- A study of curing characteristics of CO2-absorption calcium silicate cement with respect to CO2 concentration
Hwa-Song Seoka,b, Jin-Sang Choc, Ki-Yeon Moonc,* and Chang-Woo Honga
aSchool of Civil, Urban, and Environmental Engineering, Korea National University of Transportation, 50 Deahak-ro, Chungju-si, Chungbuk 27469, Korea
b7-9, Dongsuwon-ro, 46Beon-gil, Gwonseon-gu, Suwon-si, Gyeonggi-do, 16672, Korea
cDepartment of Research and Development, Korea Institute of Limestone and Advanced Materials, 18-1 Udeok-gil, maepo-eup, Danyang, Chungbuk 27003, KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
CSC is an eco-friendly, low-energy cement-based material, the use of which can reduce CO2 emissions by up to 70% over the entire course of the manufacturing and curing processes when compared to typical cement mixes. In the present study, the ability of CSC to realize its desired properties, i.e., being cured by the carbonation reaction, was examined. More specifically, CSC samples were cured at varying CO2 concentrations (0, 5, 10, 20%), and their ability to implement their desired properties was studied at each curing time (5, 10, 24, 48, 96 h). In doing so, the potential for utilizing CSC in practice was assessed.
The experimental results showed that with higher CO2 concentration, the carbonation reaction of CSC became accordingly faster. The compressive strength was measured to be over 56 MPa at curing 7 days, indicating that these CSC samples provided excellent early-stage strength. Also, all samples subjected to carbonation curing exhibited a much higher curing 7 days compressive strength regardless of the CO2 concentration in the curing atmosphere when compared to Type 1 cement in accordance with the Korean Industrial Standards. This indicates that these materials have a high potential for extended applications
Keywords: CO2 absorpbing, Calcium silicate cement, CO2 concentration, Carbonation, Curing condition
In order to reduce greenhouse gas emissions in the industrial sector, it is necessary to reduce greenhouse gas emissions emitted during the production process. In the case of the cement industry, about 90% of the carbon dioxide generated in the manufacturing process is emitted from the clinker manufacturing process, and since high calcination temperature for the manufacture of clinker is essential, there is a limit to reducing greenhouse gas emissions from the manufacturing process.
Calcium silicate cement that cures by absorbing CO2 (hereinafter referred to as “CSC”) is defined as calcium silicate-based cement and lime calcium silicate cement types. In its manufacturing process, the sintering tem- perature is set to about 1,200oC, which is approximately 200oC lower than that of typical cement mixes, thereby leading to a reduction in energy consumption. Also, the ratio of its main mineral phases, Ca and Si, is set to 1:1 or 3:2 [1], and thus the CO2 emissions are less when compared to ordinary portland cement [2]. In addition, its ability to cure by absorbing CO2 may allow for a roughly 30% reduction of CO2 during the curing process. This means that, over the entire course of the manufacturing and application processes, up to 70% of CO2 emissions can be cut when compared to ordinary portland cement [2, 3].
In theory, the main mineral phases of CSC include CS (CaO · SiO2, pseudowollastonite) and C3S2 (3CaO · 2SiO2, rankinite). However, depending on the raw material mixing ratios and sintering characteristics, other cement mineral phases, such as C2S and C3S, may be included, along with the remaining unreacted SiO2. Here, the mineral phases that react with CO2 are CS and C3S2, and compressive strength of up to over 70 MPa may be achieved through reactions represented by Eqs. (1) and Eq. (2) below [1-4].
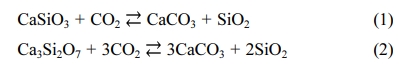
In the present study, CSC was fabricated using domestic raw materials and examined with regard to its ability to implement desired properties. In doing so, the potential for its commercialization was assessed.
