- Design and manufacturing of lanthanum phosphate (LaPO4)-based crucible for high temperature applications
Hussein Alrobeia,*, Azeem Hafiza and Rizwan Ahmed Malikb
aDepartment of Mechanical Engineering, Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia
bDepartment of Metallurgy and Materials Engineering, Faculty of Mechanical and Aeronautical Engineering, University of Engineering and Technology, Taxila, 47050, PakistanThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This paper focuses on making a novel Lanthanum Phosphate-based durable and cost-effective crucible for high temperature applications such as fabrication processes of advance functional materials. A crucible is designed and fabricated by using three parts die following by powder metallurgical technique. Stepwise sintering was used to sinter the crucible. The synthesized crucible was then exposed to several tests to assess its structure, surface topography, durability, thermal expansion, and thermal shock resistance. Thermogravimetric analysis revealed a smaller mass loss of about 9.7%. Scanning electron microscope (SEM) analysis showed a spherical structure with pores enough to make a compact structure. It is also observed that the Lanthanum Phosphate-based crucibles have high non wettability after pouring out the molten magnesium and found thermal shock resistant. Interestingly, the crucible retained its original shape without failure up to 4 cycles. These results show that the synthesized Lanthanum Phosphate-based crucible is promising for high temperature applications
Keywords: Sintering, Crucible, Lanthanum phosphate, Powder metallurgy
Engineering ceramics with good mechanical properties over a wide temperature range are an opt choice for high temperature applications like synthetization process of advance functional materials used in electronic, automotive, aerospace industry etc. [1-7]. Among these, a crucible made up of ceramic (in the form of a cup) is used at high temperatures to melt metals or other functional materials. Although traditional crucibles have been made of clay, these can also be made of any substance which can endure temperatures that are high enough to melt or change their components [8]. In this situation, ceramic composite crucibles are made by high pressure melting and stir casting and then sintered to its sintering temperature based on the component or powder content to enhance its property [9, 10]. Relative to other traditional crucibles, lanthanum phosphate crystal finds a good alternative casting in foundry. Furnace crucible come in various materials including silicon-carbide, clay-graphite, and more. For standard foundry operations, these materials can withstand high temperatures. The additional advantage of silicon carbide is that it is an extremely robust material. Several typical crucible shapes include the bilge and shape “A are available [11, 12]. Lanthanum ceramic phosphate also has excellent non-wetting properties. It is therefore also being studied in the area of metal melting regions. The metallurgist's biggest issue is the titanium melting [13]. The nature of titanium is extremely reactive and requires a passive atmosphere for the melting of titanium, although advanced configurations are available, the percentage of molten metal loss is significantly higher because of low crucible reactivity used for the melting of metal [14-16].
