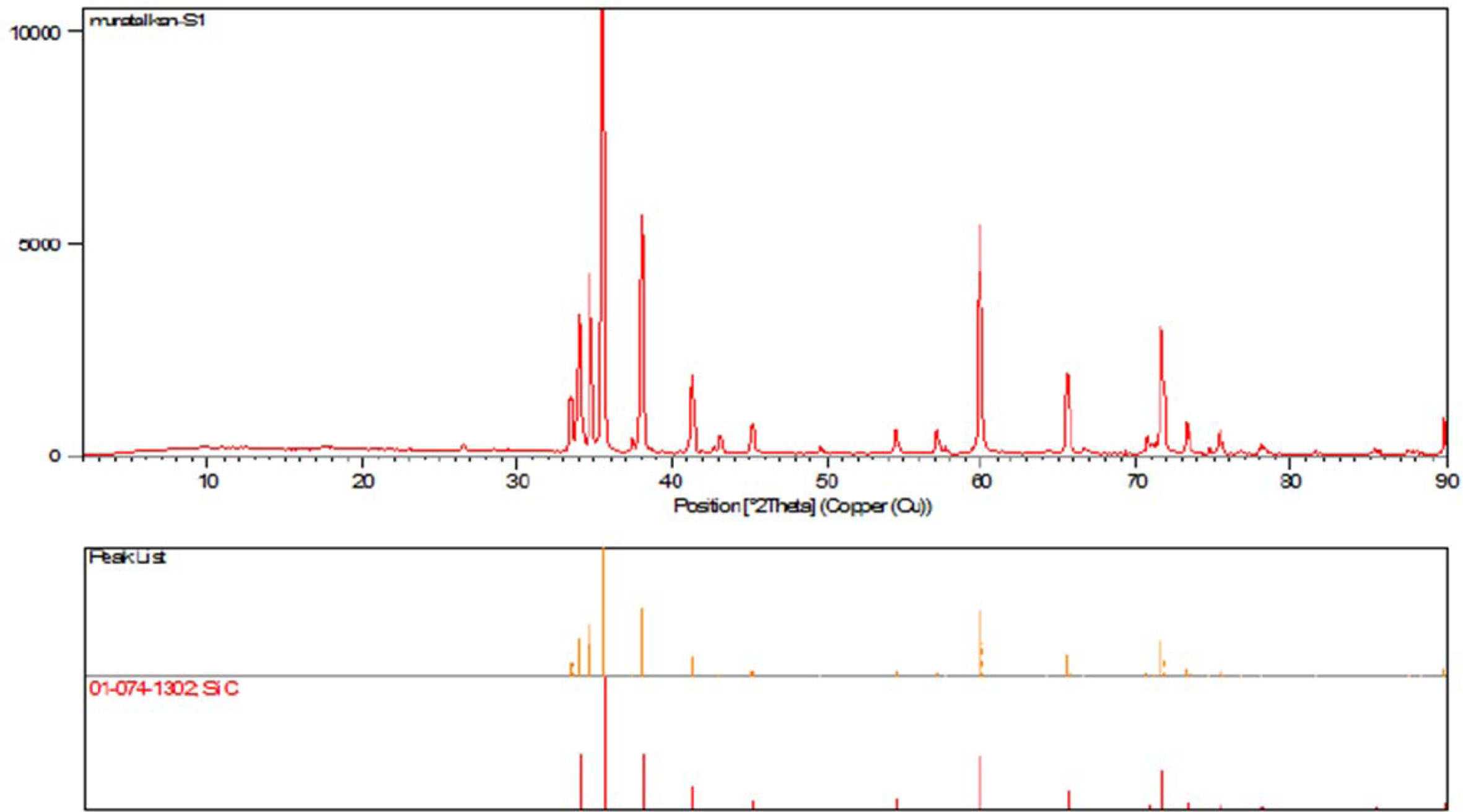
|
Fig. 1 XRD results of SiC sample. |
In the studies carried out to produce SiC filter, the mixture consisting of gum arabic and bentonite was absorbed into the polyurethane sponge to obtain the desired structure. The final product should only consist of the SiC ceramic structure. TGA analysis was used to determine the heating regime to be used to remove the polyurethane sponge and arabic gum from the structure without destroying the ceramic structure. According to the findings presented in Fig. 2, gum arabic is removed from the structure at 500 oC while the polyurethane sponge is removed at 600 oC. Fig. 2 shows that there was no change in SiC structure at these temperatures where pyrolysis and sintering took place. Data obtained in pyrolysis and sintering experiments also support the results of the TGA analysis.
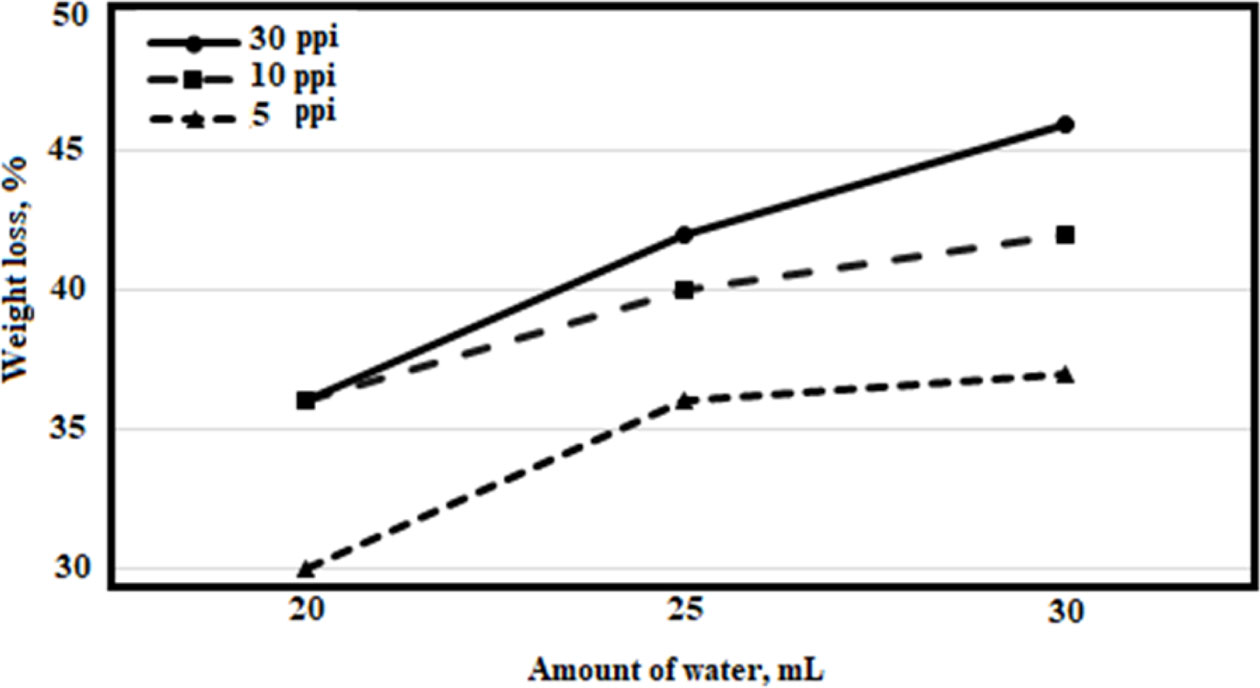
In the first stage of the experiments, sludges that contain different amounts of water (20 ml, 25 ml, 30 ml) were prepared. Prepared sludges were absorbed into polyurethane sponges with different porosities (5 ppi, 10 ppi, 30 ppi). Then, the samples were held at 600 oC for 1 hour and subjected to pyrolysis. Samples were removed after the oven was cooled to room temperature, weight losses were calculated, and the results are given in Fig. 3.
