- Synthesis and characterization of cobalt oxide nanoparticles by using industrial pulp as impregnated precursor
Soo-Jong Kim*
Department of Advanced Materials & Chemical Engineering, Halla University, 28 Halladaegil, Wonju, Kangwon 26404, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cobalt oxide (Co3O4) nanoparticles were prepared using impregnation method associated with the liquid phase synthesis. Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) was used as a starting material, and industrial pulp was used as impregnated matrix. As a result of heat treatment of cobalt oxide precursors impregnated with pulp for one hour at a temperature range of 380-800 oC, cobalt oxide nanoparticles with a particle size of 100-500 nm were obtained. The crystallization process, morphology, and thermal decomposition were studied as a function of firing temperatures. The synthesized powders were evaluated by Scanning Electron Microscope(SEM), X-ray diffractometer(XRD), Thermo gravimetric analysis(TG) and Differential scanning calorimetry(DSC). It is found that the formation of Co3O4 crystal phase is generated at about 380 oC. The crystal phase of Co3O4 is maintained to a temperature of about 800 oC, and the size of the crystals has increased as the temperature increases. This Co3O4 crystal phase was changed to a CoO crystal phase at 900 oC
Keywords: Nanoparticle, Cobalt oxide, Liquid phase synthesis
Cobalt oxides have been studied extensively due to a variety of applications in sensor, electromagnetic materials, photocatalyst, electrochromic device, and lithium ion battery. In addition Cobalt oxides have been used for R, G, B pixel barrier rib of flat panel display [1-5]. Cobalt oxide is a p-type semiconductor with direct optical band gaps at 1.48 and 2.19 eV. Co3O4 has a stable normal spinel structure. As a mixed valence compound, its formula is written as CoO∙Co2O3. Where Co2+ ions occupy the tetrahedral 8a sites and Co3+ occupy the octahedral 16d sites [6-9]. An industrial