- Characteristics of porous B4C ceramic fabricated by the microwave sintering technique
Chuan Suna,b,†, Xiaogang Lub,†,*, Yunbo Chenb, Lingli Zuob and Yunkai Lic
aNational Joint Engineering Research Center for Abrasion Control and Molding of Metal Materials, & Henan Key Laboratory of High-temperature Structural and Functional Materials, Henan University of Science and Technology, Luoyang 471003, China
bState Key Laboratory of Advanced Forming Technology and Equipment, Beijing National Innovation Institute of Lightweight Ltd, Beijing 100083, China
cSchool of Materials Science and Engineering, Beijing Institute of Technology, Beijing 10081, ChinaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Porous B4C ceramic was produced through microwave sintering technique by using pure boron carbide powder. A thermal insulation container of our own design, was adopted during sintering process to reduce the heat loss. The effects of the relative density of green body on the porosity, mechanical property and microstructure of the as prepared samples were investigated. The application of microwave sintering technique was helpful to the preparation of the porous B4C ceramic
Keywords: Boron carbide, Microwave sintering, Microstructure, Mechanical property, Porous ceramic
Boron carbide shows outstanding physical and mechanical properties including extreme hardness and dystectic point, great wear and impact resistance, good chemical stability as well as wonderful neutron absorptivity [1-6]. Furthermore, B4C possesses an excellent wet-ability for melting aluminum to prepare ceramic-matrix composites [7-12]. Thus, B4C ceramic is a suitable skeleton material to fabricate ceramics-metal composites adopting an economic method called “melt infiltration” [13-16], in which molten aluminum infiltrates into porous B4C ceramic skeleton at the temperature above the melting point of Al. So far, the B4C/Al composite materials have been successfully applied in many fields which require high strength, high modulus and high abrasive properties, such as abrasives, nozzles, neutron absorbents, automotive breaks and body armor [16-26].