When the weight losses were analyzed, it was seen that the highest loss rate was obtained as 46% with a 30 ppi sponge and 30 mL water content. For each pore size, the weight loss rate increased with an increasing amount of water. When the mixtures containing 20 mL of water were examined, it was seen that weight loss was 36% for 30 ppi and 10 ppi mixtures, and weight loss was 30% for 5 ppi mixture. As the amount of water increased, the difference between samples with 30 ppi and 10 ppi pore size increased by 4%. In the sample with 5 ppi pore size, the maximum weight loss was calculated as 37% and the other sample was far behind the two samples.
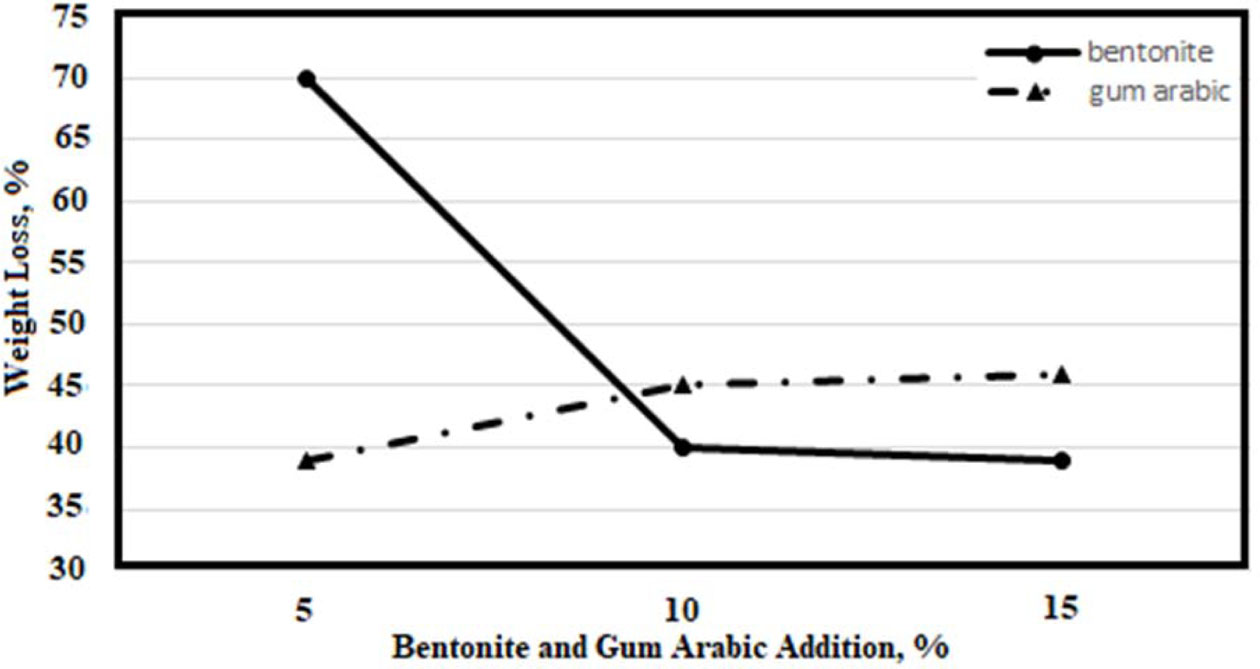
After examining the effect of the increased amount of water in the first stage of experimental studies, in the second stage, the effects of bentonite and gum arabic additions were investigated. 5%, 10% and 15% bentonite and gum arabic were added to the mixture using a 10 ppi sponge and the results are given in Fig. 4.
It is seen in Fig. 4 that the amount of weight loss decreases by increasing the amount of bentonite. This is because bentonite has a high water holding capacity and does not move away from the structure at 600 ºC. According to the results of the TGA, gum arabic removal temperature