- Preparation and characterization of zirconia ceramics with oxides addition
A. Rittidech*, S. Wantrong and S. Chommi
Department of Physics, Faculty of Science Mahasarakham University, Mahasarakham 44150, Thailand
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This research investigated the relationship between the quantitative phase crystal structure and mechanical properties of ZrO2 ceramic addition of Y2O3, MgO and BaCO3 at 0.0, 2.0, 4.0 and 6.0 mol%. Ceramic samples were prepared using mixed oxide method under normal sintering at 1,600 oC with dwell time for 120 min . ZrO2-Y2O3 and ZrO2-MgO ceramics were obtained with bulk densities values between 5.324-5.722 g/cm3 while ZrO2-BaCO3 ceramic showed densification values about 4.412-4.827 g/cm3. It was found that ZrO2-Y2O3 and ZrO2-MgO ceramic showed higher fracture toughness values than ZrO2-BaCO3 ceramics. Refinement of lattice parameter using Rietveld analysis in ceramic samples revealed the percentage of fraction phase ratios of m-ZrO2, t-ZrO2 and c-ZrO2. The refinement parameters result in sample ceramic which are Y2O3 addition between 4-6 mol% obtained a high ratio of t-ZrO2 phase and the result supported optimal mechanical properties. ZrO2-Y2O3 ceramic showed a higher lattice stain value compared with the additions of other ZrO2 oxides and it was found that lattice strain increase with high ratio of t-ZrO2 phase. Sample ceramics had crystallite size values between 56.65-82.30 nm. SEM micrographs revealed morphology and average grain sizes. All samples grains were spherical in shape combined with irregular shape and were gray in color and were obtained with an average grain size between 0.63 -2.18 µm. It was found that the ZrO2-Y2O3 ceramic showed small crystallize size and size of grains. The optimal condition for addition of oxide were found in ceramics of ZrO2-Y2O3 and ZrO2-MgO and confirmed that good mechanical properties were obtained from a high ratio of t-ZrO2 phase and fine grain size
