- Enhancing the electrical properties of multiferroic materials based on variations in BiFeO3-BaTiO3 weight ratio
Dwita Suastiyantia,* and Yuli Nurul Maulidab
aDepartment of Mechanical Engineering, Institut Teknologi Indonesia, Puspiptek Raya Street, Serpong, South Tangerang, 15314 Indonesia
bDepartment of Chemical Engineering, Institut Teknologi Indonesia, Puspiptek Raya Street, Serpong, South Tangerang, 15314 IndonesiaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
BiFeO3is an example of a multiferroic material widely used for electronic devices, although there have been reports on impeding current leakage problems arising from non-stoichiometry. This challenge is complicated by the presence of only the BiFeO3 phase. The aim of this research, therefore, is to synthesize multiferroic ceramic materials with a combination of BiFeO3 and BaTiO3. Furthermore, an increase in electrical properties have been implicated in the materials’ elevated Magneto Electric (ME) value. The synthesis was performed using the sol-gel method, comprising basic materials of Bi5O(OH)9(NO3)4, Fe(NO3)3.9H2O, C6H8O7, Ba(NO3)2, TiO2, and aquabidestilate. Moreover, the calcination and sinter temperatures used were 350 and 700 oC, respectively, while the varied sintering times include 2, 4, and 6 hours. In addition, the modified weight ratio of BaTiO3:BiFeO3 were 1:1, 1:2 and 2:1. The formed phases are evaluated using X-Ray Diffraction Test, while other characterizations include electrical properties test and particle size measurement. Therefore, the study outcome has the potential to produce ceramic powder with particle size 72-81 nm, and samples sintered at 700 oC for 6 h, with a weight ratio of BaTiO3: BiFeO3 = 2:1, possessed the most significant electrical properties
Keywords: Nanomultiferroic, Current leakage, Magnetoelectric, Non stoichiometry, Electrical properties
Multiferroic materials are well known to have multiple physical property. These include the ability to generate an electric voltage in response to the administration of an external magnetic field. Therefore, it is possibly to change the magnetization process using an electric field, or develop an electric polarization through a magnetic field. In addition, both properties are potentially affected by the introduction of stress.
