- Natural hydroxyapatite obtained from pufferfish teeth for potential dental application
Servet Ahmet Doğdua,*, Cemal Turana, Tolga Depcib and Deniz Ayasc
aMolecular Ecology and Fisheries Laboratory, Faculty of Marine Sciences and Technology, Iskenderun Technical University, Iskenderun, Turkey
bThe Graduate School of Engineering & Sciences, Iskenderun Technical University, Iskenderun, Turkey
cFaculty of Fisheries, Mersin University, Yenişehir Campus, Mersin, TurkeyThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Hydroxyapatite and associated calcium phosphate ceramic materials are commonly applied as implant resources because of their close resemblance in structure with natural bone. In the present study, the elemental composition and potential usage of pufferfish teeth were examined as natural bioceramic for dental applications. The teeth of pufferfish Lagocephalus sceleratus were removed from the fish, rinsed with deionized water, measured, and kept in the furnace at 105 oC. The dehydrated teeth were pulverized with the planetary ball. ICP-MS, TTX, XRD, and nanoindentation test analyzes were made on the obtained hydroxyapatite. In the ICP-MS analysis, the elemental composition of the teeth was 52% calcium, 39% phosphate, %2.5 Mn, %1.5 Mg, %1 Ti, %0.8 V, and %3.2 the others. Ca/P atomic ratio value was 1.32. The present study preliminary revealed that the use of pufferfish teeth can be a natural alternative source for biomedical and other industrial purposes since it has no economic value and is very abundant in the world marine waters. On the other hand, the required tests are mandatory to be accomplished before any human use
Keywords: Blue biomaterials, Pufferfish, biomedical, bioceramic, natural Hydroxyapatite
Biomaterials are resources with unique properties that make them an accurate instrument for local contact with living tissues [1]. Biomaterials should have specific features such as biocompatibility, serializability, func- tionality, and manufacturability [2]. The marine organism is getting favoured to obtain the biomaterials for bio- medical applications [3,4]. Bioceramic materials have found widespread application in the fields of bone tissue, biomedical, dentistry and medicine with their adaptability and functionality [5, 6] Biologically originated ceramic materials are much more preferred that make them proper for intimate contact with living tissues [1, 7]. Essentially, Hydroxyapatite (HA) from bones or biomass in which the main element is calcium in its chemical structure can be synthesized to use in biomedical applications [9-12]. HA and calcium phosphate ceramic materials are commonly applied as implant materials because they have perfect bioactivity, osteoconductivity and osteoinductivity [13, 14]. Therefore, natural HA has been used in dental treatment, having better metabolic activity and a sincerer response to the environment rather than the synthetic one [15]. The natural HA is used as a filling material, replacing missing or damaged teeth tissues and also to support tissue regeneration and to stay healthy teeth tissue [7].
Teeth are recognized as the hardest and most completely mineralized tissue in the human body. Teeth decay, as well as dental diseases, negatively affect both the general health status and the condition of an individual [16]. Natural teeth are function-oriented bilayer compounds with dentin forming the central part to maintain the overlying enamel. Whereas the outer enamel shows excellent stiffness, and the inner dentin receives extensive impacts and pressures [9, 18, 19].
Marine species have a vast range of features by which there is a wide range of potential applications within the biomedical discipline [1]. Invertebrates as marine species are the most common sources of bio- materials for therapeutic and medical diagnostic purposes such as sponges, soft corals, sea fans, nudibranchs, bryozoans, tunicates [1, 7, 20]. On the other hand, a notable amount of other marine species is commercially processed and by which their many wastes, e.g., skin, head, viscera, fins, and scales, are generated [8, 9]. Pufferfishes are commonly found in tropical and subtropical marine waters and include 28 genera and approximately 184 species all over the world marine waters within the Tetraodontidae family [21-24]. The Tetraodontidae family is famous for the occurrence of the powerful marine paralytic toxins in their body called tetrodotoxin (TTX). According to the current European legislative requirements [26], poisonous fish of the family Tetraodontidae must not be placed on the market. However, it is important to find and add value to their nontoxic body parts in a safe way to control their growing populations in the Mediterranean [19]. Pufferfish’ teeth are known to have a very strong structure and the use of this feature in bioceramic applications would generate important gains. Moreover, pufferfish teeth are similar to human teeth in terms of characteristic properties [19] that can be very good natural bioceramic to the dental industry.
Therefore, in the present study, we investigated teeth of pufferfish Lagocephalus sceleratus for its usability as biologically originated ceramic material in dental treatments of the natural applications.