Unfortunately, B4C is highly difficult to densification resulting from the strong covalent B-C bonding, almost no plasticity and the great difficulty to grain boundary sliding in the solid state [27, 28]. In the sintered raw material, B2O3 covering the surface of B4C particle also impedes the densification process [28, 29]. Plenty of research has been devoted to the sintering densification of B4C in the past [26, 29-33]. In order to obtain adequate densification and proper porosity, the basically required temperature for preparing B4C and its valuable ceramic skeleton is as high as 2,100 oC [34]. This apparently restricts the promising application of B4C/Al composite materials because of the high cost to prepare porous B4C precursor by conventional sintering [35].
Microwave sintering method is fundamentally different from its counterparts in which heat is delivered to the sintered samples by thermal radiation
and conduction. In contrast, microwave energy acts directly on the sintered materials by molecular interaction in the microwave electromagnetic field [36-39]. Generally, uniformity is an apparent advantage of microwave sintering, mainly because the thermal energy is directly coupled into the sample rather than transferred from an additional heating source [40, 41]. The overall heating rate in microwave furnace is 50-100 oC/min (according to different applied power). Considering the slow heating rate (about 15 oC/min) during the conventional sintering method, there is a conspicuous shortening in the process time, which leads to proper porosity
. In addition, the sintering temperature is lower than conventional method. Actually, B4C has been successfully densified below 2,000 oC by microwave sintering [42]. However, to the best of our knowledge, porous B4C ceramic prepared by microwave sintering has not been reported in previous literature.
In the present study, a porous B4C ceramic with uniform structure and suitable porosity
was obtained by microwave sintering from commercial raw material. Moreover, the influences of relative density of green body on the porosity and mechanical strength of obtained B4C ceramic have been investigated.
B4C powder (W1.5, Mudanjiang Boron Carbide Company, China) was employed as the starting material. The B4C powder has a B:C ratio of approximately 4, a mean grain size of 1.5 μm, a purity of 97%, an oxygen content of 1.7 wt.% and a carbon content of 1.3 wt.%. Pelleting was conducted by a 200-mesh screen. Green body with a size of φ20 mm × 10 mm were uniaxially pressed using a hydraulic pressure tablets machine (FW-4A, Nanjing NanDa Instrument Plant, China) by different mechanical pressures. Then the compacts were processed by different cold isostatic pressures (GTM-26867, Avure Technologies Incorporated, USA) to give green bodies of varying relative densities.
