- Effects of ZrO2 on the properties and dry-pressing preparation of mullite based crossing network structure
Weixia Dong*, Yan Liang, Qifu Bao, Xingyong Gu and Jing-wei Yang
Department of Materials Science and Engineering, Jingdezhen Ceramic Institute, Jingdezhen 333001, P. R. China
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Mullite based crossing network structure were dry-pressing prepared by using waste gangue and calmogastrin as the main raw material. Effects of ZrO2 on the properties of the samples were studied. The samples were characterized by X-ray diffraction, scanning electron microscopy and the corresponding energy dispersive analysis. The experiment results showed that when ZrO2 content was 20%, the sample reached 123.21 MPa of bending strength, 123.75% of bending strength retention rate and the coefficient of thermal expansion of 5.023×106/oC-1. It could be attributed to the crossing network structure of the mullite, which produced a lot of pore porosity to accommodate a certain expansion deformation and thus alleviate the heat stress. Moreover, micro cracks around the porosity can be formed in the main crack tip region, which decreases local elastic strain energy and restrains the crack propagation and thus enhances the materials properties
Keywords: Waste gangue, Mullite based crossing network structure, ZrO2, drying preparation, Bending strength
Mullite materials have been widely used in metallurgy, chemical industry, petroleum, machinery manufacturing, energy and other industrial areas [1-3]. Its performance improvements play an important role in the comprehensive development, the quality and energy consumption of the products [1]. With the development of ceramics, metallurgy and building materials industries, mullite industries keep a good growth momentum. However, due to the mining, processing and technical level, the comprehensive utilization of resources is not high. Especially, the mineral resources e.g. the high-quality raw material resource are more and more scarce. How to saving and comprehensive utilization of the resources have been an important topic. Therefore, the rational utilization of natural mineral raw materials, such as powder ore, low grade ore, etc have become an important target to realize the sustainable development of mullite industry [2, 3].
Mullite is a kind of typical silicon aluminium silicate, and the typical chemical composition is 3Al2O3·2SiO2. Due to its high melting point, low linear expansion coefficient, high creep activation energy, excellent thermal shock stability and corrosion resistance, etc., mullite has achieved outstanding importance as a material for both traditional and advanced ceramics. However, mullite phase exist very rarely in nature due to its high-temperature and low-pressure conditions. In recent years, mullite material are prepared by high-temperature solid phase sintering method [3]. However, high temperature sintering makes the mullite grains grow continuously, resulting in a decrease in the mechanical properties and initial properties of the sample. At the same time, when the temperature drops sharply, a large stress occurs between mullite crystals because of the different linear expansion direction of mullite, which causes the destruction of the sintered body. Therefore, the toughening of mullite ceramics is particularly important. Toughened mullite ceramics can improve the performance of single-phase mullite ceramics to a certain extent, thereby improving its bending strength, thermal shock resistance and other properties [4, 5].
In this paper, mullite based networking structures are dry-pressing prepared by adding zirconium oxide (ZrO2) using waste gangue as the main raw materials. ZrO2 exhibit high fracture toughness and bending strength at room temperature [6, 7]. Moreover, ZrO2 particles react with silicon oxides to form the ZrSiO4 crystals filled in the pore between acicular and long mullite crystals, which is helpful for the bending strength and thermal shock resistance of the samples. Therefore, in order to improve the bending strength and thermal shock resistance of mullite materials, ZrO2 are introduced in mullite networking structure. Effects of zirconium oxide contents on the properties of mullite based networking structure are discussed.
Sample preparation
The chemical composition of waste gangue (%): Al2O3 26.68, SiO2 65.21, TiO2 1.24, K2O 3.16, Na2O 0.43, CaO 0.53, Fe3O4 0.24, IL 16.7. The other chemicals are analytical grade. The mullite precursor composition was prepared by mixing 83.38% waste gangue and 30.54% Al(OH)3 with the addition of mineralizers (2% V2O5 and 3% AlF3). Batches were prepared by drying mill for 30 min. The grinding material was alumina ball. The sieved powder was to pass 120 mesh and granulated with 6% water, then aged for 24 h. The processed powder was uniaxially pressed under 20 MPa in a steel mold. The samples were put in the oven to dry at 90 oC, followed by placing them on the alumina rollers of an electric furnace to heat at 1,400 oC with a heating rate of 3 oC/min for 1 h. Samples with rectangular shape of 70×10×10 mm were prepared for the bending strength test (Scheme 1).
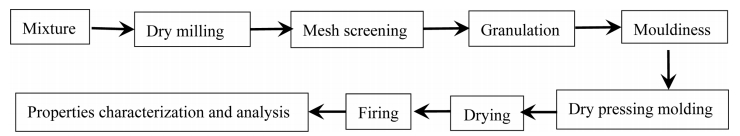
Scheme 1. Illustration of the synthesis process.
Characterization
The bending strength of the rectangular-shaped tested samples were characterized by Test Machine (Shenzhen Reger Instrument Co. Ltd. China). The crystal phase of the samples were characterized by X-ray diffraction (XRD, Bruker D8-Advance, German) in a 2q range from 5o to 70o by using Cu-Kα radiation (λ = 0.15420 nm) operating at 50 kV and 40 mA. The investigation of the microstructures and compositions of the sample powders was done by field emission scanning electron microscopy (FESEM, JSM-6700F, Japan) with an energy dispersive X-ray spectroscopy (EDS) system operating at an accelerating voltage of 5.0 kV or 15.0 kV. Etching solution like hydrofluoric acid solution was used to remove the glassy surface of the fired sample, so that the morphology of the crystal can be revealed. Moreover, RPZ-01 (PANalytical B.V., Holland) was used to analyze the thermal expansion coefficient of the samples. The sintering properties of the fired samples were evaluated based on the Archimedes principle. Thermal shock resistance was characterized by analyzing high bending strength of the samples. The process is as follows: the samples were placed in the furnace heating up to the required temperature of 1,400 oC. After 60 min, the samples are quickly taken from the furnace and then naturally cool in air. After cooling, the high-temperature bending strength of the samples are characterized. The bending strength retention rate of the samples (%) are calculated by means of the following equation [8]:

P0 is the bending strength of the fired sample at room temperature, Pt is the bending strength of the sample fired at 1,400 oC for 60 min.
