- Enhanced the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode by ZrO2 coating
Sung-Joo Joa,#, Hyun-Soo Kimb,#, Do-Yeong Hwanga, Bong-Soo Jinb, Seong-Ju Simb, Jae-Soo Shina and Seung-Hwan Leea,*
aDepartment of Advanced Materials Engineering, Daejeon University, Daejeon 34520, Republic of Korea
bNext-Generation Battery Research Center, Korea Electrotechnology Research Institute, Changwon, 641-120, South Korea
ZrO2 coated Ni-rich
LiNi0.8Co0.1Mn0.1O2 cathode has
enhanced electrochemical performance and stability compared with the pristine.
The discharge capacity of both samples have no significant difference. However,
the 1.77 wt% ZrO2 coated LiNi0.8Co0.1Mn0.1O2
material delivers enhanced charge-discharge cycling (capacity retention of 97.1
% after 100 cycles at 0.5 C) while the capacity retention of pristine is 94.8 %
under the same condition. Based on these, we can infer that the ZrO2
coated LiNi0.8Co0.1Mn0.1O2 material
shows great cyclability, originated from suppressing undesirable side reaction
and decrease effectively electrolyte decomposition reaction. Therefore, the ZrO2
coated Ni-rich LiNi0.8Co0.1Mn0.1O2
cathode can be considered as an effective strategy for long-life Li-ion
batteries.
Keywords: ZrO2, LiNi0.8Co0.1Mn0.1O2, electrochemical performances
Li-ion batteries (LIBs) have attracted a lot of attention
for electric vehicles (EVs), mobile devices, plug-in hybrid electric vehicles
(PHEVs), hybrid electric vehicles (HEVs) and residential energy storage