The primary objective of this study is to develop lanthanum phosphate-based ceramic crucible and com- pare it with the most appropriate materials for the crucibles manufacture. Lanthanum Phosphate is selected as the main contrast with other current crimson material. Lanthanum phosphate crucibles are to be manufactured and analysed in different ways to evaluate durability, cost efficiency, thermal stability, compression strength, thermal expansion, and other variables [17-20].
The study was based on a problem identification of non-durable crucibles. Crucibles now-a-days made of graphite, silicon carbide etc, have limited applications. The problem identification that we found and try to improve are as follows. Graphite is fragile that can only be used for a restricted number of implementations. Silicon carbide-based crucibles are very durable but involve very high production costs [21]. During the molten metal melting, silica leaches from the crucibles at high temperatures. It causes the percentage (%) of silica content to increase in final product. Because of these factors, a highly durable, cost effective material is required with adequate life cycles, thus reaching Lanthanum Phosphate's choice [19, 22, 23].
Due to these existing scenarios or the limitations of other crucibles, an attempt is made to improve and to come up with a better chemical compound to best suit the crucibles applications. The primary objective of this study is the development of cost effective and durable lanthanum phosphate-based ceramic crucible via three parts die following by utilizing stepwise sintering to sinter the crucible. The synthesized crucible was then analysed through XRD, SEM, Thermogravimetric analysis, Non wettability testing and Thermal resistance analysis to check its suitability for high temperature applications. According to best of our knowledge, there is a few reports are available on the designing, optimization and synthesis of lanthanum phosphate-based ceramic crucible via three parts die following by utilizing stepwise sintering.
Crucible should have properties focusing especially on heat transfer and how much the material reacts to the temperature increase. To obtain the required pro- perties, the fabrication of crucible should follow certain steps and methodologies (a) Determination of the optimized crucible design (b) The design of die for the manufacture of the crucible (c) Crucible manufacture using lanthanum phosphate powder (d) Analysis of specimens of produced crucibles. The fabrication of crucibles includes steps from designing of die to what temperature the crucible must be sintered. CMC (Car- boxymethyl Cellulose) and Polyvinyl Alcohol (PVA) was found to be the correct binder [24, 25]. The die is made from mild steel as a die material and manufactured on CNC. In this work, crucible sintering is done at 1500 oC.
CAD Design
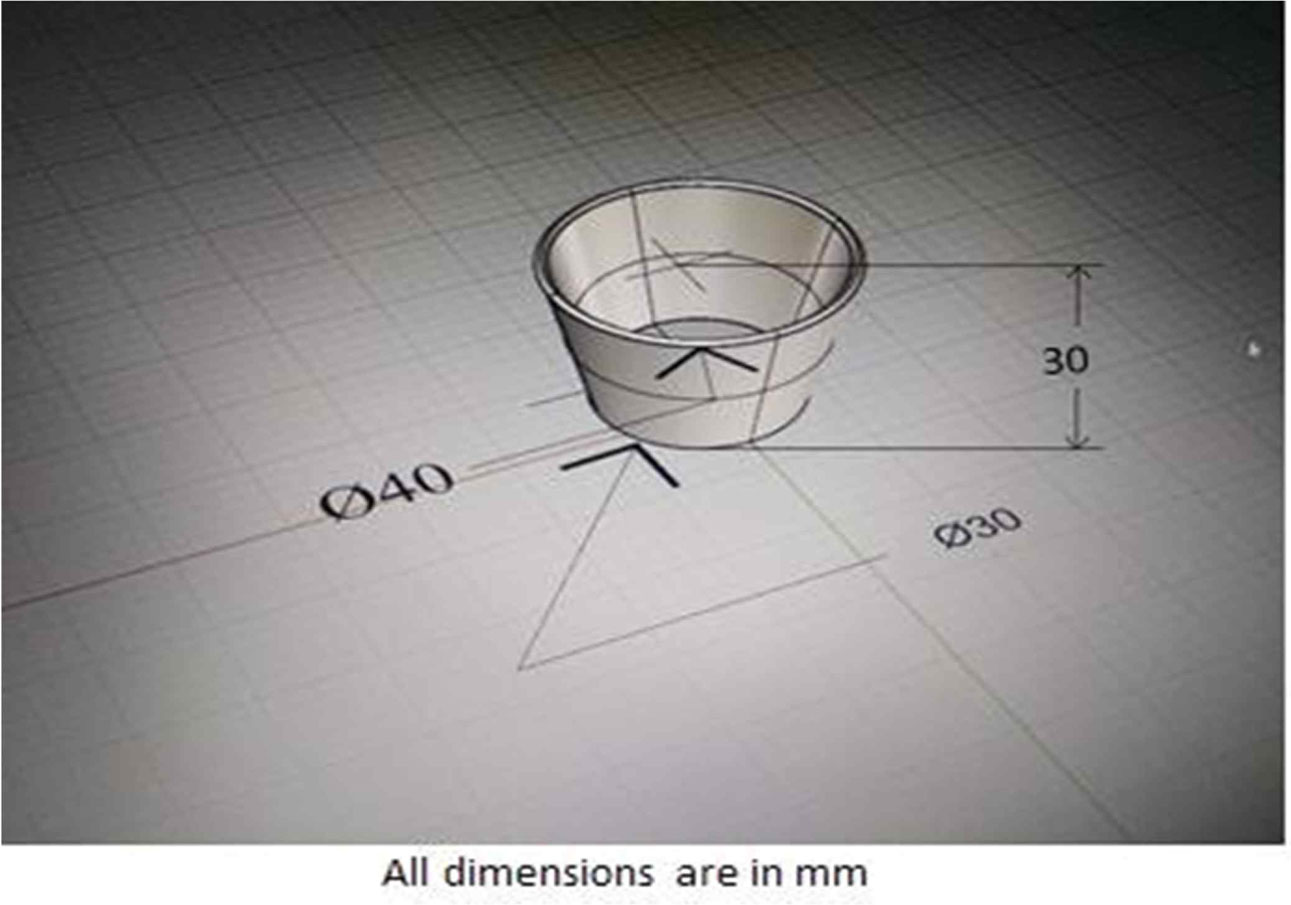
