- Preliminary assessment of recycling Malaysia’s Electric Arc Furnace (EAF) steel slag waste as one of raw materials for geopolymer ceramic product
Siti Koriah Zakariaa, Nur Iman Najwa Abd Rahmana, Siti Zuliana Salleha, Nurulakmal Mohd Sharifb, Anasyida Abu Semanb, Mustaffa Ali Azhar Taibc, Julie Juliewatty Mohameda, Mahani Yusoffa, Abdul Hafidz Yusoffa, Mardawani Mohamada, Mohamad Najmi Masria, Arlina Alia and Pao Ter Teoa,*
aFaculty of Bioengineering and Technology, Universiti Malaysia Kelantan, Jeli Campus, 17600 Jeli, Kelantan, Malaysia
bSchool of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Engineering Campus, 14300 Nibong Tebal, Penang, Malaysia
cDivision of Advanced Ceramic Materials Technology, Advanced Technology Training Center (ADTEC) Taiping, 34600 Kamunting, Perak, MalaysiaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This research work investigated the suitability of electric arc furnace (EAF) slag as one of raw materials for geopolymer ceramic product. Two sets of different body formulations were used; EAF slag with and without presence of China clay were prepared in alkaline medium. In addition, this work also studied the effect of varying curing temperature on the properties of EAF slag waste-based geopolymer. The chemical and mineralogical of this geopolymer are characterized by X-Ray Diffraction (XRD), X-Ray Fluorescence (XRF) and Fourier Transfer Infrared Spectroscopy (FTIR). The physical properties included water absorption, apparent porosity, and bulk density are well reported where phase analysis and functional groups present in the geopolymer supported the observations. Furthermore, the compressive strength obtained was in line with the result observed in physical properties
Keywords: EAF steel slag waste; geopolymer ceramic; alkaline-activated material
Recently, development of green and sustainable ceramic product is getting prominent and gaining much attention from worldwide researchers. A ceramic product is considered ‘green and sustainable’ if it is made from industrial solid wastes, non-hazardous, recyclable and cheaper in cost [1]. Several researchers have proposed on the potential utilization of industrial wastes such as blast furnace slag from iron making plant [2-4], glass wastes [5, 6], fly ash [7-9], sewage sludge [10-12] and EAF steel slag from carbon steel making plant [13-17], as partial replacement of raw materials into green ceramic product. The idea of reusing the solid wastes for ceramic body is widely reported as those wastes are found to have close chemical composition to the natural resources (clay, flux and filler) required for conventional ceramic manufacturing. The recent waste utilization approach is much preferable as it can resolve problems associated with over-limit storage of industrial wastes and reduce exploration of natural resources for ceramic products to continuously sustain the nature.