Preparation of teeth samples
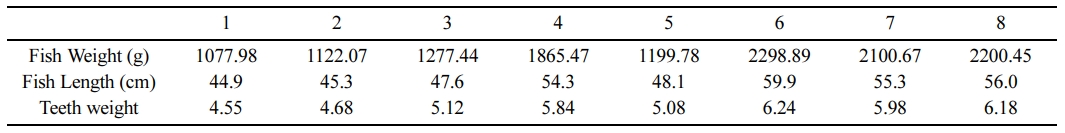
The specimens of pufferfish Lagocephalus sceleratus were captured from Iskenderun Bay, the northeastern Mediterranean, and then transferred to the laboratory. The teeth of each fish were removed from the fish (Fig. 1), and measurements of the eight fish and their teeth were given in Table 1. The teeth samples were put into a 30% H2O2 solution in sterile plastic containers for 1 hour at ~0.75 ml/min under vigorous stirring, then the solution was filtrated, and the residual soft tissues and blood tissue on the teeth samples were gently removed using a brush and H2O2 solution. The collected teeth specimens were rinsed with deionized water and kept in the furnace at 105 oC for overnight. The dehydrated material was crushed using the ball mill (Retsch, PM100), and the obtained powder form teeth were washed several times with 30% H2O2 solution in sterile plastic cups and then filtered and dried in the incubator at 105 oC overnight.
Metal analysis
The powdered teeth samples were weighed (ranged from 0.08 to 0.10 g) and placed in glass tubes using a plastic spoon. The teeth completely dissolved with a solution prepared from HCl (2 mL, Merck, Hydrochloric acid fuming 37%), HNO3 (6 mL, Merck, Nitric acid 65%) and H2O2 (2 mL, Merck, Hydrogen peroxide 30%). All the samples were melted until 150 oC on the hot plate. The prepared solution was examined with an inductively coupled plasma mass spectrometer (ICP-MS, Agilent, 7500ce Model, Japan) to define metals in the teeth structure. The ICP-MS experimental tuning was as follow: radiofrequency, 1500 W; plasma gas flow rate, 15 L min-1; auxiliary gas flow rate, 1 L min-1; carrier gas flow rate, 1.1 L min-1; spray chamber T, 2 oC; sample depth, 8.6 mm; sample start flow rate, 1 mL min-1; nebuliser pump, 0.1 rps; extract lens, 1.5 V. Macro (Na, Mg, P, K, Ca) and trace elements (Co, Cu, Zn, Mo, Ni, Se). The potentially toxic metals (Cd, Pb, As, Cr) in all teeth specimens were calculated as μg metal g-1 dry weight. Determination of the metals was conducted with the High Purity Multi-Standard (Charleston, SC 29423). The elements and potentially toxic metals were diluted using standard solutions for calibration curves. The ready solutions were ensured a content of lead, cadmium, arsenic, and chromium in preparation of 1-50 ppb (0.001 to 0.050 mg/L) for the toxic metals and content of copper, iron, and zinc in preparation of 1-50 ppm (1 to 50 mg/L) for the macro and trace elements.
TTX analysis
For the control of existence of the TTX in the teeth’s, 1 g of the teeth sample in the powder form was added into the 3 mL of a methanol solution containing 1% acetic acid. Then, the solution in the plastic container was placed into an ultrasonic bath. The samples held at room temperature for 15 min were centrifuged (4500 rpm, 4 oC, 20 min) after which the supernatant phase was separated, and 3 mL of methanol containing 1% acetic acid was added again to the residue. The supernatant obtained after the second centrifugation step was combined with the supernatant previously separated, and the resulting solution was completed to 7 mL. The final solution was homogenized with the vortex, and then 1 mL solution passed through a C18 cartridge (3 mL/500 mg; Supelco-57012) conditioned with 6 mL of methanol and 6 mL of water with the aid of a vacuum manifold. After passing the sample, 10 mL of methanol was passed through the cartridge. The final solution was made up of 12 mL with methanol and homogenized with a vortex. Then the evaporation was carried out using an evaporator, and the residue dissolved in 1 mL of methanol was transferred (after filtration with 0.45 μ membrane filters) to the vials for analysis [27]. The presence of the TTX in the pufferfish teeth was investigated with the LC-MS-MS system composed of an Agilent 6460 triple quadrupole mass spectrometer coupled to an Agilent 1200 HPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA).
XRD analysis
X-ray powder diffraction (XRD) was used to identify phase compositions and the crystallinity of the pufferfish teeth. The XRD patterns were recorded by Rigaku Miniflex 600 Diffractometer with Cu Kα (40 kV, 15 mA, λ = 1.54050 Å) radiation. Scanning was conducted between 10 deg < 20 < 70 deg (with 0.01-deg and 0.05-deg steps and 1 deg/min rate).
Scanning electron microscope (SEM)
The morphologic structure of pufferfish teeth powder was identified with a scanning electron microscope (JSM-6390LV, NTC, Tokyo, Japan) through an accelerating voltage of 10 KV.
Nanoindentation test
Nanoindentation test is applied to find the mechanical properties of composite fill materials. This method comprises an in-situ scanning probe microscopy (SPM) imaging facility that allows post-test observation of the samples. The probe is used for indent and image eliminating the complicated situation of detecting the same part with different tools or combining two separate tools such as an SEM and a nanoindenter to labour together [28]. Alpha-Dent® Self Cure Hybrid Composite Kits were used as composite fillers in the test units added to pufferfish teeth powder, and seven sample sets were created for the analyzes.