The proposed CAD design was to make only a sample piece with small diameter and height as follows; top diameter = 40 mm, bottom diameter = 30 mm, thickness = 4 mm, height = 30 mm as shown in Fig. 1. Six-part die design for the crucible with the base that could be compressed first to a limit and then the walls and base is compressed together to make the compressive bond (green strength) But it turned out to be complex and waste of material and production cost. In case of two-part die, it seems impossible to remove the lanthanum phosphate in its green strength. Then three parts of die with less complexity were designed by cutting off the base (see Fig. 2) which cost least materials and pro- duction. The three parts die are a base for easy removal of the crucible, a center piece as the body which is hollow for the lanthanum phosphate to be compressed which is also the female part and a top piece which was the male part or the ram which went into the center piece where the force was acted upon. The base and center piece of the die was made from mild steel and the male part was made using aluminium to reduce production cost. Also, the compressive force range was only up to100kN [26].
Binder preparation.
Poly vinyl alcohol (PVA) was used in this study as a binding agent as it has excellent film shapes, emulsifies and adhesives characteristics [27, 28]. Preparation process is as follows: (1) In distilled water, 2 to 5% weight per volume of PVA powder is blended. (2) The mixture is completely mixed to prevent lumps from forming. (3) The mixture is then taken at a temperature of around 80 oC in a water bath. (4) In the water bath, the mixture is mixed continually for around 30 min to create a uniform mixture.
Study of Lanthanum Phosphate
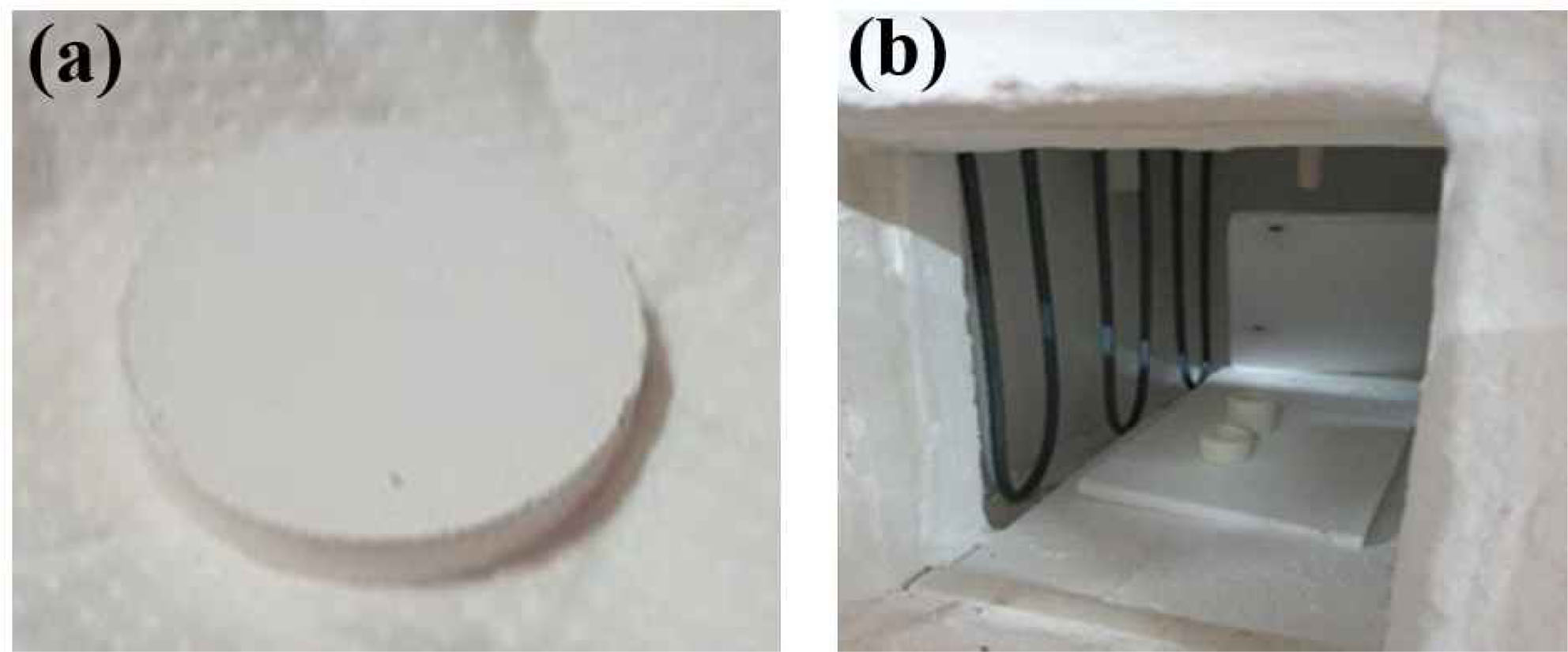
In order to study lanthanum phosphate (LaPO4) and PVA mixture, and to further test its properties a pellet was manufactured using pellet die of 35 mm diameter as shown in Fig. 3(a). The pellet was broken down to macro pieces and tested to different properties. The pellet preparation and mixture details are: Take and press 2% of the weight of PVA powder per volume to stop lumps. The compressed powder is kept at 80 C for 30 minutes in a hot air oven. The powder is mixed and compressed with LaPO4. The mixture is fed into the die to fill the base segment fully. The die is put in a com- pression testing machine and approximately 20 KN of pressure is applied. Afterwards, the pellet was sintered to 1300 oC in box furnace (see Fig. 3(b)).
Die pressing
The die machined and smoothed was then lubricated for the pressing process and filled with the lanthanum phosphate mixture with PVA. The procedure for pressing with 2% PVA are: 40 g lanthanum phosphate powder is added to the prepared PVA binder. In order to avoid lumps, the mixture is completely mixed in a quartz bowl. To eliminate excess moisture in the mixture, the mixture is then fed into a hot air oven held at 80 oC for around 30 min. The mixture is then poured into the die base and compressed into a compression test machine. Compression takes place in stages between 5 and 100 KN. After each compression stage, the powder is introduced to make sure complete powder filling in the die. The crucible is separated from the die after the compression process has been completed. Stepwise sintering of the crucible was done at a rate of 5 oC per minute up to 1000 oC and above 1000 oC, a rate of 3 oC per minute up to 1300 oC was applied.
|
Fig. 1 Crucible CAD design. |