Although Badiee et al. [13], Sarkar et al. [14] and our preliminary study; Teo et al. [15]; Teo et al. [16] have managed to incorporate the EAF slag in ceramic product via conventional high temperature firing (1,150 oC - 1,180 oC), they mutually agreed that the higher iron oxide content in EAF slag (25 - 42.4 wt.%) would cause vigorous fluxing action in the ceramic. It was reported that iron oxide strongly contributes to formation of excessive glassy phase and lower densification point to the level at which gases as still being liberated during firing process. This vigorous glassy phase would then seal pores and trap gases, leading to surface defects such as bloating and blistering upon cooling of the ceramic [18]. Apart from that, another challenge is the entrapped gases are would also remain as closed porosity in the ceramic body upon cooling. Therefore, combination of these drawbacks, surface defects and closed porosity would in turn deteriorate physical (water absorption and apparent porosity) and mechanical properties (compressive strength) of the ceramic product and these make the utilization of EAF slag in ceramic is rather more challenging as compared to other solid wastes.
Owning to these, geopolymerization is regarded as a potential route to surpass drawbacks of conventional high temperature (1,150 oC to 1,180 oC) firing process in EAF slag based ceramic product. The term ‘‘geopolymer” was first used by Davidovits [19] to describe a family of mineral binders closely related to artificial zeolites. These structures consist of a polymeric Si-O-Al (silico-oxide) functional group, similar to that found in zeolites. Generally, geopolymerization involves alkaline activation of functional groups in the product curing process, and low firing temperature (500 oC to 1,000 oC) is employed. Most of up-to-date journals merely reported on formation of geopolymer chains from pure clay using sodium hydroxide (NaOH) alkaline medium as geopolymerization activation agent. Several researchers have reported on development of geopolymer structural clay product such as ceramic tile and brick from clay (mandatory raw material) and solid wastes such as waste ceramic [20], dust ceramic [21] and fly ash [22, 23]. From the studies, they have mutually agreed that geopolymerization route integrated with low firing temperature (500 oC to 1,000 oC) is capable to yield ceramic product which have comparable physical and mechanical properties with conventional ceramic product.
Nevertheless, none of research work has been reported on developing of geopolymer chains from combination of EAF slag waste and clay. Previous FTIR (Fourier Transform Infra Red) analysis has revealed that the slag generally consists of functional groups such as silico-aluminate (-Si-O-Al-O-) and/or ferro-silico-aluminate (-Fe-O-Si-O-Al-O-) while clay consists of silico-oxide (silico-oxide: -Si-O-Si-O) functional group [24, 25]. Subsequently, through alkaline activation of curing process, there is a high likelihood for the functional groups (from EAF slag and clay) to chemically react and form a new geopolymer network in the geopolymer ceramic product. Therefore, the purpose of this work is to preliminary investigate the potential utilization of EAF slag into geopolymer ceramic including different body formulations and curing parameters. The results discussed will be based on the physical and mechanical properties, as well as mineralogical property of the EAF slag waste-based geopolymer ceramic.