In addition, magnetoelectric properties occur due to the presence of couplings between magnetizable materials, not with standing the regularity level of the magnetic moment and polarization, as well as the electric moment. Therefore, the two physical quantities, including namely magnetization and polarization have the potential torise based on the influence of a specific force field [1]. Conversely, coupling is known to occur either through coupling between paramagnetic and ferroelectric, para-electric and ferromagnetic, or inter-phase combinations, with possible regular magnetic and electric dipole moments. Moreover, the indirect methods include coupling instigated through strain on the material phase. This magnetoelectric (ME) effect is characterized by the presence of a new physical quantity, termed a constant representing the coupling activity between both fields in the materials, and is known as the linear ME coupling coefficient [2].
The multiferroic materials are known to possess several properties, including magnetoelectricity, magneto elasticity, and piezoelectric interrelated with one another. The bulk system’s composite structure between both phases on the nanometer phase scale facilitates interac- tions between piezoelectric and inner magnetization [3]. Therefore, material systems are expected to produce large coupling constants and open opportunities for multifunctional device applications, including magnetic-electric transducers, actuators, and smart sensors [4].
Furthermore, multiferroic material of BiFeO3 are known to possess a magnetoelectric coupling characteristic. This manipulates the ferroelectric polarization by magnetic fields or control antiferromagnetic vector orientation by electric fields [5, 6]. Moreover, this may be applied to spintronic devices because it handles magnetization of ferromagnets and also exchange coupling to an anti- ferromagnet ferroelectric through electric field, therefore a single BiFeO3 material phase has many advantages. The structure, Curie and Neel temperature of this chemical has been stated by many researchers [7-10], however