applications. This is because LIBs have an advantage of high operating voltage
and energy densities, power performance, low price, long lifetime
and good stability compared to traditional batteries [1]. However,
LIBs suffer from severe potential deposition and capacity fading when
charge-discharge cycling. The energy density, lifetime, material
cost and stability are essential criteria for assessing if they can
be used in practical application in LIBs. Because the energy density of the
LIBs is extremely dependent on the cathode material, thus, significant efforts
have been conducted for high-performance cathode material [2].
In Particular, the layered nickel-rich oxide LiNi1-x-y CoxMnyO2 (x
> 0.8, NCM) cathodes have drawn substantial interest [3]. Among various
cathode materials, Nickel-rich NCM shows good electrochemical performance and it is considered as one
of most effective cathode materials owing to low price and elevated electrochemical
performances [4-5]. The superior energy density,
excellent thermal stability and cyclability of Ni-rich NCM made it an ideal
candidate for next-generation cathode material. However, high Ni content also
results in irreversible capacity and poor cycling performance owing to the
similar ionic radius of Ni2+ and Li+ [6]. Especially, at
higher temperature, the structural instability can lead to rapid performance
fading of Ni-rich NCM [7]. To overcome these problems, various strategies such as TiO2,
SiO2 and carbon coating and/or V, B and Mo doping [8-13] have been
performed.