|
Fig. 2 The three-parts of the final die. |
|
Fig. 3 (a) Lanthanum phosphate pellet, (b) Box Furnace |
X- Ray diffraction test
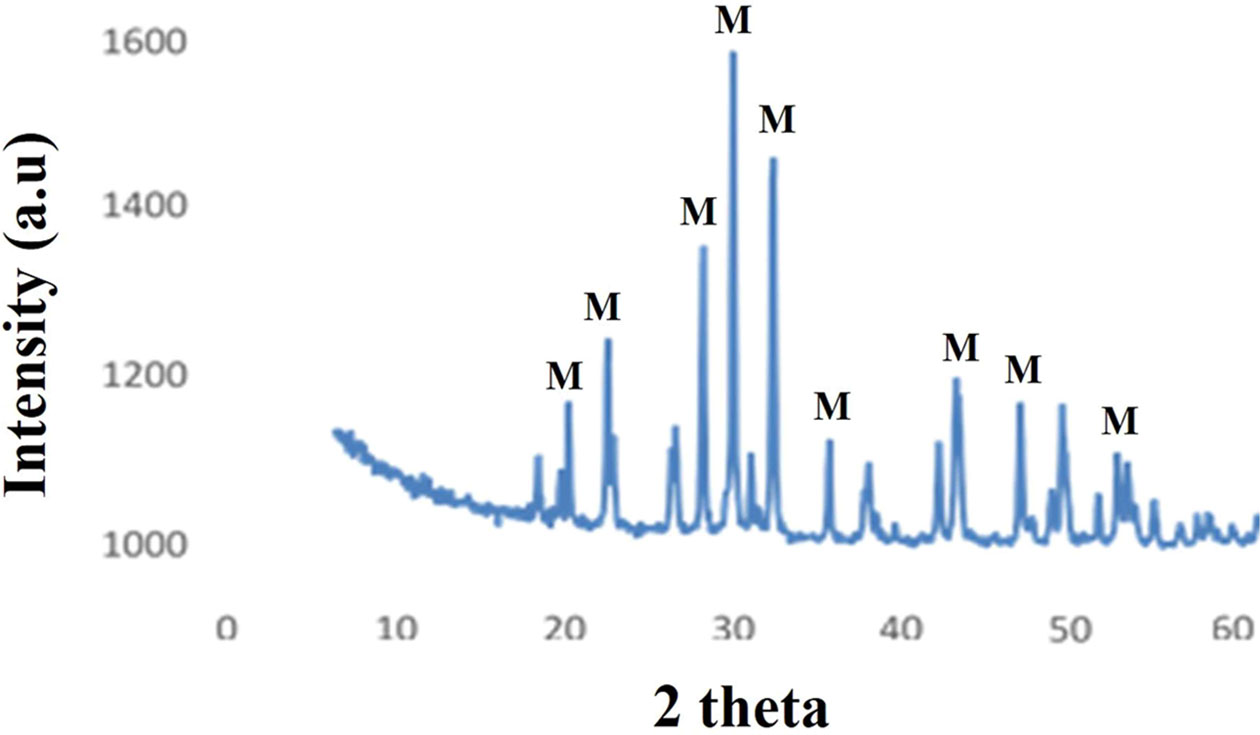
X-ray powder diffraction (XRD) is utilized for the identification of crystalline phases of the gel powder dried at 80 oC. The phase patterns obtained for lanthanum phosphate powder after heat treatment at 1300 oC is shown in Fig. 4. The lanthanum phosphate powder calcined at 1300 oC showed a stable monoclinic phase. The diffraction peaks were indexed to (200), (120) and (012) crystal planes of monoclinic LaPO4 appearing at 2θ values of 26o, 28o and 30, respectively. The similar observations were also observed by Sankar et al. [29] at higher calcination temperature of 1300 oC. The lanthanum phosphate exhibits hexagonal and monoclinic phase, when calcined at 400 and 800 oC temperatures, respectively. The complete transformation of monoclinic phase to stable monoclinic phase of lanthanum phosphate occurred at temperatures higher than 1200 oC.
Thermogravimetric analysis (TGA)
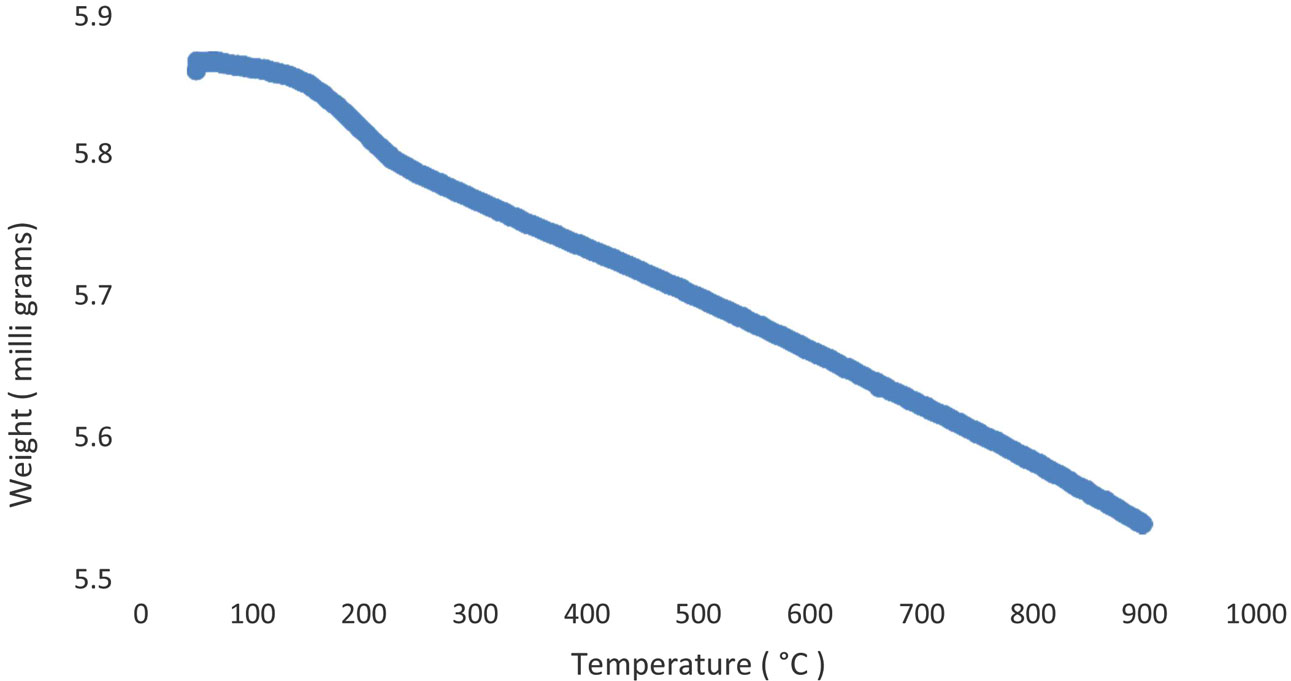
Thermogravimetric analysis (TGA) is a thermal analysis process that calculates the sample mass as the temperature changes over time. TGA is conducted on dried samples to study the weight loss from ambient temperature to 1000 oC. As shown in Fig. 5, a total weight loss of ~9.7% was observed in samples. The weight loss below 200 oC corresponds to the loss of adsorbed moisture. However, the weight loss above 200 oC corresponds to the removal of hydration water as well as PVA. The PVA decomposes and evaporated at around 500 oC. M.A. Ahmadzadeh et al. [30] reported that weight loss in TGA curves starting from room temperature to 500 oC corresponds to the decompositions of hydrated water and moisture.