the possession of multiple phases have some complications with presence of impurities including Bi2Fe4O9 and Bi25FeO40 from the synthesis. These phases are marked with low ferromagnetic value, increased current leakage, decreased magnetoelectric coupling, and resistivity [11-13]. In addition, several research have supported the use of only BiFeO3 powders, even conventional solid-state reaction and sol-gel technique do not produce a single phase of BiFeO3 [14, 15] but based on chemical routes especially sol-gel method is better and simpler to use [16]. The solid-state reaction method was discovered to produces coarser powders than other technique used, and also decreased the reproducibility function [17-19]. Furthermore, to help prevent impurity phases, different technologies including synthesis of thin films or nano structures, ABO3 perovskite material’s formation (bismuth ferrite & barium titanate), and BiFeO3 compounds [20-22] were all developed. The doping A site added to ABO3 perovskite through Ca, La, Sr, Pb, and Ba ion is a good technology for having only BiFeO3 phase set to the property enhance- ment. Moreover, Bismuth ferrite has a spin structure with 62 nm wavelength [23], When analyzed by G-type cycloidally all other methods have to be declared [24]. The highest magnetization values were discovered at Bi1−xAxFeO3 multiferroics doped with largest of ionic radius (Ba2+, Pb2+). Also, the impact of diamagnetic ions variations on Bi0.8A0.2FeO3 (A = Ca, Sr, Pb, Ba) compounds properties were declared using this chemical. This doping method of BiFeO3 with large ionic radius achieves better multiferroic properties [25]. Meanwhile, reason for selecting x = 0.2 among all Bi1-xBaxFeO3 compounds is based on the fact Bi0.8Ba0.2FeO3 shows the highest value of magnetoelectric coupling, increased fatigue resistance, increased ferroelectric hysteresis loop, better multiferroic properties and high activation energy. The impact of Ba doping and calcination temperature on Bi1−xBaxFeO3 multiferroic characteristics with structure were been obtained.