Microwave sintering was experimenting with a 2.45 GHz microwave multimode applicator (MMT-101, GAMA Microwave Technology Company, USA) in a flowing argon atmosphere. All the specimens were sintered from room temperature to 1,700 oC within 20 minutes with a soaking time of 10 min. A complex system of thermal isolation was adopted, as shown in Fig. 1. The samples were placed into an alumina crucible arranged with SiC rods. The crucible was placed into a quartz container filled with graphite filler. The heating regime was determined together by the microwave absorption of sample, the structure of thermal isolation and the performance parameters of the sintering equipment [39-43]. The surface temperature of the sintering sample was measured using a thermocouple (below 1,000 oC) or an optical pyrometer (above 1,000 oC).
Archimedes method with xylene as the immersion medium was using to measure the relative densities of the green bodies. Porosity and average pore size of as-prepared B4C ceramic skeleton was measured using a mercury porosimetry (Auto Pore IV 9500, Micromeritics Instrument Corporation, USA). The phases in the samples were characterized by X-ray diffractometry (X' Pert PRO MPD, PANalytical, Netherlands) with CuKα radiation (λ-1.5406Å) operated at 40kV and 40mA. And the scanning speed was 1o/min for 2θ, divergence and scattering slit widths were set as 1o while the receiving slit width was set as 0.36 mm. Sintered pellets were cut, polished with 2500-grit paper and then gold-coated for microscopic observation. The microstructure was detected by the scanning electron microscope (SEM) (S-4800, Hitachi-hitec, Japan). The flexural strength at room temperature of sample was determined by three-point flexural test on a universal testing machine (E45, Mechanical Testing and Simulation incorporated, USA) with a loading rate of 0.5 mm/min. The dimension of the test piece was 15 mm×15 mm×10 mm. Hardness test was performed by a Rockwell indentor (HR-210MR, Mitutoyo Instrument Company, Japan) with a load of 60 kg. All results were repeated for five times, and then take an average. CAS numbers for all chemicals mentioned above are listed in Table 1.