Materials
Two different types of raw materials were used in this research, whereby the raw and waste materials were mixed at a different ratio. The raw material used in this work is China clay (supplied by Kaolin Malaysia Sdn. Bhd.) and EAF slag waste. The EAF slag was supplied by a local Malaysian steel manufacturer. Meanwhile, the alkaline medium used was sodium hydroxide (NaOH) pellet (Brand: MERCK).
Sample preparation
Fine powder of EAF slag was obtained by crushing it for 6 min using high energy ring milling. Then, NaOH solution was prepared by dilution NaOH pellets in distilled water to produce 2 M alkaline activator solution. After that, two different compositions denoted as Composition 1 and 2 were prepared with different ratios of waste and raw materials according to the formulation as tabulated in Table 1. Each composition was homogeneously mixed with a constant amount of prepared alkaline solution by using a Heidolph mixer for 5 min.
After the mixing process, the mixed pastes were compacted using cylindrical polyvinyl chloride mould with a dimension of 3 cm ´ 2 cm (diameter x height). In the next step, the samples were hermetically sealed for 24 h for pre-curing at room temperature. Finally, the samples were demoulded and post-cured at different curing temperatures; 25, 60 and 80 oC. The experimental procedures were summarized in Fig. 1.
Characterizations
The characterizations performed in this research included chemical analysis, physical behaviour and mechanical properties determination in order to investigate the properties of Malaysia’s electric arc furnace (EAF) steel slag waste. These properties were expected to influence the performances of EAF slag-based geopolymer.
X-Ray Fluorescence (XRF) analysis (Bruker S2 Ranger X-Ray Fluorescence spectrometer) was used to determine the chemical compositions. X-ray diffraction (XRD) measurement (Bruker D2 Phaser X-ray diffractometer) was used to determine the crystallinity of the geopolymer sample. The XRD diffractograms of the raw and waste materials were obtained by using Cu Kα radiation and the samples were step-scanned with 2ϴ in the ranges between 10-80o. Thermo Fisher Scientific’s Fourier Transform Infrared (FTIR) spectrometer was used to analyse the functional groups present. Spectral analysis was performed over the range 2,500-500 cm-1.
The physical properties such as water absorption, apparent porosity, and bulk density were evaluated in accordance with Malaysian Standard (MS ISO 10545-3:2001). Meanwhile, American Standard for Testing and Materials (ASTM C39) was used to determine the compressive strength.