Among them, in this work, we synthesized well-crystallized
ZrO2 coated LiNi0.8Co0.1Mn0.1O2
(NCM811) in order to stabilize the structure and improve electrochemical performances. Consequently,
the ZrO2 coated NCM811 can be considered for advanced cathode
material.
Co-precipitation method was used to synthesize the Ni-rich
NCM811 powders. the Ni-rich NCM811 powders were
synthesized by using co-precipitation method NiSO4-6H2O,
CoSO4-7H2O and MnSO4-H2O were
prepared to assemble Ni0.8Co0.1Mn0.1(OH)2
precursor. The chelating agent were comprised of NaOH and NH4OH
solution. The as-prepared spherical Ni0.8Co0.1Mn0.1(OH)2
precursor was used to mix with LiOH-H2O at a molar ratio
1.05 : 1. After that, the mixture was fired at 480 oC for 5 h. Then
the mixture was cooled down at 750 oC in air for 15 h. For surface
coating, all samples of ZrO2 coated Ni-rich NCM811 were synthesized
exploiting different ratios of ZrO2: Ni-rich NCM811 and then heated
at 500 oC in air for 5 h.
The cathodes were prepared by mixing Ni-rich NCM811
powder, conductive carbon black binder and polyvinylidene fluoride in the
weight ratio of 96 wt%: 2 wt%: 2 wt%. Afterwards, N-Methyl pyrrolidinone (NMP)
solvent was included in the cathodes. The 2032 coin cells were compounded with
Lithium metal disc in argon-gas-filled glove box as an anode. The electrolyte was
consisted of 1 M LiPF6 in ethylene carbonate, dimethyl carbonate,
and ethyl methyl carbonate (EC:DMC:EMC 1:1:1 in volume). The polyethylene (PE,
20 μm) was used as a separator.