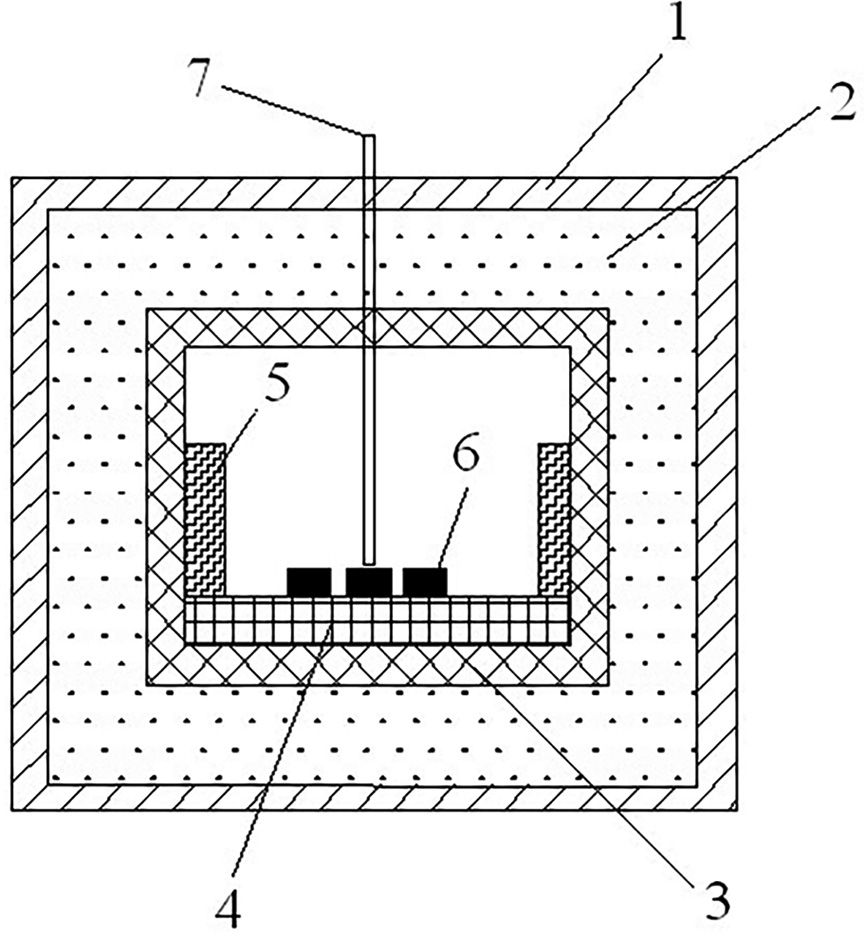
|
Fig. 1 Schematic drawing of the attemperator: 1. quartz container, 2. graphite fiber filler, 3. alumina crucible, 4. BN plate, 5. SiC rod (assistant heat materials), 6. specimens, 7. optical pyrometer view port or thermocouple in platinum tube. |
X-ray diffraction
The XRD patterns of the specimen before (a) and after (b) microwave sintering are shown in Fig. 2. Besides B4C, carbon and B2O3 are detected phase according to curve a. After microwave sintering, the diffraction peaks associated with free graphite reduce greatly and no B2O3 is found according to curve b. This phenomenon indicates that some C react with B2O3 and generate B4C. The reaction temperature is about 1350 oC according to the research of G. Arslan [44]:
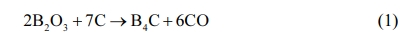
Porosity and mechanical property
Microwave sintering of samples are conducted from the green bodies with different relative densities ranging from 35% T.D. to 60% T.D. The relationship between the relative density of starting body before sintering and the porosity/bending strength of porous B4C is illustrated in Fig. 3. The porosity of specimens decreases with the increase in relative densities. This is because the high-density of green body can promote mass transport during the process of sintering. Besides, the porosity curve declines rapidly when the relative density of starting body is below 50% T.D. and then becomes smooth and slow while the relative density continues increasing. Contrary to the porosity, the increasing relative density leads to an increase in the bending strength of samples as shown in Fig. 3. The flexure strength can reach 64 MPa when the relative density of green body is 60% T.D. Macro-Hardness, which is also an important property of porous ceramics used for melt infiltration [45], increases with the relative density of green pressing. The highest value of Rockwell hardness obtained is 75.9 HRA.
Generally, a decrease of porosity in material improves mechanical strength. For the case of porous ceramics, mechanical properties and porosity are contradictory, a decreasing porosity usually results in an improvement of bending strength and hardness [46, 47].