is 500ºC. This is the reason for the increase in the percent loss with an increasing amount of gum arabic in Fig. 4. This is the reason for the increase in the percent loss with increasing amount of gum arabic in Fig. 4.
The weights of the polymeric sponges before and after the mud coating were determined. The amount of mud accumulated in the polyurethane sponge was determined according to Eq. (1).
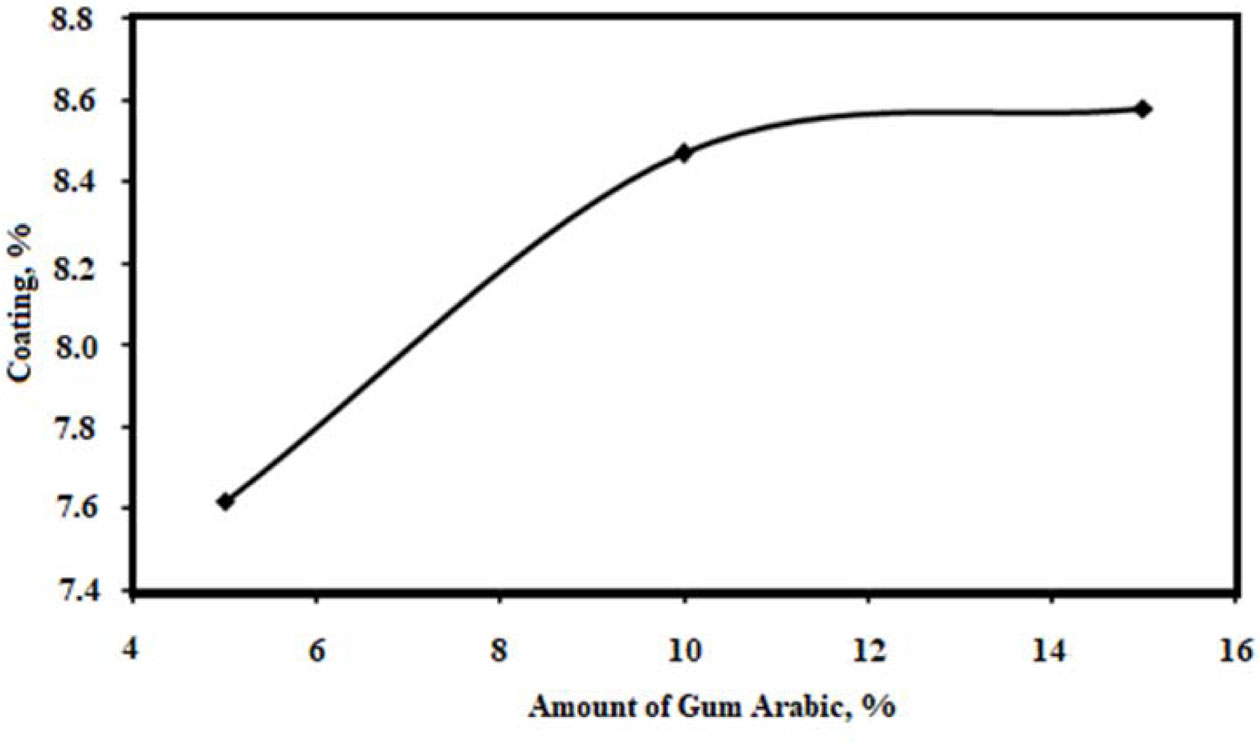
Fig. 5 shows the effect of gum arabic on the coating of the ceramic sludge on the polyurethane sponge (10 ppi). As the amount of gum arabic is increased, the amount of coating increases. However, since the amount of gum arabic is directly proportional to the viscosity of the sludge, the viscosity increases, and the increase in the gum arabic amount causes a decrease in the porosity amount in the structure.
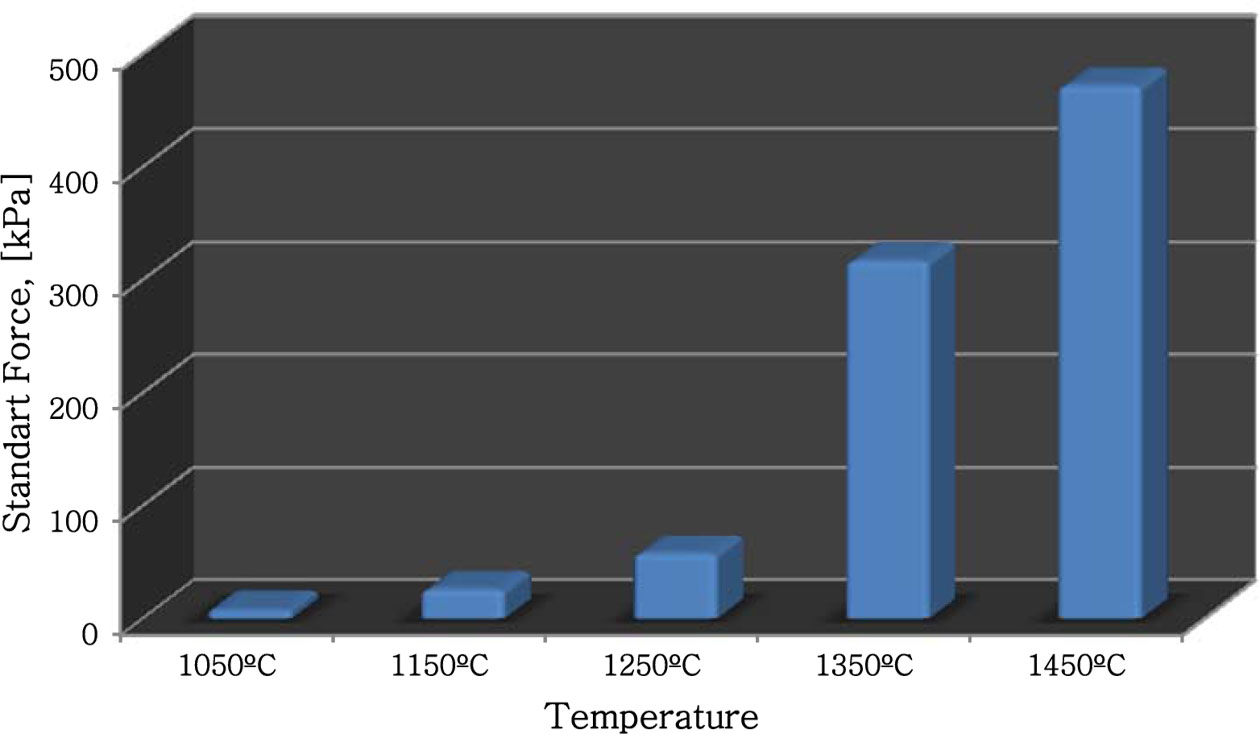
In the next step, the samples were subjected to com- pression test according to ASTM C1424 standard, and the maximum load they could bear are given in Fig. 6. According to Fig. 6, sufficient compression strength could not be obtained in the samples sintered at 1,050 ºC and 1,150 ºC. However, in samples sintered at 1,350 ºC and 1,450 ºC, the compression strength increased to 317 kPa and 472 kpa respectively. Therefore, the sintering temperature of 1,450 ºC, where the highest strength was obtained, was determined as the optimum parameter.