The XRD (Philips, X-pert PRO MPD) was used to measure the
crystalline phase of samples. The FE-SEM (Hitachi
S-4800) was prepared to observe the morphology of the
pristine and ZrO2 coated samples. The electron diffraction
spectroscopy (EDS) was used in order to identify the component elements on the
surface of particles. The electrochemical performances were mea- sured with an equipment
(TOSCAT-3100, Toyo system). The Electrochemical Impedance
Spectroscopy (EIS) was measured with frequency range from 1 MHz to 100 mHz and
the amplitude of the AC signal of 10 mV.
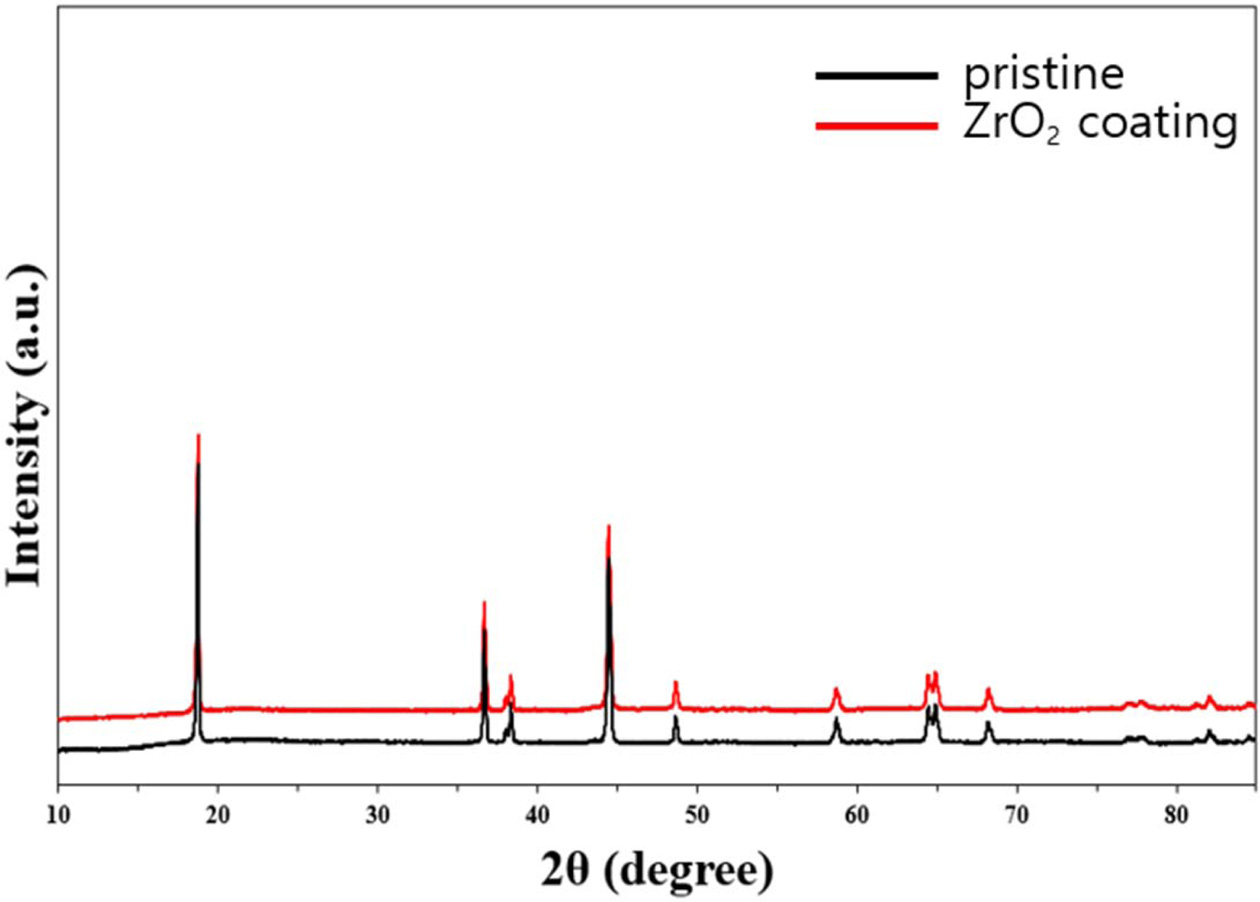
Fig. 1 shows the X-ray diffraction (XRD) spectra of the
pristine and ZrO2 coated NCM811. The shape of all samples is
identical without secondary peaks since ZrO2 coating do not affect
the crystal structure of NCM811 materials. Overall, main diffraction peaks are
indexed as a layered oxide structure in the hexagonal α-NaFeO2
structure with a space group of R-3m [14]. Both obvious splitting of the
(018)/(110) and (006)/(102) peaks suggests well-ordered layered structure.
Also, the ratio of the intensities of (003) and (104) peaks (I(003)/I(104)) are
regarded as index of cation mixing [15]. The cation ordering is resulted from
the similar radius of Ni2+ (0.69 Å) and Li+ (0.76 Å) at
the 3a site [16]. The I(003)/I(104) of all samples are above 1.2, demonstrating
superior cation ordering, which is regarded as an one of the important factor
for good electrochemical performance.