Furthermore, sizes of nano and single phase particles is critical to get the effect of Magnetoelectric coupling (coupling ME) to enable magnetic and electrical properties become stronger simultaneously in one material. This strong contact between nano particles in single-phase materials is certain to cause interactions around crystal surfaces. The average size of material phases on nano- meter scale causes the interacting surface fraction to increase with decreasing grain measurement.
Based on practical applications, all this chemicals are hampered by current leakage problems arising from non-stoichiometry because an outcome of only one BiFeO3 phase is complicated. The aim of this research is therefore to synthesize multiphase ceramic material with a combination of BiFeO3 and BaTiO3. This therefore shows the effect of adding BaTiO3 on the electrical properties of ceramics.
The ferroelectric materials especially BaTiO3 are very important, and therefore continuous investigation are embarked on to expose the unique potential reasons including having dielectric, pyroelectric, piezoelectric, and electro-optic properties. Meanwhile, this material has been extensively studied and is applied to multiferroic material engineering. The particle size in nanometers was obtained with sol-gel method to help synthesize ceramic powders. This process is known as wet chemical method and is easily embarked on without agglomeration and equally produce nanoparticle powders size.
The Sol-gel method was used to produce BiFeO3-BaTiO3 ceramic. This possibly generates powders in nanoparticle size and reduces the incidence of agglo- meration. In addition, the method adopted involve easily obtained simple laboratory equipment. The goal research goal was achieved using weight variations of BaTiO3: BiFeO3 = 1:1, 1:2, and 2:1.
Preparation
The research involved the use of chemical compounds Bi5O(OH)9(NO3)4, Fe(NO3)3.9H2O, C6H8O7, Ba(NO3)2, TiO2, and H2O,with 99.99% purity, obtained from Merck product (pro analysis). Therefore, the variations in barium titanate and bismuth ferrite weight ratio were1:1, 1:2, and 2:1.
Heating solution
The solution comprising the afore mentioned chemical compound was dissolved using H2O. This was then heated at 85-90oC for 6 hours using a hot plate, while a magnetic stirrer was used to achieve homogeneity. The solution was very runny at the initiation of stirring, became thick and finally turns into gel. This form served as the basic ingredient for making powder after completing several heating processes.