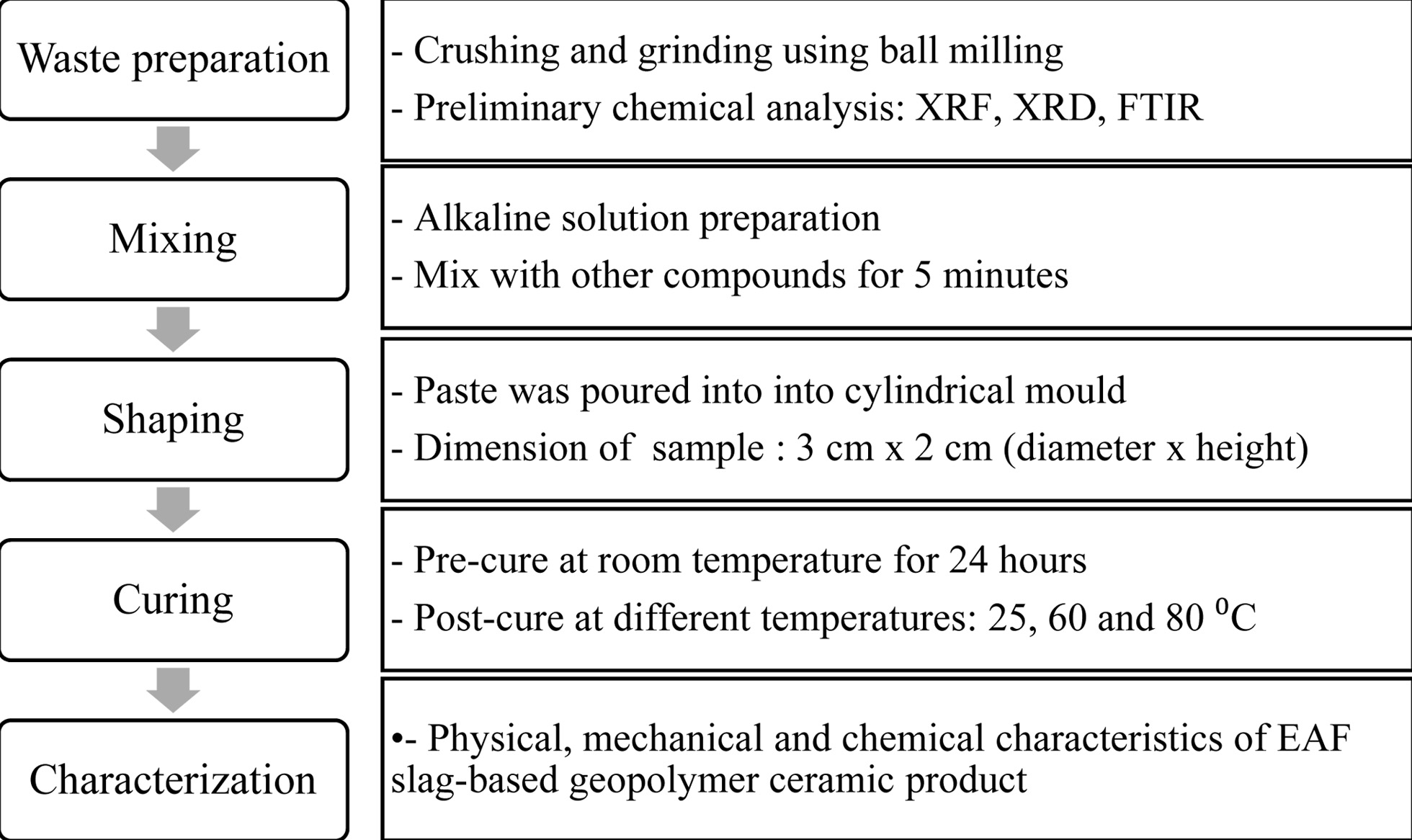
|
Fig. 1 Flow chart of experimental procedure |
The chemical composition of raw materials; EAF slag and China clay obtained from XRF analysis is tabulated in Table 2. It can be seen that EAF slag contains a higher percentage of CaO and Fe2O3. Normally EAF contains about 15~30% of iron [26]. This indicates that the high content of both oxides in EAF slag is suitable for acting as a fluxing agent for geopolymerization [27]. Meanwhile, China clay composed of a high content of SiO2, Al2O3 and K2O, which is normally known as the aluminosilicate sources for geopolymer fabrication [28-30].
Fig. 2(a) and (b) shows the XRD diffractograms for both raw materials. This diffractogram confirmed the presence of larnite (2CaO·SiO2), hematite (Fe2O3), wustite (FeO), gehlenite (Al2O3·2CaO·SiO2) and quartz (SiO2) in EAF slag as shown in Fig. 2 (a) which agree with COD 9012789, COD 9015065, COD 9009766, COD 1011002 and COD 9011493 respectively. The presence of these crystalline phases is expected to be beneficial to the mechanical properties of the resultant geopolymer [18]. No apparent peak is available that represents alumina is obtained in XRD diffractograms for EAF. This is consistent with the data obtained previously in XRF analysis, where the amount of Fe2O3 is very low. Meanwhile, China clay composed of kaolinite (Al2O3.2SiO2.2H2O) and quartz (SiO2) as depicted in Fig. 2(b) that are in accordance with COD 9009230 and COD 9000775, respectively.
FTIR spectra with the functional groups present in EAF slag and China clay is illustrated in Fig. 3 (a) and (b), respectively. Dakhane et al. [31] have reported that vibration modes of silica bonds can be observed in the range between 400 to 1,200 cm-1. In this work, Fig. 3(a) shows that the prominent peaks of EAF slag at 850.37 and 882.75 cm-1 which represents the symmetric stretching of Si-O bond and asymmetric stretching vibration bond of Si-O-T (T=Si or Al). The other functional groups present in EAF slag can be seen at 982.75 to 1,474.41 cm-1. These peaks attributed to the asymmetric stretching vibration of Si-O/Al-O, respectively [32].
The absorption bands at 3,687.98 cm-1 and 3,620.28 cm-1 as shown in Fig. 3(b) are assigned to the stretching vibration of OH groups in China clay. A similar observation was reported by Sore et al. [33] where the stretching vibration of OH groups of china clay are located at a range between 3,688-3,620 cm-1. The other groups such as vibrations of Si-O, symmetric Si-O-Si and asymmetric stretching band of Si-O-Al of kaolinite at 1,112.35, 1,026.98, and 994.60 cm-1 [34, 35] are observed in China clay.