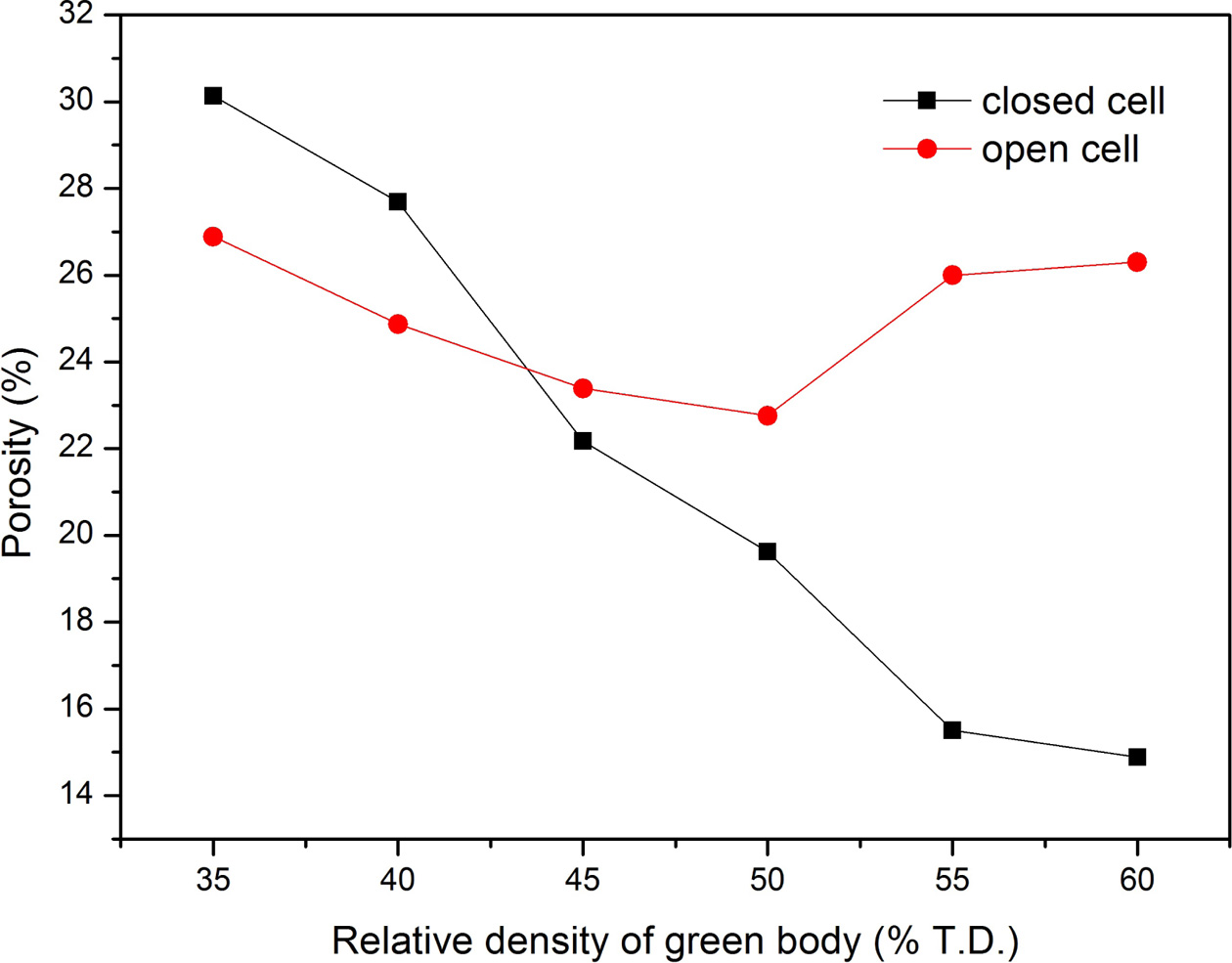
Changes of closed and open porosity percentage of the specimens are respectively shown in Fig. 4. The percentage of closed cell decreases significantly from 30.1 vol.% to 14.9 vol.%, while the percentage of open cell decreases first and then increases when the relative density of green pressing is higher than 50% T.D. Furthermore, closed cell is dominated when the relative density before microwave sintering is less than 43.2% T.D. Open cell accounts for the majority of total pore while the relative density before microwave sintering is greater than 43.2% T.D. For porous B4C ceramic skeleton with a sufficient strength, it is clear that the more open pores, the more favorable for the infiltration of melting aluminum. As shown in Fig. 4, the percentage of open cell reaches to a maximum point (26.3 vol.%) when the relative density of green body is 60% T.D. According to the previous data on bending strength and hardness, a green body with high relative density is preferred.
Microstructure
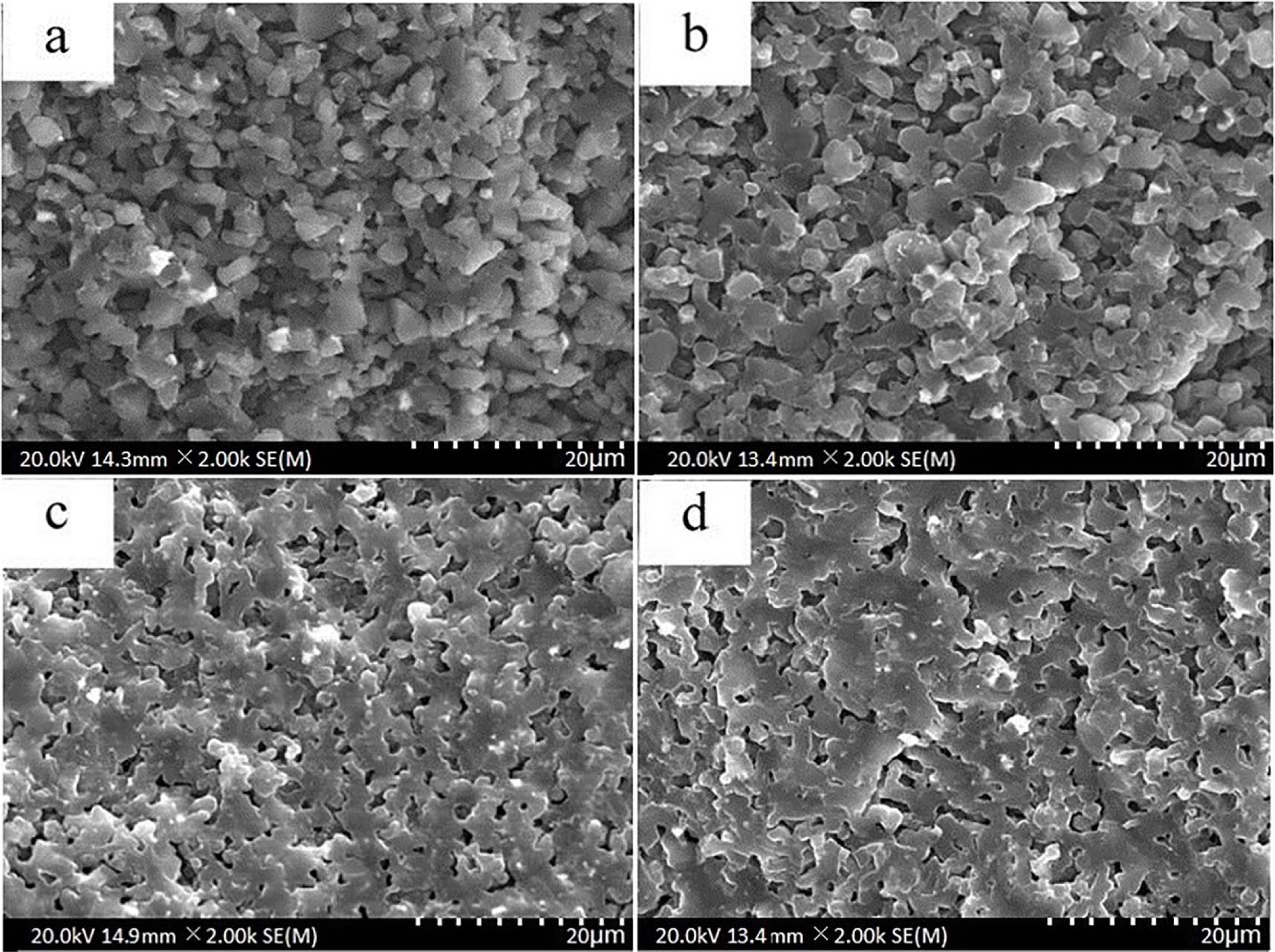
The properties of prepared ceramics skeletons are remarkably influenced by the pore size and morphology [48-50]. Fig. 5 shows the microstructure of porous B4C samples sintered from green pressing with different relative densities (35% T.D., 45% T.D., 50% T.D. and 60% T.D.). A limited sintering can be appreciated from Fig. 5(a), a sample sintered from the pressing gets the lowest relative density. Porosity is considerably large because the distance between boron carbide particles is long and the structure basically remains its initial states. When the relative density of green pressing increases to 45% T.D. (Fig. 5(b)), obvious sintering necks are found. With the further increasing in the relative density, the well-sintered samples with uniform structure and micron pores which are nearly round in shape are obtained (Fig. 5(c) and (d)). This appearance is probably resulted from the shortening of the diffusion distance because of the increase in the relative density of green body.
Average pore size
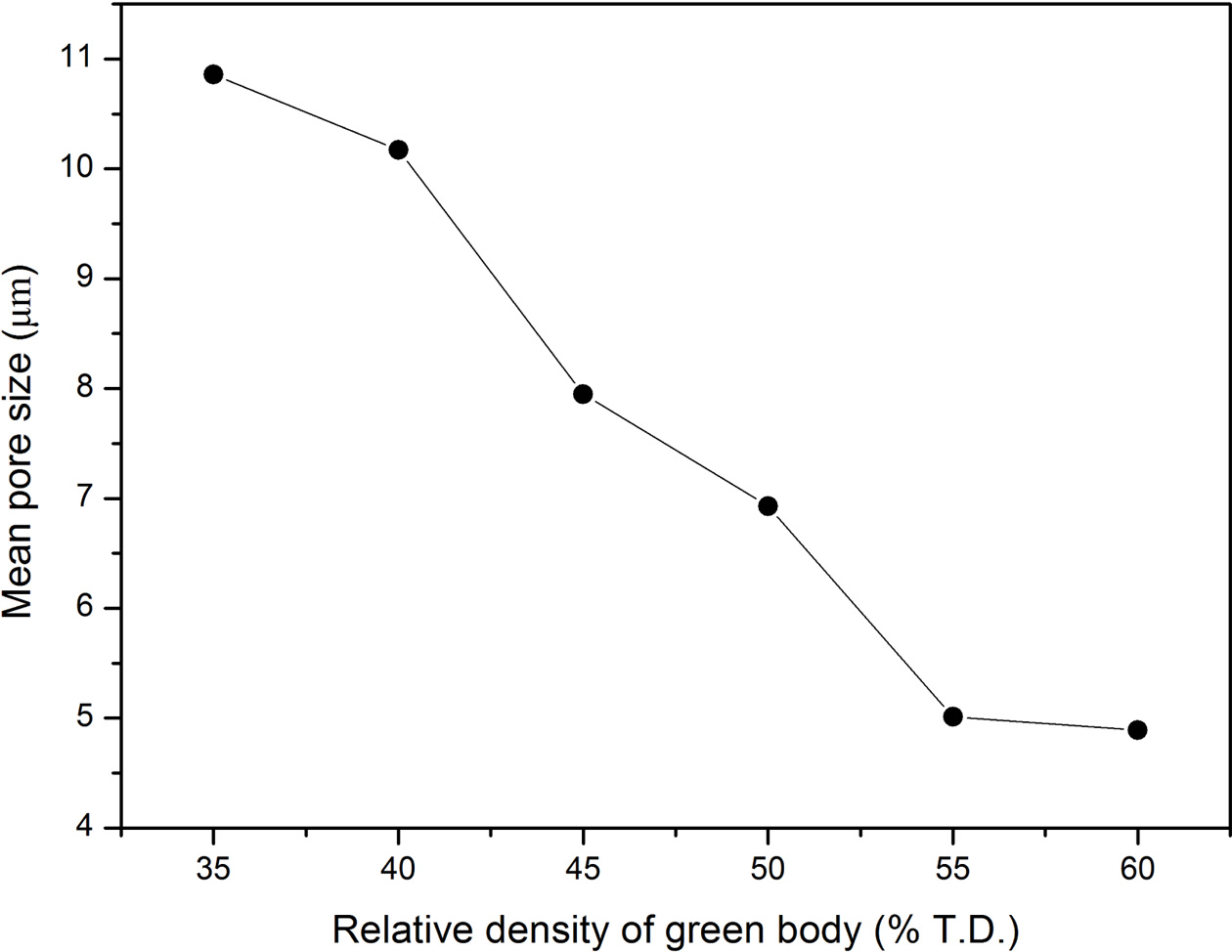