Effects of ZrO2 on the phase of mullite based crossing network structure
Fig. 1 shows XRD patterns of the samples with different ZrO2 contents. Without the addition of ZrO2, the pure mullite (JCPDS15–0776) is obtained. With the increase of ZrO2 contents, the peak intensities of mullite phase at 25.96o decrease, however, those of ZrSiO4 phase (JCPDS72–0402) and a small amount of m-ZrO2 (JCPDS 03–0515) increase, indicating ZrSiO4 phase and a small amount of m-ZrO2 increase, which is due to the increase of ZrO2 contents. It can be seen from A12O3-SiO2 phase diagram [9] that the sample is composed of SiO2 and mullite solid solution. The formation of ZrSiO4 is due to the reaction with the abundant SiO2 and ZrO2, which will be later discussed in the next section.
Effects of ZrO2 on the microstructure of mullite based crossing network structure
Fig. 2 shows the SEM images of the sample with the addition of different ZrO2 contents. Fig. 2(a, b) shows the sample is composed of well-developed mullite columnar with crossing network structure, which provides an enough interspace for the growth of mullite without the addition of ZrO2. When ZrO2 content is 20%, the sample exhibits well-developed mullite columnar structure (Fig. 2(c)). From the enlarged SEM image (Fig. 2(d)), many uniform particles fill in the pores due to the formation of the mullite crossing network. Increasing ZrO2 content to 25%, the mullite columnar is poor, and the uniform particles increase and cluster together, the sample is dense which is harmful for the growth of mullite (Fig. 2(e, f)). And there are some isolated spherical pores existing in the fractured surface of the samples, which indicate the existence of the liquid phase and the viscous flow mechanism [8]. The existence of viscous flow can also be verified from the EDS analysis (Table 1) and SEM images (Fig. 3).
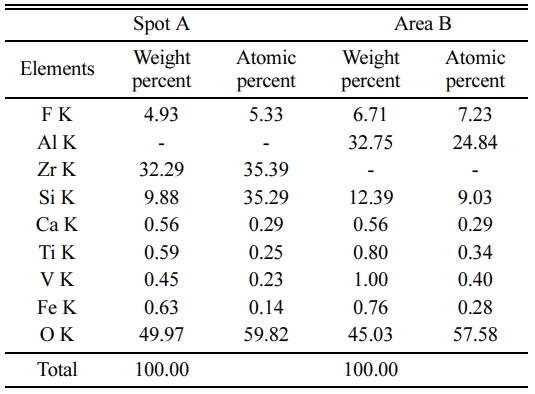
Fig. 3 EDS spectrum of the sample with the addition of 20% ZrO2. It can be seen that the columnar particles contain Ca, Fe, F, V, Ti, Al, Si and O elements, Zr elements are not detected. However, small particles are only composed of Ca, Fe, F, Zr, V, Ti, Si and O elements. Combining with XRD and EDS results, it could be concluded that columnar particle maybe be mullite phase [6], and small particles maybe be ZrSiO4 and m-ZrO2 phase. To further confirm the composition with different shape particles, element quantitative analysis for A and B spots are analyzed as shown in Table 1. The corresponding EDS spectrum shown in Table 1 reveal that the spot A for columnar particle consist of Al and Si contents with an atomic ratio of about 3:2, which are close to the stoichiometry of mullite, and the spot B for small particles consist of Zr and Si contents with an atomic ratio of about 1:1, which are close to the stoichiometry of ZrSiO4. The glass around the mullite crystals and ZrSiO4 contains elements of Ca, Fe, F, V, Ti, indicating the glass phase forms from a liquid containing impurities at firing temperature, which may be from the composition of waste gangue, V2O5 and AlF3. The liquid phase significantly increases by extensive incorporation of liquid during firing at 1,400 oC, while the viscosity of liquid phase favors the growth of mullite crystals and promotes the formation of ZrSiO4 particles due to the reaction with ZrO2 and SiO2 (Fig. 1) via viscous flow mechanism, which is similar with our previous report [8]. Thus a positive effect on the bending strength of the sample is exerted, conversely, In addition, the interlocking texture constructed by mullite crystals also favors the bending strength of the samples.