Calcination process
The final product was calcined at 350 oC (240 minutes). This procedure was aimed to reduce useless ingredient, including carbon, hydrogen, and nitrogen, which convert into impurities of the ceramic powder. Furthermore, agglomerization is prevented by pulverizing until having to generate the smallest grain of powder.
Sintering process
The crushed powder was then sintered at 700 oC for 2, 4, and 6 h, in order to ensure complete crystallization, before a finally crushing.
Characterization
The result analysis involve the use of synthesis, where several tests were employed. These include X-Ray Diffraction (XRD)of Pananalytical, Type : E’XPERT PRO, for electrical properties assessment (laboratory team made by using BaTiO3 as standard material), and the particle size were subsequently measured (nanoparticle size analyzer nano trac wave II type). Particularly, the wet method was used for PSA (Particle Size Analyzer), which involves the incidence of dispersion to avoid aglomerisation and. Finally, the measured sizes were single, and the evaluation was performed in the form of narration and graphics.
The diffraction of X-Ray test is done to determine phase types formed as shown in Fig. 1, 2 and 3. These figures proves ceramic powder with sintering time of 2 hours has a different diffraction pattern, especially in ranges of 2 theta all within 20-30 degrees. In addition, for low sintering temperatures (less than 1000 oC), changes in diffraction pattern are noticed at above 2 hours sintering time. Meanwhile all ceramic powders show a diffraction pattern where the sintering time fails to change at 4 to 6 h, and are equally crystalline.
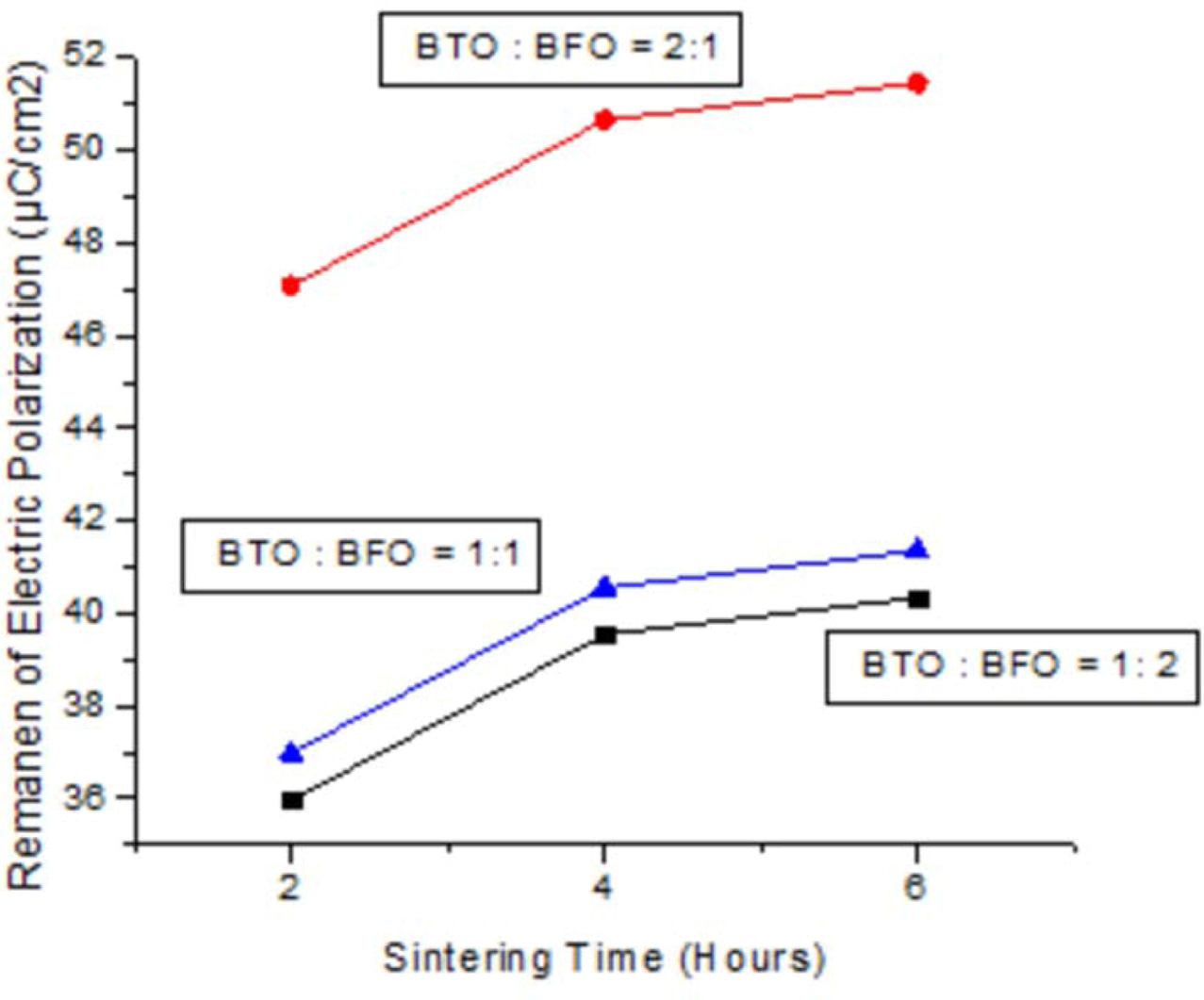
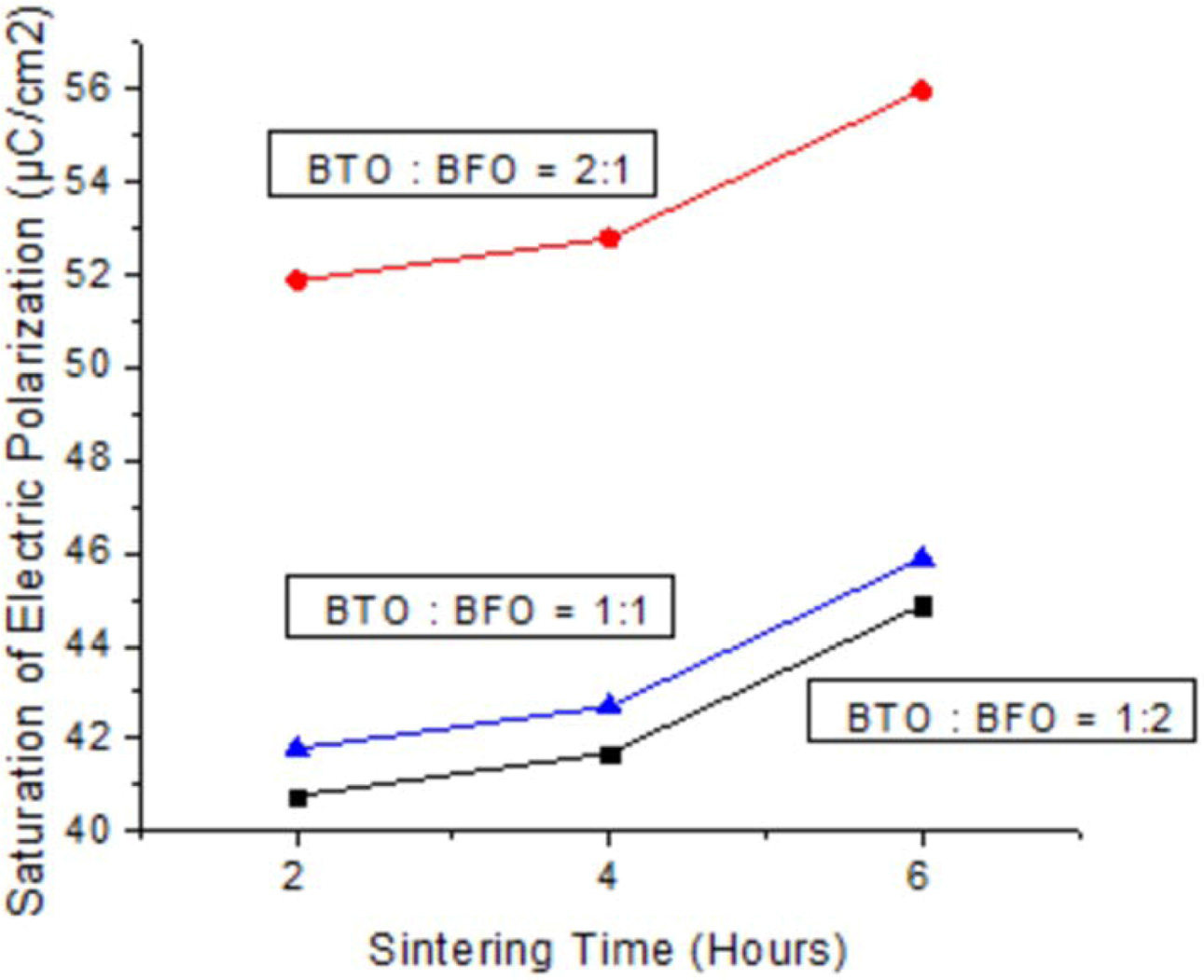