Fig. 6 illustrates the mean pore size of the open cell in porous B4C ceramic measured by mercury intrusion method. When the relative density of the specimen before sintering is low, the mean size of open cell of the final sample is about 10.9 μm. As the relative density increases, the mean pore size drops sharply to 5.0 μm and then stays flat.
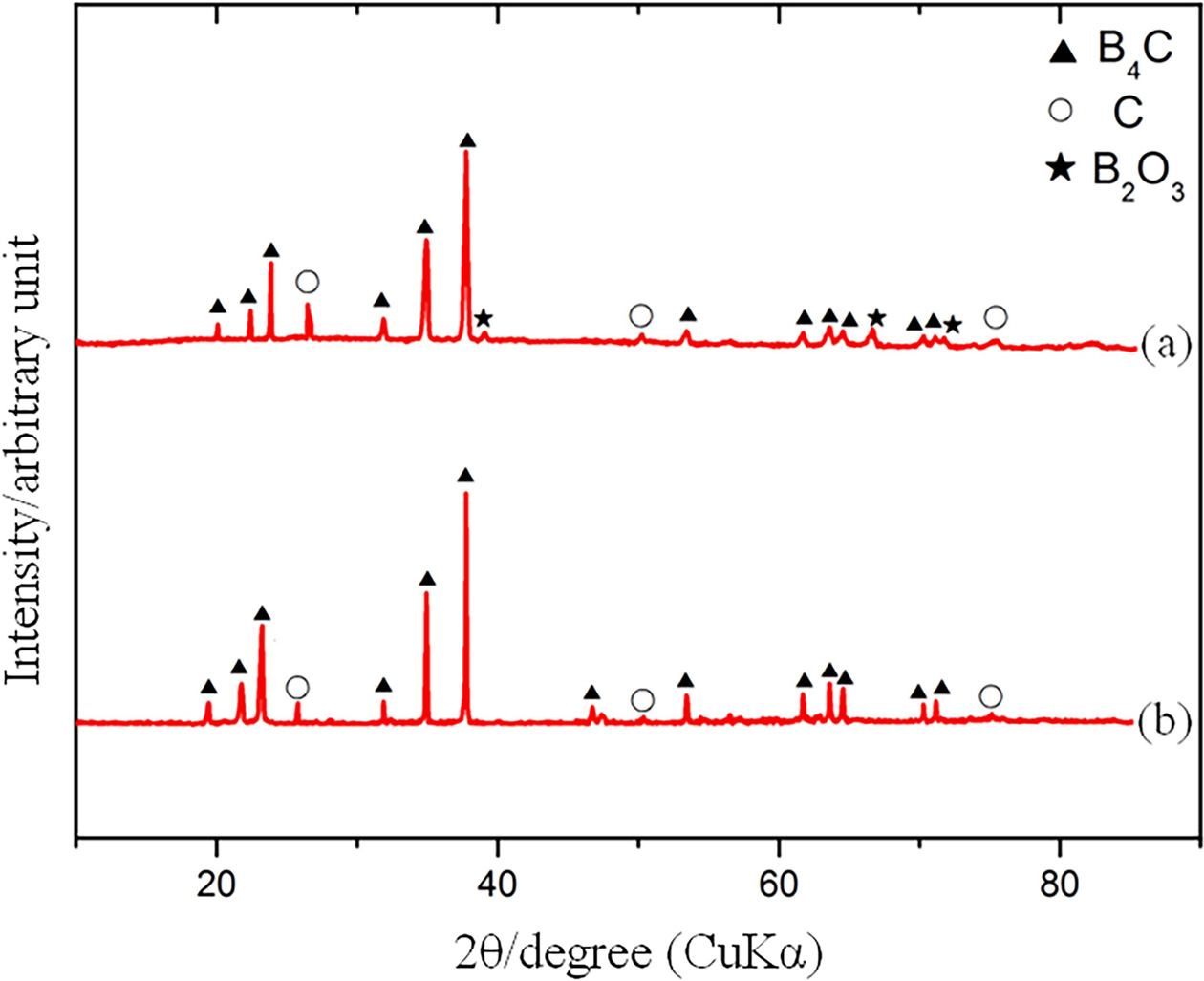
|
Fig. 2 XRD pattern of the sample before (a) and after (b) microwave sintering. |
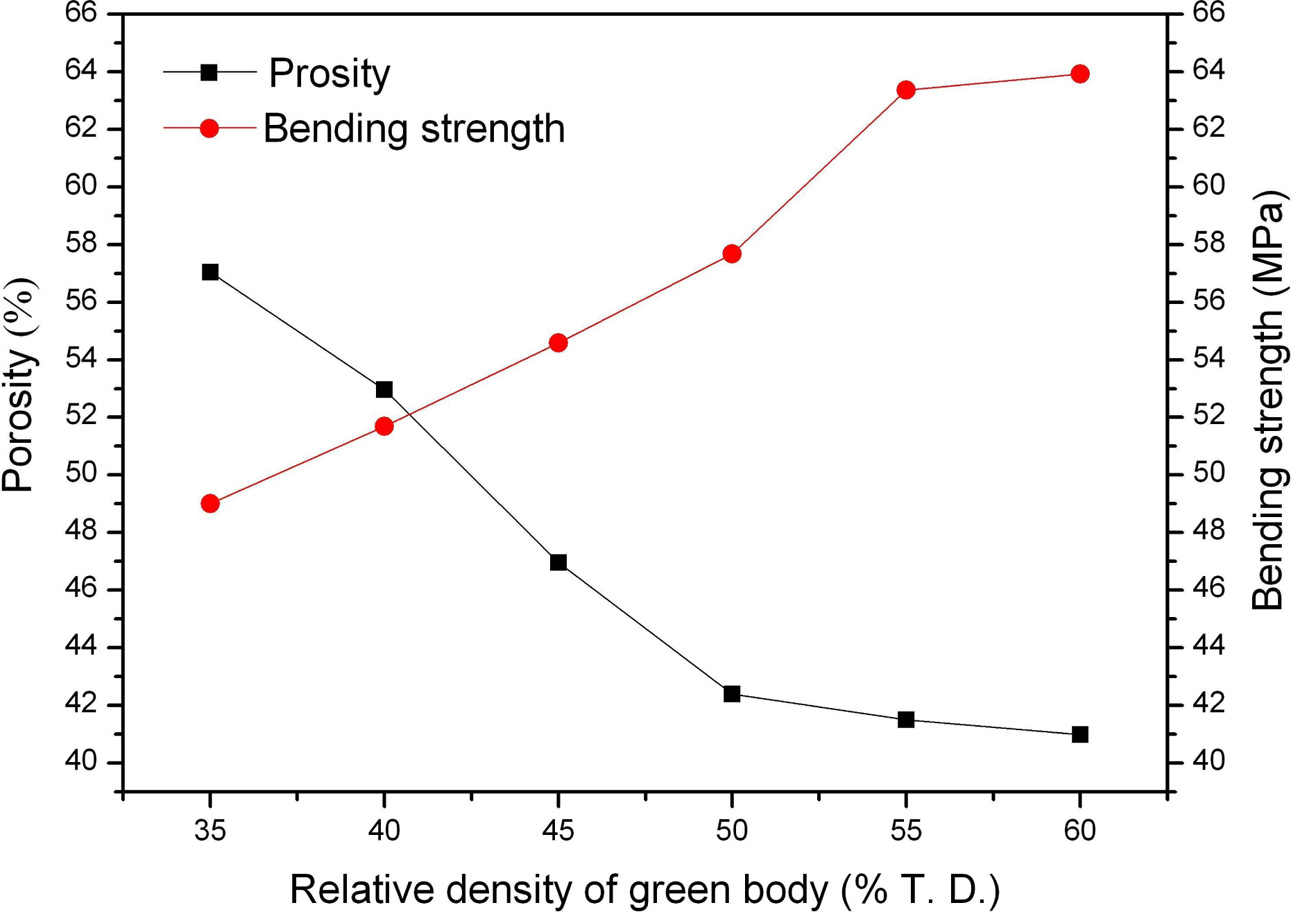
|
Fig. 3 Porosity and bending strength changes as a function of the relative density of green body. |
|
Fig. 4 Closed porosity percentage and open porosity percentage changes as a function of the relative density of green body |
|
Fig. 5 SEM micrographs of the polished fracture of B4C samples prepared from green bodies with different relative densities: (a) 35% T.D. (b) 45% T.D. (c) 50% T.D. (d) 60% T.D.. |
|
Fig. 6 Mean pore size of open cell of samples sintered from green bodies with different relative densities. |
Porous boron carbide ceramics with homogenized structure were prepared by microwave sintering method with a heat insulating apparatus. The sintering process lasted just 30 min. The porosity of porous B4C ceramic varied from 40.9 vol.% to 57.1 vol.% by adjusting the relative density of green body. Open cell was dominated when the relative density of green body was more than 43.2% T.D. The sample exhibiting open porosity of 26.3 vol.% and bending strength of 64 MPa was obtained when the relative density of green body was 60% T.D. The average pore size decreased with an increasing in the relative density of green pressing, which was based on the data measured by mercury intrusion method.
It is demonstrated that microwave sintering is an effective method to fabricate porous B4C ceramics, and this framework material with a certain extent of open porosity and strength could be an appropriate precursor skeleton to make wonderful composite. On the side, porous B4C may have exciting potential applications in the treatment of waste gas/water and catalysis field for its large specific surface area.
This work was supported by Open Fund of National Joint Engineering Research Center for Abrasion Control and Molding of Metal Materials (HKDNM201812), and Technology Development Fund Project of China Academy of Machinery Science and Technology (212004Q9).
- 1. W. Zhang, Prog. Mater. Sci. 116 (2021) 100718.
-