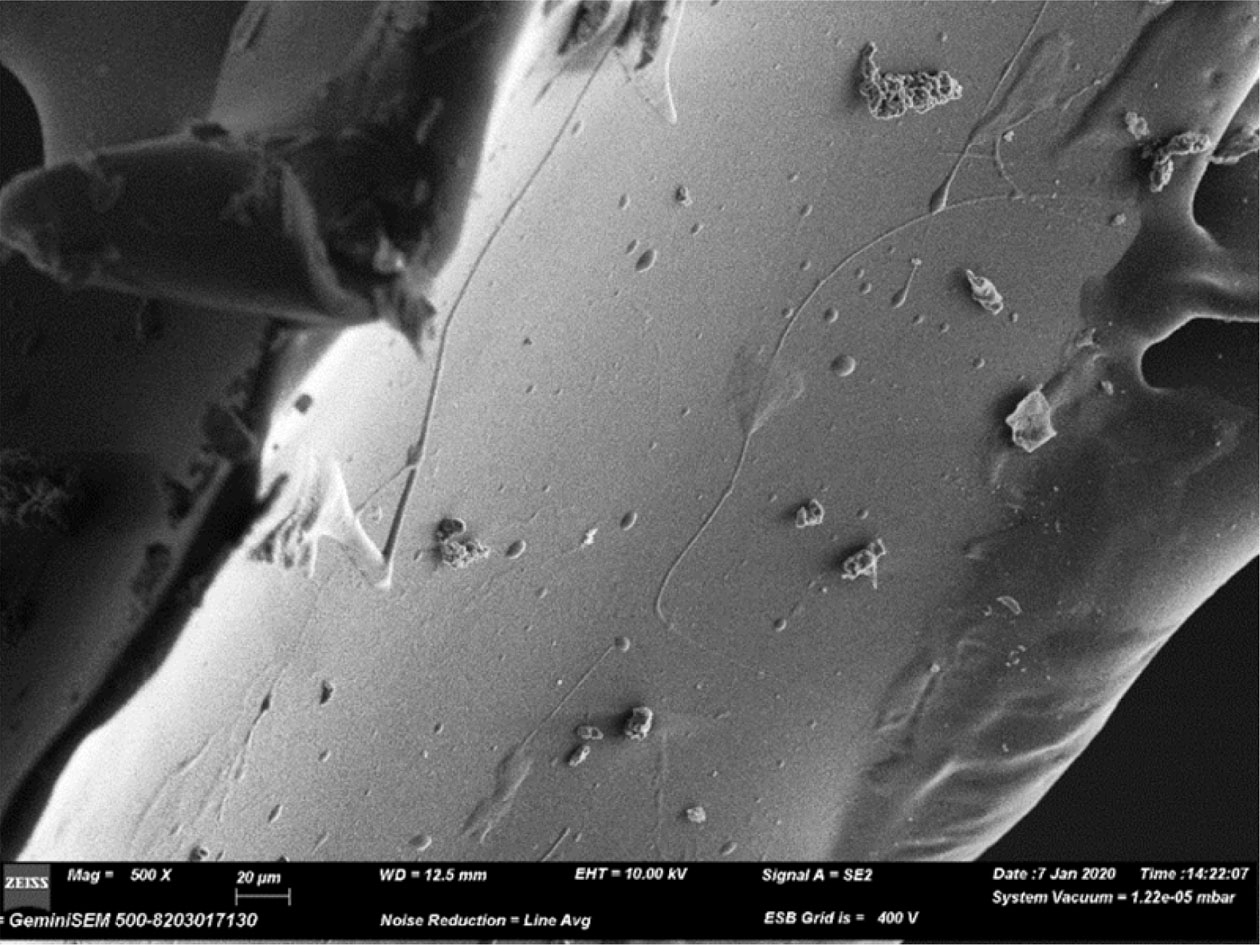
After examining the removal of binding materials from the structure, microstructure studies of the samples were performed. In microstructure studies, polyure- thane sponge was initially examined, and the results are given in Fig. 7. When the SEM image of the poly- urethane sponge is examined, it is seen that there is enough roughness to hold the sludge. EDS results of the polyurethane sponge are given in Table 2, and no impurities were found in the structure.
The polyurethane sponge was impregnated with silicon carbide, bentonite and gum arabic, and pyrolysis was applied at 600 oC for 1 h. SEM image of the pyrolysis sample is given in Fig. 8, and EDS results are presented in Table 3.
It is seen in Figure 8 that the SiC particles are successfully attached to the sponge. When the EDS results given in Table 3 are examined, it is seen that bentonite and gum arabic components were completely removed from the structure.
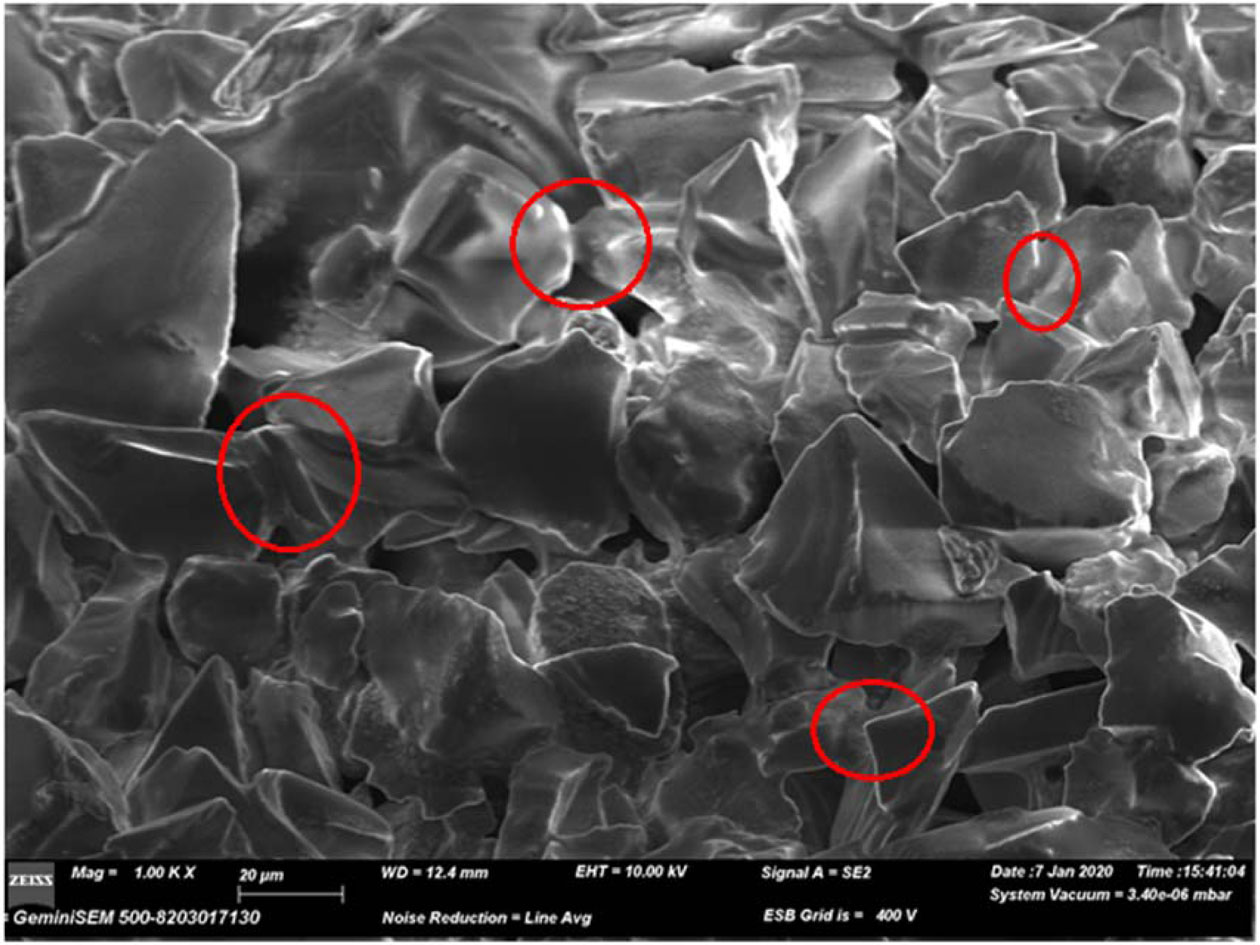
Experiments continued with the sintering process. Samples were sintered at 1,050 oC, 1,150 oC, 1,250 oC, 1,350 oC and 1,450 oC for 1 h. Fig. 9 shows the SEM image of the sample sintered at 1,050 oC, and Table 4 shows the EDS results of the same sample.