The results of electrical properties test (specifically remanen, coercivity, and saturation) are shown in Fig. 4, 5, and 6. These diagrams represent the highest electrical properties owned by ceramic powder and sintered for 6 hours at 700 oC with weight ratio of BaTiO3 : BiFeO3 = 2 : 1. After 6 h, research should be done again to determine the change in electrical properties. Moreover, ceramic powder with weight ratio of BaTiO3 : BiFeO3 = 2 : 1 at all sintering time has electrical properties value higher than other mass percentage. This is because the addition of BaTiO3 (ferroelectric material) was observed to be 66% more than BiFeO3 therefore spikes an increase in electrical properties of ceramics. The balanced weight ratio of BaTiO3 and BiFeO3 causes values of electrical properties to be between the other calculations. Meanwhile, BiFeO3 is a ferroelectric and antiferromagnetic material at room temperature, with non-collinear rotation. The spins were spiral-shaped with a wavelength of 62 nm, and linear magnetoelectric effects were recorded at zero. However, the reaction is also caused by chemical substitution and mixture of other electric or magnetic compounds [26], through the introduction of epitaxial thin films limits. The BaTiO3 materials when added to alloy ceramic improves electrical properties and therefore become the basis of application in flat screens (television industry) and screen displays on cameras. This indicated a linear decrease of remanen behaviors with increasing temperature for hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 composites through solid state reaction route [27]. The decrease in dielectric constant of (Ba0.7Sr0,3) TiO3 at 200 kHz or higher frequency is caused by the substantial decline in polarization or internal space charge [28]. Furthermore, electrocaloric properties of Ba(Sr, Ti)O3/K(Ra, Nb)O3 shows highest recorded value near the temperature where residual polarization suddenly changed, and increased as applied electric field amplified [29]. This residual polarization intensity of SBT sintered in air atmosphere is 2Pr = 15.3 µC/cm2 while oxygen atmos- phere is 2Pr = 16.6 µC/cm2. This is because the samples sintered in oxygen atmosphere has large lattice distortion, lower oxygen vacancy concentration and larger a-axis oriented grains, therefore are favorable for increase in residual polarization and reduction of coercive field [30].
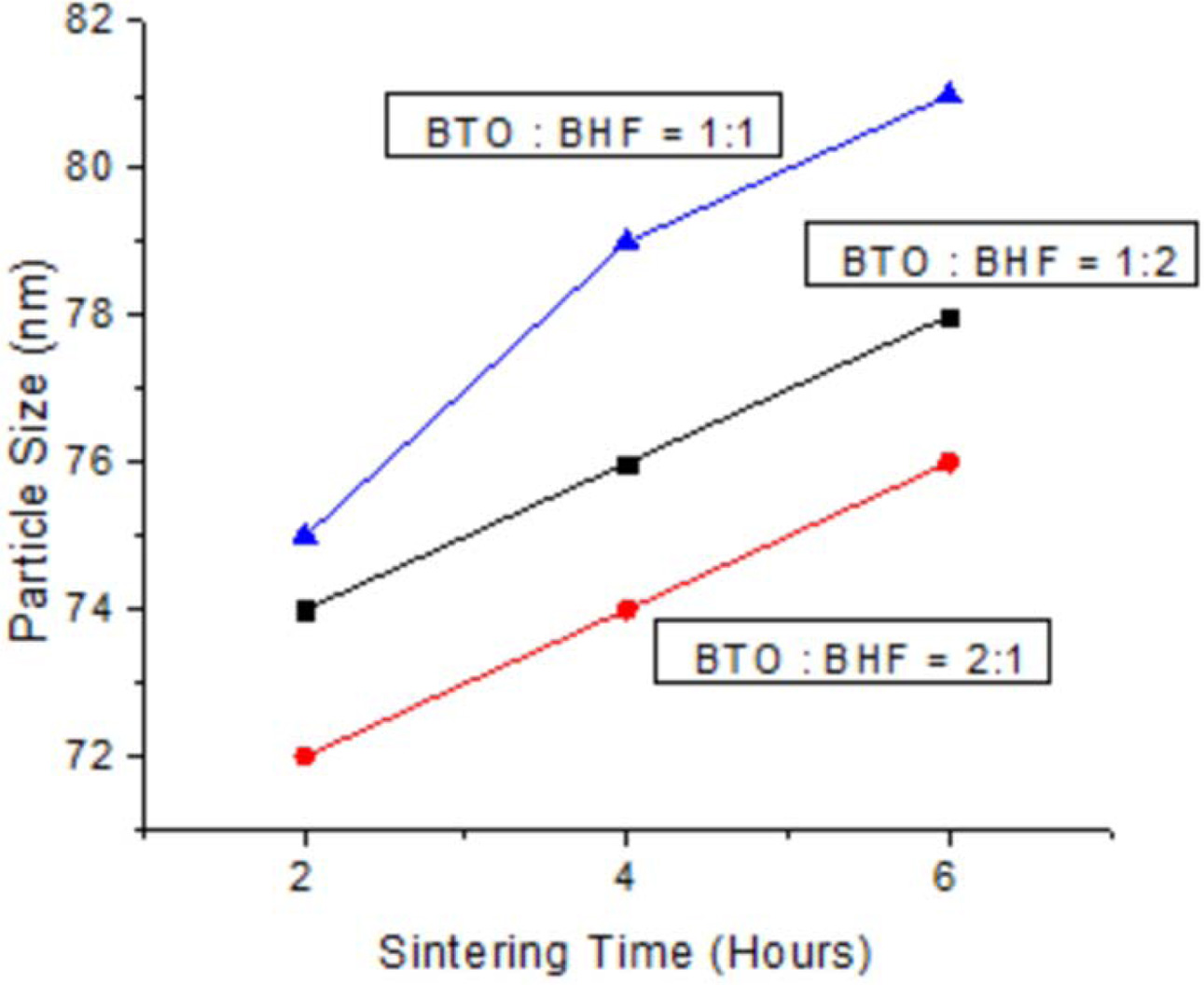