|
Fig. 1 The external appearance of teeth of L. sceleratus, (b) Powdered teeth, (c) The final form of the filled teeth |
The TTX in the teeth was below the detectable limit (0.1 µg/g). The elemental composition of the teeth obtained from the eight pufferfish specimens were done separately, presented in Table 2. The elemental composition and the metal ratios of the teeth were very close between the specimens. On average, the main structure of teeth consists of 52% calcium and 39% phosphate, %2.5 Mn, %1.5 Mg, %1 Ti, %0.8 V and %3.2 others.
According to the ICP-MS analysis, the filler material can be determined as a binder and compatible with the powdered teeth. The Ca/P atomic ratio value in pufferfish was detected to be 1.32, which is relatively higher than the stoichiometric HA. The detected higher molar Ca/P ratio can be related to the existence of carbonate ions substituting the phosphate, which is validating the existence of B-type carbonate HA [29]. The detected HA in pufferfish teeth is typical of the mineral phase of biological apatites in which the carbonates can strongly change Ca/P ratio [30]. Lee et al. [31] reported that HA from cuttlefish bone was between 1.64-1.70 for two different mixing ratios of the calcined cuttlefish bone to phosphoric acid. Bahrololoom et al. [32] investigated natural HA Ca/P ratios from various animal bones and reported them to be between 1.46-2.01. Nazarpak et al. [33] detected the Ca/P ratio from synthetic HA as 1.62. Latif et al. [34] extracted natural HA from Thunnus thynnus and found the Ca/P ratio as 1.60. In human bovine bones, the Ca/P ratio was reported to be 1.68 in HA [35]. The detected Ca/P ratio in pufferfish is within the range of the acceptable limits of HA [36, 37] which validate its usage as a natural HA source in dental applications.