Effects of ZrO2 on the porosity and bending strength of mullite based crossing network structure
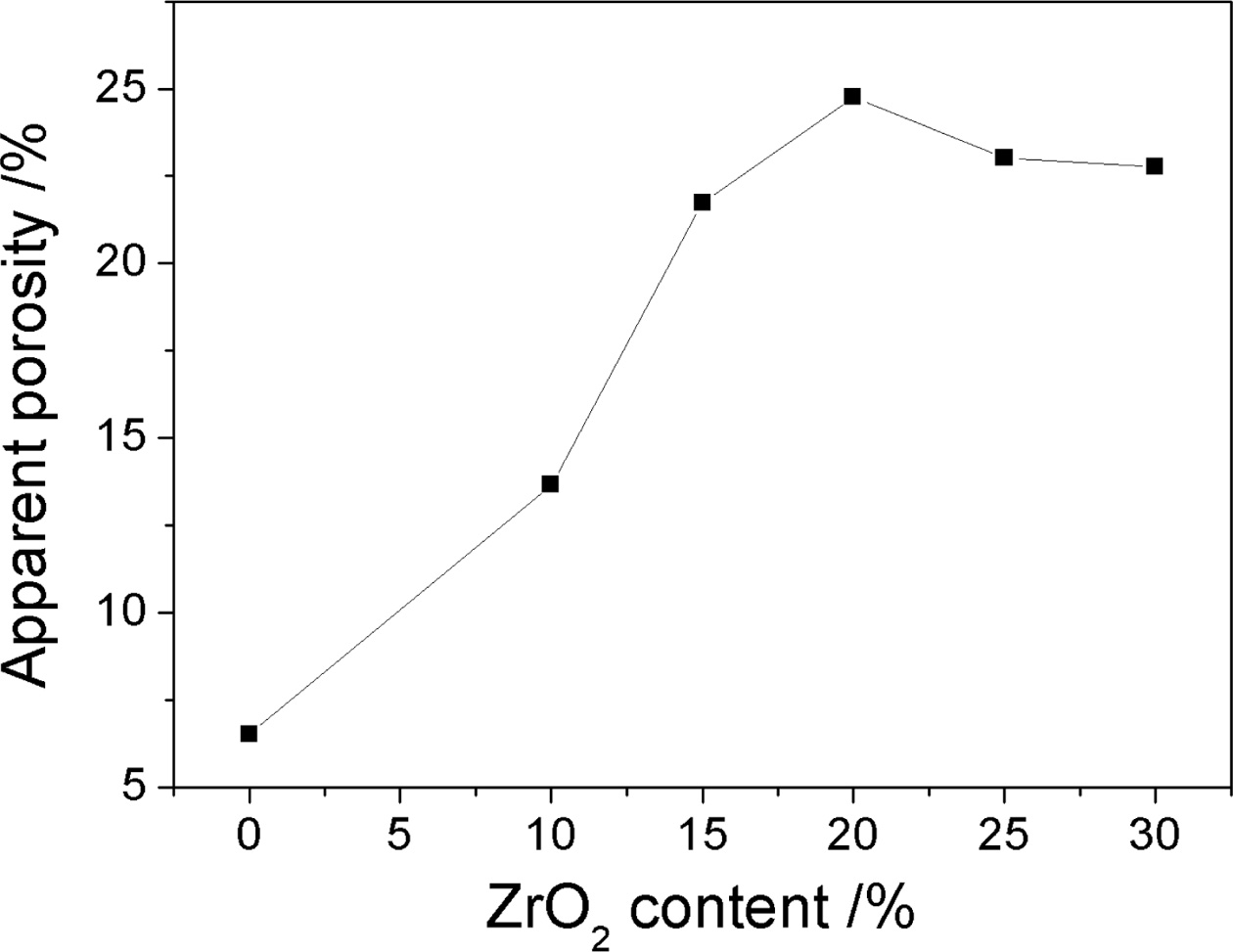
Fig. 4 shows the porosity curves of the samples with different ZrO2 contents. It can be obviously seen that the porosity of the sample increases with the increase of ZrO2 contents, and when ZrO2 contents are more than 15%, the increase in the porosity of the sample becomes slowly. Zirconia toughening is used to monoclinic phase into a tetragonal zirconia inducing microcrack toughening, which is one of the three kinds of toughening mechanisms. After adding zirconia, zirconium oxide would transform from the monoclinic zirconia to tetragonal zirconia at 1,170 oC with around 7% of the volume change during the transformation and thus makes the samples produce many tiny micro cracks, which toughen the properties of the material [11, 12]. At the same time, the binary or multiple liquid produced from the reaction among the impurity e.g., CaO, TiO2 and Fe2O3 of waste gangue, AlF3, V2O5, Al2O3 and SiO2 [13, 14], which is helpful for the growth of the network structure constructed by long and accular mullite crystals (Fig. 2). So the porosity of the samples increased gradually. Further increasing zirconia contents to 25%, zircon phases increase (Fig. 1(d)), then grow up and gather themselves together, and thus prevent the growth of the mullite. Therefore mullite columnar crystal is short and the network structure of mullite decreases (Fig. 2(e, f)), and thus the densification degree of the sample increases, which are consistent with XRD and SEM results, so the porosity of the sample decreases.