The sol-gel analysis used in this research produced nano-sized powder, as shown in Fig. 7. This diagram equally shows the success of this experiment to produce a ceramic powder with nanoparticle for all BaTiO3 : BiFeO3 weight ratios. The outcome of this experiment proves coarse powder is recorded at 81 nm (for sintering time of 6 h), and the finest was 72 nm (for sintering time of 2 h). These particles get coarser as sintering time increases due to the addition of heat energy capable of growing larger particles. Furthermore, weight ratio of BaTiO3 : BiFeO3, produced the smallest particle at the percentage of 2 : 1. The nanometer-scale causes interacting surface fraction to increases with decreasing grain size and relates with better electrical properties.
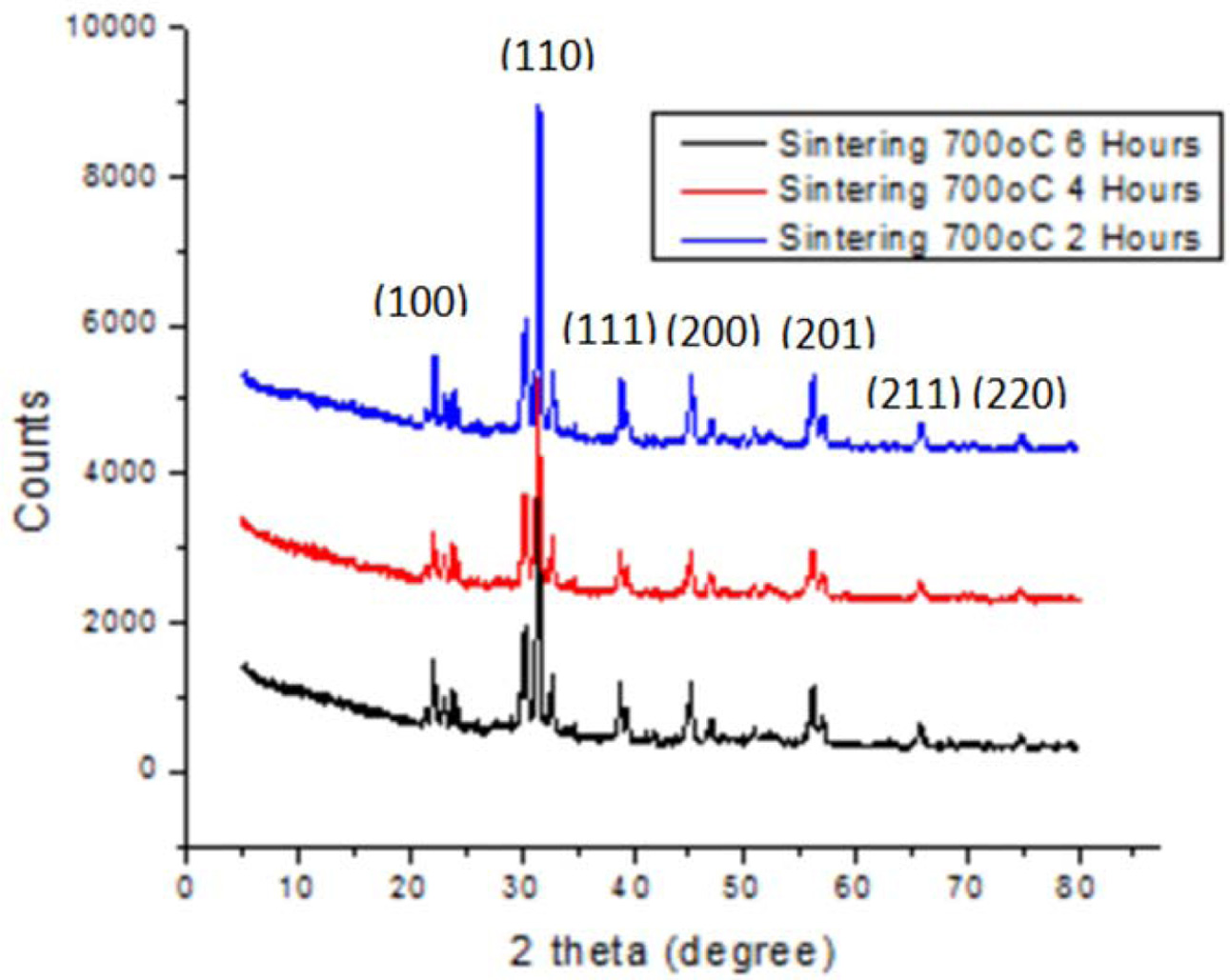
|
Fig. 1 XRD pattern for ceramic with sintering 700 oC for 2, 4 and 6 h and ratio of BaTiO3:BiFeO3 = 1:1. |
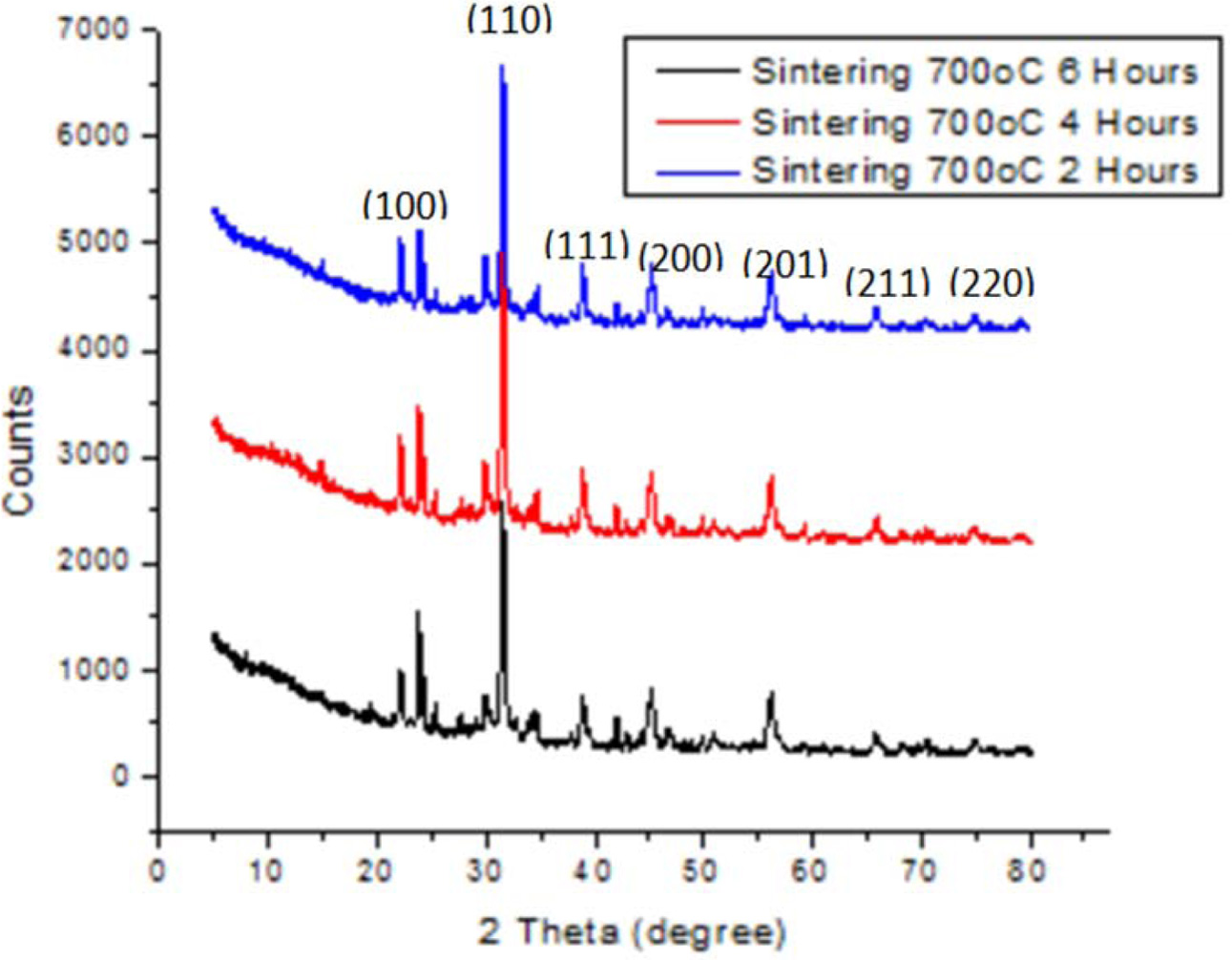
|
Fig. 2 XRD pattern for ceramic with sintering 700 oC for 2, 4 and 6 h and ratio of BaTiO3:BiFeO3 = 2:1. |
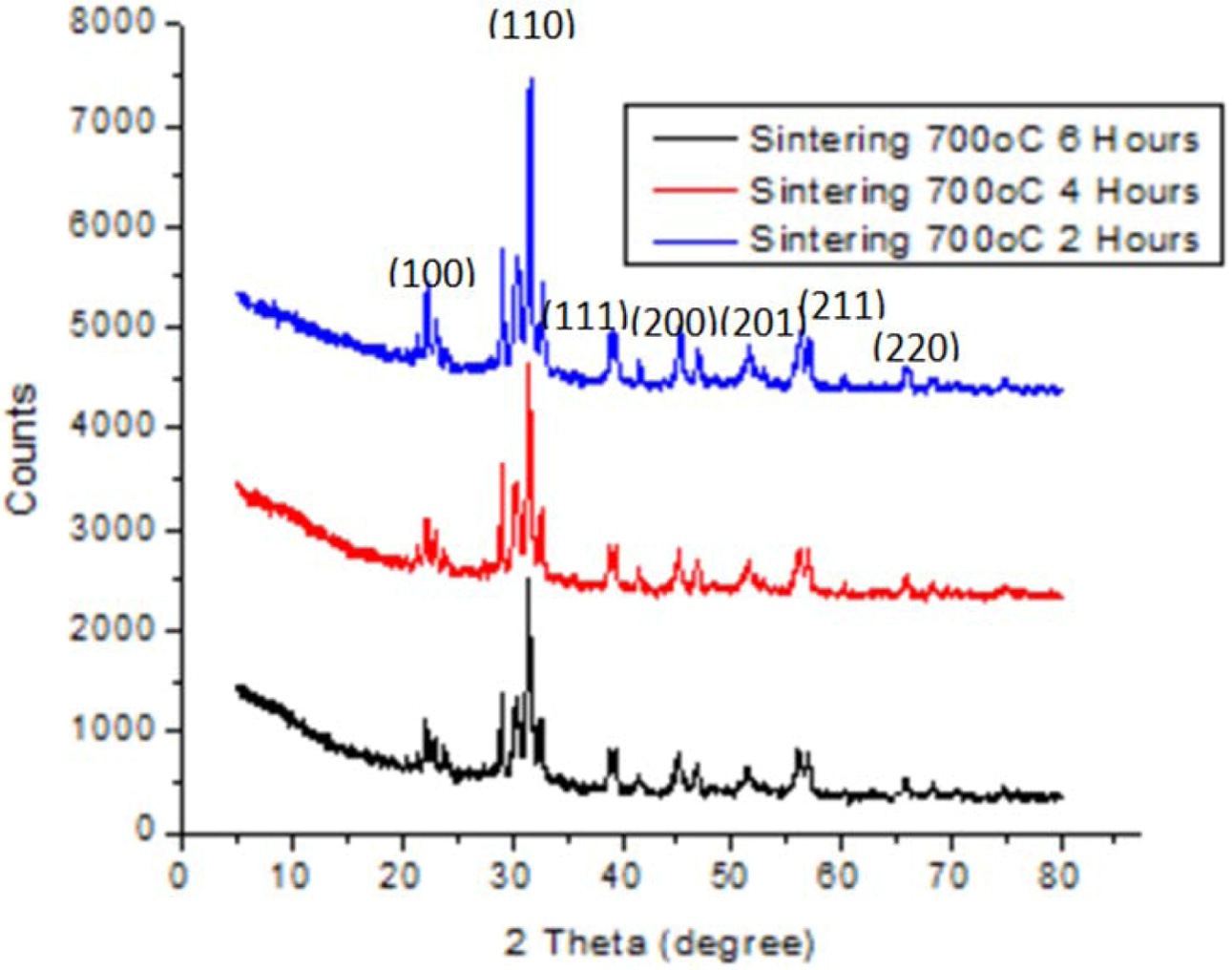
|
Fig. 3 XRD pattern for ceramic with sintering 700 oC for 2,4 and 6 h and weight of BaTiO3:BiFeO3 = 1:2. |
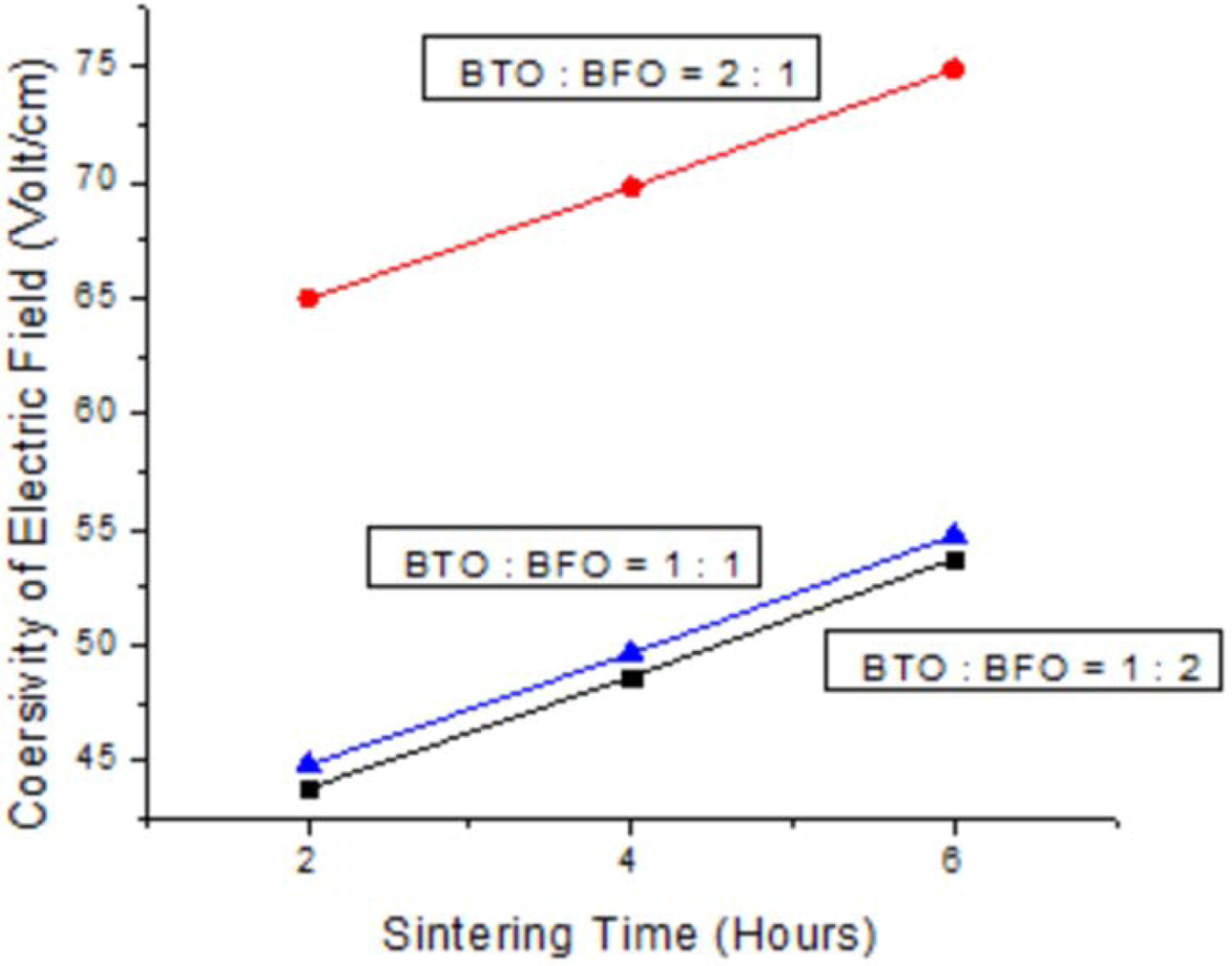
|
Fig. 4 Sintering time vs coersivity of electric field. |
|
Fig. 5 Sintering time vs remanen of electric polarization. |
|
Fig. 6 Sintering time vs saturation of electric polarization. |
|
Fig. 7 Sintering time vs particle size. |
Firstly, all parameters of this research produce ceramic powder in the nanoparticle, with size particle of 100 nm or more. Secondly, the powder in nano size causes an increase in interacting surface fraction, therefore electrical properties (remanen, coercivity, and saturation) amplifies. Finally, the finest particle and highest electrical properties value belonged to ceramic powder, sintered at 700 oC for 2, 4, and 6 h with a weight ratio of BaTiO3 : BiFeO3 = 2:1.
The authors are grateful to Kemenristekdikti, Republic of Indonesia for their guide in this project under letter of 057/KP/LPKT-ITI/IV/2019.
- 1. K.S. Martirosyan, E. Galstyan, S.M. Hossain, Y.J. Wang, and D. Litvinov, Mater. Sci. Eng. 176[1] (2011) 8-13.
-