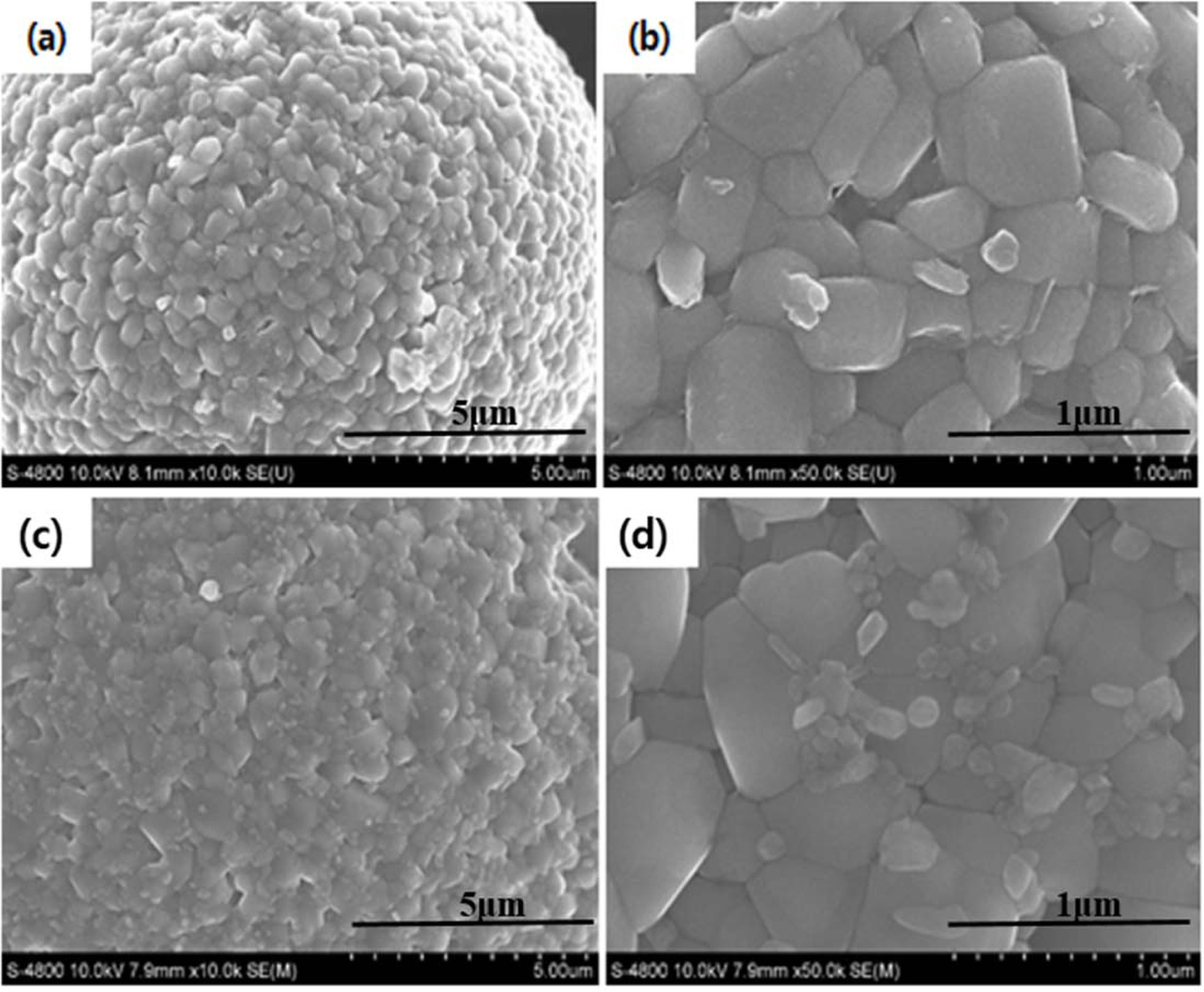
Fig. 2 shows the FESEM images of (a-b) pristine and (c-d)
ZrO2 coated NCM811 cathode materials. The pristine and ZrO2
coated NCM811 sample have similar the morphology (particles size and shape)
[17]. All samples have spherical morphology secondary particles with
the average size of 15 ~ 20 μm, which is composed
of numerous primary particles about 300 ~ 500 nm in diameter. The
benefit of spherical secondary particle can achieve both high tap density and
energy density. The surface of ZrO2 coated NCM811 shows slightly
rough as compared to pristine NCM811.
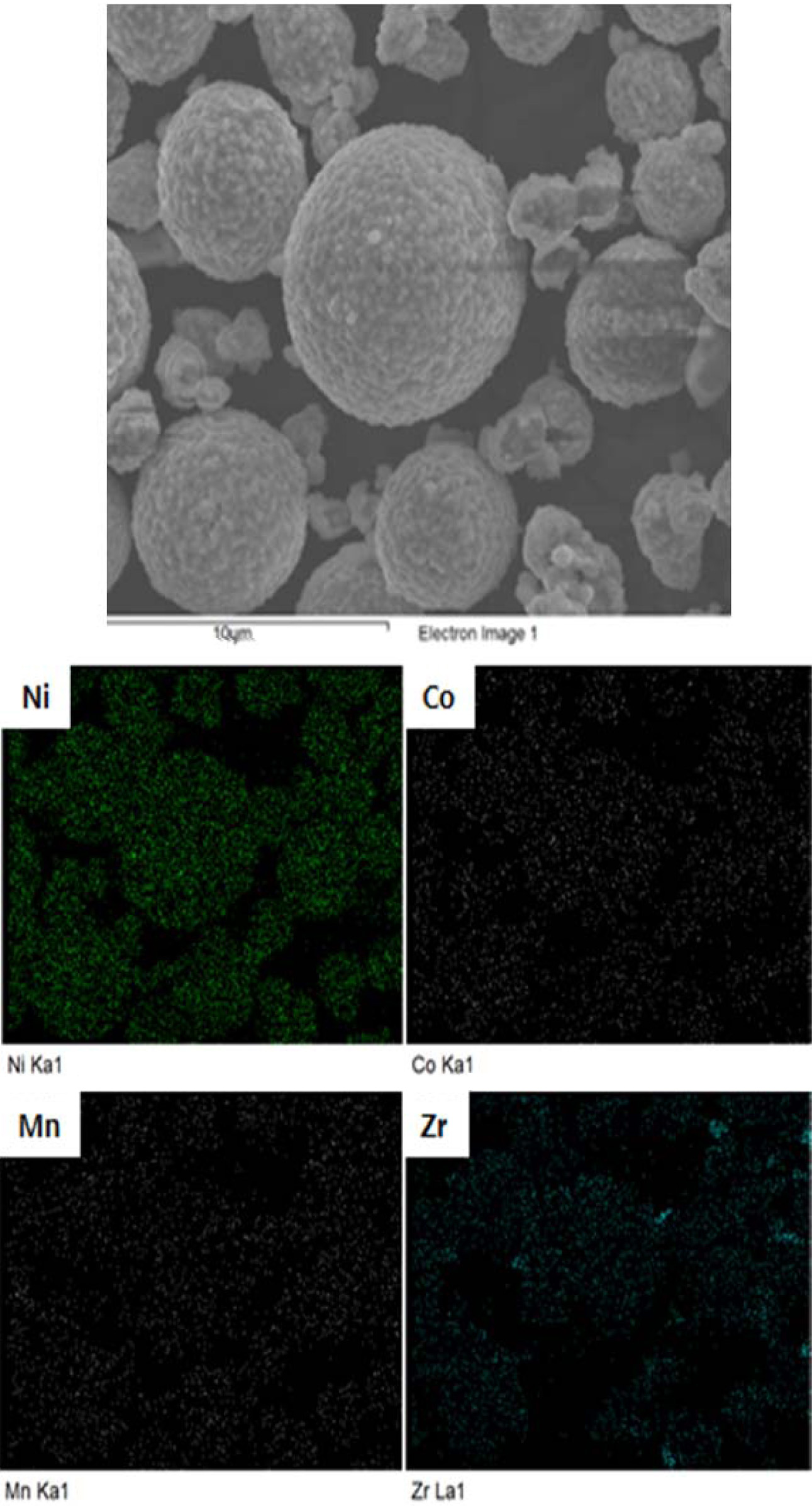
EDS (Energy Dispersive X-ray Spectroscopy) spectrum analysis
of ZrO2 coated NCM811 sample are conducted, as shown in
Fig. 3. It can be confirmed that the Ni, Co, Mn and Zr are equally distributed
and the amount of ZrO2 is 1.77 wt%. The ZrO2 coating can
decrease the contact area between the cathode and the electrolyte, resulting in
suppressing the electrolyte degradation while charge-discharge is cycling [18].
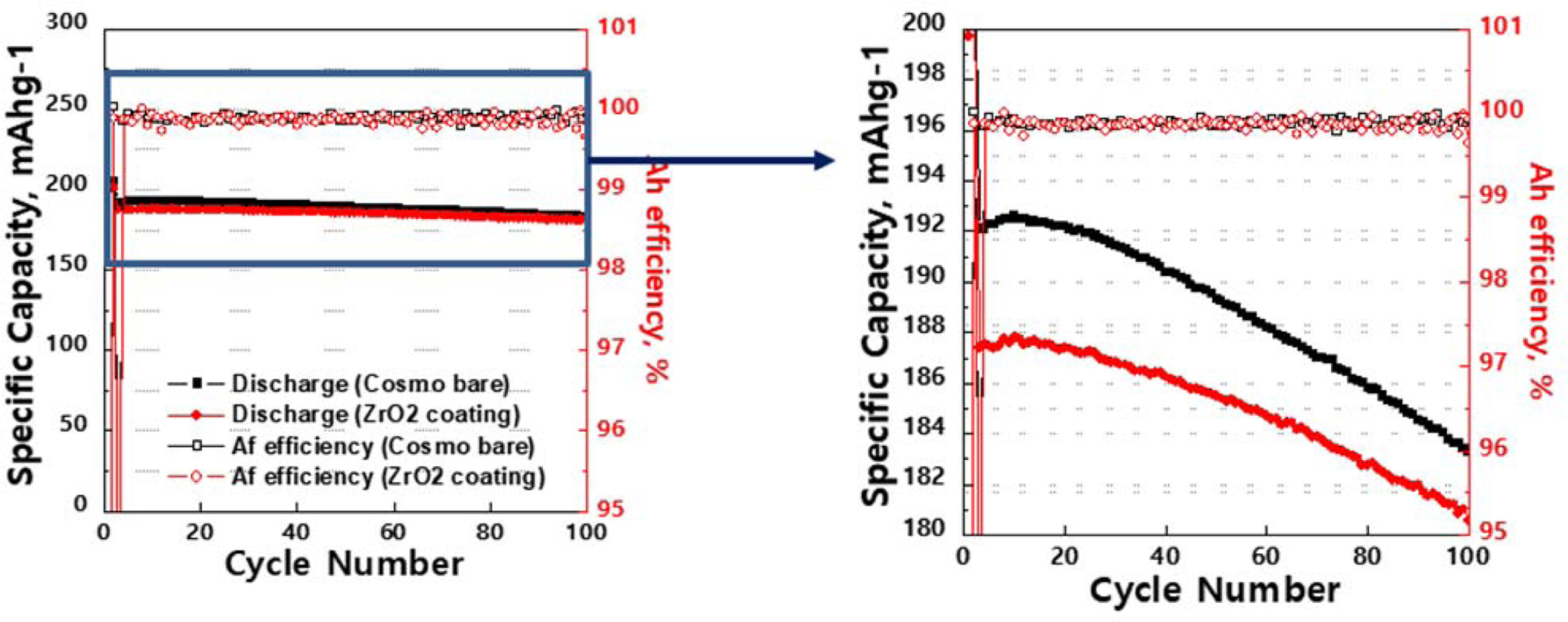
In order to investigate the long-term performance, Fig. 4
presents the cycle performance of the pristine and ZrO2 coated
NCM811 with vinylene carbonate (VC) and propane sultone (PS) additives. The
cycle performances are measured at 0.5 C in the voltage between
3.0 and 4.3 V. It has been proved that additives can increase
the cyclability for both samples since additive is designed to form the