Keywords: ZrO2, Y2O3, MgO, BaCO3, Rietveld refinement
ZrO2 has attracted extensive attention for decades because it exhibit excellent characteristics such as low thermal conductivity and good mechanical properties under high temperature conditions. These properties have led to the use of zirconia-based components in many applications such as automobile engine part, medical devices and cutting tool. Zirconia is a polymorphic metastable material that exists in three crystallographic phases: monoclinic phase (up to 1,170 oC), tetragonal phase (1,170-2,370 oC), and cubic phase (2,370-2,680 oC) [1]. Zirconia is generally stable at room temperature in monoclinic phase. Zirconia in the tetragonal form at room temperature has the best mechanical properties among the forms [2]. The tetragonal (t-ZrO2) to mono- clinic(m-ZrO2) martensitic transformation occurring during cooling after sintering is detrimental to sintered zirconia integrity as this process is accompanied by a large increase in volume, leading to disintegration by crack formation and propagation [3]. The high temperature polymorphs of pure zirconia cannot be retained by quenching to room temperature [4]. Many reports are attempt to synthesize for a metastable tetragonal phase formation in the zirconia at low temperature [5-7].
Stabilizing oxides such as CaO, Co, Y2O3 and MgO are used to stabilize in the cubic and tetragonal zirconia form at room temperature. Previous works reported [8-10] effects of zirconia ceramic stabilizing oxides on the phase structure, microstructure and mechanical properties. The mechanism of t-ZrO2 to m-ZrO2 transition is typical of partially stabilized zirconia and commonly supported by many studies of the transformation strength process, which has been widely, discussed [11, 12]. However, the study of the relationship of quantitative analysis in crystal zirconia stabilizing oxides ceramic with XRD patterns on its characteristics is of interest to study.