According to the SEM image, the neck formation between SiC powders begins at 1,050 ºC. Therefore, it is necessary to reach higher temperatures to reach higher density values. According to EDS data, the amount of oxygen increased, and a denser structure was obtained compared to the results obtained in the pyrolysis process.
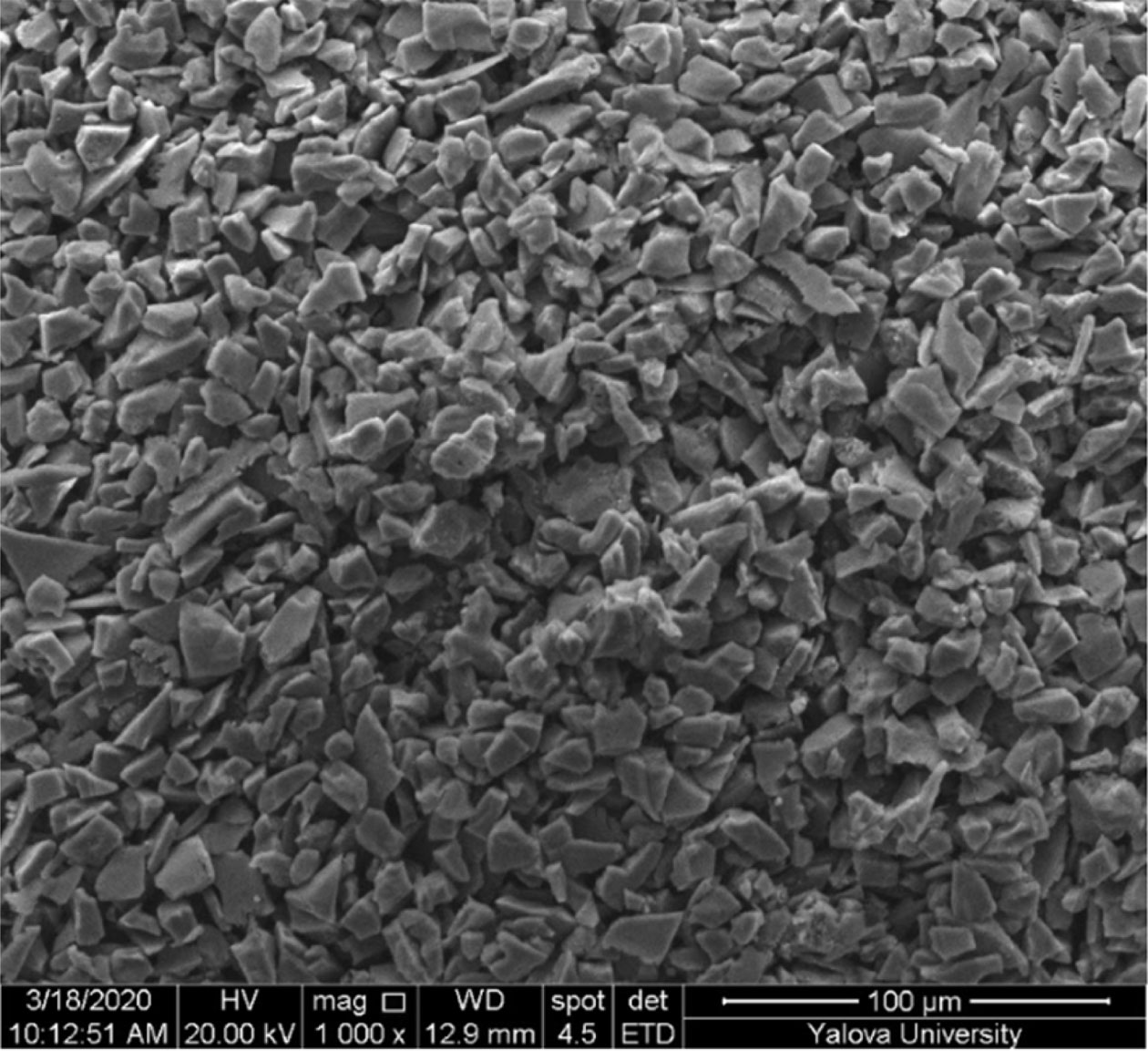
Then, samples are sintered at higher temperatures and the effects of high temperature on the structure were examined. SEM image of the sample sintered at 1,450 oC is given in Fig. 10. According to Fig. 10, condensation was completely realized, and the grains were fully connected.
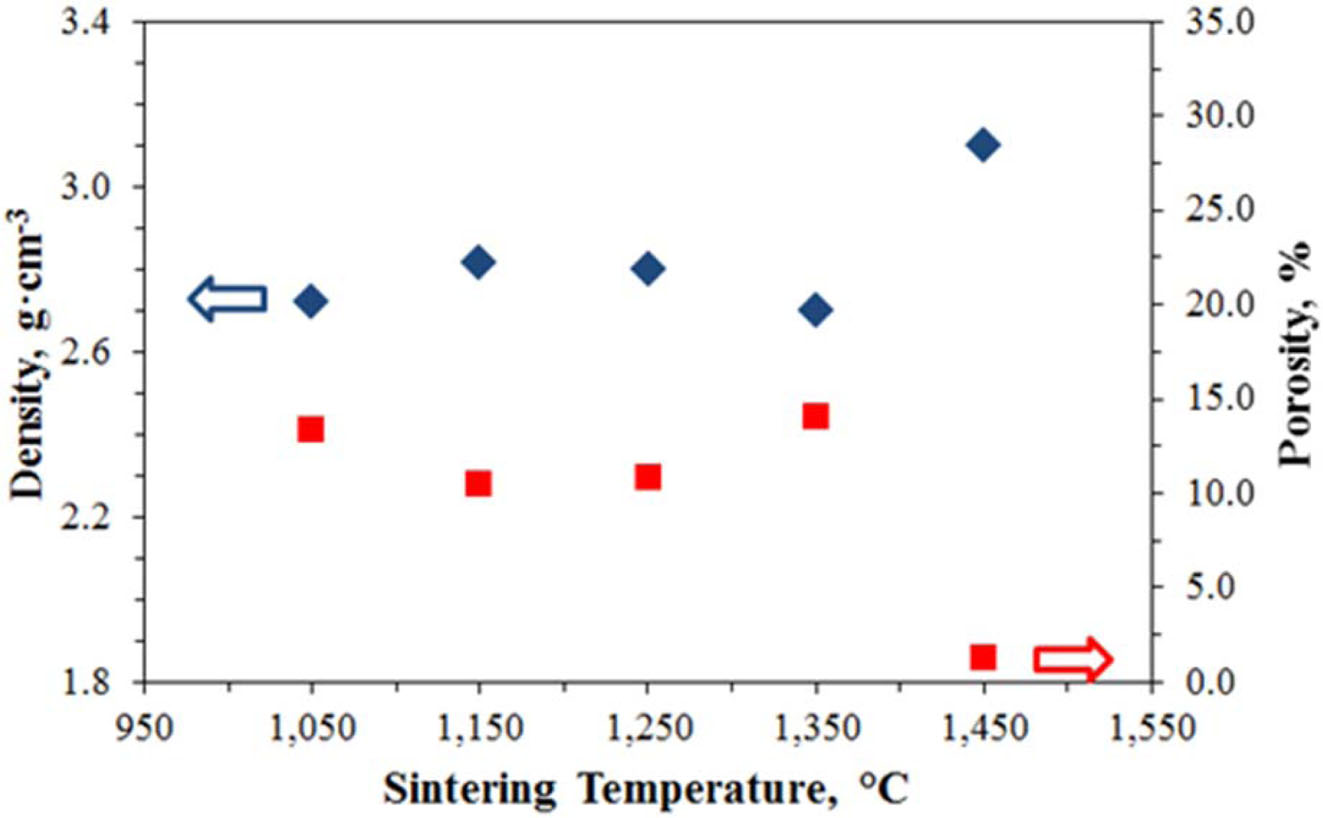
After the microstructure analysis of the samples, their density was measured and the porosity amounts were calculated accordingly. The density values of the samples at varying temperatures are given in Fig. 11. According to Fig. 11, bentonite or SiO2 sourced liquid phase sintering was performed up to 1,350 ºC, and SiC was sintered after 1,350 ºC. The porosity values according to the density sample are determined from Eq. (2). Fig. 10 also shows the porosity values of the samples. Accordingly, the porosity value decreases from 14.08% to 1.24% after 1350ºC. It proves that the sintering process is completed at 1450ºC. The density calculated here shows not the grade of the general skeleton, but the density of the material absorbed on the structure.
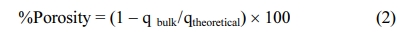
After the production of SiC filters was carried out by replication method, their effects on casting applications were investigated. SiC filtered and unfiltered castings were performed in the production of CuSn12 alloy performed in Benzesik casting, and the findings are given in Table 5.
When Table 5 is examined, it is clearly seen that there is a decrease in the amount of all materials except copper, with the effect of the filter. Accordingly, the amount of copper, which has the highest percentage among the alloying elements, increased with the filtering process. Analysis results show that the filter successfully reduces the amount of impurities in the alloy.
|
Fig. 2 TGA results for SiC, gum arabic, polyurethane sponge and bentonite. |
|
Fig. 3 Weight losses for different amounts of water. |
|
Fig. 4 Weight losses for different amounts of bentonite and gum arabic. |
|
Fig. 5 Effect of gum arabic on the coating of the ceramic sludge on the polyurethane sponge. |
|
Fig. 6 Effect of temperature on the compression strength of the samples. |
|
Fig. 7 SEM image of polyurethane sponge. |