The nanostructure of teeth powder replacing materials is generally associated with decent bioactivity and osteoconductivity that cause an increase of osteoblast functions [38]. High levels of Na, Mg, K and Sr elements in the pufferfish teeth were detected (Table 2) which are important in the behaviour of biological apatites for their contribution to metabolism in the human cell adhesion. In bone metabolism and osteoporosis, Na and Mg have an important role [29, 39] that Mg is crucial in cell proliferation and function since the cells can not proliferate in the lack of extracellular Mg due to the resultant reduction in DNA, RNA and protein synthesis [35]. Moreover, Sr is linked with decreasing bone resorption and accelerating bone formation, causing the prevention of the risk of fractures [40].
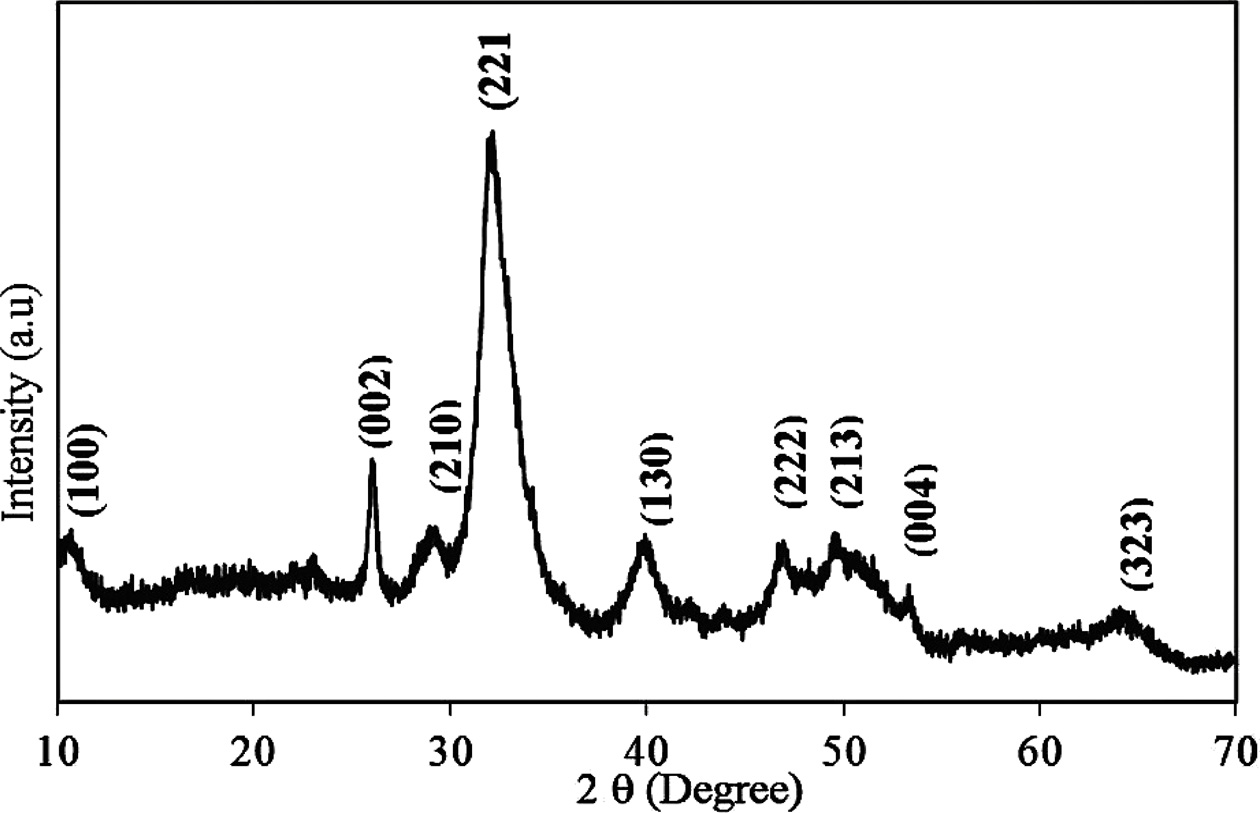
An analysis of the powder XRD patterns of the pufferfish teeth given in Fig. 2 indicated that all intense reflections could be attributed to the hydroxyapatite with a Ca5( PO4)3(OH) chemical formula. The patterns of the teeth perfectly match with the data presented in the ICDD (PDF2.DAT) Card No: 01-089-4405. Cell parameters of the teeth were found as follows: a = 9.451(1), b = 18.823(1), and c = 6.926(2), which are very close to the ICDD card. The XRD patterns showed that the composition of the pufferfish teeth is very compatible with the human teeth. The broad structure seen in the XRD patterns indicates the biologically mineralized low crystalline hydroxylapatite with an organic matrix [32,41]. The XRD analysis supports the result of the elemental analysis. The main composition of the teeth is calcium and phosphate. The other heavy metals were not detected in the XRD pattern because of the concentration. The heavy metals (Vanadium (V), Chromium (Cr), Cobalt (Co), Nickel (Ni), Copper (Cu), Zinc (Zn) Arsenic (As), Molybdenum (Mo), and Lead (Pb) were below the limit established by ASTM Standards for an organic bone for surgical implants [29]. The existence of nontoxic measurements of heavy metals revealed that the impacts of obtained powders are non-cytotoxic and similar to that of the marketable HA.
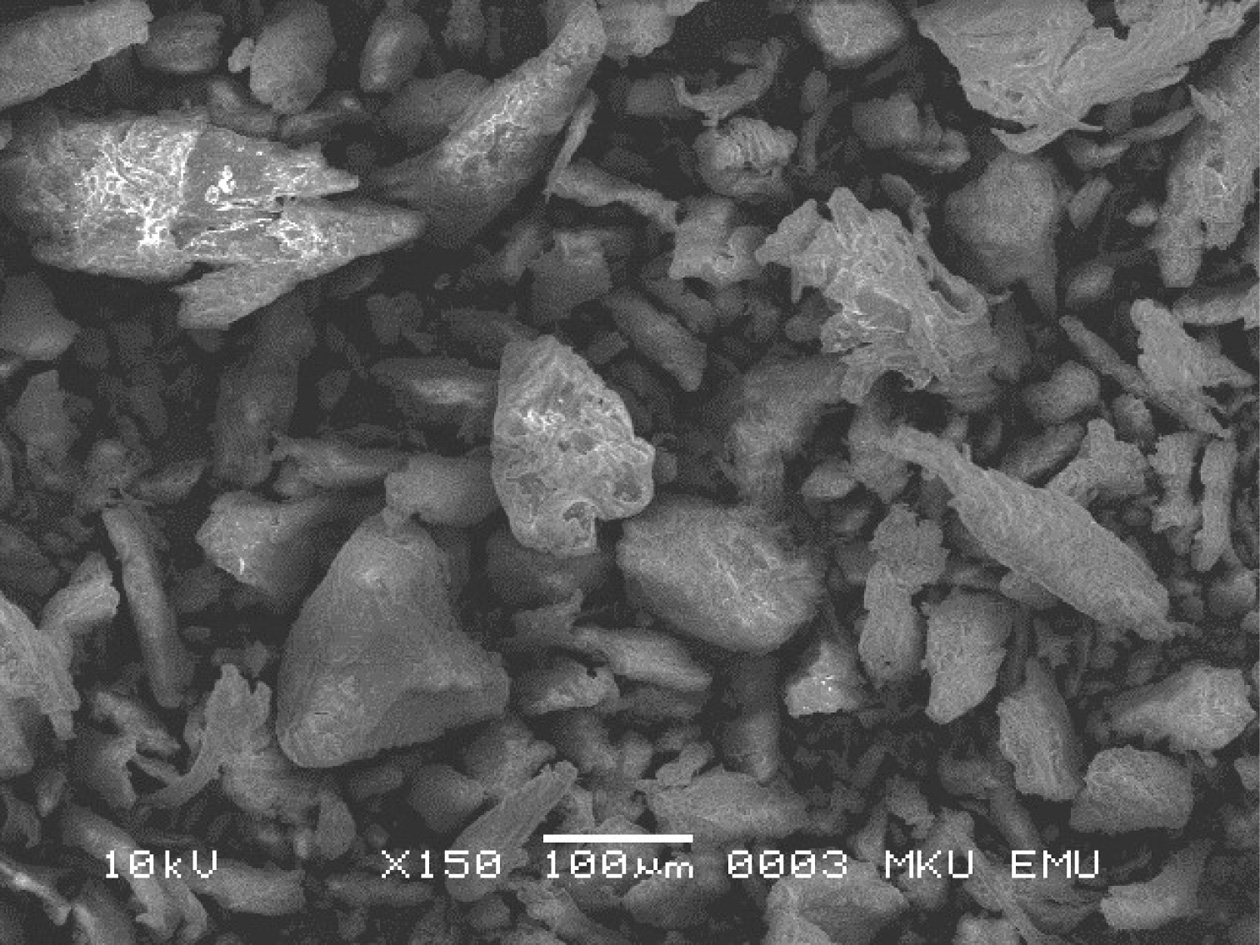