passivating layer on the surface, which can deactivate the active “catalytic”
centers [19]. The ZrO2 coated sample exhibits lower capacity fading
than pristine sample after 100 charge-discharge cycles. This is because ZrO2
coating could stabilize the interface between NCM811 and
electrolyte. The ZrO2 coated and pristine NCM811 have
capacity retentions of 97.1% and 94.8%, respectively, after 100 cycles.
We can confirm that the ZrO2 coating effectively delay
the capacity fading. In other words, it is demonstrated that the ZrO2
coating on the Ni-rich NCM811 leads to superior cycle performance. This is
because the bond dissociation energies of Zr-O, Ni-O, Co-O and Mn-O have 760,
391.6, 368 and 402 kJ·mol-1
at 25 oC. The excellent cyclability of ZrO2 coated
Ni-rich NCM811 is closely related to the stable crystal structure, resulting
from stronger iono-covalent of Zr-O [20].
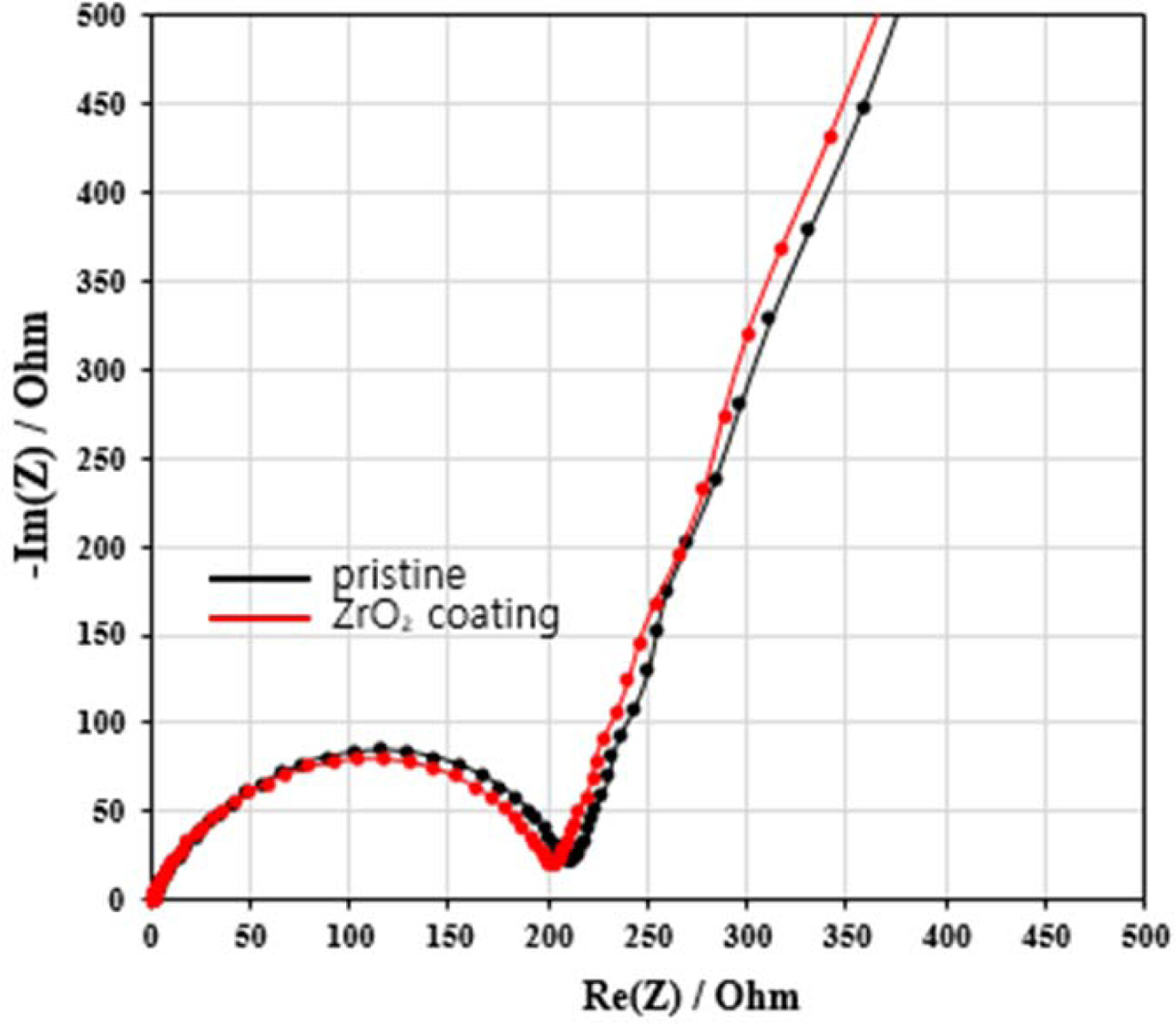
Electrochemical Impedance Spectroscopy (EIS) measurements are used in order to
investigate the charge-transfer kinetics of the electrodes. Nyquist plots of
the pristine and the ZrO2 coated NCM811 are measured after
first cycle in the voltage range 3.0 – 4.3 V. Usually,
Nyquist plot is composed of semicircle and one slope. Fig. 5 shows the EIS
curves of the pristine and ZrO2 coated NCM811. All samples have same
electrolyte resistance (Re) in the high-frequency because we used
same electrolyte. The medium-frequency semicircle is contributed to the
resistance of Rct at the interface between the surface of the