FTIR spectra represent the functional groups present in the EAF slag-based geopolymer with different com- positions is depicted in Fig. 4. The determination of Si-O-Si and Si-O-Al bonds geopolymer can be observed at different peaks for different compositions. For instance, the peaks are located at 882.75 and 982.83 cm-1 for sample denoted as 1T25 meanwhile the peaks are slightly shifted to lower bands for samples denoted as 1T60 and 1T80. A similar trend can be observed in Composition 2 when the post-curing temperature is beyond 20 oC and keep increasing. The shifted peaks can be related to the changes of the silicate network as a consequence of the curing process [36]. It is suggested that the polycondensation of these bonds occurred in the alkaline environment [37].
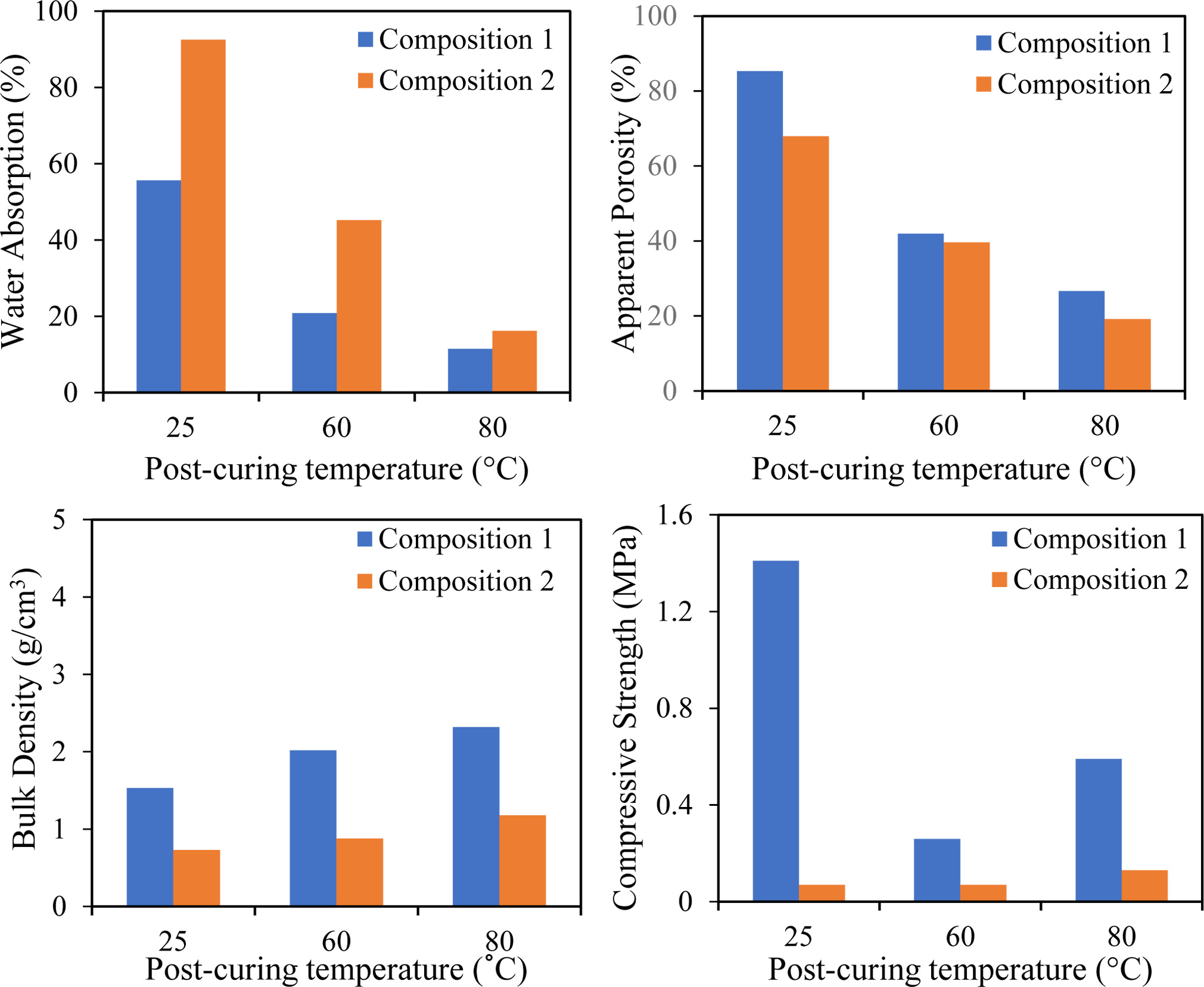
The physical properties of for EAF slag-based geopolymer with different compositions and post-curing temperatures are shown in Fig. 5(a-c). The water absorption for Composition 1 is lower than Composition 2. This indicates that the water accessibility in Composition 1 is unfavourable which can be related to the low apparent porosity and high bulk density as supported in Fig. 5(b) and (c), respectively.
In addition, it can be observed that the percentage of water absorption for any compositions keep decreasing with temperature and Composition 1 showed significant low values of water absorption as compared to Composition 2. In general, low water absorption with increasing post-curing temperature can be elaborated with the condensation process within the matrix that occurred after gelation [33].
Fig. 5(b) and (c) also show that the apparent porosity and bulk density of EAF slag-based geopolymer are influenced by the post-curing temperature. This indicates that temperature control the raw material dissolution and gel condensation thus consequently the formation of pores and density of geopolymer sample. Sore et al. [33] explained that dissolution of raw materials and condensation of the gel are slow at low temperature which leads to porosity formation and caused low densities. A similar observation is obtained where the apparent porosity is high; meanwhile, bulk density is low at 25 oC. Again, a similar trend can be seen where less porosity formation and thus resulted in high-density geopolymer at high temperature.
Compressive strength for both compositions with various post-curing temperatures is plotted in Fig. 5(d). The value of compressive strength of Composition 1 is higher than Composition 2 irrespective of post-curing temperature. This observation can be explained by the formation of the granular structure in the EAF slag-based geopolymer sample. As mentioned previously, China clay was added in Composition 2, and the alkaline medium was used as an activator in this work. It was reported that China clay has low reactivity with the alkaline solution, which causes limited surface area for China clay dissolution and thus unable to provide Si4+ and Al4+ ions for the further reaction [36]. China clay used is not pre-heated, which also causes a decrement of the mechanical strength of the final products [38]. On top of that, Abdullah et al. [36] explained that the homogeneity of the geopolymer mixtures is crucial in order to attain high strength and additional water for mixing can detrimental to the mechanical strength.