Characteristics of raw materials
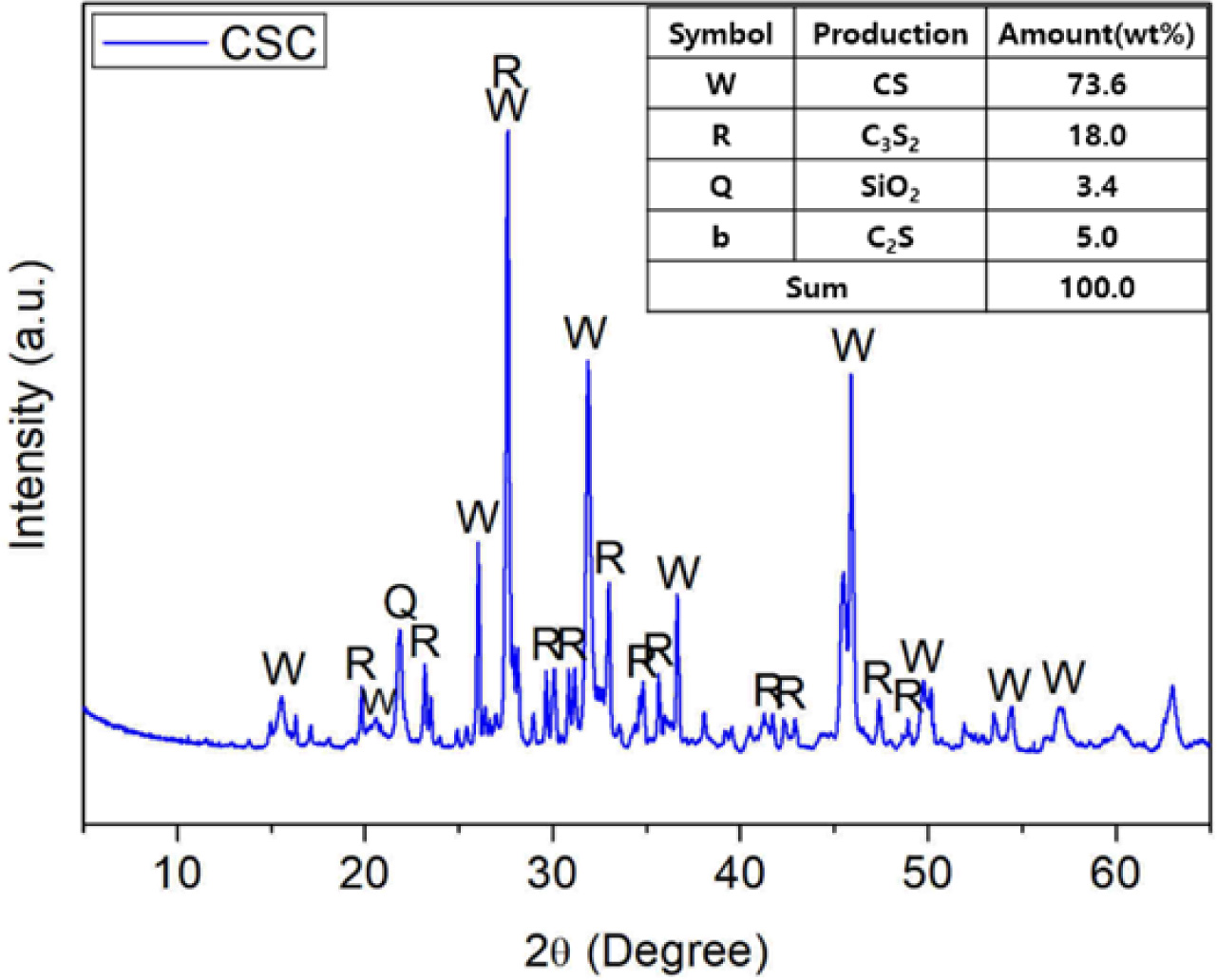
In the present study, CSC clinker was produced using domestic limestone and siliceous raw materials, as shown in Fig. 1. It was then crushed into particles with a size of about 10 μm, which is similar to the particle size range of ordinary Portland cement. As a result, CSC samples were obtained, as shown in Fig. 2. The main mineral phases of these CSC samples included CS, C3S2, C2S, and unreacted SiO2, which largely coincided with the results of previous overseas studies that reported the mineral characteristics of CSC, as shown in Fig. 1 [2-4]. Also, the proportion of CS and C3S2 combined, which directly contributes to the carbonation reaction, was found to be 91.5%. This figure was considered to be high enough to achieve sufficient durability improvement. It was found that a small amount of SiO2 was used when mixing raw materials remained. As reported in other similar studies, it was less likely that SiO2 could be fully used up during the manufacturing process despite attempting to adjust the mixing molar ratio of raw materials [4-6]. Also, it was confirmed that its presence did not negatively affect CSC realizing its properties.