method of producing Co3O4 is to heat Co(OH)2 in air to a high temperature. In addition, it is synthesized by coprecipit- ation by adding carbonate, ammonia, sodium hydroxide, etc. to a salt of cobalt. Also, a method of manufacturing by hydrothermal synthesis has been reported [10-12]. Fig. 1 shows the steps of manufacturing the cobalt oxide (Co3O4) obtained industrially. In this process, CoSO4 obtained by solvent extraction of cobalt ore was precipitated with sodium hydroxide to prepare cobalt hydroxide. After drying the cobalt hydroxide, and heated to a high temperature in air to produce a cobalt oxide (CoO, Co3O4). This method has the disadvantage of using a large amount of solvent in the step for obtaining the cobalt hydroxide. It can also be seen that the shape and size of the cobalt oxide particles synthesized by this method are uneven as shown in Fig. 2. Various liquid-phase syntheses of CoO and Co3O4 nanoparticles have been reported [13-17]. The precipitation operation common to these methods, since by using a large amount of solvent includes a step for obtaining a cobalt hydroxide precipitate is recovered and the processing cost of the solvent is a problem. By synthesis method used in this study is heat treated by impregnating a hydrate of a metal salt or metal salt in a polymer matrix to produce the ceramic fine particles, it is possible to easily manufacture the ceramic multi-component particles [18, 19]. The pulp used as a cellulose source in the impregnation operation and there is a microfibrillar structure of a size below 1 μm. Therefore, the shape of the particles produced after the heat treatment is a powder which particle size is uniform, depending on the microstructure of the pulp. This synthesis method can easily control the particle size different from the type of the matrix or by changing the firing conditions [20]. And it has the advantage that you do not use strong alkaline organic solvents used in other synthesis methods, such as co-precipitation