The trend of compressive strength of Composition 1 is inconsistent with temperature, but it can be noticed that the compressive strength is higher at 25 oC than at any temperature. It has been reported by Yunsheng et al. [29] where the addition of EAF slag beyond 50.00% will deteriorate the strength of geopolymer. However, the percentage of EAF slag used in this work was 92.00%, and the compressive strength obtained at 25 oC is 1.41 MPa for sample denoted as 1T25. This indicates that EAF slag has the potential of a great filler in enhancing the mechanical properties of geopolymer [36].
However, the compressive value is decreased at higher post-curing temperature for Composition 1. The gel within the mixture contracted without transforming into semi-crystalline form due to the dehydration and excessive shrinkage caused the low compressive strength value at high temperature. The other factors that influence the compressive strength of the geopolymer are the size of slag [39] and the addition of water during the mixing process. Huang et al. [40] explained that small size of slag will able to distributed in the gap of large particles and the hydration degree of small particles was bigger than that of large particles when the reaction started. Thus, calcium silicate hydrate gel of small particles and large particles has coalesced, and a dense structure was formed. This formation was responsible for high compressive strength.
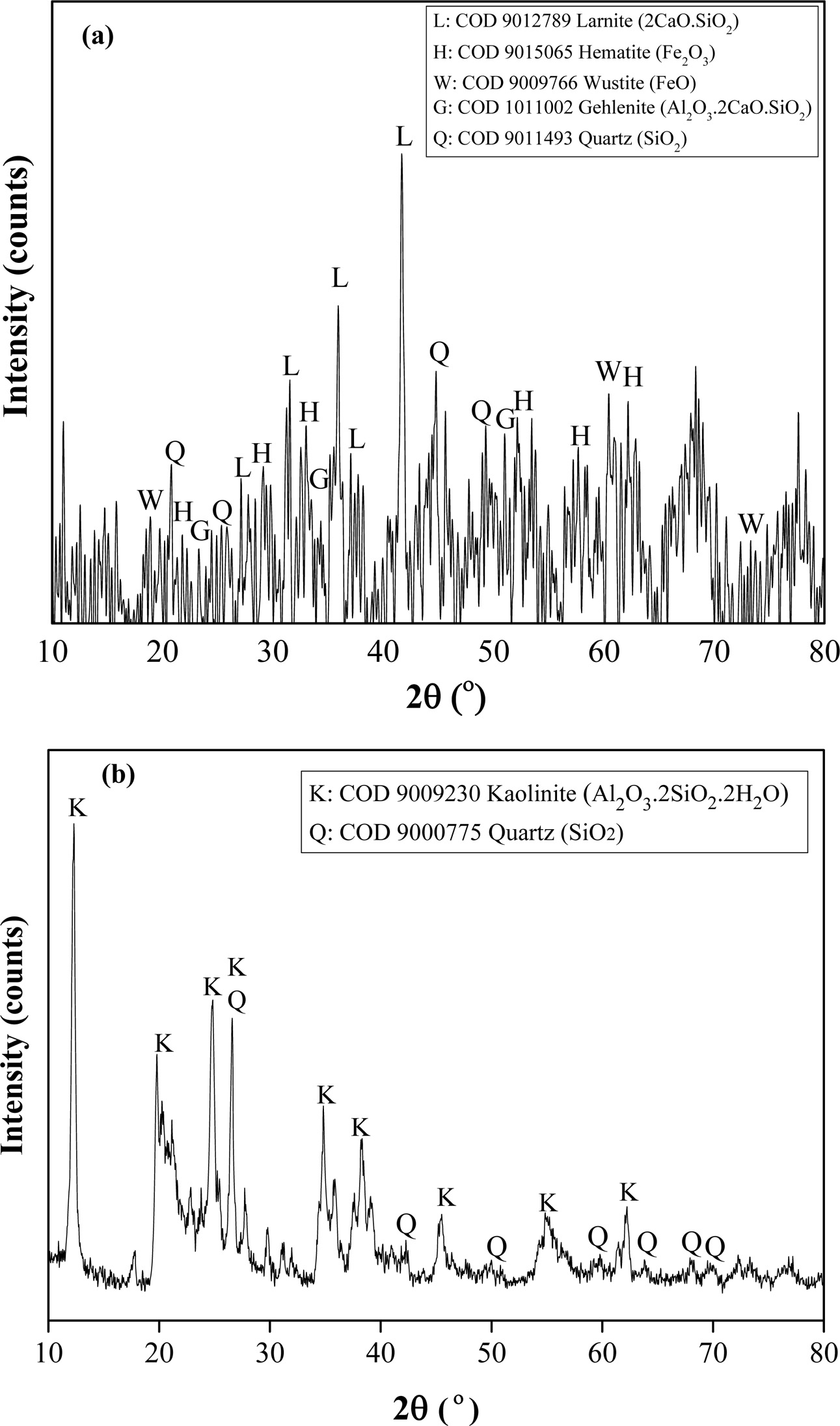
|
Fig. 2 XRD pattern of (a) EAF slag and (b) China clay. |
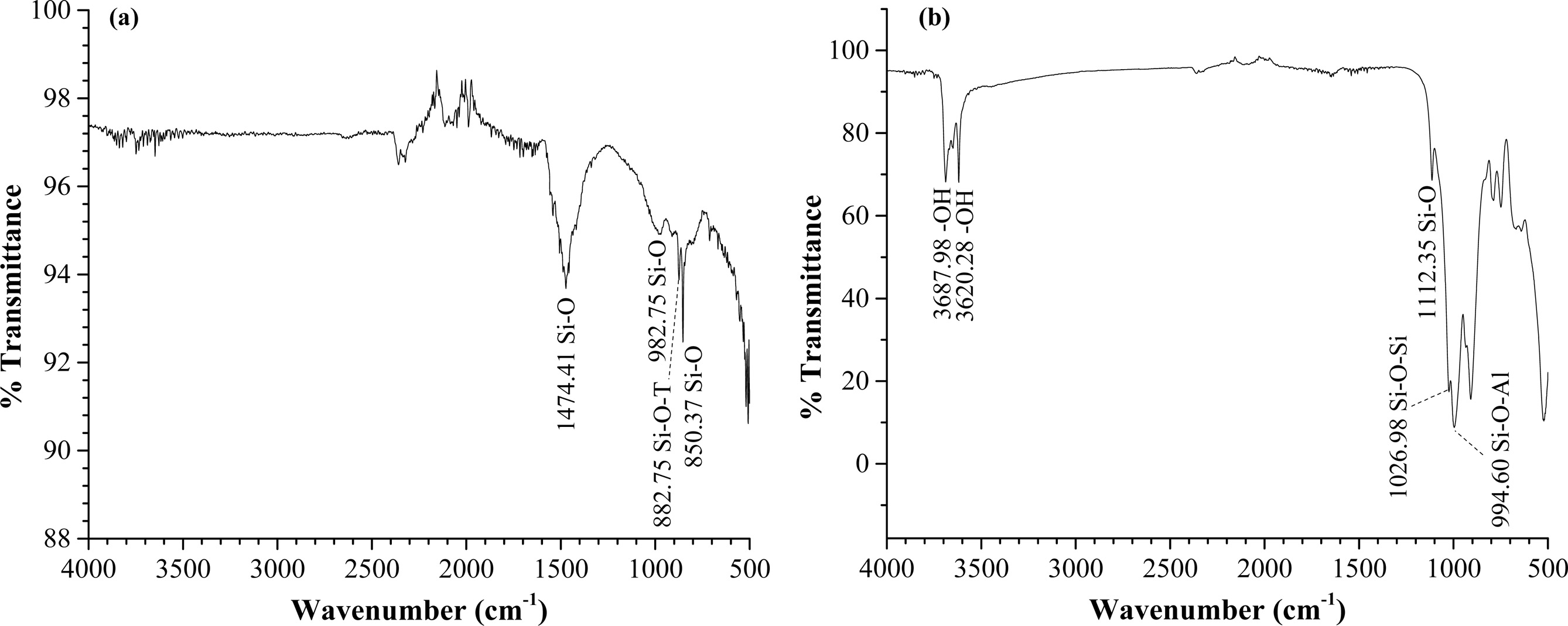
|
Fig. 3 FTIR Spectra of Geopolymer Ceramic (a) Composition 1 and (b) Composition 2. |
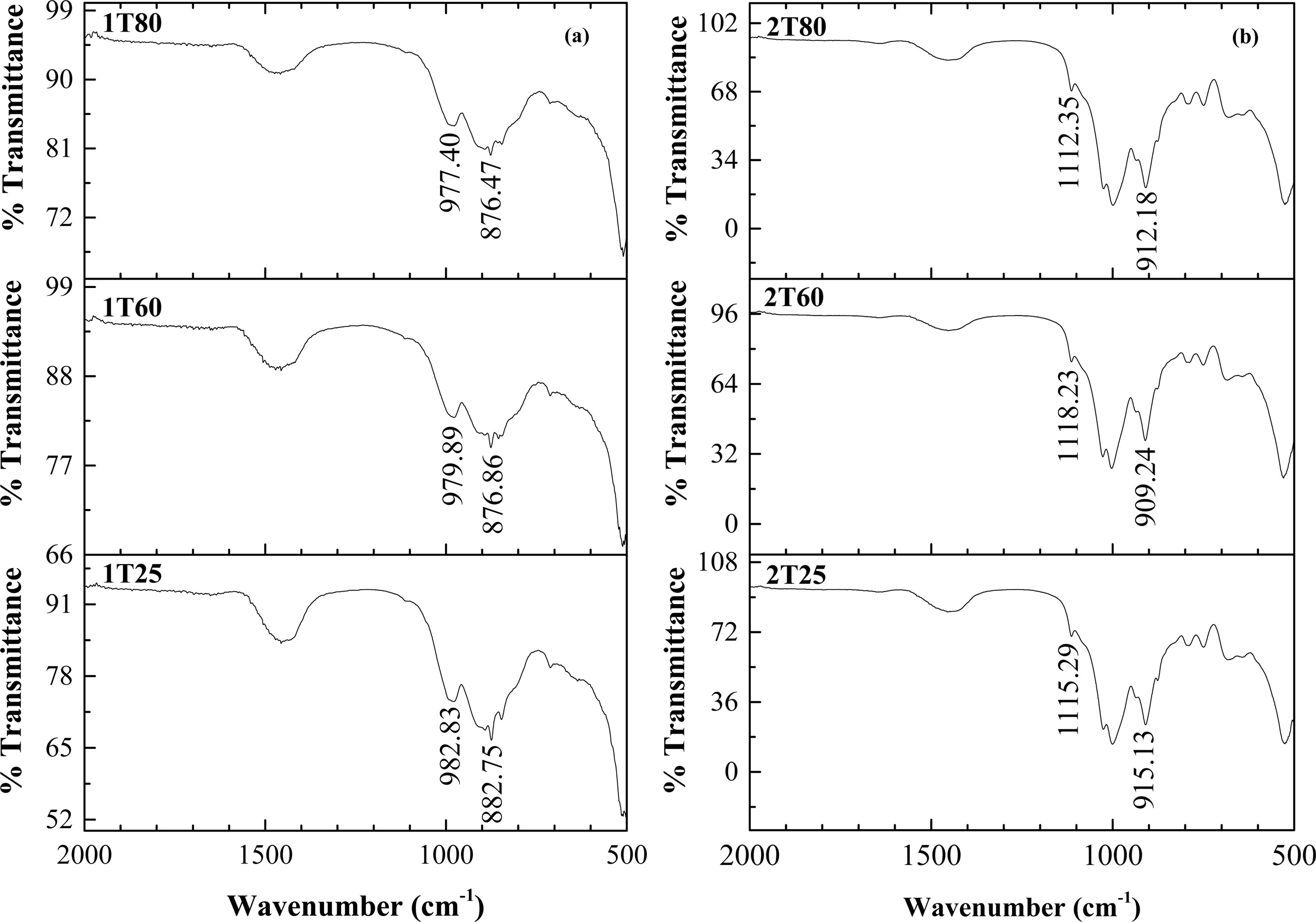
|
Fig. 4 FTIR Spectra of Geopolymer Ceramic (a) Composition 1 and (b) Composition 2. |
|
Fig. 5 Properties of EAF slag based geopolymer ceramic with (Composition 2) and without (Composition 1) presence of china clay (a) Water absorption, (b) Apparent porosity, (c) Bulk density and (d) Compressive strength. |
In overall, the results obtained in this work have proven the presence of geopolymer functional groups in the EAF slag based ceramic product. Thus, EAF slag is suitable to be utilized as one of raw materials for geopolymer ceramic product. Future study would focus on fine tuning of body formulation and curing parameter to further optimize and improve the physical (water absorption, apparent porosity and bulk density) and mechanical (compressive strength) properties of the EAF slag based geopolymer ceramic product.
The authors would like to thank Fundamental Research Grant Scheme (FRGS), Ministry of Higher Education Malaysia (Grant No: R/FRGS/A1300/01687A/00/2018/00549), as well as the Universiti Malaysia Kelantan’s Postdoctoral (Research) Scheme (Reference Number: Siti Zuliana Salleh_UMK.PC/B01/08/03/500-19/5/2), for the funding awarded to this project.
- 1. D. Gabaldón-Estevan, E. Criado, and E. Monfort, J. Clean. Prod. 70 (2014) 242-250.
-