Experimental methods
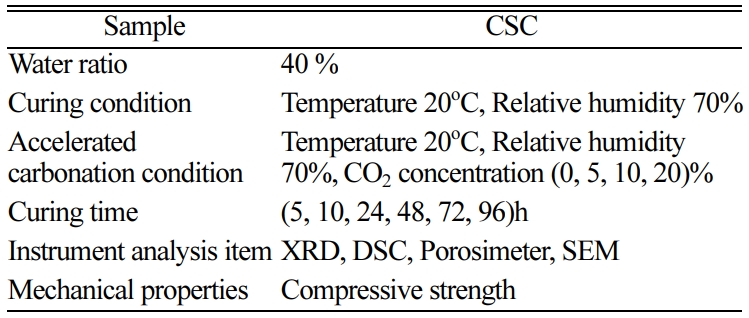
CSC paste was prepared to examine its ability to realize desired properties with respect to the curing conditions. Subsequently, it was subjected to carbonation curing while varying the CO2 concentration. The test conditions are summarized in Table 1. When preparing this CSC paste, the water ratio was set to 40%, which is the level that allows for the fabrication of samples in the form of a paste. Immediately after the mixing process, these samples were put on each reagent plate and spread widely with a thickness of about 100 mm and then subjected to curing in a carbonation chamber. This carbonation chamber operated at a temperature of 20oC and a humidity of 70%, and the CO2 concentration was set to 0, 5, 10, and 20%. At each curing time, samples were withdrawn from the chamber and pretreated, and their physical characteristics were examined using XRD, DSC, SEM, and porosity measurement.
Compressive strength test specimens were molded in a cement specimen mold with width, length, and height of 40 mm, 40 mm, and 160 mm, respectively. It was impossible to de-mold the molded samples right after the molding process was complete, and thus they were dried and cured in the atmosphere for about 24 h and then de-molded to be cured inside the carbonation chamber. Here, the operating conditions of the carbonation chamber were set to be the same as the curing conditions of the paste. At curing 7 days, the compressive strength was measured at a loading rate of 144 kN/min in accordance with KS L ISO 679.