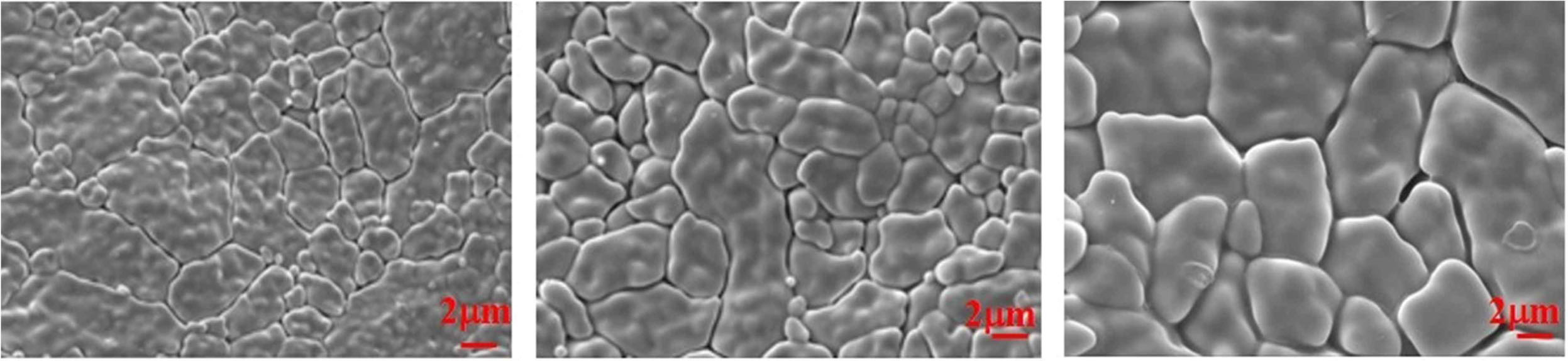
Scanning electron microscope (SEM) analysis
SEM analysis is an important research technique that uses a direct electron beam to develop detailed images of the surface topography of the specimen [31-33]. The Fig. 6 shows the morphology as well as grain structure of lanthanum phosphate calcinated at 1300 oC.
From the images obtained, it is clear that the pellet prepared in this method showed spherical grain structures with the stability. Also, there is enough pores made by the binder which makes the pellet high heat resistant. The spherical structure with pores enough to make a compact structure proves to be successful.
Non wettability test
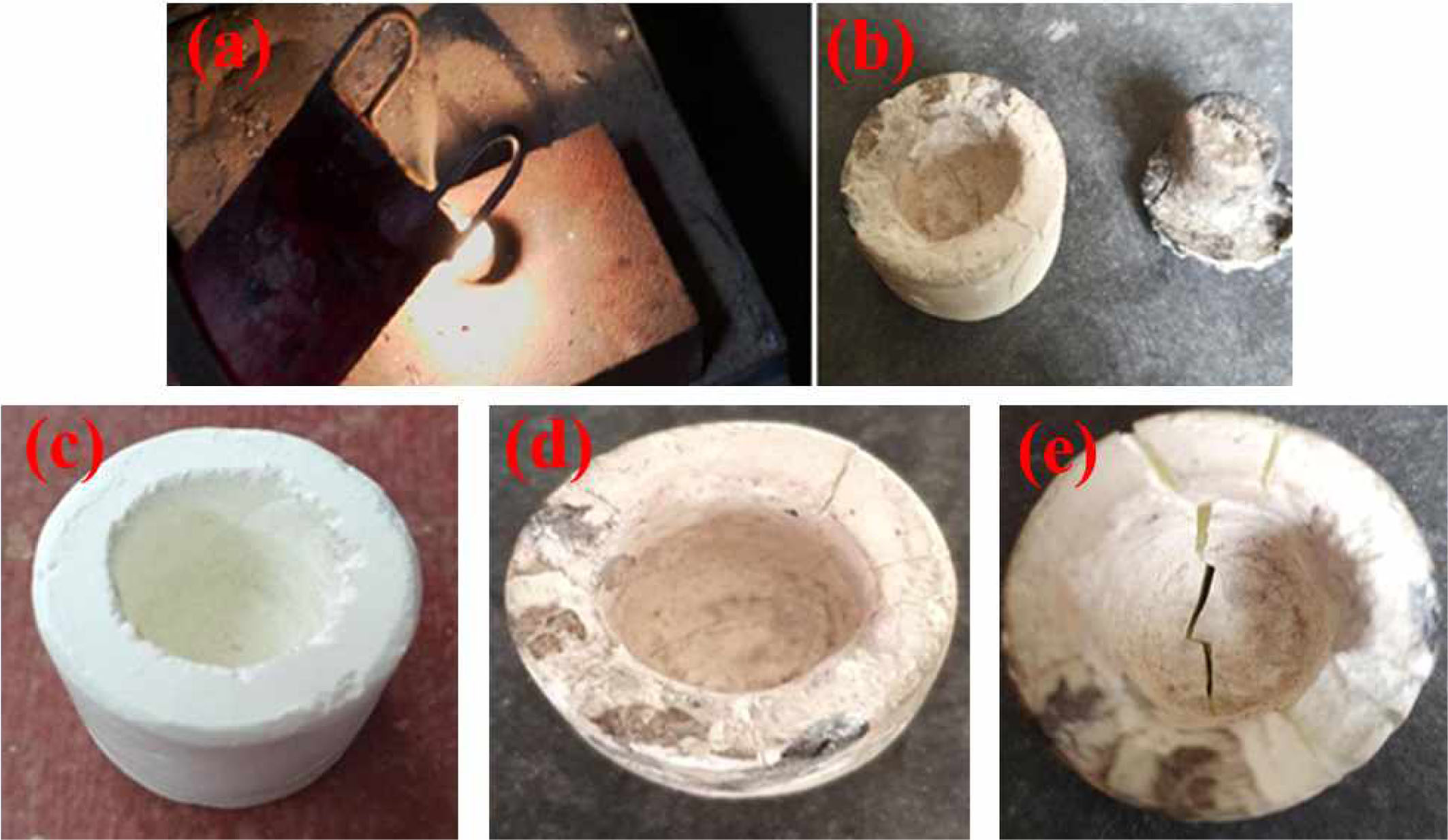
Metal removal from the crucible is almost complete. It is thus proven that Lanthanum Phosphate is extremely non-wettable. The crucible remains unchanged after removal of the metal and can therefore be used for additional procedures.
For non-wettability testing molten magnesium was poured into the crucible (see Fig. 7(a,b)). Then the molten metal was poured out of the crucible to analyze any loss of iron poured. The crucible was found to have high non wettability after pouring out the molten magnesium. Nearly all the metal found in the crucible was released without any damage.
Thermal resistance
Molten metal was poured on to the crucible without preheating the crucible to check the thermal shock resistance. The crucible was found intact after pouring molten metal directly into the crucible as room tem- perature. The shape of the crucible remained unchanged even after pouring the molten magnesium (see Fig. 7(c, d)). After the process, a little of the molten material seems to have remained on the surface of the crucible.