- 2. H. Palneedi, V. Annapureddy, S. Priya, and J. Ryu, Actuators 5[1] (2016) 9.
-

- 3. M.M. Vopson, D. Zhou, and C. Caruntu, Applied Phys. Lett. 107[18] (2015) 182905.
-

- 4. D. Burdin, D. Chashin, N. Ekoonomov, L. Fetisov, Y. Fetisov, and M. Shamonin, J. Phys. D Appl. Phys. 49[37] (2016) 375002.
-

- 5. V. Gorige, R. Kati, D.H. Yoon, and P.S. Anil Kumar, J. Phys. D Appl. Phys. 49[40] (2016) 405001.
-

- 6. V.A. Khomchenko D.V. Karpinsky, and J.A. Paixão, J. Mater. Chem. C. 5[14] (2017) 3623-3629.
-

- 7. S.M. Selbach, T. Tybell, M.A. Einarsrud, and T. Grande, Chem. Mater 19[26] (2007) 6478-6484.
-

- 8. T. Zhao, A. Scholl, F. Zavaliche, K. Lee, M. Barry, A. Doran, M.P. Cruz, Y.H. Chu, C. Ederer, N.A. Spaldin, R.R. Das, D.M. Kim, S.H. Baek, C.B. Eom, and R. Ramesh, Nature Mater. 5[10] (2006) 823-829.
-

- 9. A. Jaiswal, R. Das, K. Vivekanand, P. Mary Abrahan, S. Adyanthaya, and P. Poddar, J. Phys. Chem. C. 114[5] (2010) 2108-2115.
-

- 10. E. Mostafavi and A. Ataie, J. Mater. Sci. Technol. 31[8] (2015) 798-805.
-

- 11. T. Ramesh, V. Rajendra, and S.R. Murthy, J. Mater. Sci. Mater. Electron. 28[16] (2017) 11779-11788.
-

- 12. B. Bhushan, D. Das, A. Priyam, N. Vasanthacharya, and S. Kumar, Mater. Chem. Phys. 135[1] (2012) 144-149.
-

- 13. X. Zhang, Y. Sui, X. Wang, J. Tang, and W. Su, J. Appl. Phys. 105[7] (2009) 07D918.
-

- 14. M. Valant, A.K. Axelsson, and N. Alford, Chem. Mater. 19[22] (2007) 5431-5436.
-

- 15. M. Bernardo, T. Jardiel, M. Peiteado, A. Caballero, and M. Villegas, J. Eur. Ceram. Soc. 31[16] (2011) 3047-3053.
-

- 16. S. Sharma, V. Singh, R. Kotmala, and R.K. Dwivedi, J. Mater. Sci.: Mater. Electron. 25[4] (2014) 1915-1921.
-

- 17. M.M. Kumar, V. Palkar, K. Srinivas, and S. Suryanarayana, Appl. Phys. Lett. 76[19] (2000) 2764-2766.
-

- 18. S. Hussain, S.K. Hasanain, G.H. Jaffari, S. Faridi, F. Rehman, T.A. Abbas, and S.I. Shah, J. Am. Ceram. Soc. 96[10] (2013) 3141-3148.
-

- 19. G. Achenbach, W. James, and R. Gerson, J. Am. Ceram. Soc. 50[8] (1967) 437.
-

- 20. G. Dong, G. Tan, W. Liu, A. Xia, and H. Ren, J. Mater. Sci. Technol. 30[4] (2014a) 365-370.
-

- 21. G. Dong, T. Tan, Y. Luo, W. Liu, H. Ren, and A. Xia, Mater. Lett. 118 (2014b) 31-33.
-

- 22. F. Wan, X. Bai, K. Song, J. Zheng, X. Lin, and C. Cao, J. Mater. Sci. Technol. 33[9] (2017) 1061-1066.
-

- 23. L. Zhang, W. Ren, X. Luo, and Z. Zhang, J. Mater. Sci. Technol. 35[5] (2019) 764-768.
-

- 24. E. Mostafavi and A. Ataie, J. Mater. Sci. Technol. 31[8] (2015) 798-805.
-

- 25. K.M. Mishra and R.N. Mahaling, Chin. J. Phys. 56[3] (2018) 965-973.
-

- 26. W. Eerenstein, N.D Mathur, and J.F. Scott, Nature. 442[7104] (2006) 759-765.
-

- 27. Y. Yang, X. Liu, S. Feng, X. Kan, Q. Lv, F. Hu, J. Ni, C. Liu, and W. Wang, J. Ceram. Process. Res. 21[4] (2020) 416-424.
-

- 28. M.S. Kwon, S.G. Lee, K.M. Kim, and S. Choi, J. Ceram. Process. Res. 20[4] (2019) 395-400.
- 29. M.S. Kwon, S.G. Lee, K.M. Kim, and S. Choi, J. Ceram. Proc. Res. 20[6] (2019) 603-608.
-

- 30. F. Zhang, C. Li, H. Li, X. Guo, and S. Fan, J. Ceram. Process. Res. 19[2] (2018) 101-104.
 This Article
This Article
-
2021; 22(1): 61-65
Published on Feb 28, 2021
- 10.36410/jcpr.2021.22.1.61
- Received on Jul 12, 2020
- Revised on Oct 13, 2020
- Accepted on Oct 23, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Dwita Suastiyant
-
Department of Mechanical Engineering, Institut Teknologi Indonesia, Puspiptek Raya Street, Serpong, South Tangerang, 15314 Indonesia
Tel : +62-85697163727 - E-mail: dwita_suastiyanti@iti.ac.id






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.