particles and the electrolyte [21]. The straight line of low frequency region
is the Warburg impedance (W). There is a gap between pristine and ZrO2
coated Ni-rich NCM811 for Rct. The Rct of ZrO2
coated NCM811 is lower than the Rct of pristine. After first cycle,
the Rct of pristine is 211.5 Ω, while the Rct of ZrO2
coated NCM811 is 203.7 Ω. This is because the ZrO2 coating layer
suppresses the formation of SEI due to accelerating the Li+ movement
at the surface of cathode [21]. Based on this, we can infer that ZrO2
coating can reduce side reaction, cell impedance increases and Rct.
Therefore, the better cycle performance of ZrO2 coating can be
explained by lower Rct.
|
Fig. 1 |
|
Fig. 2 |
|
Fig. 3 |
|
Fig. 4 |
|
Fig. 5 |
In this work, we prepare the ZrO2 coating on
the surface of Ni-rich NCM811 cathode in order to enhance the electrochemical
performance. The ZrO2 coating has no effect on the crystal structure,
particle size and shape. It is proved that ZrO2 can not only
stabilize the NCM811 structure but also improve the electrochemical
performances due to the deceasing side reaction and the increasing
electrochemical reaction kinetics of lithium ion. These factors can enable good
cycling performance. Therefore, the ZrO2 coating lead to being
considered as an efficient way for Ni-rich NCM811 cathode.
This research was supported by the Daejeon University fund (2020)
- 1. W.S. Cho, S.M. Kim, K.W. Lee, J.H. Song, Y.N. Jo, T.E. Yim, H.T. Kim, J.S. Kim, and Y.J. Kim, Electrochim. Acta. 198 (2016) 77-83.
-