method. In this study by using a water-soluble cobalt compounds Co(NO3)3· 9H2O as the starting material was dissolved in water to prepare a cobalt salt solution. After the cobalt salt solution is impregnated to a pulp used in the polymer matrix, the cobalt oxide was prepared by heating at a lower temperature range; it was investigated by the difference between the particle formation processes according to the change in the heat treatment temperature.
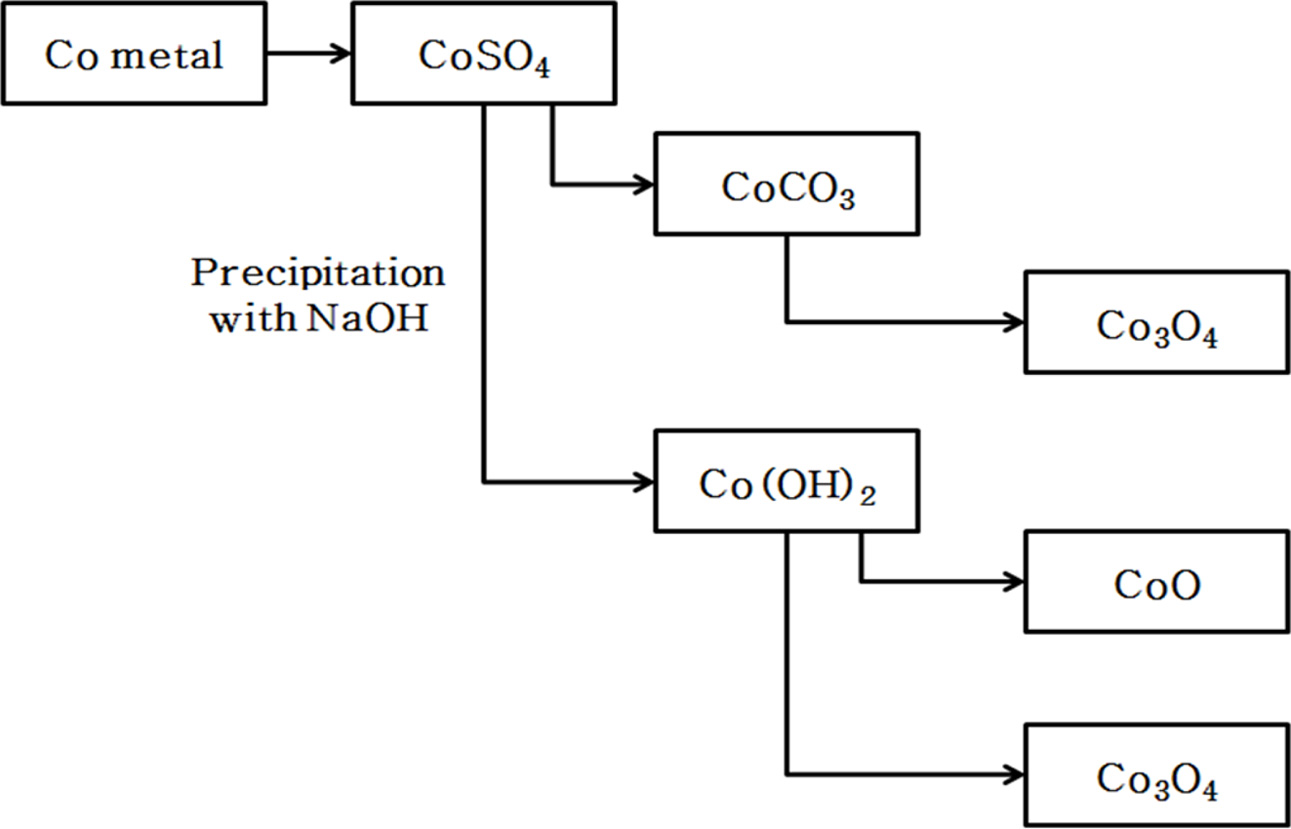
|
Fig. 1 Industrial manufacturing process of Co3O4 |
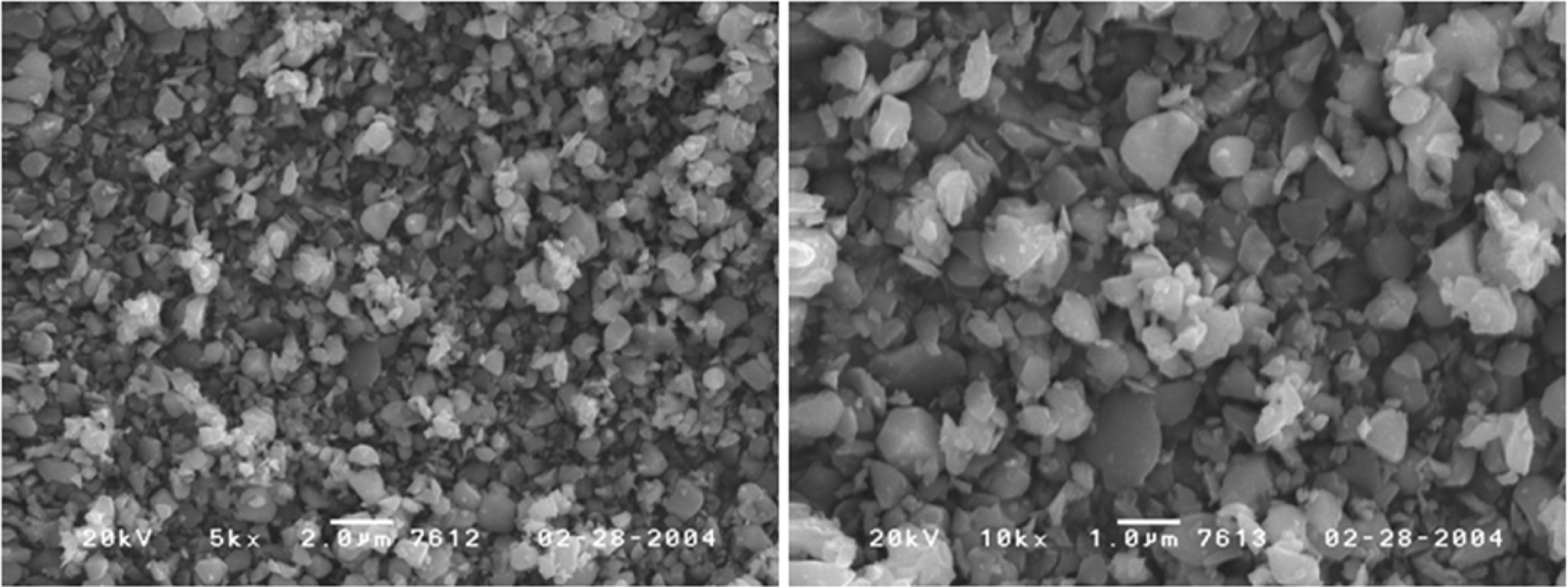
|
Fig. 2 SEM micrographs of commercial Co3O4 powders fabricated by solid reaction. |
Preparation of cobalt oxide powder
Nano-sized Co3O4 particle have been synthesized by a liquid phase precursor technique using Cobalt nitrate hexahydrate as a source of Cobalt oxide. Pulp was used as a cellulose source. Fig. 3 shows a schematic flow diagram of a cobalt oxide nanoparticles prepared by the liquid phase precursor method used in this study. Dissolving a certain amount of Co(NO3)2·6H2O in distilled water to remove the ions, and then the saturated aqueous solution was prepared by stirring for 2 h at 25 oC. The weight ratio of the saturated metal salt solution and the pulp used for the impregnation is 1:1. This impregnated mixture was dried at 80 oC for 12 h, and heat treated in the temperature range of 380-900 oC for 1hr in air with a heating rate of 5 oCmin-1.
Characterization
The X-ray Diffraction (XRD) measurements of the calcined powder samples were carried out by using a Rigaku (RINT-2000) X-ray diffractometer with mono- chromated Cu-Kα radiation (λ = 1.54 Å). XRD patterns were recorded in the 2θ range from 10° to 80° at a resolution of 0.05°. Identification of phases was carried out by comparing the diffraction patterns with the standard PDF cards. In order to measure the size, shape and distribution of Co3O4 powder particles, measure- ments were conducted using a field emission