|
Fig. 1 XRD pattern of CSC. |
|
Fig. 2 Particle size diameter of CSC. |
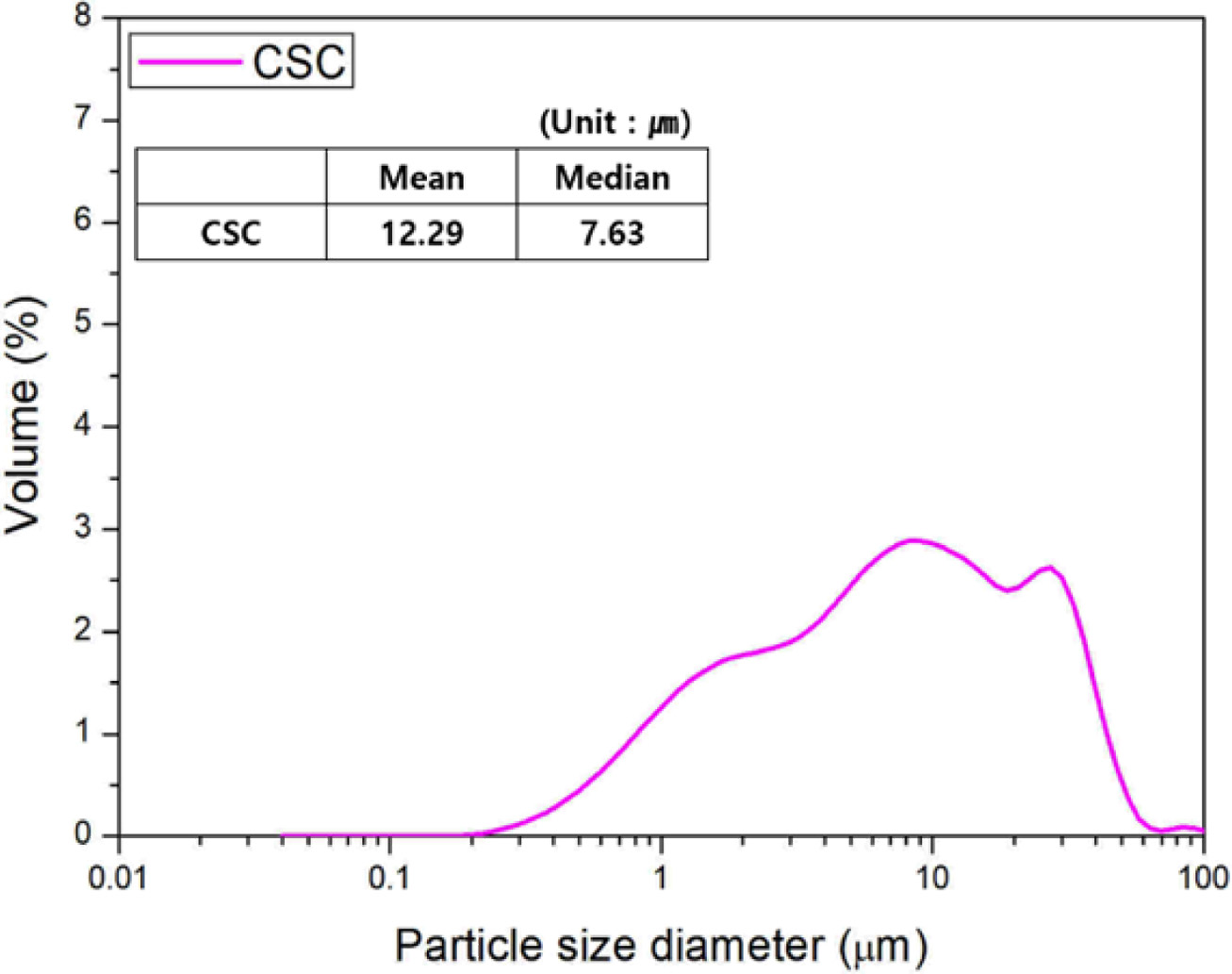
The mineral phases of the CSC paste were analyzed according to the curing conditions, as shown in Fig. 3. As the CO2 concentration increased, the CaCO3 peak near 30o was found to increase in size. This was considered to be due to the increased contribution of C3S2 and CS to the carbonation reaction caused by the increasing CO2 concentration. This was also likely related to the time of formation of CaCO3, which might vary according to the CO2 concentration. However, as shown in the mineral phase analysis results, it was impossible to ascertain how changes in the C3S2 and CS peak sizes are related to their contribution to the carbonation reaction. Also, the formation of amorphous SiO2, one of the reaction products of the carbonation reaction, was not clearly identified.
In the case of the samples cured under typical moist curing conditions without any CO2 supply, as shown in Fig. 3(a), there were no noticeable changes in the mineral phases at varying curing days, unlike in the samples subjected to carbonation curing, as shown in Figs. 3(b), (c), and (d). Also, no products of the carbon- ation reaction were found. This suggests that carbonation curing is critical to CSC being able to realize its desired properties. It is thus necessary to determine the optimal curing conditions that will allow CSC to realize its desired properties [7, 8].