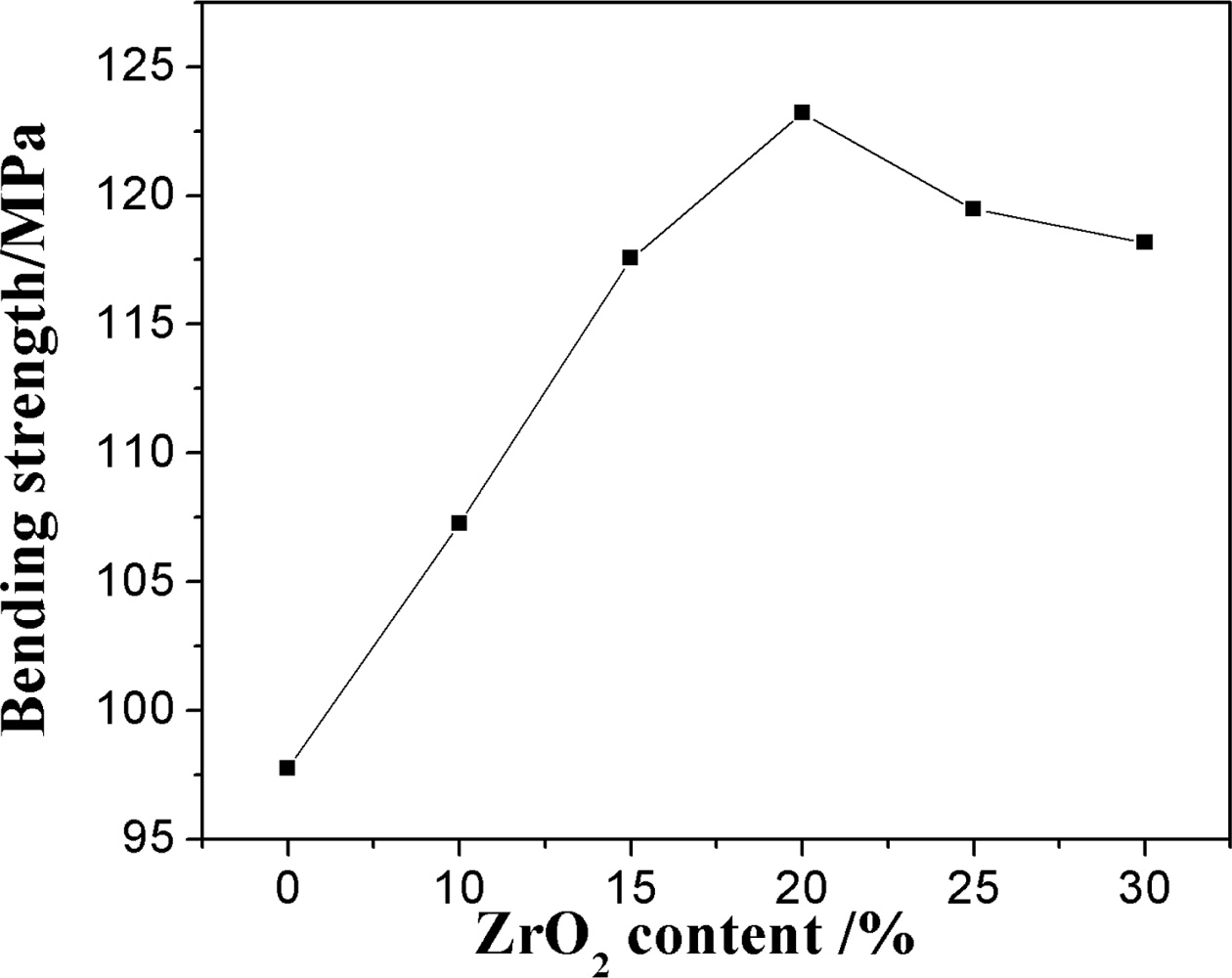
Fig. 5 shows the bending strength of the sample with the various ZrO2 content. The bending strength of the samples increases with the increase of ZrO2 contents firstly and then decreases. It is surprising that the sample prepared by adding 20% ZrO2 has the maximum value of 123.21 MPa, although the porosity of the sample by adding 20% ZrO2 is higher than that of the others (Fig. 4). ZrO2 content affects the bending strength of the samples by controlling the growth of network structure constructed by mullite crystals, crystallinity, the micro cracks based on the phase transition of zirconia, and porosity of the samples. It is known that the the transfor- mation of zirconia reduces some micro cracks formation, which consumes its elastic energy consumption and thus improves the bending strength of the samples [17]. For the growth of network structure constructed by mullite crystals, with the increase of ZrO2 content (Fig. 2), well-developed mullite columnar with crossing network structure become poor and the mullite crystals decrease, the defects of the crystal increase, thus the bending strength of the samples decreases. As ZrSiO4 phase and m-ZrO2 cystallinty increase keeping the mullite almost unchanged, more ZrSiO4 crystals filled in the interspace of the mullite networking, and the transformation of zirconia, which improved the the bending strength of the samples [17]. On the other hand, as for the porosity, it is common that the fracture surface energy of the samples tends to decrease with the increase of the porosity and the pore exists as a defection, so the samples with the smaller porosity have the larger bending strength. However, it is worth pointing out that in the presence of the high stress gradient due to the the zirconia transformation in the present experiment, the pore between the mullite networking could accommodate the deformation and prevent crack propagation, which improves the bending strength of the samples [16]. Accordingly, when ZrO2 content is ≤ 20%, the increase of bending strength can be ascribed to the enhancement of the ZrSiO4 phase and m-ZrO2 crystallnity, well-developed mullite columnar with crossing network structure and increase of porosity with increasing ZrO2 content (Fig. 2, Fig. 3 and Fig. 4). However, when ZrO2 is more than 25%, the ZrSiO4 crystallinity and porosity decrease dramatically (Fig. 1(d) and Fig. 4), lots of ZrSiO4 particles incline to aggregate itself and grow into continuous beaded structure, which would destroy the mullite network (Fig. 2(e, f)) and decrease the porosity of the sample, so the bending strength of the sample decreases.