scanning electron microscope (FE-SEM, model JSM-6700, JEOL). For a more accurate comparative analysis of particle size and particle size distribution, the magnification of all samples was uniformly set to 30,000x, 50,000x, and 100,000x. All samples were covered with a thin gold layer. The thermal analysis of the impregnated precursor was determined by differential scanning calorimeter (Scinco DSC N-650) and thermogravimetric analysis (Scinco TGA N-1000). The heating rate of the sample was 10 oC min-1, with a flowing N2 atmosphere.
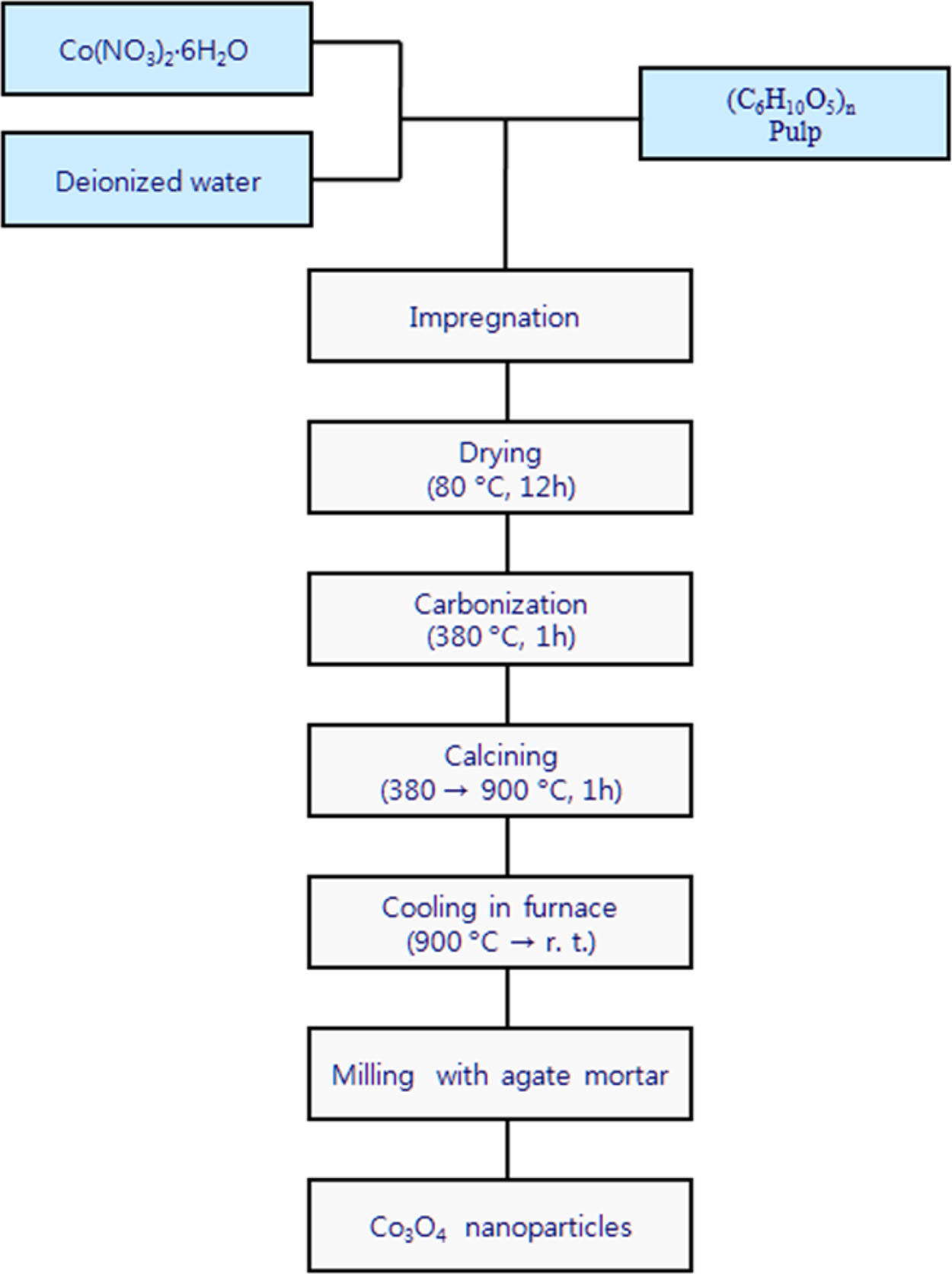
|
Fig. 3 Experimental procedure of preparing Co3O4. |
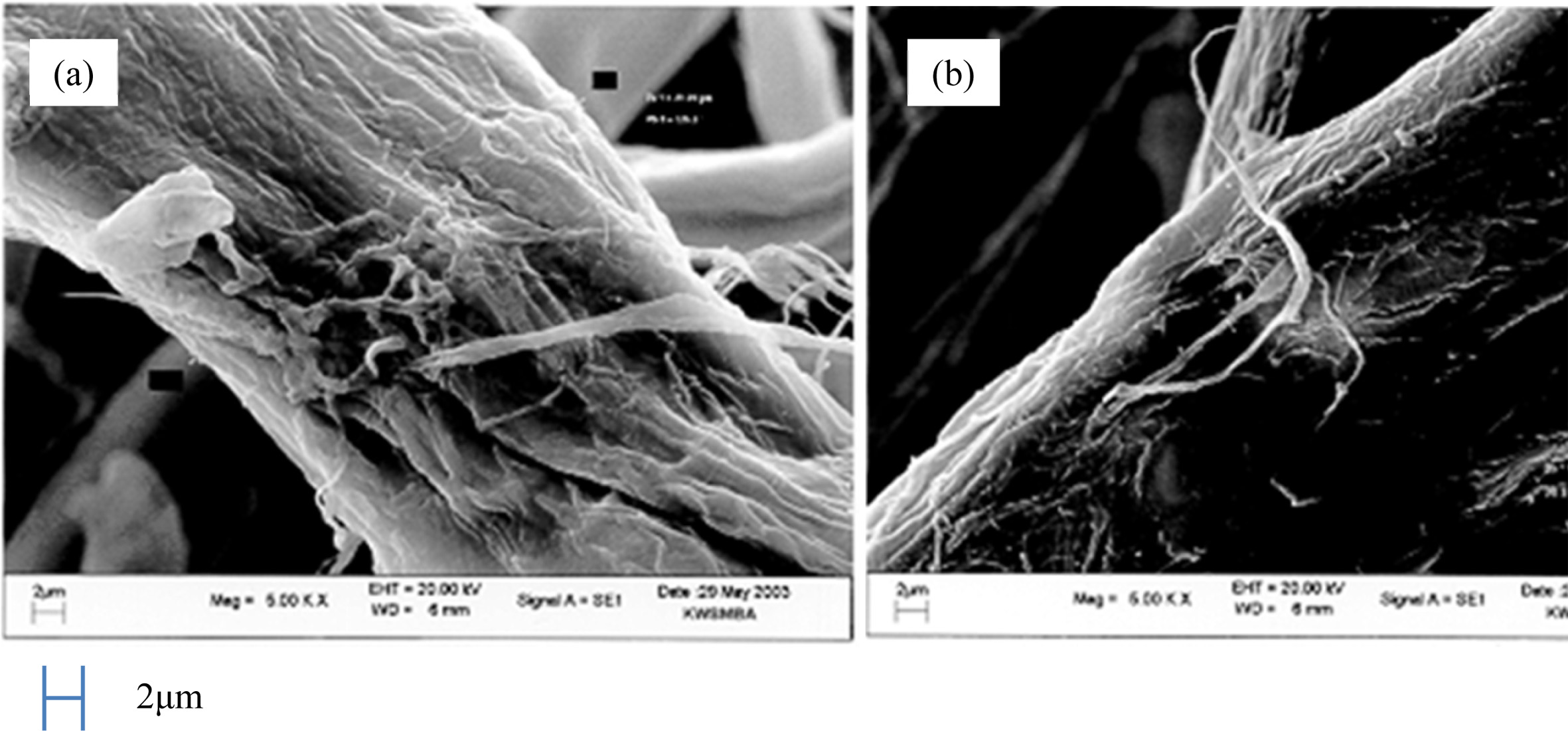
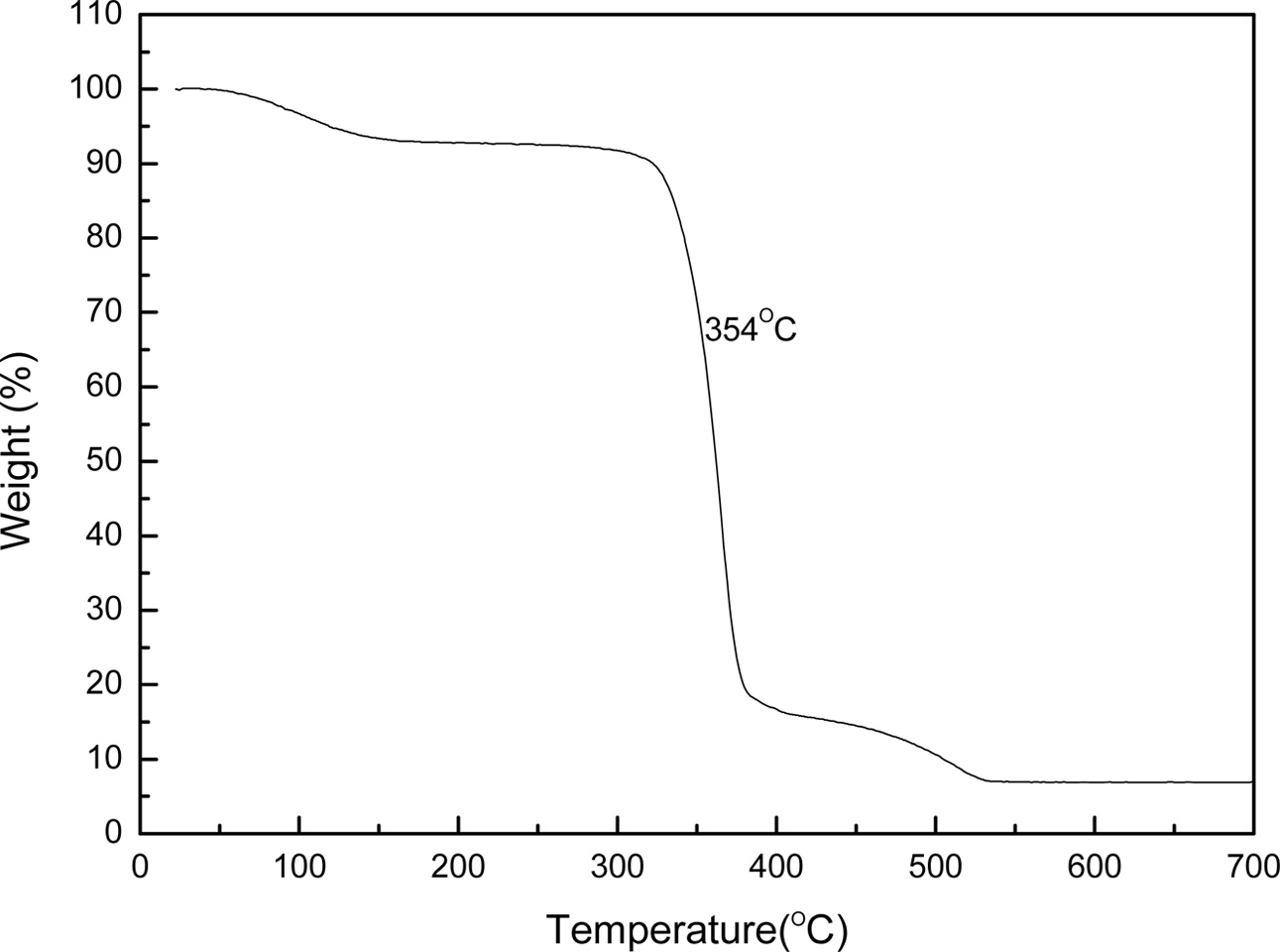
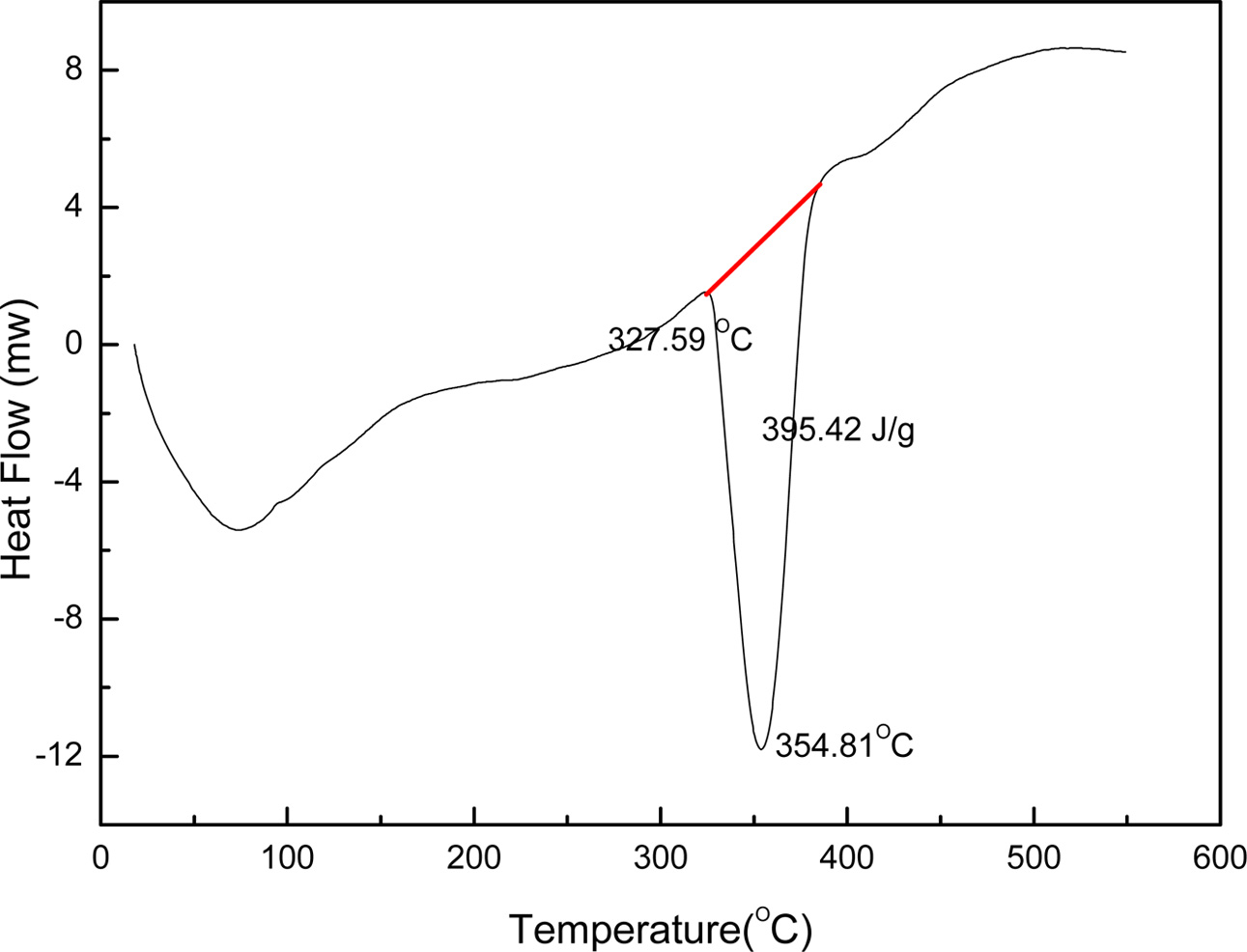
In this study, liquid phase precursor method was used to prepare Co3O4 nanoparticles. Pulp, a cellulose poly- mer having a microfibril structure, was used as an impregnation medium. Dissolving the cobalt nitrate in deionized water, it was impregnated in pulp with a micro fibril structure of microscopic size less than 1 μm. The dissolved cobalt nitrate aqueous solution is stirred sufficiently to uniformly disperse the pulp. The particle size of the Co3O4 fine particles obtained by heat treatment was uniform, and this Co3O4 particle was excellent crystallinity. Fig. 4 shows the micro structure change of pulp before and after impregnation. Fig. 4(a) shows the pure pulp prior to impregnation, Fig. 4(b) shows the microstructure of the pulp that was dried by impregnating it with an cobalt nitrate aqueous solution. Raw material solution of the cobalt nitrate solution, as shown in Fig. 4(b) it can be seen that it is uniformly impregnated in the fine structure of the polymer micro fibrils. Fig. 5 shows a process to perform the impregnation operation. It was impregnated by changing the weight ratio of the cobalt nitrate solution and the pulp to 0.5-1.5. A cobalt salt aqueous solution with a weight ratio of pulp 1:1 or less, the amount