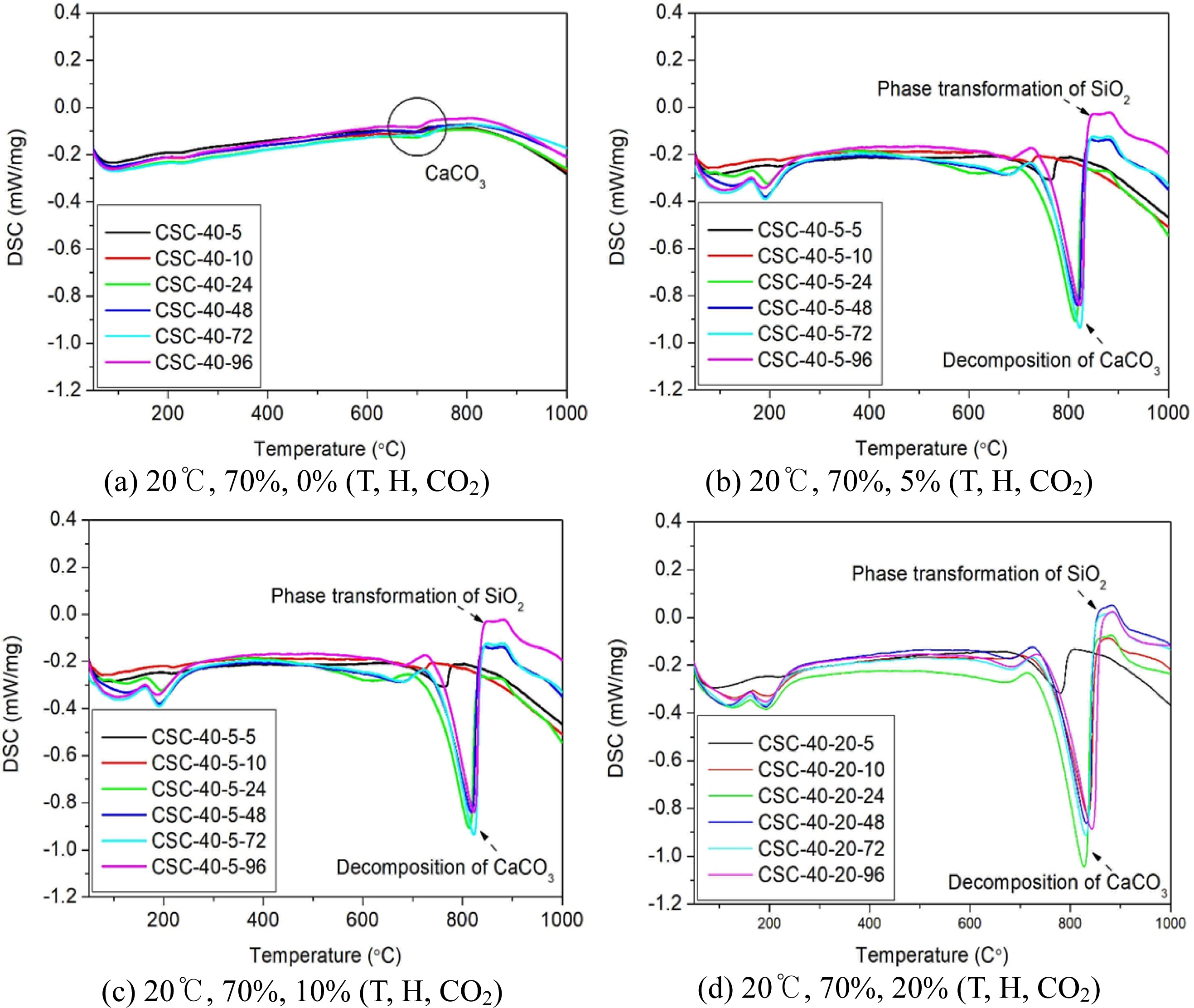
For the relative quantification of products of the carbonation reaction, differential scanning calorimetry (DSC) was performed, as shown in Fig. 4. It was found that the CaCO3 peak around 800oC increased in size with increasing CO2 concentration and curing time [7]. Also, the onset of CaCO3 formation was earlier when the CO2 concentration was higher, which suggested the relationship between the CO2 concentration and the kinetics of the carbonation reaction [8].