|
Fig. 8 SEM image of the pyrolysis sample. |
|
Fig. 9 SEM image of the sintered sample at 1,050 ºC. |
|
Fig. 10 SEM image of the sintered sample at 1,350 ºC. |
|
Fig. 11 Density and porosity values of samples. |
In conclusion, SiC filters developed for use in the casting process was successfully produced by the replica method. In the experimental studies, the effects of sponge porosity, varying water and binder amounts on production parameters were determined. Accordingly, the highest weight loss in the experiments was determined as 46% by adding 30 mL of water to a 30 ppi sponge. Afterwards, the strength values of the samples were examined, and 472 kPa strength was obtained in the sample sintered at 1,450 ºC. When SEM images of the samples were examined, it was seen that neck formation started at 1,050 ºC, and densification was completed at 1,450 ºC. Then, porosity and densifi- cation rates were calculated and 1,450 ºC sintered sample gave the best results with 3.101 g/cm3 densification and 1.24% porosity values. Finally, the filtering effect of the sample sintered at 1,450 ºC was observed in the actual casting process. It has been observed that the filter can both preserve its structure at the casting temperature and perform the filtration process successfully.
- 1. G.Q. Jin, X.Y. Guo, Microporous Mesoporous Mater. 60[1-3] (2003) 207-212.
-