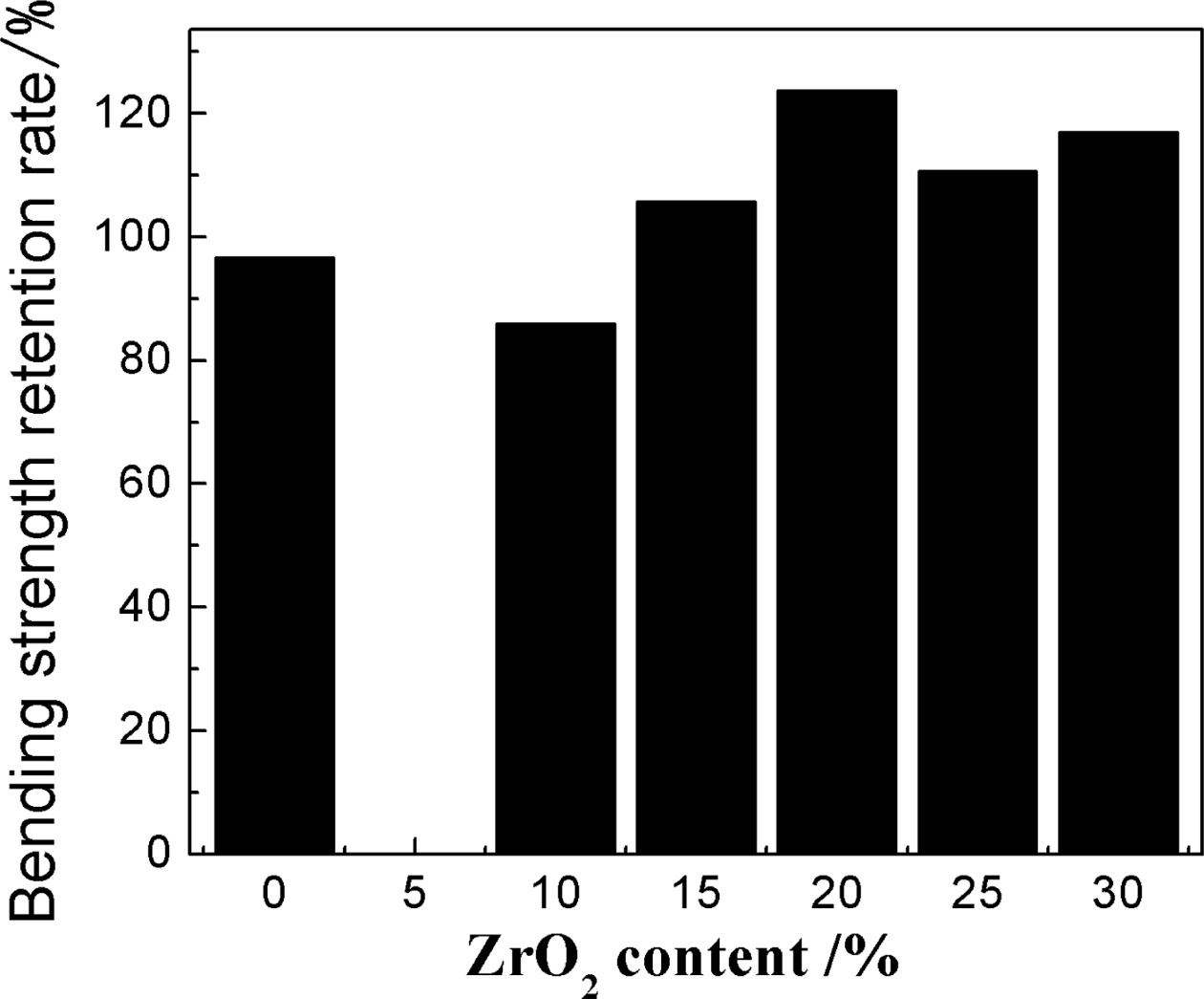
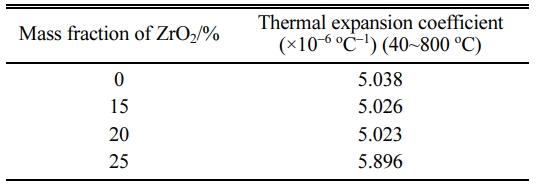
Fig. 6 shows the bending retention analysis diagram of the sample with the various addition of ZrO2. Table 2 shows the thermal expansion coefficient (40~800oC) of the samples with the addition of 20% ZrO2 and without ZrO2. It can be seen that from Fig. 6 and Table 2, with the increase of ZrO2 contents (≤ 20%) obviously enhanced the thermal shock resistance of the samples and lower the thermal expansion due to the micro cracks based on the phase transition of zirconia, the increased zircon content and porosity of the samples (Fig. 5). When ZrO2 contents is 20%, the bending retention strength of the sample is 123.75% and the coefficient of thermal expansion decrease. It is well known that thermal expansion coefficient of zircon, zirconia and mullite is 4.51×106/oC-1, 10.3×10-6/oC-1 and 5.32×106/oC-1, respectively [18]. After adding ZrO2, from the result of XRD, it is obvious that zircon is produced which is higher than m-ZrO2 content (Fig. 1). Although the thermal expansion coefficient of zirconia is large, based on experimental result (Table 2), it indicates that when ZrO2 contents is suitable (≤ 20%), the micro cracks due to the phase transition of zirconia, the increased zircon content and porosity of the samples are dominated as main factors for lowering the thermal expansion coefficient in our experiment. Therefore, the thermal expansion coefficient of the samples is lowered. When the thermal expansion coefficient of the sample is smaller, the volume change of materials caused by temperature is smaller and thus the corresponding temperature stress is smaller and thermal shock resistance is better, therefore, the bending retention of the sample is more than that of the sample without ZrO2. At the same time, it maybe attribute to microcracks origined in the changes in the volume of the trans- formation of zirconia crystal, and mullite with the well-perfect interlaced network structure produced a lot of porosity which could accommodate a certain expansion deformation and thus reduce the thermal stress. In common, porosity is found in other intergranular and micro cracks are slightly more originated on the grain boundary or near other microstructure defects, hence porosity can form local network in the main crack tip region, which decreases local elastic strain energy and restrains the crack propagation, thus improves the material bending retention [9-14]. However, further increasing ZrO2 content, due to decreased porosity and poor mullite interlaced network structure of the samples, the large thermal expansion coefficient of zirconia is dominated as main factors, Thus, the thermal expansion of the samples increases.