Usable cycles
It is observed that the number of cycles went up to four cycles of molten metal poring and cooling. The crucible sample can be used successfully without failure for up to 4 cycles. After four procedures, deep cracks emerged at the base of the crucible as displayed in Fig. 7(e).
|
Fig. 4 XRD Lanthanum Phosphate Graph results. |
|
Fig. 5 TGA Graph result for Lanthanum Phosphate. |
|
Fig. 6 Morphology and Grain structure. |
|
Fig. 7 (a), (b) Non-wettability, (c) Crucible before and (d) after poring molten metal, (e) Crack after 4 cycles. |
As efficiency in both heat transfer and cost is a major factor in industries. Our study was to bridge the gap between efficiency and production for crucibles in the future. A novel Lanthanum Phosphate-based crucible was designed and fabricated by using stepwise sintering process. Scanning electron microscope (SEM) analysis showed a spherical structure with pores enough to make a compact structure. The synthesized crucible showed good durability and thermal shock resistance. Interestingly, the crucible retained its original shape without failure for up to 4 cycles. Thus, coming up with a better solution for crucibles rather than conventional crucibles like graphite, Silicon Carbide and Alumina Crucibles is introduced.
This project was supported by the Deanship of Scientific Research at Prince Sattam bin Abdul aziz University, under the research project no. 2020/01/17063.
- 1. S. Ahmed, B. Li, and L. Zou, Adv. Appl. Ceram. 119[7] (2020) 407-413.
-