Therefor the aim of this research is to investigate effects of oxides doping on characterization of zirconia ceramics such as physical properties, phase composition, crystalline structure, microstructure, mechanical properties. Rietveld refinement was used to calculate significant quantities in crystal to describe the relationship between crystal structure and ceramics properties.
Powders with ZrO2 addition of Y2O3, MgO and BaCO3 at 0.0, 2.0, 4.0 and 6.0 mol% were prepared from ZrO2, MgO, Y2O3 and BaCO3 as precursors and isopropyl alcohol as solvent. All the ten different batches were then ball milled for 24 h. After ball-milling, drying in electronic furnaces and sieving with 120 mesh, the resulting powders were calcined at 1,200 oC, with dwell times for 120 min and heating/cooling rates of 10 oC/min. The powders were then pressed at 3 MPa into form pellets having 1.5 cm diameter using a hydraulic press and sintered in an alumina crucible at a temperature of 1,600 oC for 120 min with heating rate of 10 oC/min. The sintering experiments were carried out in an electrical furnace (Nabertherm, Germany). The bulk densities of sintered sample were calculated using Archimedes’ method and measured percentage of linear shrinkages. Phase identification was then performed using an X-ray diffractometer (XRD; Bruker model D8 advance). Next, Rietveld refinement of the XRD patterns of all samples was carried out by TOPAS software. Microstructural analysis was performed by using Scanning Electron Microscopy (SEM) (JEOL JSM-840A) of sintered samples on a polished surface. The micro hardness of the bulk ceramics was measured using a micro scan from Vickers and Knoops (FM- 700e type D, Future Tech., Japan).
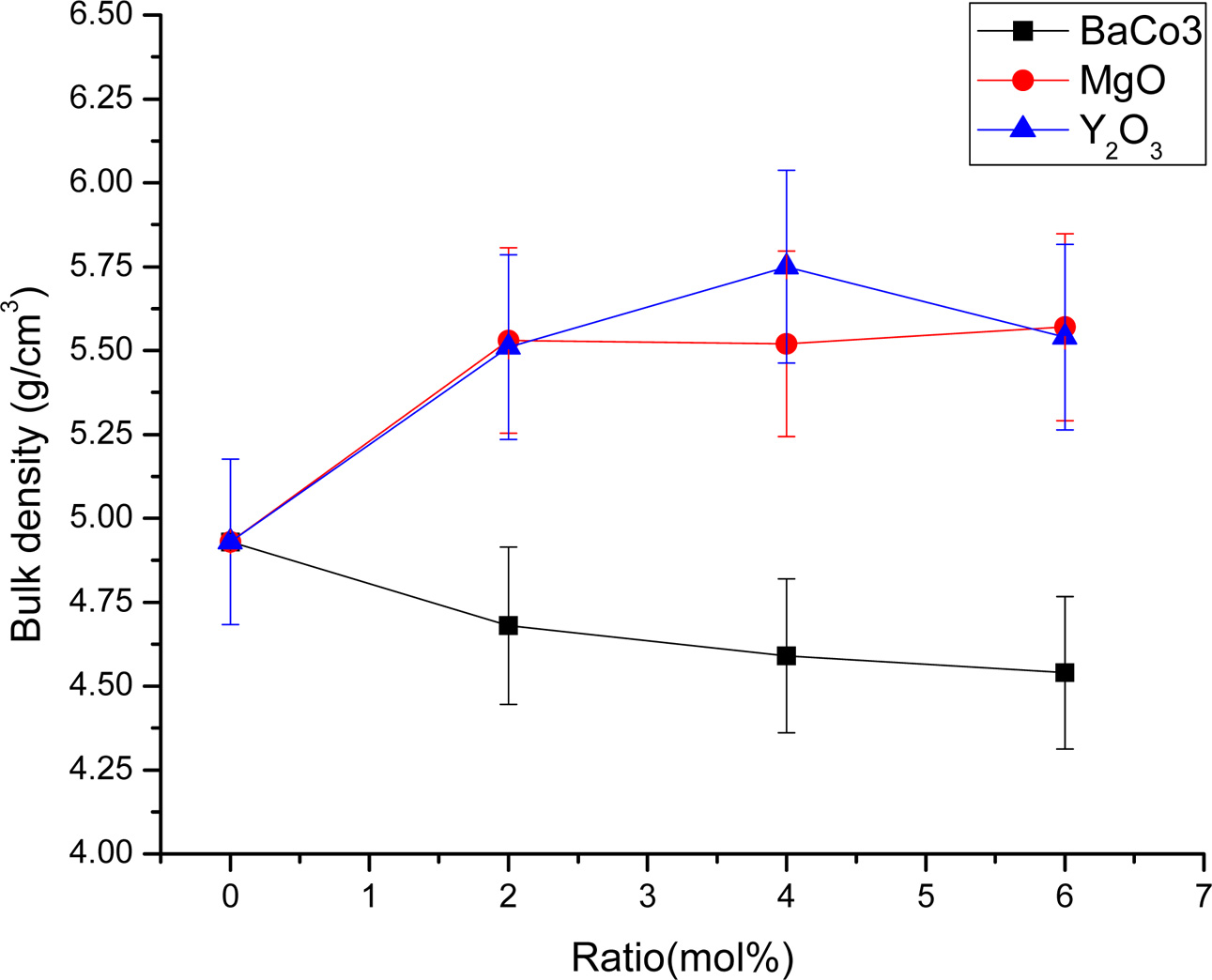
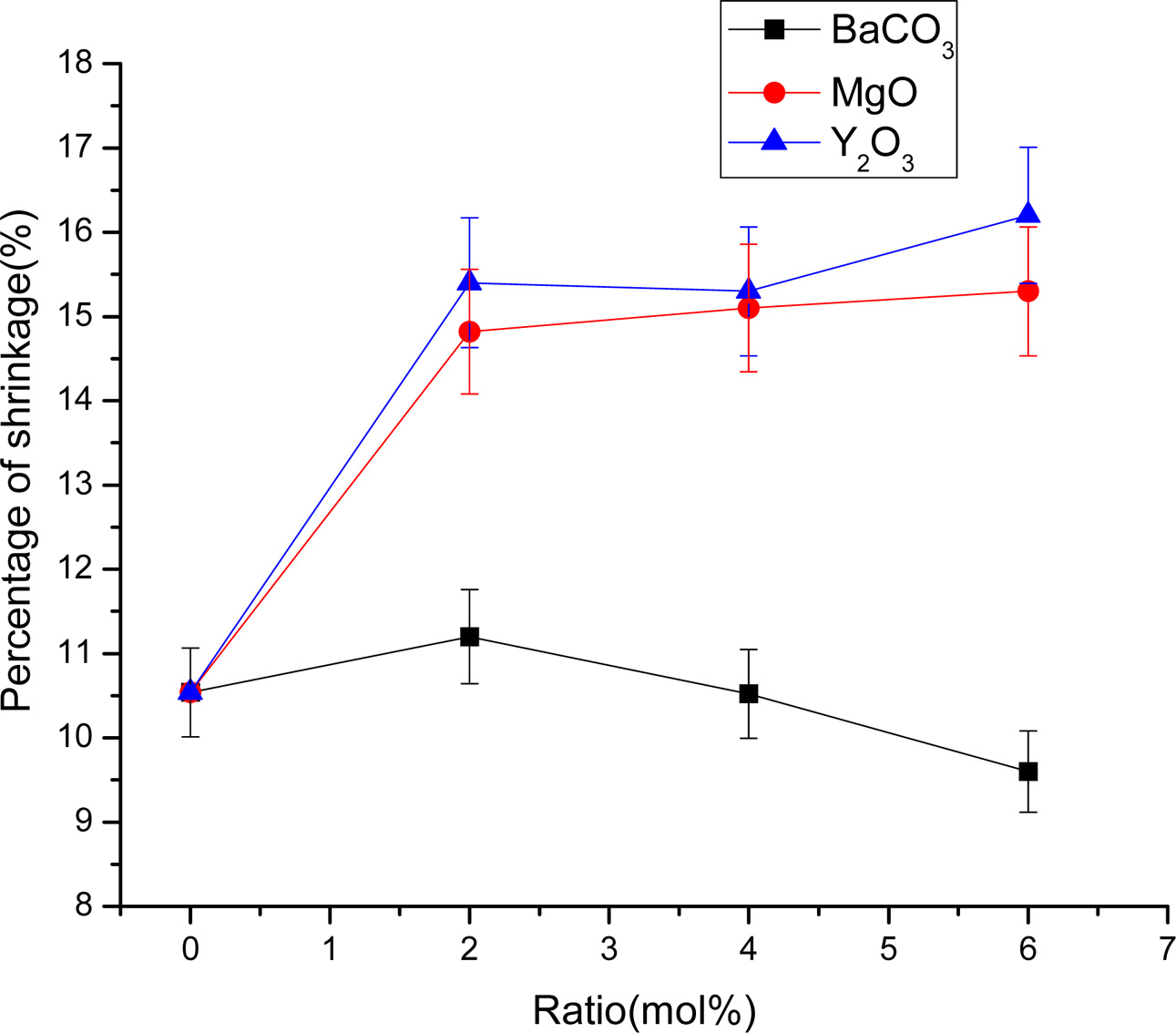