For the samples subjected to carbonation curing, as shown in Figs. 4(b), (c), and (d), it was expected that the final amount of the reaction products might vary according to the curing atmosphere where the CO2 concentration varied. However, the volume of reaction products was found to be not significantly affected by CO2 concentration. In general, the carbonation reaction starts from the sample surface, where any surfaces in contact with CO2 are preferentially subjected to densifi- cation caused by the crystalline growth of mineral phases. However, when the reaction proceeds to some extent, it becomes difficult for CO2 to enter the inside of the sample because its crystalline structure has been densified [2, 9, 10]. Therefore, it is difficult to confirm the immediate effect of the CO2 concentration on the reaction results. In light of this nature of the carbonation reaction, in the present study as well, the intensity and kinetics of the reaction could be confirmed in a macro- scopic manner with respect to the CO2 concentration at the early stage of curing (24 h). At the later curing stage, however, the total amount of reaction products remained at similar levels, understandably, because the reactivity was no longer affected by the CO2 concentration.
A hyperbolic peak near 100-200oC was considered to be due to 2H4SiO4, an intermediate compound that was generated during the formation of SiO2, one of the reaction products of the carbonation reaction of CS and C3S2 [2, 4].
In an attempt to observe the crystalline growth of CaCO3, the samples cured at a CO2 concentration of 20% were examined with respect to the curing time using SEM, as shown in Fig. 5. It was found that the carbonation reaction proceeded somewhat faster in the samples cured at a CO2 concentration of 20% than in those cured at the other conditions. Even at the early stage of curing, i.e., 10 h, the formation of CaCO3 was identified. Similarly, SEM analysis results confirmed the crystallinity of CaCO3 at curing 10 hours. At the early stage of curing, the degree of crystallinity appeared to be low, but as the curing time increased, the crystallinity became more evident, and the cured microstructure became increasingly densified. This was a macroscopic confirmation of durability improvement achieved in the cured CSC body by the carbonation reaction.
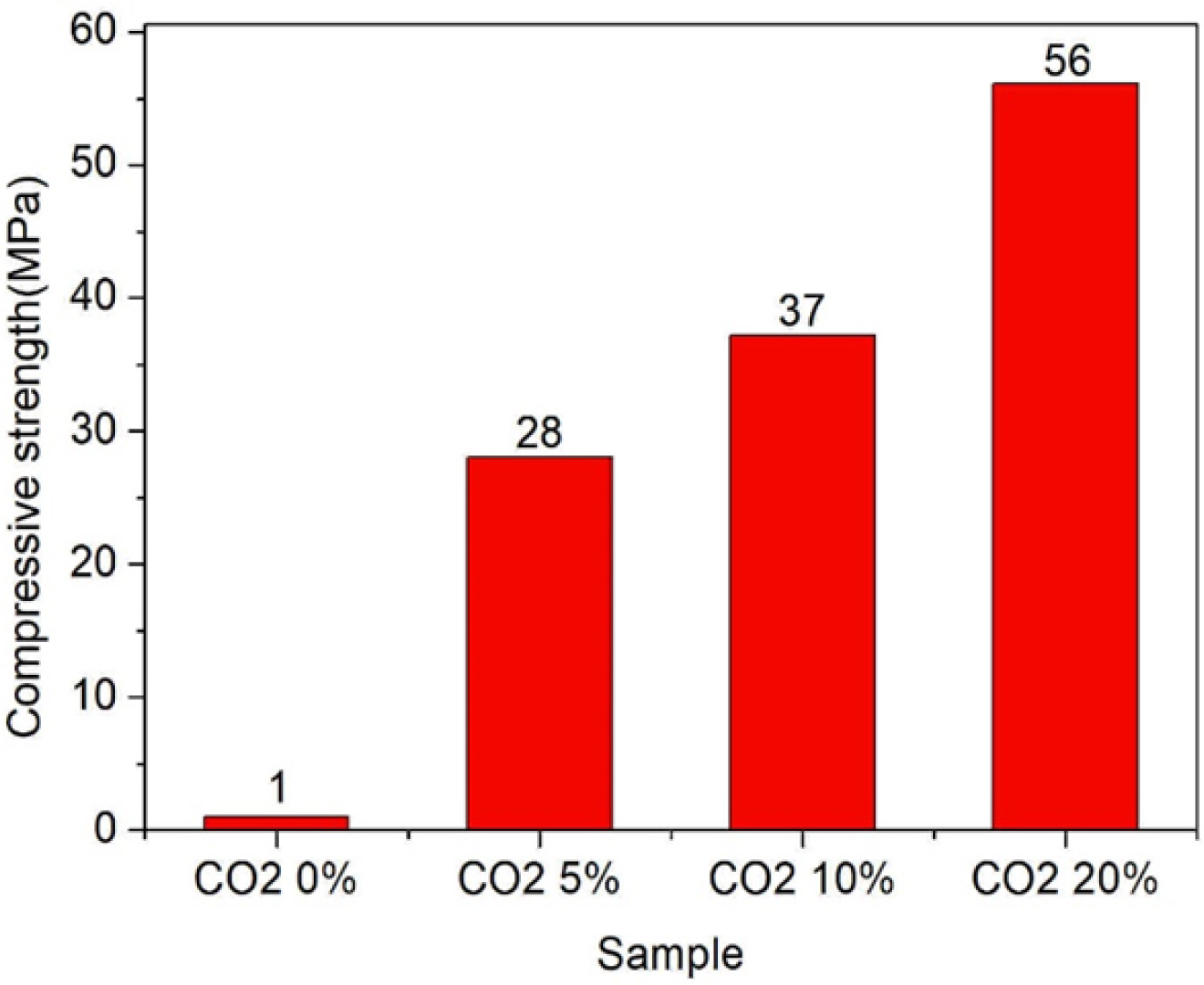