The oval and plate-shaped particles were observed at the structure in the SEM image of the sample given in Fig. 3. Initially, the pufferfish teeth were light off-white and yellow, which indicates the existence of collagen and other organic parts in the HA. When teeth were examined with the above methods, the yellow colour was missed, which sign precise exclusion of collagen and other organic moieties. The size and morphologic structure of the crystals take a significant part in the biomechanical functioning of pufferfish teeth since the hierarchical assembly and the orientation of the HA crystals in the collagen support the high hardiness of the teeth. As the SEM micrographs in Fig. 3 showed that the obtained powders reveal a stick shape, which is possibly shaped by the fusion of fundamental blocks [42], presenting a preferential crystalline orientation. The teeth particles were similar to that of nano fish-bone powders prepared using the method of 600 ºC pretreatment combined with dry media milling reported by [29]. The extent and shape of powders attained from biogenic HA can provide significant features such as good bioactivity and flexibility [43, 44].
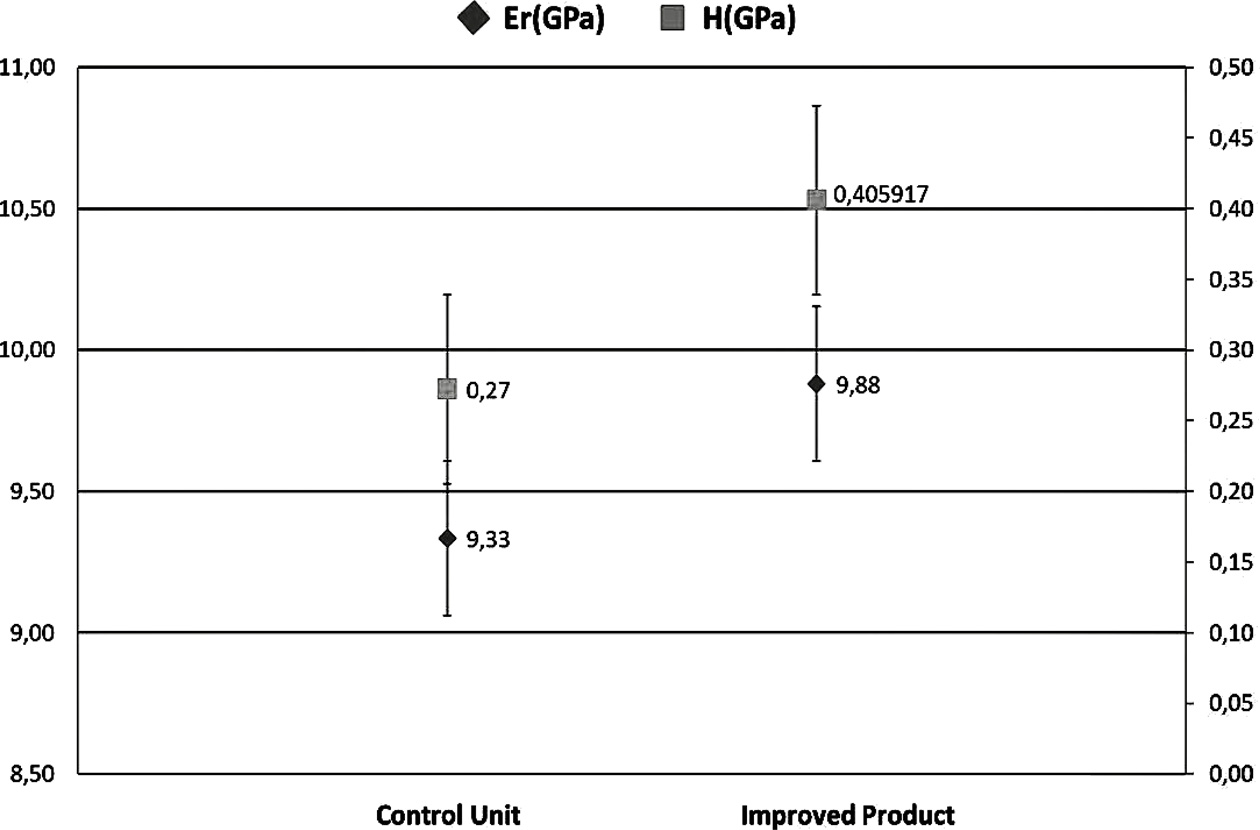
In nanoindentation analysis, a total of 14 samples were prepared, including seven samples for the control group and seven samples for improved product samples. A total of 5 indentations were applied under loads of 50N in all samples. The mean value of the control samples for elastic modulus (Er) and hardness test (H) were found to be 9.33 and 0.27, respectively (Fig. 4). The mean value of elastic modulus (Er) and hardness test (H) for the improved product groups were 9.88 and 0.40, respectively. The most applied mechanical properties measured are hardness and modulus of elasticity [45]. It can be concluded that pufferfish teeth have advantages such as being organic, low cost, durable, bacteria-resistant, stain-resistant due to the presence of titanium and vanadium in their structure. Aesthetics, stiffness, strength, functionality, suitability, and compatibility of the pufferfish teeth are provided here as effective and simple in the filling, implant, and denture treatments that provide the production of natural teeth filling materials.
|