of pulp than the cobalt salt solution is the cause of degradation of purity after firing because of the presence in excess. On the other hand, when the weight ratio of the cobalt salt solution and the pulp is 1:1 or more, an excessive amount of unimpregnated aqueous solution is present. Therefore, even after the impregnated pulp is dried, cobalt nitrate crystals remain on the surface of the pulp. They are the cause of action of the non-uniform distribution of particles because it failed to enter the micro fibril structure of the pulp. Therefore, In this study, the weight ratio of the aqueous solution of cobalt nitrate and pulp 1:1 was carried out to determine the impregnation operation. In order to examine the thermal weight decrease properties of the impregnated mixture, Thermogravimetric analysis (TGA) results is shown in Fig. 6. Between 120 oC and 200 oC showed a weight loss due to evaporation of the crystal water in the precursor. At temperatures between 340 oC and 380 oC after showing a remarkable weight decrease due to thermal decomposition of the organic matter in the precursor, gradually modest weight loss is continued until 520 oC. A moderate weight loss appeared between 380 oC and 520 oC has shown that the organic com- ponents contained in the starting material and fibrous components of the pulp to be completely thermally decomposed, in the above 520 oC did not show a weight decrease. Fig. 7 shows the DSC curve of the impregnated mixture. As shown in Fig. 7, between 327-354 oC showed an endothermic peak due to thermal decomposition of the organic material. Therefore, the heat treatment temperature of the impregnated mixture was set to above 354 oC. A thermal decomposition of the organic material and the creation of cobalt oxide during the heat treatment is can be estimated that proceeds as shown in the following expression.
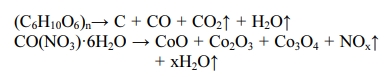
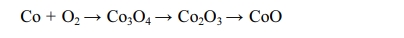
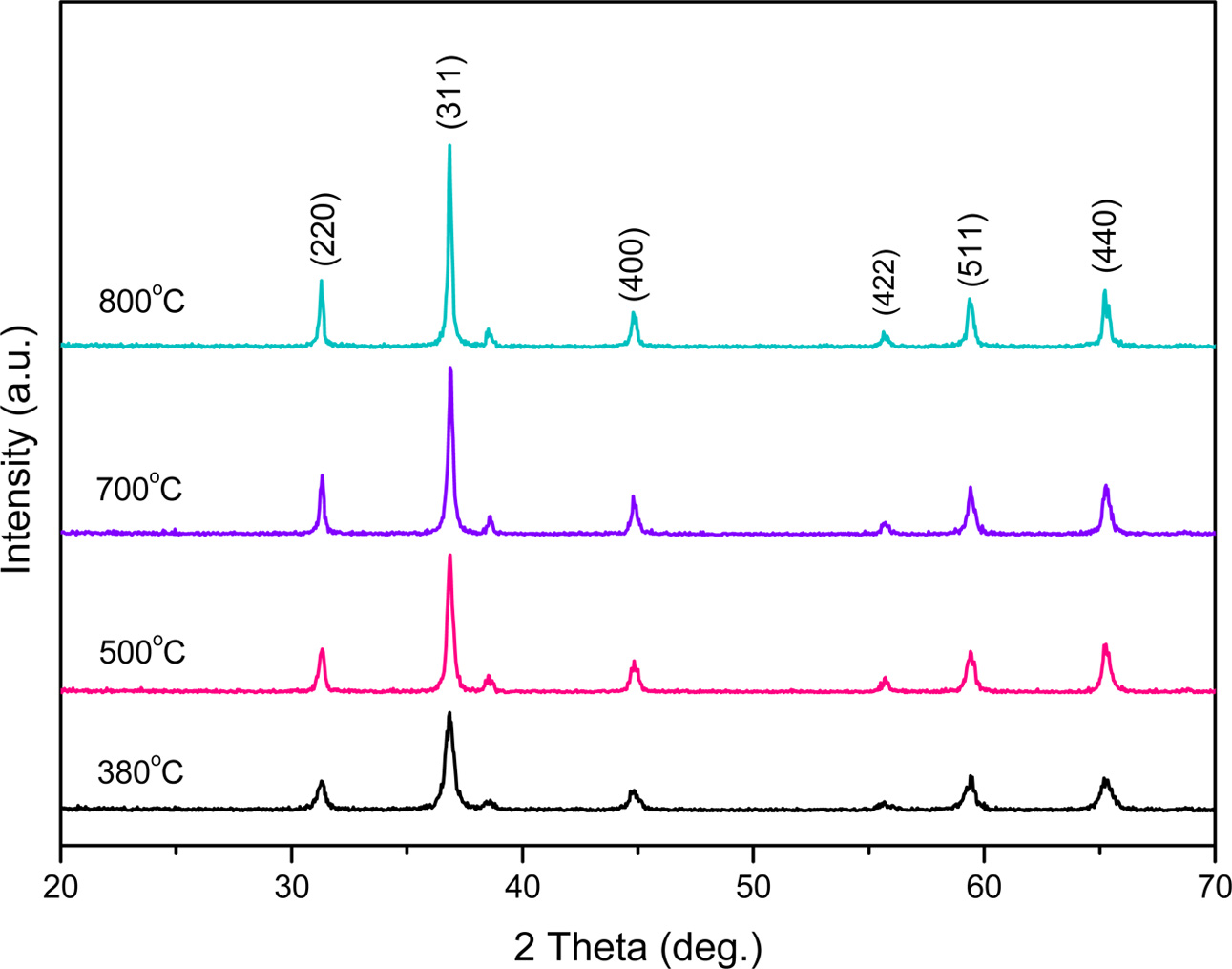