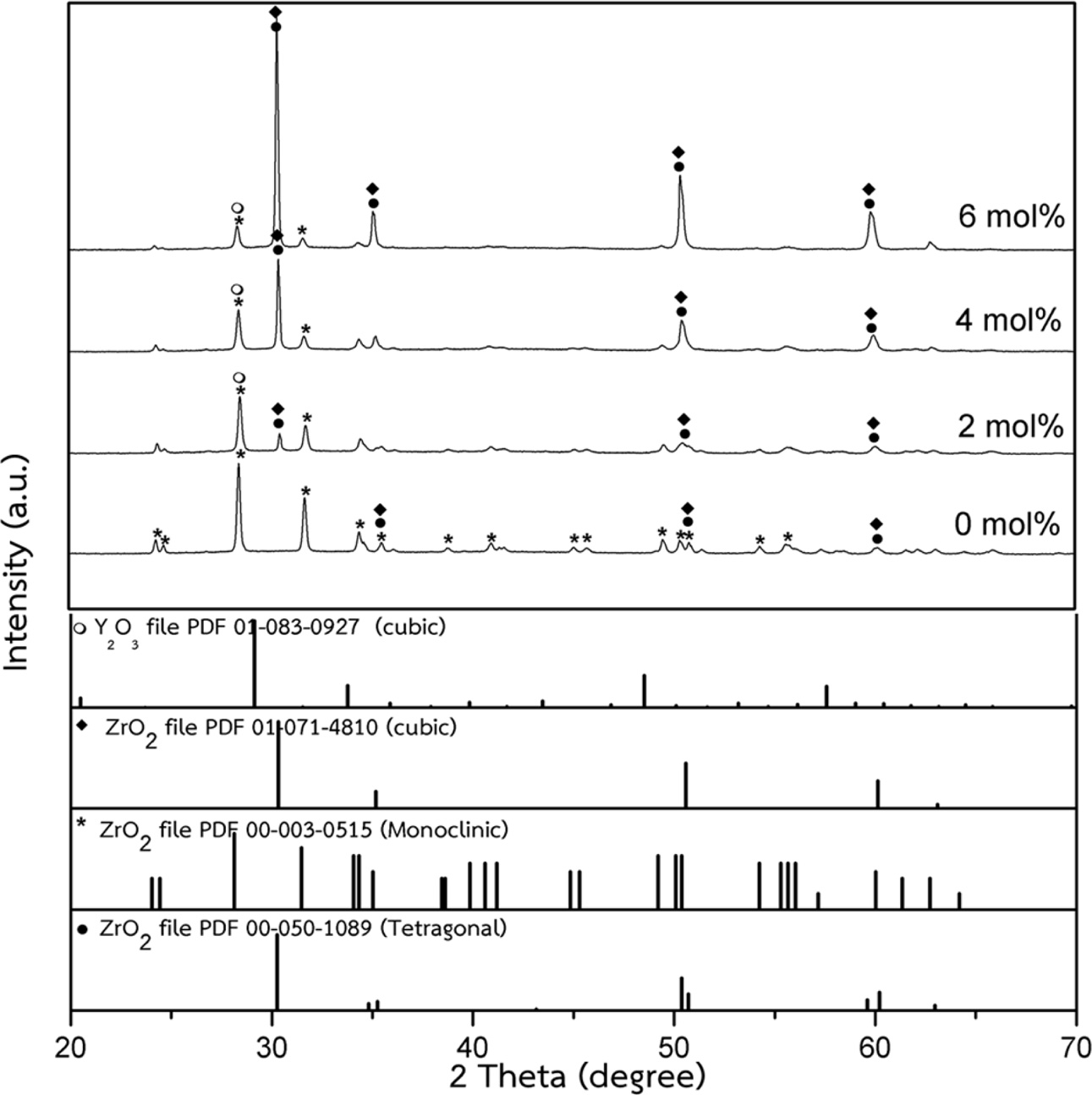
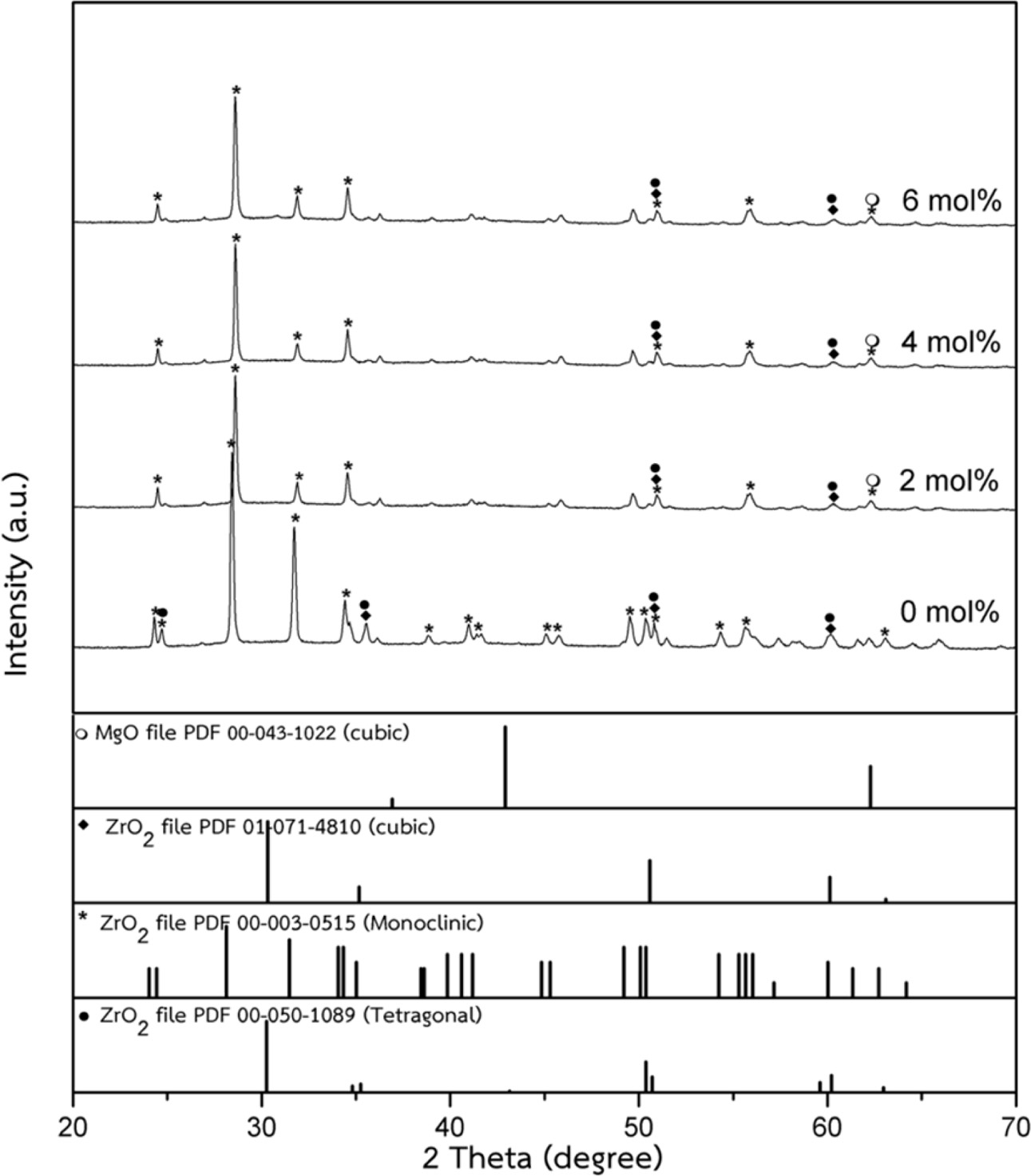
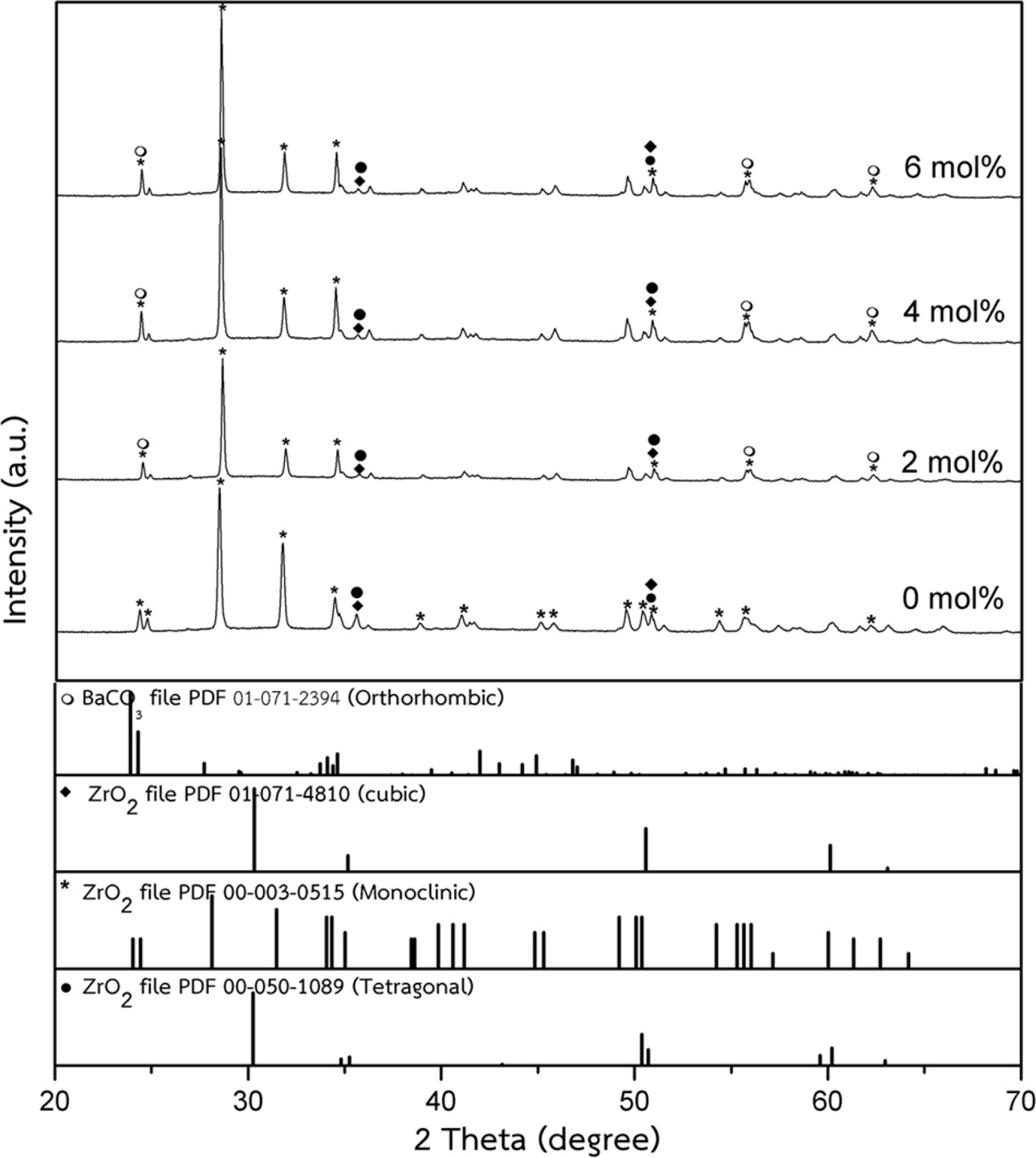
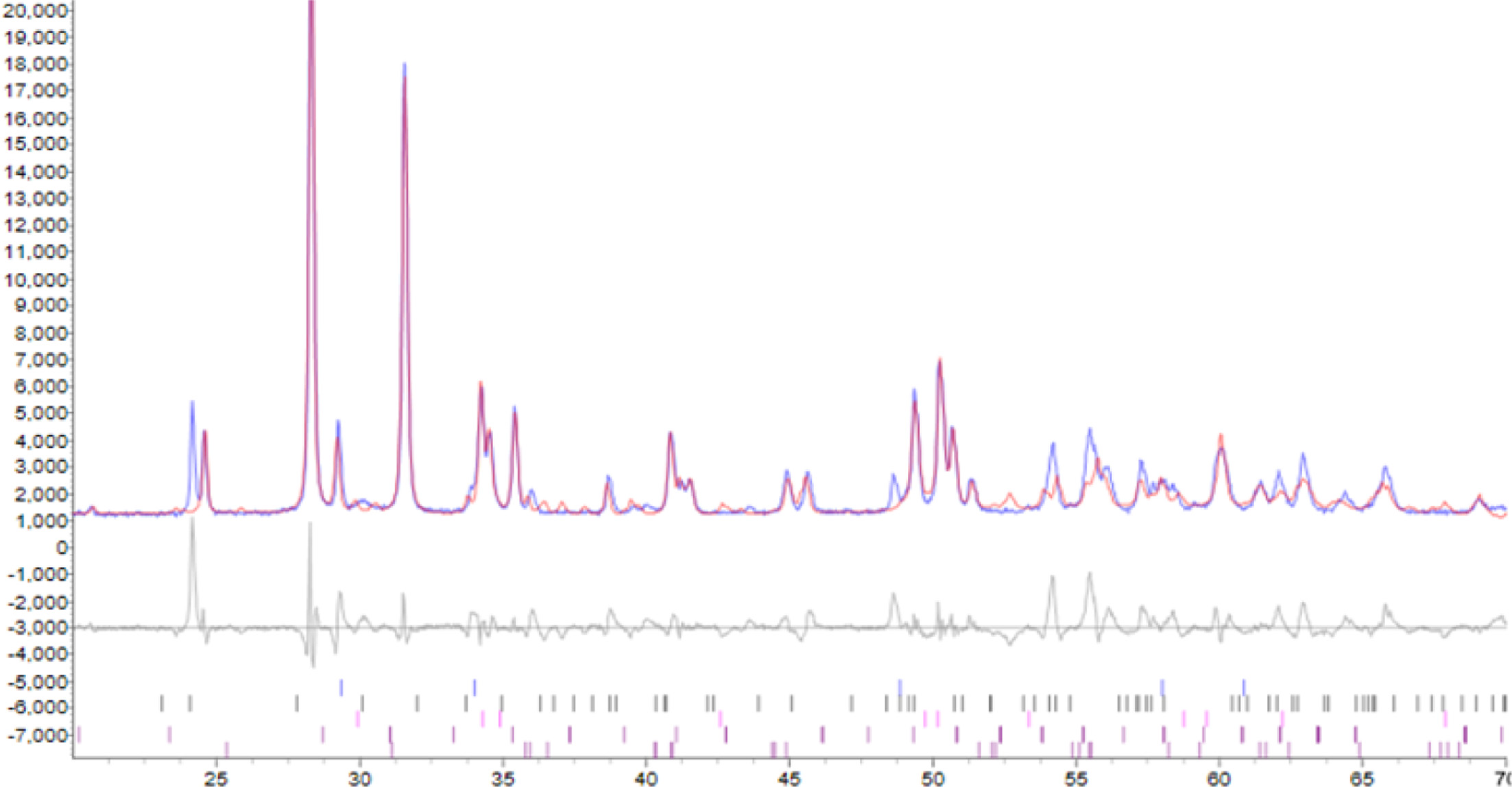
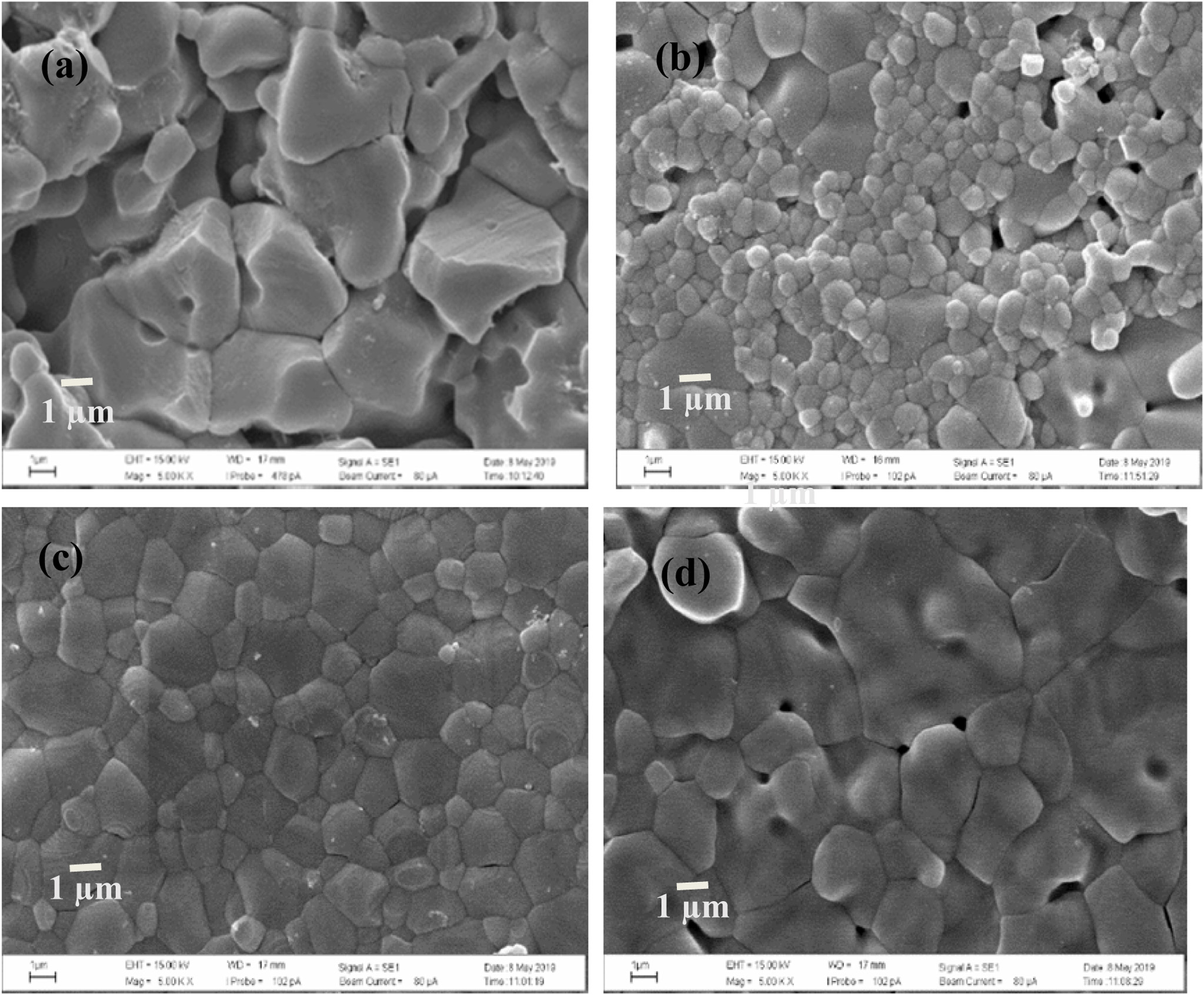
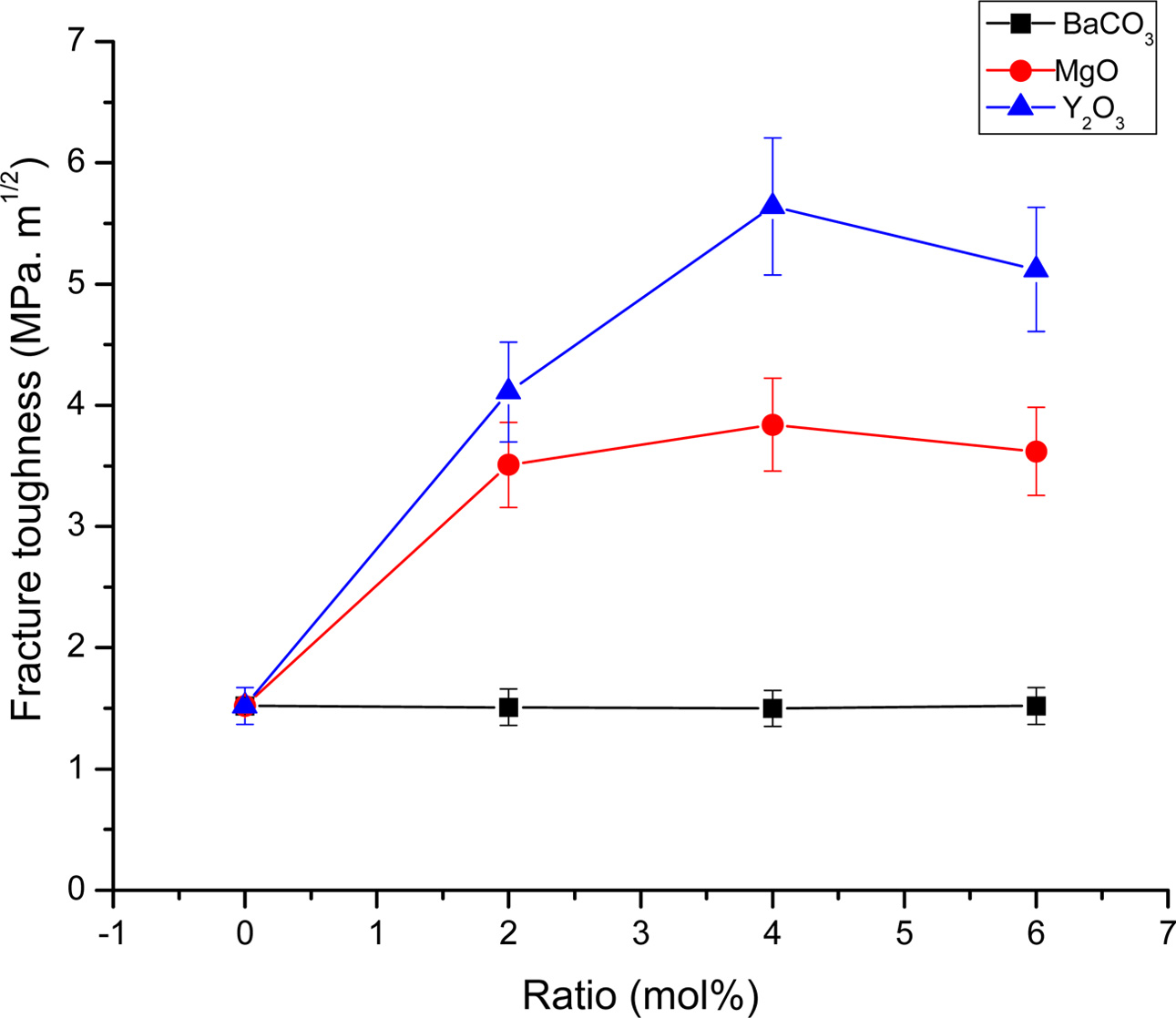
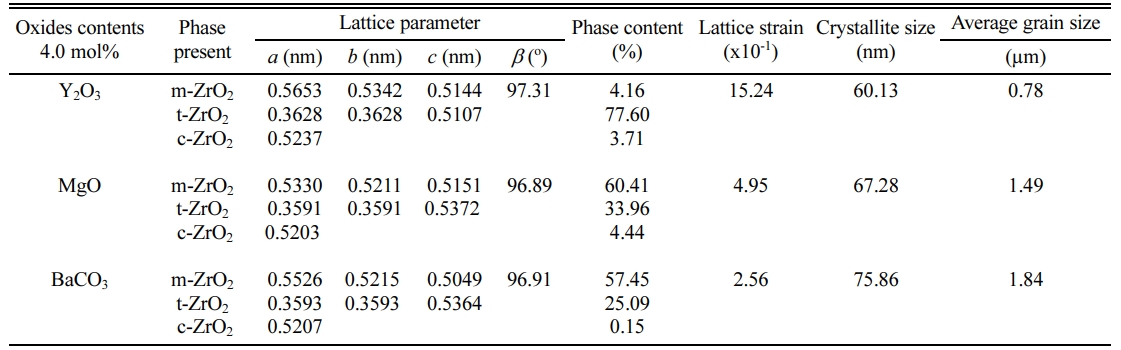
The densification and shrinkage of ceramic samples which were doped with the three oxides are shown in Fig. 1 and 2. ZrO2 ceramics with addition of Y2O3 at 0.0, 2.0, 4.0 and 6.0 mol% showed a density of between 4.93 and 5.51 g/cm2 and percentage of linear shrinkage values between 10.54 and 14.52. ZrO2 ceramics with addition of MgO at 0.0, 2.0, 4.0 and 6.0 mol% showed a density of between 4.93 and 5.75 g/cm2 and percentage of shrinkage values between 10.54 and 16.01. ZrO2 ceramics with addition of BaCO3 at 0.0, 2.0, 4.0 and 6.0 mol% were showed a density of between 4.54 and 4.93 g/cm2, percentage of shrinkage values between 9.60 and 10.54. Densities value of ZrO2 ceramics with addition of Y2O3 and MgO tended to increase with increasing added oxides while density values of ZrO2 ceramics with addition of BaCO3 tended to decrease with increasing BaCO3 content. This result may be due to the different ionic radius of Ba and Zr, therefor making it difficult for Ba2+ substitution of Zr4+. The XRD patterns of ZrO2 ceramics sample which were doped with the three oxides are shown in Fig. 3, 4 and 5. The three figures reveal the characteristic peaks of XRD patterns of ZrO2 ceramics with different oxides contents. Fig. 3 shows XRD patterns of ZrO2 ceramics with added 0.0-6.0 mol%Y2O3, which were identified using the main refraction of m-ZrO2 (011) at 24.4o, (-111) at 28.2o, (111) at 31.45o, (020) at 34.70o and t-ZrO2 (101) at 30.41o, (002) at 34.81o, (110) at 35.10o and c-ZrO2 (010) at 30.10o. Fig. 4 shows XRD patterns of ZrO2 ceramics with added 0.0-6.0 wt% MgO, which were identified as the main refraction of m-ZrO2 (011) at 24.4o, (111) at 31.45o, (020) at 34.70o and t-ZrO2 (101) at 35.10o. Fig. 5 shows XRD patterns of ZrO2 ceramics with added 0.0-6.0 mol% BaCO3, which are identified using the main refraction of m-ZrO2 (011) at 24.4o, (-111) at 28.2o, (111) at 31.45o, (020) at 34.70o and t-ZrO2 (110) at 35.10o. The results indicated the appearance of ZrO2 polymorphous crystalline phase consistent with previous reports [8, 13, 14]. The intensity of ZrO2 peaks in three polymorphous are the result of different oxides addition at varies proportions of dopants. It was found that XRD patterns in Fig. 4 and Fig. 5 are nearly similar patterns. Full pattern matching refinement of XRD patterns was performed using the TOPAS program based on the Rietveld method to obtain more detailed information on crystallographic spectra of ZrO2 ceramics with three oxides addition (Y2O3 , MgO, BaCO3 ) using 0.0, 2.0, 4.0 and 6.0 mol% and selected sample with added 4.0 mol%Y2O3, shown as the XRD refinement pattern in Fig. 6. The fitted patterns of ZrO2 ceramics with added three oxides are in good agreement with the respective experiment data, denoted by Rp, Rwp and GOF factors listed below Table 1. ZrO2 ceramics with added 4.0 mol%Y2O3 had a larger lattice parameter compared to ZrO2 ceramics with added MgO and BaCO3 ,which may be attributed to substitution to Zr4+site due to the addition of substances with similar ion sizes in agreement with the studies of M.T. Vinas et al. [9], M. Borik et al. [10]. This is clearly seen from the result of the higher tetragonality (c/a) and strain lattice in ZrO2 ceramics with added Y2O3. Moreover, when considering the percentage ratio of tetragonal phase, it was obviously related to crystallize size and average grain size of ZrO2 ceramics with oxides addition. The high tetragonal fraction of ZrO2 ceramics with small crystallize size and fine grains was found in ZrO2 ceramics with added