- 2. W. Zhang, S. Yamashita, and H. Kita, Adv. Appl. Ceram. 118[4] (2019) 222-239.
-

- 3. G.S. Samy and S.T. Kumaran, Measurement. 103 (2017) 1-9.
-

- 4. D. Davtyan, R. Mnatsakanyan, L. Liu, S. Aydinyan, and I. Hussainova, J. Mater. Res. Technol. 8[6] (2019) 5823-5832.
-

- 5. H. Roghani, S.A. Tayebifard, A. Kazemzade, and L. Nikzad, J. Ceram. Process. Res. 17[3] (2016) 170-175.
- 6. L. Nikzad, T. Ebadzadehb, M.R. Vaezia, and A. Tayebifard, J. Ceram. Process. Res. 13[5] (2012) 590-594.
- 7. W. Zhu, X.L. Cai, Z.Y. Wang, Z.W. Xu, and M. Feng, Mater. Rep. 30[27] (2016) 478-482. (in Chinese)
- 8. R. Ambigai and S. Prabhu, Aust. J. Mech. Eng. 17[2] (2019) 53-63.
-

- 9. M.H. Shojaeefard, M. Akbari, A. Khalkhali, and Parviz A, Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 232[8] (2016) 1-15.
-

- 10. Y.T. Yao and L.Q. Chen, Mater. Maunf. Processes. 31[10] (2016) 1286-1291.
-

- 11. P. Larsson, N. Axén, and S. Hogmark, J. Mater. Sci. 35[14] (2000) 3433-3440.
-

- 12. T. Murthy, S. Ankata, J.K. Sonber, K. Sairam, K. Singh, A. Nagaraj, P. Sengupta, R.D. Bedse, S. Majumdar, and V. Kain, Ceram. -Silik. 62[1] (2018) 15-30.
-

- 13. C.L. Cramer, A.M. Elliott, J.O. Kiggans, B. Haberl, and D.C. Anderson, Mater. Des. 180 (2019) 107956.
-

- 14. A. Ravanan, J.M. Vieira, B.A. Almeida, C. Ramkumar, F.J. Oliveira, A.B. Lopes, and H.Y. Wu, Mater. Today: Proc. 16[2] (2019) 374-383.
-

- 15. Y.T. Yao and L.Q. Chen, J. Mater. Sci. Technol. 30[7] (2014) 661-665.
-

- 16. X.G. Huang, C. Yin, H.Q. Ru, S.M. Zhao, Y.J. Deng, Y.J. Guo, and S. Liu, Mater. Des. 186 (2020) 108323.
-

- 17. I. Sudhakar, V. Madhu, G. Madhusudhan Reddy, and K. Srinivasa Rao, Def. Technol. 11[1] (2015) 10-17.
-

- 18. Y.N. Zan, Y.T. Zhou, Z.Y. Liu, Q.Z. Wang, W.G. Wang, D. Wang, B.L. Xiao, and Z.Y. Ma, Mater. Sci. Eng., A. 773 (2020) 138840.
-

- 19. C.L. Cramer, A.M. Elliott, J.O. Kiggans, B. Haberl, and D.C. Anderson, Mater. Des. 180 (2019) 107956.
-

- 20. L. Zhang, Z. Wang, Q.G. Li, J.Y. Wu, G.P. Shi, F.F. Qi, and X. Zhou, Ceram. Int. 44[3] (2018) 3048-3055.
-

- 21. S. Karabulut, H. Karakoc, and R. Citak, Composites, Part B. 101 (2016) 87-98.
-

- 22. H.S. Chen, W.X. Wang, Y.L. Li, J. Zhou, H.H. Nie, and Q.C. Wu, Mater. Des. 94 (2016) 360-367.
-