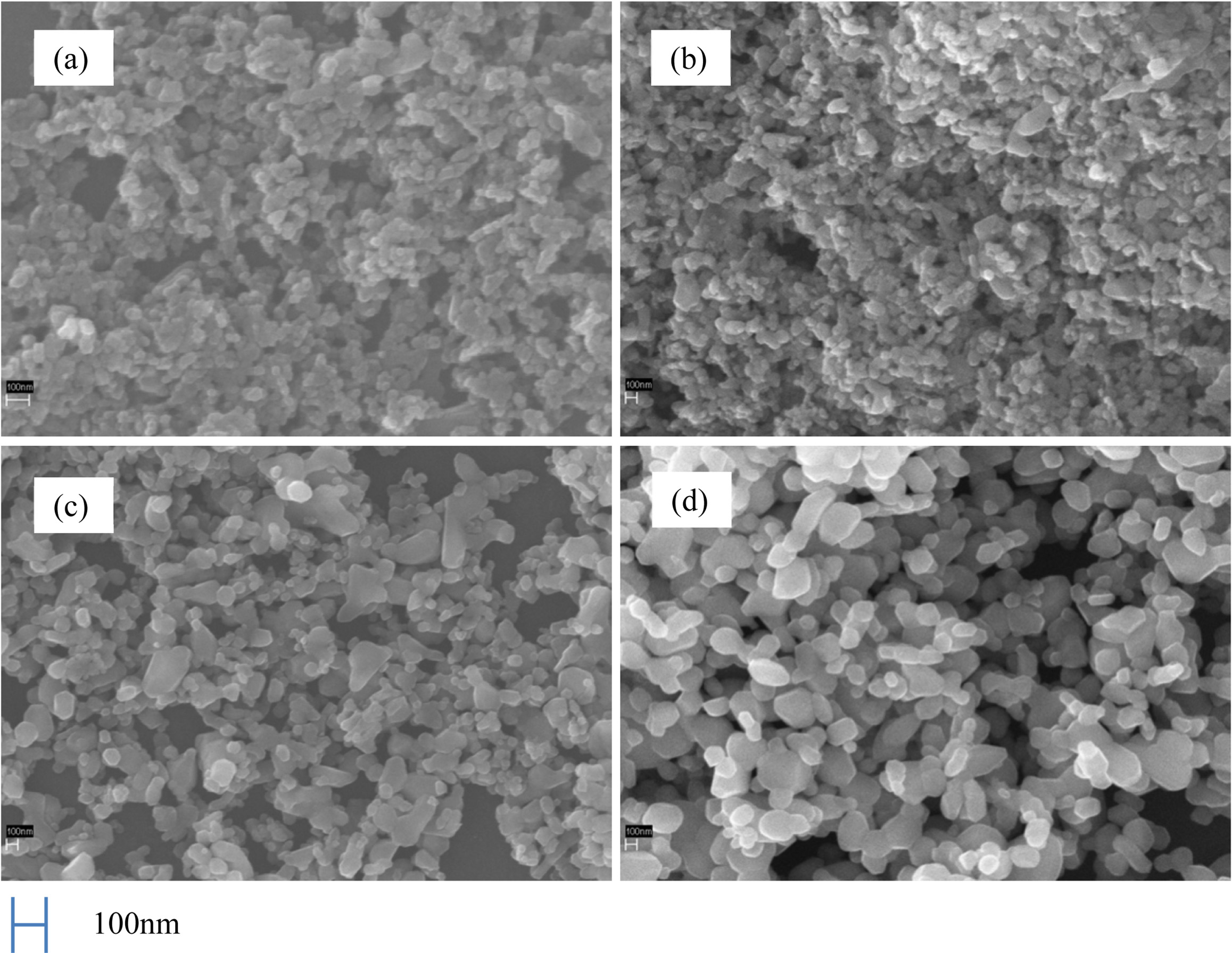
Fig. 8 shows the XRD patterns of Co3O4 powders calcined at 380 oC, 500 oC, 700 oC and 800 oC, respec- tively. As shown in the figure, the diffraction peaks (2θ = 31o, 37o, 44o, 56o, 59o, 65o) of Co3O4 were distinctly observed. As the primary peaks exhibiting high diffraction intensity were in good agreement with the standard JCPDS card No. 43-1003, the crystalline phase of the synthesized Co3O4 is confirmed. The heating rate and the calcination time for all experiments were fixed at 5 oC/min and 1 h, respectively. At 380 oC, the crystalline phase of Co3O4 started to form, and with increase in the calcination temperature from 500-800 oC, the intensity of the peaks also increased, indicating progress in crystallization. Fig. 9 shows SEM images of the Co3O4 powder prepared by calcination of the impregnated precursor, which measures the surface structure of the prepared Co3O4 particles at a fixed calcination time of 1 h and by the variation of tem- perature from 500-800 oC. As can be observed in the XRD pattern in Fig. 8, at 500 oC, Co3O4 crystals had already formed. We can see that most of the particles exhibit a granular morphology. Fig. 9(a) the sample was calcined to 500 oC was the start of Co3O4 crystal phase are generated, Fig. 9(b) in the sample calcined at 600 oC, the size of the growing up of the particle was grown to the 100-200 nm, Fig. 9(c) calcined at 700 oC, the growth of the particles were further expanded. In Fig. 9(d), with the increase in the calcination temperature to 800 oC, crystallinity increased, with growth in the particle size (100-500 nm). From the above results, such as Co3O4 crystal phase is being formed from a relatively low temperature in about 350 oC, and was available a uniform fine powder prepared in the nano- meter order was confirmed that single-phase Co3O4 is generated in a temperature range of 380-800 oC.
|
Fig. 4 SEM photographs of (a) pure pulp and (b) pulp impregnated with solution of cobalt nitrate. |