Y2O3, supporting previous works [15-17]. The micro- structure of ZrO2 ceramics is shown in Fig. 7, where Fig. 7(a) is a micrograph of pure ZrO2 ceramic and Fig. 7(b-d) are micrographs of ZrO2 ceramics with three different oxides addition using a ratio of 4.0 mol%. Microstructural evaluation was performed, i.e., uniformly sized grains with well-packed, continuous grain structure and spherical shape combined with irregular shape in gray color. By applying the linear intercept method [18] to these SEM images, grain sizes were estimated for these samples as given in Table 1. It can be seen that ZrO2 ceramics with no added oxide exhibited large grains with average grain sizes in the range of 2.65-2.91 mm. While, ZrO2 ceramics with addition of the three oxides showed average grain sizes between 0.63-2.18 mm. Comparing the grain sizes of pure ZrO2 ceramics and ZrO2 ceramics added oxides, it is found that the grain of ZrO2 ceramics with addition of oxides had smaller sizes than grain sizes of pure ZrO2 ceramics. Thus, the optimal contents and type of stabilizing oxides is an important parameter for development of ceramic microstructures. The mechanical properties of ZrO2 ceramics were investigated by measuring micro- hardness by Knoop and Vickers techniques and then calculating the fracture toughness values. The fracture toughness of ZrO2 ceramics as a function of different type and concentration of oxide additions is shown in Fig. 8. The toughness values of ZrO2 ceramics with additions of Y2O3 and MgO were in the range 3.51-5.64 MPa m1/2. Pure ZrO2 ceramics and ZrO2 ceramics with additions of BaCO3 had toughness values that were not much different and in the range 1.52-1.58 MPa m1/2. This result indicated that the mechanical properties are related to grain size and phase trans- formation, which increase as grain size decrease and high fraction of tetragonal phase, in agreement with previous reports by J. Vleugels et al. [19] and Chun-Feng Hu et al. [20]. The highest of fracture toughness value was found in ZrO2 ceramics with 4 mol% Y2O3 added, which corresponds to high ratio of tetragonal phase by XRD refinements and optimal microstructure.