- 2. Q. Hou, G. Cao, P. Wang, D. Zhao, X. Cui, S. Li, and C. Li, J. Alloy. Comp. 747 (2018) 796-802.
-

- 3. J.Z. Kong, Y. Chen, Y.Q. Cao, Q.Z. Wang, A.D. Li, H. Li, and F. Zhou, J. Alloy. Comp. 799 (2019) 89-98.
-

- 4. A.Y. Kim, F. Strauss, T. Bartscch, J.H. Teo, T. Hatsukade, A. Mazilkin, J. Janek, P. Hartmann, and T. Brezesinski, Chem. Mater. 31 (2019) 9664-9672.
-

- 5. C.S. Yoon, H.H. Ryu, G.T. Park, J.H. Kim, K.H. Kim, and Y.K. Sun, J. Mater. Chem. A. 6 (2018) 4126.
-

- 6. S.J. Sim, S.H. Lee, B.S. Jin, and H.S. Kim, Sci. Rep. 9 (2019) 8952.
-

- 7. X. Li, K. Zhang, M. S. Wang, Y. Liu, M. Z. Qu, W. Zhao, and J. Zheng, Sustain. Energy Fuels. 2 (2018) 413-421.
-

- 8. Y.H. Cho, Y.S. Lee, S.A. Park, Y.I. Lee, and J.P. Cho, Electrochim. Acta 56.1 (2010) 333-339.
-

- 9. S.H. Lee, G.J. Park, S.J. Sim, B.S. Jin, and H.S. Kim, J. Alloy. Comp. 791 (2019) 193-199.
-

- 10. Q. Hou, G. Cao, P. Wang, D. Zhao, X. Cui, S. Li, and C. Li, J. Alloy. Comp. 747 (2018) 796-802.
-

- 11. S.J. Sim, S.H. Lee, B.S. Jin, and H.S. Kim, Sci. Rep. 9 (2019) 8952.
-

- 12. L. Pan, Y. Xia, B. Qiu, H. Zhao, H. Guo, K. Jia, Q. Gu, and Z. Liu, J. Power Sources. 327 (2016) 273-280.
-

- 13. Y. Li, Q. Su, Q. Han, P. Li, L. Li, C. Xu, X. Cao, and G. Cao, Ceram. Int. 43 (2017) 3483-3488.
-

- 14. G. Shang, Y. Tang, Y. Lai, J. Wu, X. Yang, H. Li, C. Peng, J. Zheng, and Z. Zhang, 423 (2019) 246-254.
-

- 15. J. Yang and Y. Xia, J. Electrochem. Soc. 163 (2016) 2665-2672.
-

- 16. J.Z. Kong, Y. Chen, Y.Q. Cao, Q.Z. Wang, A.D. Li, H. Li, and F. Zhou, J. Alloy. Comp. 799 (2019) 89-98.
-

- 17. F. Schipper, H. Bouzaglo, M. Dixit, E. M. Erickson, T. Weigle, M. Talianker, J. Grinblat, L. Burstein, M. Schmidt, J. Lampert, C. Erk, B. Markovsky, D.T. Major, and D. Aurbach, Adv. Energy Mater. 8.4 (2018) 1701682.
-

- 18. Z. Wang, E. Liu, L. Guo, C. Shi, C. He, J. Li, and N. Zhao, Surf. Coat. Technol. 235 (2013) 570-576.
-

- 19. Z.D. Li, Y.C. Zhang, H.F. Xiang, X.H. Ma, Q.F. Yuan, Q.S. Wang, and C.H. Chen, J. Power Sources. 240 (2013) 471-475.
-

- 20. S. Chang, Y. Chen, Y. Li, J. Guo, Q. Su, J. Zhu, G. Cao, and W. Li, J. Alloy. Comp. 781 (2019) 496-503.
-

- 21. J.W. Seok, J. Lee, T. Rodgers, D.H. Ko, D.H. Ko, and J.H. Shim, Trans. Electr. Electron. Mater. 20 (2019) 548-553.
-

 This Article
This Article
-
2020; 21(6): 731-735
Published on Dec 31, 2020
- 10.36410/jcpr.2020.21.6.731
- Received on Jul 30, 2020
- Revised on Sep 2, 2020
- Accepted on Oct 2, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seung-Hwan Lee
-
Department of Advanced Materials Engineering, Daejeon University, Daejeon 34520, Republic of Korea
Tel : +82-42-280-2414 - E-mail: shlee@dju.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.