Fig. 2 X-ray diffraction patterns of powdered L. sceleratus teeth. |
|
Fig. 3 SEM images of teeth of L. sceleratus in powder form. |
|
Fig. 4 Comparison of the improved product and control unit with the elastic modulus (Er) and hardness test (H) |
|
Table 2 The elemental composition of the teeth specimens of pufferfish L. scelaratus (µg g-1 dw). |
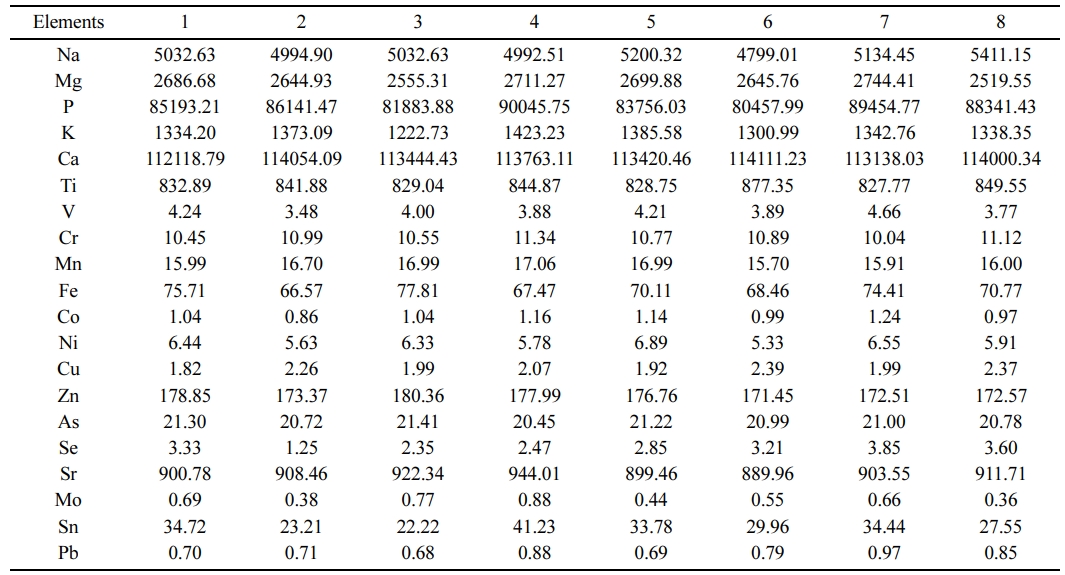
Ind: individual, dw: a dry weigh |
HA was obtained from many natural sources such as pig and bovine bone [46-48]. However, there are a few researchers who used fish-bone as a raw material [32, 34, 49-51]. Thermal calcination coupled with the milling process [52] and the alkaline hydrolysis method [47] has been generally used for the segregation of nano- structured HA from bovine bone. Here, we conducted the alkaline hydrolysis method for the segregation of HA from the teeth of pufferfish L. sceleratus to find a natural marine source. In previous studies, the HA has so far been isolated by the thermal calcination method from fish-bone of Pseudoplatystoma corruscans, Paulicea lutkeni, P. fasciatum and T. obesus [49, 51, 53, 54], therefore, this is the first study for the isolation of HA from the teeth of L. sceleratus. The present study provides a natural, original and alternative product for the production of fillers, implants, bone powder, and dentures for biomedical and other industrial purposes since it has no economic value and is very abundant in the world marine waters. On the other hand, the required tests are mandatory to be accomplished before any human use.
This study is produced from Servet Ahmet Doğdu’s PhD thesis. Thanks to the Iskenderun Technical University for supporting the Research Project (2019LTB-01) and the Scientific & Technological Research Council of Turkey (TUBITAK-2211/C National PhD Scholarship Program for Priority Areas) and Council of Higher Education for 100/2000 PhD scholarship program for support.
- 1. A. Srivastava, A. Srivastava, and A. Srivastava, P. Chandra, in “Marine Biomaterials in Therapeutics and Diagnostic” (Springer, 2015) p.1247-1263.
-