|
Fig. 1 Density of ZrO2 ceramics with different stabilizing oxides additions. |
|
Fig. 2 Shrinkage ZrO2 ceramics with different stabilizing oxides additions |
|
Fig. 3 XRD patterns of ZrO2 ceramics with added 0.0-6.0 mol% Y2O3. |
|
Fig. 4 XRD patterns of ZrO2 ceramics with added 0.0-6.0 mol% MgO. |
|
Fig. 5 XRD patterns of ZrO2 ceramics with added 0.0-6.0 mol% BaCO3. |
|
Fig. 6 Rietveld refinement of ZrO2 ceramics with added 4.0 mol% Y2O3 |
|
Fig. 7 SEM micrographs of ZrO2 ceramics with oxides added: (a) 0.0 mol%, (b) 4.0 mol% Y2O3 (c) 4.0 mol% MgO (d) 4.0 mol% BaCO3. |
|
Fig. 8 Fracture toughness of ZrO2 ceramics with different stabilizing oxides additions |
|
Table 1 Parameters obtained from Rietveld analysis, percentage of fraction phase, lattice parameter, lattice strain, crystallize size and average grain sizes of ZrO2 ceramics with added 4.0 mol% stabilizing oxides. |
In the present work, ZrO2 ceramics with addition of three oxides (Y2O3, MgO, BaCO3 ) at 0.0, 2.0, 4.0 and 6.0 mol% were prepared by solid state reaction method. The effects of addition of different oxides and concen- trations on the properties of ZrO2 ceramics were studied. This samples had phase compositions of polymorphous combine with t-, c- and m- ZrO2 phases. Quantitative analysis from XRD pattern using Rietveld technique was performed to explain the crystal structure. The relationship between the tetragonal phase ratio, densifi- cation, grain size and mechanical properties is discussed.
This research project was financially supported by Mahasarakham University, Thailand.
- 1. A. Behbahani, S. Rowshanzamir, and A. Esmaeilifar, Proced. Engin. 42 (2012) 908-917.
-