Fig. 6 shows the results of compressive strength measurement at curing 7 days with respect to the CO2 concentration. When the CO2 concentration was 5, 10, and 20%, the compressive strength was measured to be 28, 37, and 56 MPa, respectively, which indicated that the compressive strength increased with increasing CO2 concentration. For Type 1 (typically referring to Portland cement) in accordance with the Korean Industrial Standards (KS), the curing 7 days compressive strength and curing 28 day compressive strength are required to be 22.5 MPa and 42.5 MPa [11], respectively. In the present study, the CSC samples subjected to carbonation curing exhibited a higher curing 7 days compressive strength than Type 1 cement under the KS regardless of the curing conditions. Also, the samples cured at a CO2 concentration of 20% showed a much higher compressive strength at curing 28 days than Type 1 cement under the KS. However, the curing 7 days compressive strength of the samples subjected to moist curing, where the CO2 concentration was 0%, was measured to be 1 MPa or lower, which caused difficulty in measuring compressive strength in practice. These results indicated that carbonation curing was critical to CSC being able to implement their desired properties [7, 8]. Based on this nature, it will be possible to design the properties of CSC by adjusting the curing conditions. Also, it was found that these CSC samples were able to provide mechanical properties that were equivalent to or higher than those of Type 1 cement under the KS. Therefore, these materials are considered to have a high potential for extended applications, such as blocks, ACL, panels, and rapid-hardening cement.
|
Fig. 3 XRD patterns of CSC paste with CO2 concentration. |
|
Fig. 4 DSC curves of CSC paste with CO2 concentration. |

|
Fig. 5 DSC curves of CSC paste with curing at CO2 concentration 20%. |
|
Fig. 6 Compressive strength of CSC with CO2 concentration at curing of 7 days. |
In the present study, CSC was fabricated using domestic raw materials, and its physical behavior was observed while varying the CO2 concentration in the curing atmosphere.
1) In the samples subjected to moist curing where the CO2 concentration was 0%, almost no reaction products were found regardless of the curing time. Also, the compressive strength at curing 7 days was measured to be 1 MPa, which indicated that those samples had practically failed to implement their desired properties.
2) The mineral phases of CSC were analyzed according to the CO2 concentration. It was found that the CaCO3 peak increased in size with increasing CO2 concentration. Here, CaCO3 was a reaction product of the carbonation reaction. However, it was difficult to clearly confirm the formation of amorphous SiO2.