- 2. H. Ehrlich, in “Biological Materials of Marine Origin” (Springer, 2010) p.1-436.
-

- 3. I. Vasile-Antoniac, I.G. Lesci, A.I. Blajan, G. Vitioanu, and A. Antoniac, Key Engineering Materials, 672 (2016) 276-292.
-

- 4. Y.S. Lim, Y.J. Ok, S.Y. Hwang, J.Y. Kwak, and S. Yoon, Mar. Drugs. 17[8] (2019) 467.
-

- 5. M. Mojahedian, F. Fahimipour, K.L. Larsen, M. Kalantar, F. Bastami, and N. Omatali, J. Ceram. Process. Res. 17[11] (2016) 1138-1142.
- 6. J.H. Lee, H.J. Choi, S.Y. Yoon, B.K. Kim, and H.C. Park, J. Ceram. Process. Res. 14[4] (2013) 544-548.
- 7. D.F. Williams, Biomaterials 30[30] (2009) 5897-5909.
-

- 8. S.K. Kim and E. Mendis, Food Res. Int. 39[4] (2006) 383-393.
-

- 9. T.P. Boaventura, A.M. Peres, V.S. Gil, C.S. Gil, R.L. Oréfice, and R.K. Luz, Quím. Nova 43[2] (2020), 168-174.
-

- 10. W. Pon-On, P. Suntornsaratoon, N. Charoenphandhu, J. Thongbunchoo, N. Krishnamra, and I.M. Tang, Mater. Sci. Eng. C 62 (2016) 183-189.
-

- 11. L. Tang, S. Chen, W. Su, W. Weng, K. Osako, and M. Tanaka, Process Biochem. 50[1] (2015) 148-155.
-

- 12. A. Yücel, K. Onar, C. Turan, T. Depci, and M.E. Yakıncı, in “TIPTEKNO16, 27-29 Ekim 2016” (Antalya, 2016) p.348-350.
- 13. S.M. Best, A.E. Porter, E.S. Thian, and J. Huang, J. Eur. Ceram. Soc. 28[7] (2008) 1319-1327.
-

- 14. J. Venkatesan, B. Lowe, P. Manivasagan, K.H. Kang, E.P. Chalisserry, S. Anil, D. Kim, and S.K. Kim, Materials 8[8] (2015) 5426-5439.
-

- 15. Z. Orman, S. Yucel, Y.M. Sahin, O. Gunduz, and F.N. Oktar, Acta Phys. Pol. A. 135[5] (2019) 1089-1092.
-

- 16. R.J. Block, M.K. Horwitt, and D. Bolling, J. Dent. Res. 28[5] (1949) 518-526.
-

- 17. N.S. Goel, I. Rozehnal, and R.L.A. Thompsoni, Remote Sens. Environ, 36[2] (1991) 73-104.
-

- 18. W.D. Jung, R.W. Jung, and A.R. Loudermilk, U.S. Patent No. 6,239,868 (2001).
- 19. S.A. Doğdu, C. Turan, and D. Ayas, NESciences 4[3] (2019) 308-314
-

- 20. E.B. Tuna, Y. Oshida, B. Ozen, E. Gjorgievska, and T. Tuzuner, Biomed Res. Int. 2017 (2017) 2520536.
-

- 21. K. Matsuura, Ichthyol. Res. 62[1] (2015) 72-113.
-

- 22. M. Farrag, A.A. El-Haweet, E.S. Akel, and M.A. Moustafa, BioInvasions Rec. 5[1] (2016) 47-54.
-

- 23. C. Turan, M. Gürlek, D. Ergüden, A. Uyan, S. Karan, and S.A. Doğdu, NESciences 2[3] (2017) 55-66.
-

- 24. C. Turan, M. Gürlek, N.Başusta, A. Uyan, S.A. Doğdu, and S. Karan, NESciences 3[3] (2018) 333-358.
-

- 25. A.R. Kosker, F. Özogul, D. Ayas, M. Durmus, Y. Ucar, J. M. Regenstein, and Y. Özogul, Chemosphere 219 (2019) 95-99.
-

- 26. European Commission (2004). EU Report 29 April 2004, 854/2004/EC.
- 27. M. Silva, J. Azevedo, P. Rodriguez, A. Alfonso, L.M. Botana, and V. Vasconcelos, Mar. Drugs. 10[12] (2012) 712-726.
-

- 28. P. Mondal, S.P. Shah, and L. Marks, Cem. Concr. Res. 37[10] (2007) 1440-1444.
-