- 2. A. Zampieri, H. Sieber, T. Selvam, G.T.P. Mabande, W. Schwieger, F. Scheffler, M. Scheffler, P. Greil, Adv. Mater. 17[3] (2005) 344-349.
-

- 3. K. Koumoto, M. Shimohigoshi, S. Takeda, H. Yanagida, J. Mater. Sci. Lett. 6 (1987) 1453-1455.
-

- 4. C. Liu, X. Meng, X. Zhang, C. Hong, J. Han, W. Han, B. Xu, S. Dong, S. Du, Ceram. Int. 41[9] (2015) 11091-11096.
-

- 5. K.Y. Lim, Y.W. Kim, I.H. Song, Ceram. Int. 39[6] (2013) 6827-6834.
-

- 6. M. Fukushima, J. Ceram. Soc. Japan. 121[1410] (2013) 162-168.
-

- 7. D.M. Lukin, C. Dory, M.A. Guidry, K.Y. Yang, S.D. Mishra, R. Trivedi, M. Radulaski, S. Sun, D. Vercruysse, G.H. Ahn, J. Vučković, Nat. Photonics. 14 (2020) 330-334.
-

- 8. E.M. Huseynov, T.G. Naghiyev, U.S. Aliyeva, Phys. B Condens. Matter. 577 (2020) 411788.
-

- 9. S.M. Ali, Eng. Sci. Technol. an Int. J. 22[4] (2019) 1125-1135.
-

- 10. A.H. Lu, W. Schmidt, W. Kiefer, F. Schüth, J. Mater. Sci. 40[18] (2005) 5091-5093.
-

- 11. T. Nagano, K. Sato, T. Saitoh, Y. Iwamoto, J. Ceram. Soc. Japan. 114[1330] (2006) 533-538.
-

- 12. T.E. Wilkes, M.L. Young, R.E. Sepulveda, D.C. Dunand, K.T. Faber, Scr. Mater. 55 (2006) 1083-1086.
- 13. H. Suda, H. Yamauchi, Y. Uchimaru, I. Fujiwara, K. Haraya, Desalination. 193[1-3] (2006) 252-255.
-

- 14. P. Krawiec, S. Kaskel, J. Solid State Chem. 179[8] (2006) 2281-2289.
-

- 15. M. Fukushima, Y. Zhou, H. Miyazaki, Y.I. Yoshizawa, K. Hirao, Y. Iwamoto, S. Yamazaki, T. Nagano, J. Am. Ceram. Soc. 89[5] (2006) 1523-1529.
-

- 16. H.P. Martin and G. Standke, J. Adler. Adv. Eng. Mater. 10[3] (2008) 227-234.
-

- 17. J.S. Lee, S.H. Lee, S.C. Choi, J. Alloys Compd. 467[1-2] (2009) 543-549.
-

- 18. H.S. Nam, J. Ceram. Process. Res. 20[4] (2019) 379-387.
- 19. Y.J. Joo, K.Y. Cho, C.J. Kim, J. Ceram. Process. Res. 20[5] (2019) 563-569.
- 20. S.M. So, H.W. Hwang, S.H. Yi, J.S. Park, K.H. Kim, K.H. Lee, J. Park, S.G. Lee, J. Ceram. Process. Res. 21[S1] (2020) 16-22.
-

- 21. S.H. Ahn, K.W. Nam, J. Ceram. Process. Res. 18[11] (2017) 767-776.
- 22. J. Adler, Int. J. Appl. Ceram. Technol. 2[6] (2005) 429-439.
-

- 23. U.F. Vogt, L. Györfy, A. Herzog, T. Graule, G. Plesch, J. Phys. Chem. Solids. 68[5-6] (2007) 1234-1238.
-

- 24. K. Ohno, in Proceedings of the 2nd International Congress on Ceramics, July 2008, edited by K. Komeya, Y.-B. Cheng, J. Tatami and M. Mitomo (Trans Tech Publications, 2008) p.207-210.
- 25. S. Wood, A.T. Harris, Prog. Energy Combust. Sci. 34[5] (2008) 667-684.
-

- 26. G. Karagiannakis, C.C. Agrafiotis, C. Pagkoura, A.G. Konstandopoulos, D. Thomey, L. De Oliveira, M. Roeb, C. Sattler, Int. J. Hydrogen Energy. 37[10] (2012) 8190-8203.
-

- 27. A.R. Studart, U.T. Gonzenbach, E. Tervoort, L.J. Gauckler, J. Am. Ceram. Soc. 89[6] (2006) 1771-1789.
-

- 28. A. Najafi, M. Khoeini, A. Jamshidi, Ceram. Int. 46[10] (2020) 15935-15942.
-

- 29. J.H. Eom, Y.W. Kim, S. Raju, J. Asian Ceram. Soc. 1[3] (2013) 220-242.
-

- 30. C. Voigt, E. Jäckel, F. Taina, T. Zienert, A. Salomon, G. Wolf, C.G. Aneziris, P. Le Brun, Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 48[1] (2017) 497-505.
-

- 31. L.N.W. Damoah, L. Zhang, Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 41 (2010) 886-907.
-

- 32. C. Chelladurai, N.S. Mohan, D. Hariharashayee, S. Manikandan, P. Sivaperumal, Mater. Today Proc. 37 (2020) 386-394.
-

- 33. U.T. Riedel, J.E. Morgan, C.A. McMahon, F.J. Guild, W. Bleck, Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 213[4] (1999) 427-430.
-

- 34. X. Dang, M. Wei, R. Zhang, K. Guan, B. Fan, W. Long, J. Ma, H. Zhang, Process. Appl. Ceram. 11[2] (2017) 106-112.
-

- 35. K. Kornaus, G. Grabowski, R. Marian, A. Gubernat, Process. Appl. Ceram. 11[4] (2017) 329-336.
-

 This Article
This Article
-
2021; 22(5): 510-516
Published on Oct 31, 2021
- 10.36410/jcpr.2021.22.5.510
- Received on Dec 6, 2020
- Revised on May 17, 2021
- Accepted on Jun 3, 2021
 Services
Services
Shared
 Correspondence to
Correspondence to
- Mehmet Bugdayci
-
Chemical Engineering Department, Faculty of Engineering, Yalova University, 77200, Yalova, Turkey
Tel : +90535 695 72 68 Fax: +90226 815 5319 - E-mail: mehmet.bugdayci@yalova.edu.tr











 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.