- 2. S. Ghosh, M. Das, S. Chakrabarti, and S. Ghatak, Ceram. Int. 28[4] (2002) 393-400.
-

- 3. E. Karamanova, G. Avdeev, and A. Karamanov, J. Eur. Ceram. Soc. 31[6] (2011) 989-998.
-

- 4. Z. Bayer Ozturk, and E. Eren Gultekin, Ceram. Int. 41[9] (2015) 12020-12026.
-

- 5. S.R. Bragança, J. Vicenzi, K. Guerino, and C.P. Bergmann, Waste Manag. Res. 24[1] (2006) 60-66.
-

- 6. V. Karayannis, A. Moutsatsou, A. Domopoulou, E. Katsika, C. Drossou and A. Baklavaridis, J. Build. Eng. 14 (2017) 1-6.
-

- 7. R. Sokolar and L. Vodova, Ceram. Int. 37[7] (2011) 2879-2885.
-

- 8. N.U. Kockal, Boletín La Soc. Española Cerámicay Vidr. 51[5] (2012) 297-304.
-

- 9. N.U. Kockal, Ceram. Int. 41[10] (2015) 14529-14536.
-

- 10. M.M. Jordán, M.B. Almendro-Candel, M. Romero, and J.M. Rincón, Appl. Clay Sci. 30[3-4] (2005) 219-224.
-

- 11. I. Merino, L.F. Arévalo, and F. Romero, Waste Manag. 27[12] (2007) 1829-1844.
-

- 12. M. Montero, M. Jordan, M. Almendrocandel, T. Sanfeliu, and M. Hernandezcrespo, Appl. Clay Sci. 43[2] (2009) 186-189.
-