- 29. M. Boutinguiza, J. Pou, R. Comesaña, F. Lusquiños, A. De Carlos, and B. León, Mater. Sci. Eng. C. 32[3] (2012) 478-486.
-

- 30. A. Antonakos, E. Liarokapis, and T. Leventouri, Biomaterials. 28[19] (2007) 3043-3054.
-

- 31. S. Lee, Y. Lee, and Y. Yoon, J. Ceram. Process. Res. 8[6] (2007) 427-430.
- 32. M.E. Bahrololoom, M. Javidi, and S. Javadpour, J. Ma, J. Ceram. Process. Res 10[2] (2009) 129-138.
- 33. M.H. Nazarpak, M. Solati-Hashjin, and F. Moztarzadeh, J. Ceram. Proc. Res. 10[1] (2009) 54-57.
- 34. A.F.A. Latif, N.A.S. Mohd Pu'ad, N.A.A. Ramli, M.S. Muhamad, H.Z. Abdullah, M.I. Idris, and T.C. Lee, Mater. Sci. Forum 1010 (2020) 584-589.
-

- 35. S. Joschek, B. Nies, R. Krotz, and A. Göpferich, Biomaterials. 21[16] (2000) 1645-1658.
-

- 36. E. Milella, F. Cosentino, A. Licciulli, and C. Massaro, Biomaterials 22[11] (2001) 1425-1431.
-

- 37. A. Shanaghi, B. Mehrjou, Z. Ahmadian, A.R. Souri, and P.K. Chu, Mater. Sci. Eng. C. 118 (2021) 111524.
-

- 38. G. Balasundaram, M. Sato, and T.J. Webster, Biomaterials. 27[14] (2006) 2798-2805.
-

- 39. M. Šarić, M. Piasek, M. Blanuša, K. Kostial, and J.Z. Ilich, Nutrition 21[5] (2005) 609-614.
-

- 40. P.J. Marie, Bone 38[2] (2006) 10-14.
-

- 41. E. Landi, G. Celotti, G. Logroscino, and A. Tampieri, J. Eur. Ceram. Soc. 23[15] (2003) 2931-2937.
-

- 42. S.J. Eppell, W. Tong, J.L. Katz, L. Kuhn, and M.J. Glimcher, J. Orthop. Res. 19[6] (2001) 1027-1034.
-

- 43. Y. Huang, Y. Wang, C. Ning, K. Nan, and Y. Han, Biomed. Mater. 2[3] (2007) 196-201.
-

- 44. J. Chevalier and L. Gremillard, J. Eur. Ceram. Soc. 29[7] (2009) 1245-1255.
-

- 45. S.M. Chung and A.U.J. Yap, Dent. Mater. 21[11] (2005) 1008-1016.
-

- 46. C.Y. Ooi, M. Hamdi, and S. Ramesh, Ceram. Int. 33[7] (2007) 1171-1177.
-

- 47. N.A. Barakat, M.S. Khil, A.M. Omran, F.A. Sheikh, and H.Y. Kim, J. Mater. Process. Technol. 209[7] (2009) 3408-3415.
-

- 48. A. Raksujarit, K. Pengpat, G. Rujijanagul, and T. Tunkasiri, Mater. Des. 31[4] (2010) 1658-1660.
-

- 49. T.M. Coelho, E.S. Nogueira, W.R. Weinand, W.M. Lima, A. Steimacher, A.N. Medina, M.L. Baesso, and A.C. Bento, J. Appl. Phys. 101[8] (2007) 084701.
-

- 50. H. Ivankovic, G.G. Ferrer, E. Tkalcec, S. Orlic, and M. Ivankovic, J. Mater. Sci. Mater. Med. 20[5] (2009) 1039-1046.
-

- 51. J. Venkatesan and S.K. Kim, Materials 3[10] (2010) 4761-4772.
-

- 52. B.M. Venkatesan and R. Bashir, Nat. Nanotechnol. 6[10] (2011) 615-624.
-

- 53. M. Ozawa and S. Kanahara, J. Mater. Sci. 40[4] (2005) 1037-1038.
-

- 54. T.M. Coelho, E.S. Nogueira, A. Steimacher, A.N. Medina, W.R. Weinand, W.M. Lima, M.L. Baesso, and A.C. Bento, J. Appl. Phys. 100[9] (2006) 094312.
-

 This Article
This Article
-
2021; 22(3): 356-361
Published on Jun 30, 2021
- 10.36410/jcpr.2021.22.3.356
- Received on Dec 2, 2020
- Revised on Mar 22, 2021
- Accepted on Apr 19, 2021
 Services
Services
- Abstract
introduction
material and methods
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Servet Ahmet Doğdu
-
Molecular Ecology and Fisheries Laboratory, Faculty of Marine Sciences and Technology, Iskenderun Technical University, Iskenderun, Turkey
Tel : +90 542 311 11 05 Fax: +90 326 613 56 13 - E-mail: servetdogdu.mfbe17@iste.edu.tr, servetdogdu@yandex






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.