- 2. F. Akram, R.A. Malik, T.K. Song, S. Lee, and M.-H. Kim, J. Eur. Ceram. Soc. 39[7] (2019) 2304-2309.
-

- 3. C. Ma, H. Guo, and X. Tan, Adv. Funct. Mater. 23[42] (2013) 5261-5266.
-

- 4. A. Hussain, A. Maqbool, R.A. Malik, J.-H. Lee, Y.-S. Sung, T.K. Song, and M.-H. Kim, Ceram. Int. 43 (2017) S204-S208.
-

- 5. T. Jiang, M. Xie, X. Wang, and X. Song, Adv. Appl. Ceram. 119[4] (2020) 212-217.
-

- 6. F. Akram, A. Hussain, R.A. Malik, T.K. Song, W.-J. Kim, and M.-H. Kim, Ceram. Int. 43 (2017) S209-S213.
-

- 7. A. Ullah, C.W. Ahn, R.A. Malik, and I.W. Kim, Phys. B 444 (2014) 27-33.
-

- 8. M.O. Adeoti, O.A. Dahunsi, O.O. Awopetu, and O.I. Oyedeji, Niger. J. Technol. 38[3] (2019) 614-620.
-

- 9. J.W. Amoroso, J. Marra, C.S. Dandeneau, K. Brinkman, Y. Xu, M. Tang, V. Maio, S.M. Webb, and W.K. Chiu, J. Nucl. Mater. 486 (2017) 283-297.
-