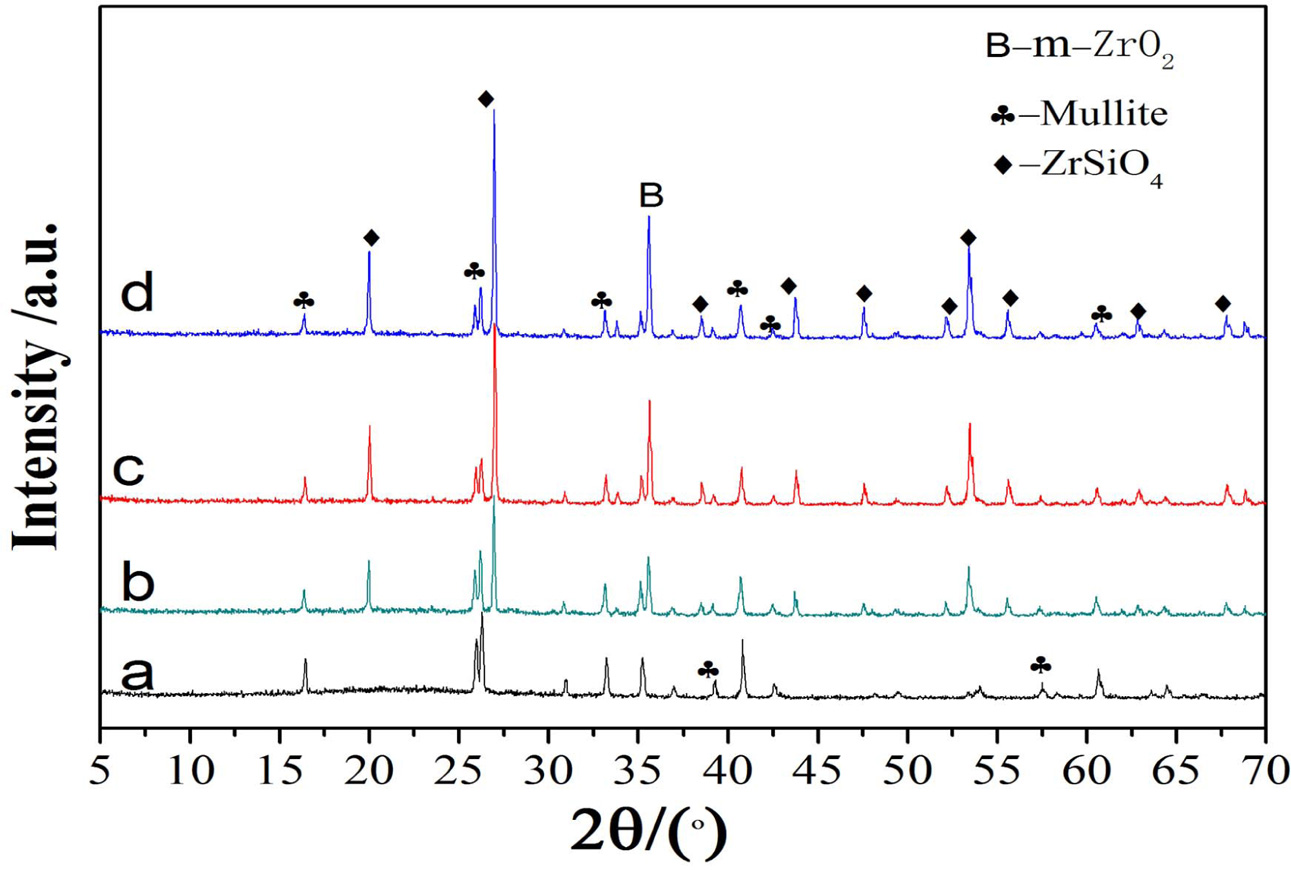
|
Fig. 1 XRD patterns of the samples with various zirconia contents: (a) 0%, (b) 10%, (c) 20%, (d) 25%. |
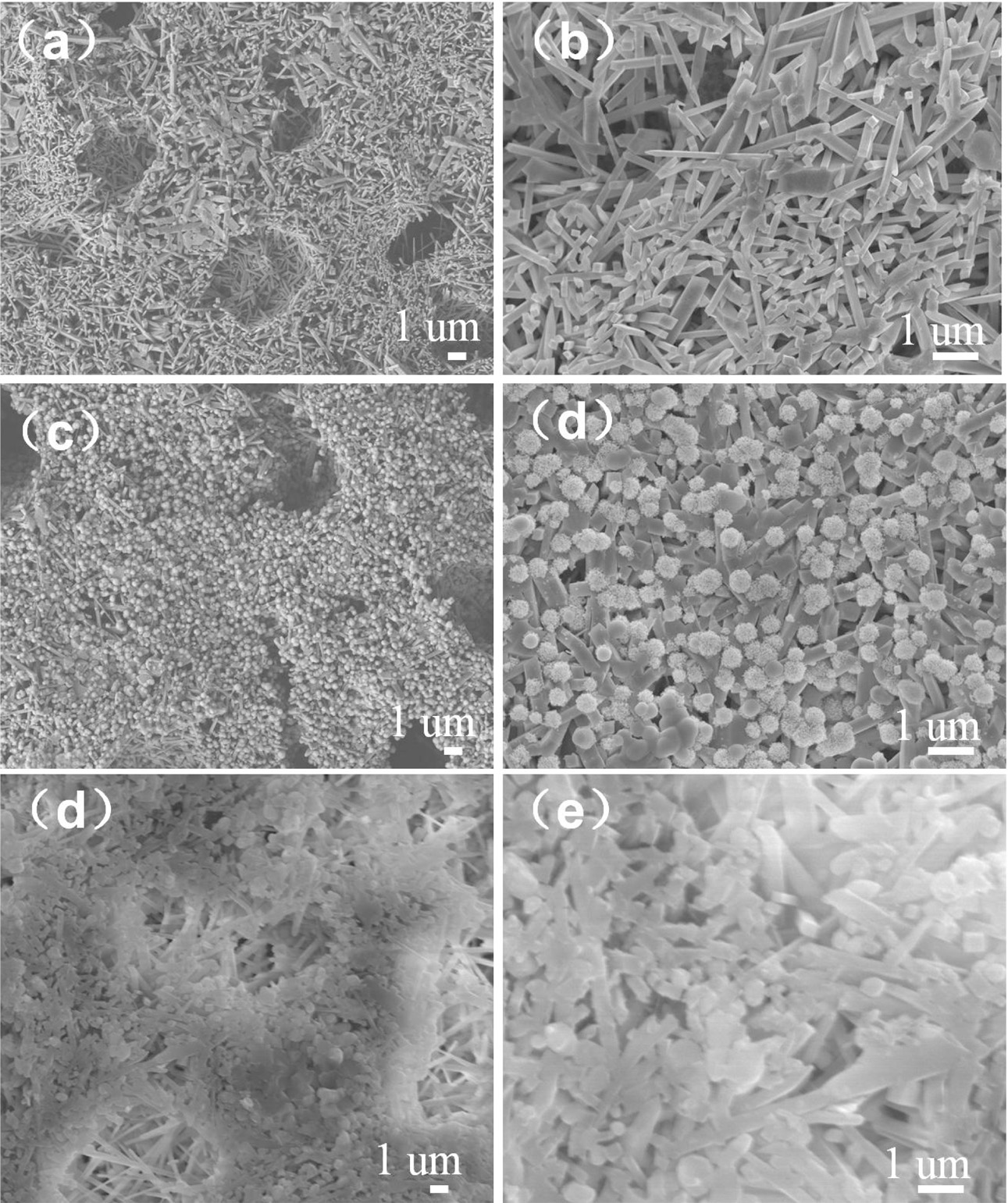
|
Fig. 2 SEM images of the samples with different zirconia: (a, b) 0%, (c, d) 20%, (e, f) 25%. |
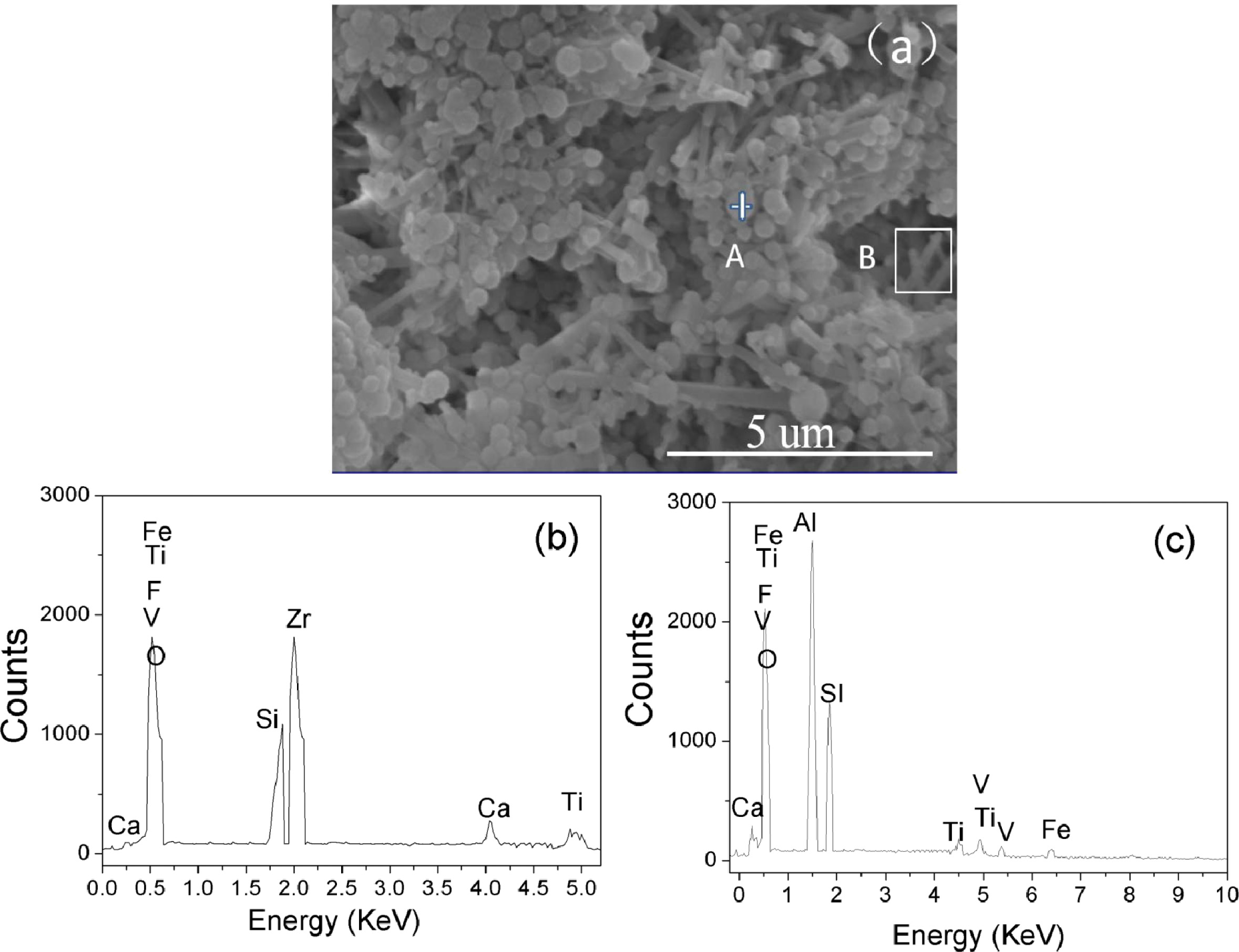
|
Fig. 3 EDS spectra of the sample with 20% ZrO2: (b) Spot A in Fig. 3(a), (c) Area B in Fig. 3(a). |
|
Fig. 4 Apparent porosity curves of the samples with different zirconium oxide. |
|
Fig. 5 Bending strength curves of the samples with different zirconium oxide. |
|
Fig. 6 Bending strength retention rate curves of the samples with different zirconium oxide. |
|
Table 2 Thermal expansion coefficient of the samples doped with zirconium oxide. |
Mullite based networking structure was dry-pressing prepared by adding ZrO2 using waste gangue and aluminium hydroxide as the main raw material. XRD pattern shows that with the increase of amount of ZrO2, mullite phases decrease, however, zircon and m-ZrO2 increase. The porosity, bending strength and the retention bending strength of the samples firstly increase and then decrease, and the thermal expansion coefficient decreases. When the zirconia content is 20%, the bending strength of 123.21 MPa, retention bending strength of 123.75%, thermal expansion