- 2. M. Ruehle, Adv. Mater. 9[3] (1997) 195-217.
-

- 3. M. Matei, E.A. Voinea, R. Rica, H. Manolea, L. Mogoanta, A. Salan, A. Rica. V.C. Dinescu, and N. Cioatera, Ceram. Inter. 45[12] (2019) 14859-14866.
-

- 4. T. Chraska, A.H. King, and C.C. Berndt, Mater. Sci. and Eng. A 286[1] (2000) 169-178.
-

- 5. M.G. Shahri, A. Shafyei, A. Saidi, and K. Abtahi, Ceram. Inter. 40[8] (2014) 13217-13221.
-

- 6. O. Gorban, S. Synyakina, G. Volkova, S. Gorban, T. Konstanyiova, and S. Lyubchik, J. Sol. Stat. Chem. 232 (2015) 249-255.
-

- 7. K.H. Lee, S.H. Ahn, and K.W. Nam, J. Ceram. Process. Res. 19[3] (2018) 183-188.
- 8. G.P. Cousland, X.Y. Cui, A.E. Smith, A.P.J. Stampfl, and C.M. Stampfl, J. Phys. and Chem. of Sol. 122 (2018) 51-71.
-

- 9. M.T. Vinas, F. Zhang, J. Vleugels, and M. Anglada, J. Eur. Ceram. Soc. 38[6] (2018) 2621-2631.
-

- 10. M. Borik, M. Gerasimov, E. Lomonova, F. Milovich, V. Myzina, P. Ryabochkina, N. Sidorova, and N. Tabbachkova, Mat. Chem. Phys. 232 (2019) 28-33.
-

- 11. B. Basu, Int. Mater. Rev. 50[4] (2005) 239-256.
-

- 12. J. Chevalier, L. Gremillardw, A.V. Virka, and D.R. Clarke, J. Am. Ceram. Soc. 92[9] (2009) 1901-1920.
-

- 13. Z. Zeng, Y. Liu, F. Qian, J. Guo, and M. Wu, J. Eur. Ceram. Soc. 39[14] (2019) 4338-4346.
-

- 14. N.F. Amat, A. Muchtar, M.S. Amril, M.J. Ghazali, and N. Yahaya, J. Mater. Res. Technol. 8[1] (2019) 1092-1101.
-

- 15. C. Piconi, W. Burger, H.R. Richter, A. Cittadini, G. Maccauro, V. Covacci, N. Bruzzesse, G. A. Ricci, and E. Marmo, Biomat. 19[16] (1998) 1489-1494.
-

- 16. C. Piconi, and G. Maccauro, Biomat. 20[1] (1999) 1-25.
-

- 17. S.T. Aruna, and K.S. Rajam, Mat. Res. Bull. 39[2] (2004) 157-167.
-

- 18. W.E. Lee, and W.M. Rainforth, in “Ceramics Microstructure Property Control by Processing” (Chapman&Hall, 1994) p.220.
- 19. J. Vleugels, Z.X. Yuan, and O.V. Der Biest, J. Eur. Ceram. Soc. 22[6] (2002) 873-881.
-

- 20. C.F. Hu, B.N. Kim, Y.J. Park, K. Morita, H. Yoshida, S. Grasso, H.B. Zhang, S.Q. Guo, and Y. Sakka, J. Ceram. Process. Res. 16[3] (2015) 281-286.
 This Article
This Article
-
2021; 22(2): 221-225
Published on Apr 30, 2021
- 10.36410/jcpr.2021.22.2.221
- Received on Sep 19, 2020
- Revised on Nov 2, 2020
- Accepted on Nov 11, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- A. Rittidech
-
Department of Physics, Faculty of Science Mahasarakham University, Mahasarakham 44150, Thailand
Tel : +66 43 754379 Fax: +66 43 754379 - E-mail: aurawan.r@msu.ac.th






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.