|
Fig. 5 Impregnation and calcination procedure of Co3O4 nanoparticle. |
|
Fig. 6 TG analysis of impregnated precursor |
|
Fig. 7 DSC analysis of impregnated precursor. |
|
Fig. 8 XRD patterns of Co3O4 powders with various calcining temperature. |
|
Fig. 9 SEM micrographs of calcined powders, (a) 500 oC, (b) 600 oC, (c) 700 oC and (d) 800 oC. |
In this study, Co3O4 nanoparticles were synthesized by the simple impregnation method. Cobalt nitrate was impregnated with pulp, a polymer matrix, and calcined to prepare Co3O4 nanoparticles. The method of synthe- sizing these Co3O4 nanoparticles is simple and inex- pensive. Also, as in the case of the synthesis method by the precipitation method, there is an advantage that the wastewater treatment of the precipitation solution is not necessary. The XRD result indicates the formation of the single phase of Co3O4. At a temperature ranging from 380 oC to 800 oC, with increasing calcinations temperature, crystallization was accelerated, and particle growth (100-500 nm) was observed.
- 1. M. Ando, T. Kobayashi, S. Iijima, and M. Harita, J. Mater. Chem. 7[9] (2000) 1779-1783.
-

- 2. N.R. Jana, Y.F. Chen, and X.G. Peng, Chem. Mater. 16[20] (2004) 3931-3935.
-