coefficient of 5.023×106/oC-1 of the sample obtain the optimum values. It indicated that moderate ZrO2 content could improve the performance of the mullite based material. SEM images exhibited that appropriate amount of ZrO2 (20%) was conducive to form ZrSiO4 particles filling into gaps in a network structure, and thus enhanced toughening effect. It will be a good application prospect as a refractory material.
The present work was supported by Educational Major Project of Jiangxi Province in China (GJJ190707 and GJJ201301).
- 1. M. Zhao and J. Liang, J. Gra. Natur. Sci. Med. 33 (2012) 17-19.
- 2. D. Xu and S. Wang, Chn. Steel. 5 (2011) 11-15.
- 3. H. Schneider, J. Schreuer, and B. Hildmann, J. Eur. Ceram. Soc. 28[2] (2008) 329-344.
-

- 4. K. Cui, Y. Zhang, T. Fu, J. Wang, and X. Zhang. Coatings. 10[7] (2020) 672.
-

- 5. C. Sadik, I. Amrani, and A. Albizane. J. As. Ceram. Soc. 2[2] (2014) 83-96.
-

- 6. M. Hamidouche, N. Bouaouadja, H. Osmani, R. Torrecillias, and G. Fantozzi, J. Eur. Ceram. Soc. 16[4] (1996) 441-445.
-

- 7. J. Guo and P. Zhu, J. CHN. Ceram. Soc. 1 (1996) 7-17.
- 8. X. Gu, P. Li, W. Dong, and T. Luo, Key. Eng. Mater. 602-603 (2014) 628-631.
-

- 9. G. Li and C. Zhang, in “Phase basement and refactory material diagrams” (Metallurgical Industry Press, 1994) p.1.