- 10. Y. Arinicheva, N. Clavier, S. Neumeier, R. Podor, A. Bukaemskiy, M. Klinkenberg, G. Roth, N. Dacheux, and D. Bosbach, J. Eur. Ceram. Soc. 38[1] (2018) 227-234.
-

- 11. A. Badolia, R. Sarkar, and S.K. Pal, T. Indian. Ceram. Soc. 73[2] (2014) 115-120.
-

- 12. K. Balamurugan, M. Uthayakumar, S. Sankar, U.S. Hareesh, and K.G.K. Warrier, in “Abrasive Waterjet Cutting of Lanthanum Phosphate-Yttria Composite: A Comparative Approach” (Micro and Nano Mach. Eng. Mater, 2019) pp. 101-119.
-

- 13. D. Bregiroux, F. Audubert, T. Charpentier, D. Sakellariou, and D. Bernache-Assollant, Solid State Sci. 9[5] (2007) 432-439.
-

- 14. D. Bregiroux, S. Lucas, E. Champion, F. Audubert, and D. Bernache-Assollant, J. Eur. Ceram. Soc. 26[3] (2006) 279-287.
-

- 15. C. Chandler, and K.J. McClellan, J. Cryst. Growth. 510 (2019) 28-31.
-

- 16. K.K. Chawla, in “Ceramic matrix composites” (Springer Science & Business Media, 2013) pp.126-161.
- 17. J. Crum, V. Maio, J. McCloy, C. Scott, B. Riley, B. Benefiel, J. Vienna, K. Archibald, C. Rodriguez, V. Rutledge, Z. Zhu, J. Ryan, and M. Olszta, J. Nucl. Mater. 444[1-3] (2014) 481-492.
-

- 18. C. Ergun, J. Mater. Process Tech. 199[1-3] (2008) 178-184.
-

- 19. Y. Fukaya, M. Kato, M. Watanabe, and K. Sasaki, Crucible. U.S. Patent Application 29/543,974 (2019).
- 20. X.H. Wang, P.L. Chen, and I.W. Chen, J. Am. Ceram. Soc. 89[2] (2006) 431-437.
-

- 21. L. Gan, J. Xin, and L. Jiao, Measurement 131 (2019) 7-12.
-

- 22. R. Ghosh, R. Sarkar, and S. Paul, Mater. Des. 106 (2016) 161-169.
-

- 23. U.S. Hareesh, K. Balamurugan, K.G.K. Warrier, M. Uthayakumar, and S. Sankar, J. Aust. Ceram. Soc. 54 (2018) 205-214.
-

- 24. N.Y. Perez-Rangel, E. Florez-Solano, and E. Espinel-Blanco, J. Phys.: Conf. Ser. 1708 (2020) 012015. doi:10.1088/1742-6596/1708/1/012015.
-

- 25. J. Hou, Y. He, J. Lian, N. Wu, X. Zhang, and J. He, J. Aust. Ceram. Soc. 56 (2020) 819-828.
-

- 26. W. Min, K. Daimon, T. Matsubara, and Y. Hikichi, Mater. Res. Bull. 37[6] (2002) 1107-1115.
-

- 27. W. Min, D. Miyahara, K. Yokoi, T. Yamaguchi, K. Daimon, Y. Hikichi, T. Matsubara, and T. Ota, Mater. Res. Bull. 36[5-6] (2001) 939-945.
-

- 28. H. Onoda, and A. Yoshida, J. Ceram. Process. Res. 13[5] (2012) 622-626.
- 29. S. Sankar, A.N. Raj, C.K. Jyothi, K.G.K. Warrier, and P.V.A. Padmanabhan, Mater. Res. Bull. 47[7] (2012) 1835-1837.
-

- 30. M.A. Ahmadzadeh, S.F. Chini, and A. Sadeghi, Mater. Des. 181 (2019) 108058.
-

- 31. C. Wang, L. Guo, and F. Ye, Mater. Des. 111 (2016) 389-393.
-

- 32. R. Wang, W. Pan, J. Chen, M. Fang, and J. Meng. Mater. Lett. 57[4] (2002) 822-827.
-

- 33. Q. Zheng, X. Wang, J. Tian, R. Kang, and Y. Yin, Mater. Chem. Phys. 122[1] (2010) 49-52.
-

 This Article
This Article
-
2021; 22(5): 499-503
Published on Oct 31, 2021
- 10.36410/jcpr.2021.22.5.499
- Received on Oct 24, 2020
- Revised on Mar 21, 2021
- Accepted on Apr 19, 2021
 Services
Services
- Abstract
introduction
methodology
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hussein Alrobei
-
Department of Mechanical Engineering, Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia
Tel : +966115884211 Fax: +966115888272 - E-mail: h.alrobei@psau.edu.sa






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.