- 23. M. Tayebi, M. Jozdani, and M. Mirhadi, J. Alloys Compd. 809 (2019) 151753.
-

- 24. Z.L. Chao, T.T. Sun, L.T. Jiang, Z.S. Zhou, G.Q. Chen, Q. Z, and G. H. Wu, Ceram. Int. 45[16] (2019) 20539-20544.
-

- 25. M. Imran, and A.R.A. Khan, J. Mater. Res. Technol. 8[3] (2019) 3347-3356.
-

- 26. P. Švec, Z. Gábrišová, and A. Brusilová, J. Ceram. Process. Res. 20[1] (2019) 113-120.
- 27. X.W. Du, Y. Wang, Z.X. Zhang, F. Zhang, W.M. Wang, and Z.Y. Fu, Mater. Sci. Eng. A. 636 (2015) 133-137.
-

- 28. K.H. Kim, J.H. Chae, J.S. Park, D.K. Kim, K.B. Shim, and B.H. Lee, J. Ceram. Process. Res. 8[4] (2007) 238-242.
- 29. R.F. Speyer and H. Lee, J. Mater. Sci. 39[19] (2004) 6017-6021.
-

- 30. M. Mashhadi, E. Taheri-Nassaj, and V.M. Sglavo, Ceram. Int. 36[1] (2010) 151-159.
-

- 31. A. Shoshin, A. Burdakov, M. Ivantsivskiy, M. Klimenko, S. Polosatkin, and A. Semenov, Fusion Eng. Des. 146 (2019) 2007-2010.
-

- 32. B.M. Moshtaghioun, D. Gomez-Garcia, A. Dominguez-Rodriguez, and R.I. Todd, J. Eur. Ceram. Soc. 36[16] (2016) 3925-3928
-

- 33. B.M. Moshtaghioun, A.L. Ortiz, D. Gómez-García, and A. Domínguez-Rodríguez, J. Eur. Ceram. Soc. 35[6] (2015) 1991-1998.
-

- 34. J.E. Zorzi, C.A. Perottoni, and J.A.H. da Jornada, Mater. Lett. 59[23] (2005) 2932-2935.
-

- 35. Y. Wang, Q. Liu, B. Zhang, J.J. Ding, H.Q. Zhang, Y.C. Jin, Z.X. Zhong, W. Wang, and F. Ye, Ceram. Int. 46[10] (2020) 17117-17121.
-

- 36. D.B. Hong, J.T. Yuan, Z.B. Yin, H.H. Peng, and Z.Y. Zhu, Ceram. Int. 46[12] (2020) 20183-20190.
-

- 37. Z.Y. Zhu, Z.B. Yin, D.B. Hong, and J.T. Yuan, Ceram. Int. 46[17] (2020) 27362-27372.
-

- 38. E. Ghasali, M. Alizadeh, T. Ebadzadeh, A.H. Pakseresht, and A. Rahbari, J. Mater. Res. Technol. 4[4] (2015) 411-415.
-

- 39. C. Singhal, Q. Murtaza, and Parvej, Mater. Today: Proc. 5[11] (2018) 24287-24298.
-

- 40. D.E. Clark and W.H. Sutton, Annu. Rev. Mater. Sci. 26[1] (1996) 299-331.
-

- 41. R.R. Souza, D. Thomazini, M.V. Gelfuso, H. Amorín, A.S. Pereira, and V.C. Sousa, J. Ceram. Process. Res. 18[1] (2017) 73-78.
- 42. R. Ruginets and R. Fischer, Am. Ceram. Soc. Bull. 74[1] (1995) 56-58.
-

- 43. J.D. Katz, R.D. Blake, J.J. Petrovic, and H. Sheinberg, MRS Online Proc. Libr. 124[12] (1988) 219-226.
-

- 44. G. Arslan, F. Kara, and S. Turan, J. Eur. Ceram. Soc. 23[8] (2003) 1243-1255.
-

- 45. X.D. Yu, Y.W. Wang, Q.S. Ma, Y. Ma, and Z.H. Chen, Rare. Metal. Mat. Eng. 36 (2007) 587-589.
-

- 46. R.W. Rice, J. Mater. Sci. 31[6] (1996) 1509-1528.
-

- 47. S.N. Chou, H.H. Lu, D.F. Lii, and J.L. Huang, Ceram Int. 35[1] (2009) 7-12.
-

- 48. T.L. Chew, A.L. Ahmad, and S. Bhatia, J. Porous Mater. 18[3] (2011) 355-360.
-

- 49. M.C. D'Arrigo, C. Siligardi, C. Leonelli, J.Y. So, and H.S. Kim, J. Porous Mater. 9[4] (2002), 299-305.
-

- 50. J.L. Yu, J.L. Yang, H.X. Li, and Y. Huang, J. Porous Mater. 19[5] (2012) 883-888.
-

 This Article
This Article
-
2021; 22(3): 340-344
Published on Jun 30, 2021
- 10.36410/jcpr.2021.22.3.340
- Received on Oct 24, 2020
- Revised on Dec 4, 2020
- Accepted on Dec 29, 2020
 Services
Services
- Abstract
introduction
experiment
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Xiaogang Lu
-
State Key Laboratory of Advanced Forming Technology and Equipment, Beijing National Innovation Institute of Lightweight Ltd, Beijing 100083, China
Tel : +0086-10-82415063 Fax: +0086-10-82415063 - E-mail: 13121200806@163.com







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.