- 3. X. Lou, J. Han, W. Chu, X. Wang, and Q. Cheng, Mater. Sci. Eng. B 137[1-3] (2007) 268-271.
-

- 4. Y. Lu, Y. Wang, Y. Zou, Z. Jiao, B. Zhao, Y. He, and M. Hong, Electrochem. Commun. 12[1] (2010) 101-105.
-

- 5. J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhro, Z. Tang, and D. Wang, Angew. Chem. 52[25] (2013) 6417-6420.
-

- 6. C. Mocuta, A. Barbier, and G. Renaud, Appl. Surf. Sci. 162 (2002) 56-61.
-

- 7. K. Sinko, G. Szabo, and M. Zrinyl, J. Nanosci. Nanotech. 11 (2011) 4127-4135.
-

- 8. A.O. Gulino, P. Dapporto, P. Rossi, and I. Fragalàa, Chem. Mat. 15[20] (2003) 3748-3752.
-

- 9. Y. Dong, K. He, L. Yin, and A. Zhang, Nanotech. 18[43] (2007) 435602.
-

- 10. K. Sink, G. Szabo, M. Zrinyi, J. Nanosci. Nanotech. 11[5] (2011) 4127-4135.
-

- 11. G. Wang, X. Shen, J. Horvat, B. Wang, H. Liu, D. Wexler, and J. Yao, Phys. Chem. C 113[11] (2009) 4357-4361.
-

- 12. T.R. Ingraham and P. Marier, Therm. Chim. Acta 1 (1970) 39-49.
-

- 13. Y. Lu, Y. Wang, Y. Zou, Z. Jiao, B. Zhao, Y. He, and M. Wu, Electrochem. Commu. 12[1] (2010) 101-105.
-

- 14. J. Pal and P. Chauhan, Mater. Charac. 61[5] (2010) 575-579.
-

- 15. X. Lou, J. Han, W. Chu, X. Wang, and Q. Cheng, Mater. Sci. and Eng. B 137[1-3] (2007) 268-271.
-

- 16. M. Rashad, M. Rüsing, G. Berth, K. Lischka, and A. Pawlis, J. Nanomat. 2013 (2013) 1-6.
-

- 17. S.J. Kim and H.Y. Kwon, J. Kor. Ins. Ele. Ele. Mat Eng. 20[8] (2007) 871-879.
-

- 18. S.J. Kim and H.S. Kwon, J. of KIEEME(in Korean), 20[8] (2007) 671-679.
- 19. Y.L. Song, S.H. Choi, S.J. Kim, Y.H. Song, T. Masaki, and D.H. Yoon, J. Ceram. Proc. Res. 17[3] (2016)202-204.
- 20. S.J. Kim and C.H. Han, J. Ceram. Proc. Res. 19[2] (2018) 130-133.
 This Article
This Article
-
2021; 22(1): 74-78
Published on Feb 28, 2021
- 10.36410/jcpr.2021.22.1.74
- Received on Jul 19, 2020
- Revised on Nov 13, 2020
- Accepted on Dec 4, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Soo-Jong Kim
-
Department of Advanced Materials & Chemical Engineering, Halla University, 28 Halladaegil, Wonju, Kangwon 26404, Korea
Tel : +82-33-760-1237 Fax: +82-33-760-1290 - E-mail: sjkim@halla.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.