3) Differential scanning calorimetry results showed that for the samples cured at a CO2 concentration of 5% and 10%, the CaCO3 peak was observed when the curing time was 24 h or longer. However, when the CO2 concentration was 20%, the onset of CaCO3 formation was earlier, i.e., when the curing time was 10 hours. These results suggested that the carbonation reaction accelerated with increasing CO2 concentration, thereby increasing the pace at which its properties were imple- mented. However, there was no significant difference in the total amount of CaCO3 formed regardless of the curing conditions.
4) Differential scanning calorimetry results showed that there was a peak near 100-200oC arising from 2H4SiO4, an intermediate compound generated during the formation of amorphous SiO2. This was indicative of the ongoing formation of amorphous SiO2 while implying that the carbonation reaction was continuing.
5) For the samples cured at a concentration of 5, 10, and 20%, curing 7 days compressive strength was 28, 37, 56 MPa, respectively. All samples subjected to carbonation curing exhibited curing 7 days compressive strength equivalent to or higher than Type 1 cement under the KS. Also, the samples cured at a CO2 concentration of 20% showed a higher compressive strength at curing 28 days age than Type 1 cement under the KS. These results suggested that the CSC samples fabricated in the present study provided excellent early-stage strength.
6) The major findings of the present study suggest that CSC requires a carbonation reaction to function properly. Based on this nature, it will be possible to design the properties of CSC by adjusting the CO2 concentration in the curing atmosphere. Thus, these materials are considered to have a high potential for extended applications as eco-friendly construction materials, such as blocks, ACL, panels, and rapid-hardening cement.
- 1. K. Svensson, A. Neumann, F. F. Menezes, and C. Lempp, Appl. Sci. 8[2] (2018) 304.
-

- 2. W. Ashraf, J. Olek, and V. Atakan, in Proceedings of the SCMT4 (4th International Conference on Sustainable Construction Materials and Technologies), August 2016, edited by Warda Ashraf (University of Navada Press, 2019) p. 7-11.
-

- 3. W. Ashraf, J. Olek, and V. Atakan, in Proceedings of the SCMT4 (4th International Conference on Sustainable Construction Materials and Technologies), August 7-11, (2016) edited by Warda Ashraf (University of Navada Press, 2019)
-

- 4. K. Wang, L. Ren, L. Yang, and J. Mater. 11[8] (2018) 1474.
-

- 5. M. Beyene, M. Beyene, and R. Meininger, in Proceedings of the 17th EMABM (17th Euroseminar on Microscopy Applied to Building Materials), May 20-23, (2019), edited by John Hughes (UWS Press, 2019) p. 214-219.
- 6. W. Ashraf, J. Olek, and J. Jain, Cem Concr Res. 100 (2017) 361-372.
-

- 7. B. Lu, C. Shi, and G. Hou, Const Build Mater. 188 (2018) 417-423.
-

- 8. W. Ashraf and J. Olek, J Mater Sci. 51 (2016) 6173-6191.
-

- 9. W. Ashraf and J. Olek, J. CO2 Util. 23 (2018) 61-74.
-

- 10. C. Villani, R. Spragg, R. Tokpatayeva, J. Olek, and W. J. Weiss, in Proceedings of the 4th ICDCS (4th International Conference on the Durability of Concrete Structures), July 2014, edit by William Jason Weiss (Purdue Scholarly Publishing Services, 2014) p. 24-26.
-

- 11. Korean standards, NO. KS L 5201 (2016) p. 1-7.
 This Article
This Article
-
2021; 22(1): 25-30
Published on Feb 28, 2021
- 10.36410/jcpr.2021.22.1.25
- Received on May 6, 2020
- Revised on Aug 19, 2020
- Accepted on Sep 11, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ki-Yeon Moon
-
Department of Research and Development, Korea Institute of Limestone and Advanced Materials, 18-1 Udeok-gil, maepo-eup, Danyang, Chungbuk 27003, Korea
Tel : +82-43-422-2096 Fax: +82-43-422-5581 - E-mail: kymoon@kilam.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.