- 10. W. Dong, Q. Bao, X. Gu, H. Shen, and J. Yang, J. Ceram. Soc. Jpn. 125[2] (2017) 75-78.
-

- 11. N.M. Rendtorff, L.B. Garrido, and E.F. Aglietti, Ceram. Int. 34[8] (2008) 2017–2024.
-

- 12. N.M. Rendtorff, L.B. Garrido, and E.F. Aglietti, Ceram. Int. 35[2] (2009) 779-786.
-

- 13. A. Gungor, O. Celikcioglu, and S. Sahin, Ceram. Int. 38[5] (2012) 4189-4194.
-

- 14. T.M. Souza, A.P. Luz, M.A.M. Brito, and V.C. Pandolfelli, Ceram. Int. 40[1] (2014) 1699-1707.
-

- 15. N.M. Rendtorff, L.B. Garrido, and E.F. Aglietti, Mat. Sci. Eng. A 498[1-2] (2008) 208-215.
-

- 16. S. Xia and X. Zhu, Refract. 4 (1998) 192-194.
- 17. G.T.M. Stam, E.V.D .Giessen, and P. Meijers, Int. J. Sol. Struct. 31[14] (1994) 1923-1948.
-

- 18. I. Yu. Prokhorov, G.Y. Akimov, and V.M. Timchenko, Refract. Ind. Ceram. 39[5] (1998) 189-197.
-

 This Article
This Article
-
2021; 22(3): 317-322
Published on Jun 30, 2021
- 10.36410/jcpr.2021.22.3.317
- Received on Sep 25, 2020
- Revised on Nov 25, 2020
- Accepted on Dec 4, 2020
 Services
Services
- Abstract
introduction
experimental process
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Weixia Dong
-
Department of Materials Science and Engineering, Jingdezhen Ceramic Institute, Jingdezhen 333001, P. R. China
Tel : +86-571-8480007 Fax: +86-798-8480007 - E-mail: weixia_dong@sina.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.