- 13. H. Badiee, A. Maghsoudipour, and B. Raissi Dehkordi, Adv. Appl. Ceram. 107[2] (2008) 111-115.
-

- 14. R. Sarkar, N. Singh, and S. Das Kumar, Bull. Mater. Sci. 33[3] (2010) 293-298.
-

- 15. P.-T. Teo, A.S. Anasyida, P. Basu, and M.S. Nurulakmal, Waste Manag. 34[12] (2014) 2697-2708.
-

- 16. P. Ter Teo, A.S. Anasyida, C.M. Kho, and M.S. Nurulakmal, J. Clean. Prod. 241 (2019) 118144.
-

- 17. P. Ter Teo, S.K. Zakaria, S.Z. Salleh, M.A.A. Taib, N. Mohd Sharif, A. Abu Seman, J.J. Mohamed, M. Yusoff, A.H. Yusoff, M. Mohamad, M.N. Masri, and S. Mamat, Metals (Basel). 10[10] (2020) 1347.
-

- 18. H. Fraser, Ceramic Faults and Their Remedies, A&C Black Ltd, London, UK, 1986.
- 19. C. Fernández Pereira, Y. Luna, X. Querol, D. Antenucci, and J. Vale, Fuel 88[7] (2009) 1185-1193.
-

- 20. Z. Sun, H. Cui, H. An, D. Tao, Y. Xu, J. Zhai, and Q. Li, Constr. Build. Mater. 49 (2013) 281-287.
-

- 21. S.K. Amin, S.A. El-Sherbiny, A.A.M.A. El-Magd, A. Belal, and M.F. Abadir, Constr. Build. Mater. 157 (2017) 610-620.
-

- 22. A. Graytee, J.G. Sanjayan, and A. Nazari, Ceram. Int. 44[7] (2018) 8216-8222.
-

- 23. Y. Rho, and S. Kang, J. Ceram. Process. Res. 21[S1] (2020) 74-80.
- 24. N.M. Piatak, M.B. Parsons, and R.R. Seal, Appl. Geo- chemistry 57 (2015) 236-266.
-

- 25. S.-G. Son, Y.-D. Kim, W.-K. Lee, and K.-N. Kim, J. Ceram. Process. Res. 14[5] (2013) 591-594.
- 26. H.S. Lim, H.S. Lee, and S.J. Kwon, J. Ceram. Process. Res. 20[4] (2019) 363-371.
- 27. S. Sorlini, A. Sanzeni, and L. Rondi, J. Hazard. Mater. 209-210 (2012) 84-91.
-

- 28. H. Madani, A.A. Ramezanianpour, M. Shahbazinia, and E. Ahmadi, Constr. Build. Mater. 247 (2020) 118441.
-

- 29. Z. Yunsheng, S. Wei, C. Qianli, and C. Lin, J. Hazard. Mater. 143[1-2] (2007) 206-213.
-

- 30. K.A. Komnitsas, Procedia Eng. 21 (2011) 1023-1032.
-

- 31. A. Dakhane, S.B. Madavarapu, R. Marzke, and N. Neithalath, Appl. Spectrosc. 71[8] (2017) 1795-1807.
-

- 32. C.L. Hwang, and T.P. Huynh, Constr. Build. Mater. 101 (2015) 1-9.
-

- 33. S.O. Sore, A. Messan, E. Prud’homme, G. Escadeillas, and F. Tsobnang, Constr. Build. Mater. 124 (2016) 301-311.
-

- 34. A.G. San Cristóbal, R. Castelló, M.A. Martín Luengo, and C. Vizcayno, Appl. Clay Sci. 49[3] (2010) 239-246.
-

- 35. J.G. van Jaarsveld, J.S. van Deventer, and G. Lukey, Chem. Eng. J. 89[1-3] (2002) 63-73.
-

- 36. M.M.A.B. Abdullah, L.Y. Ming, H.C. Yong, and M.F.M. Tahir, in: Cem. Based Mater., InTech, 2018.
-

- 37. S. Andini, R. Cioffi, F. Colangelo, T. Grieco, F. Montagnaro, and L. Santoro, Waste Manag. 28[2] (2008) 416-423.
-

- 38. C. Ferone, B. Liguori, I. Capasso, F. Colangelo, R. Cioffi, E. Cappelletto, and R. Di Maggio, Appl. Clay Sci. 107 (2015) 195-204.
-

- 39. K. Traven, M. Češnovar, and V. Ducman, Ceram. Int. 45[17] (2019) 22632-22641.
-

- 40. X. Huang, L. Yu, D.W. Li, Y.C. Shiau, S. Li, and K.X. Liu, Mater. Res. Innov. 19 (2015) S10-413-S10-419.
-

 This Article
This Article
-
2021; 22(3): 333-339
Published on Jun 30, 2021
- 10.36410/jcpr.2021.22.3.333
- Received on Oct 21, 2020
- Revised on Dec 16, 2020
- Accepted on Dec 29, 2020
 Services
Services
- Abstract
introduction
methodology
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Pao Ter Teo
-
Faculty of Bioengineering and Technology, Universiti Malaysia Kelantan, Jeli Campus, 17600 Jeli, Kelantan, Malaysia
Tel : +609-9477427 Fax: +609-9477402 - E-mail: teopaoter@umk.edu.my






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.