- The effect of titanium and zirconium oxides additives on thermal properties of magnesium aluminate spinel
Fahad Albanumay*, Naif Alqahtani, Basheer Alshammari, Haytham Alodan, Turky Alopily and Mohammed Muhawes
Material Science Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh 11442, Saudi Arabia
Magnesium aluminate spinel
(MgAl2O4) composites were prepared by mixing the
commercial Alumina and Magnesium Oxide as starting raw materials. Titanium
oxide (TiO2) and Zirconium oxide (ZrO2) were used as
additives. The mixtures were prepared as by milling of five different
combinations using zirconia balls for 1 hour each. Then Cold Isostatic Press
(CIP) at 200 MPa pressed the batches. The material properties, such as porosity
and density, and thermal expansion of the composites were characterized by
X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), and the
dilatometer measurements. MgAl2O4 ceramic composites are
composed of spinel and garnet structures. The thermal expansion coefficients
(CTE) of MgAl2O4 composites with and without TiO2
and ZrO2 additives under different temperature condition (25 oC
to 1,300 oC) were characterized for spinel thermal expansion
study and it shows that the comparison between the five different sample
combinations at 1,300 oC, adding TiO2 or ZrO2
by small percentage gives the lowest CTE (9.89E-06, 1.02E-05) respectively, but
increasing ZrO2 increases the CTE.
Keywords: Magnesium aluminate spinel, Thermal expansion, Titanium oxide, Zirconium oxide
Magnesium aluminate spinel (MgAl2O4)
has been known as a technologically vital material which has many applications
in many different fields, such as in high temperature ceramics [1], fabricating
transparent ceramics [2, 3], and catalyst support [4, 5], nuclear
waste management applications [6], humidity sensors [7] and cement castables
[8].
Magnesium aluminate spinel is an important refractory
material because of its excellent properties such as high melting
point that reach 2,105 oC, low thermal expansion,
high thermal spalling, and corrosion resistance [9]. Synthesis MgAl2O4
is very challenging from the solid-state reaction route since it needs
repetitive grinding and calcination steps. Some common methods, such as plasma
spray decomposition of oxide and hydrothermal synthesis can be used to prepare
high quality pure material. But these methods do not get a lot of attention in
the commercial circle because of the use of expensive raw
materials and the requirement of many processing steps [10].
It is important for the side walls, the checker work of
glass tank furnace regenerators and the bottom of steel-teeming ladles. Thermal
properties are important for all these applications. Increasing the temperature
of a material increases the amplitude of vibration of the atoms and results in
on overall increase in the volume of the material. This expansion is very
critical for the structural integrity and spoiling property of the material
[11]. The crystallographic structure of the MgAl2O4
spinel is simple cubic with eight formula units in one cubic unit
cell [12]. The effect of particle size distribution of the
spinel on the ceramic mechanical properties and thermal shock performance has
been previously studied and mechanical properties of composites decreased
significantly with increasing spinel content due to thermal expansion mismatch
[13].
The crystallographic structure of the MgAl2O4
spinel has been reported by Kingrey [14] and illustrated in Fig. 1. The generic
formula of this spinel group is AB2O4, which “A”
represents a divalent metal ion such as magnesium, iron, nickel, manganese and
zinc. The “B” represents trivalent metal ions such as aluminum, iron, chromium
and/or manganese. In this study, the “A” is the magnesium and “B” is aluminum
and the spinel structure is named after the mineral spinel (MgAl2O4)
[15]. The positions of the A ions are nearly identical to the positions
occupied by carbon atoms in the diamond structure. This could explain the
relatively high hardness and high density typical of this group. The
arrangements of the other ions in the structure conform to the symmetry of the
diamond structure. However, they disrupt the cleavage as there are no cleavage
directions in any member of this group.
The coefficient of thermal expansion (CTE) is a fundamental
engineering material property that used to express the dimensional change
(volume, length, etc.) of a material in response to temperature change. The
thermal expansion/ contraction behavior due to daily and seasonal temperature
changes plays an important role on the degree of opening/closing of transverse
cracks in concrete structures [16].
The objective of this study is the investigation the
influence of TiO2 and ZrO2 additives on the spinel
material properties and the thermal expansion. The MgAl2O4 spinel
without additives will be set as a baseline composite that will be compared
with the spinel with TiO2 and ZrO2 additives. Spinel has
high melting points that allow measurements to be made over wide temperature
ranges. Five different spinel compositions, were tested for thermal expansion
characteristics.
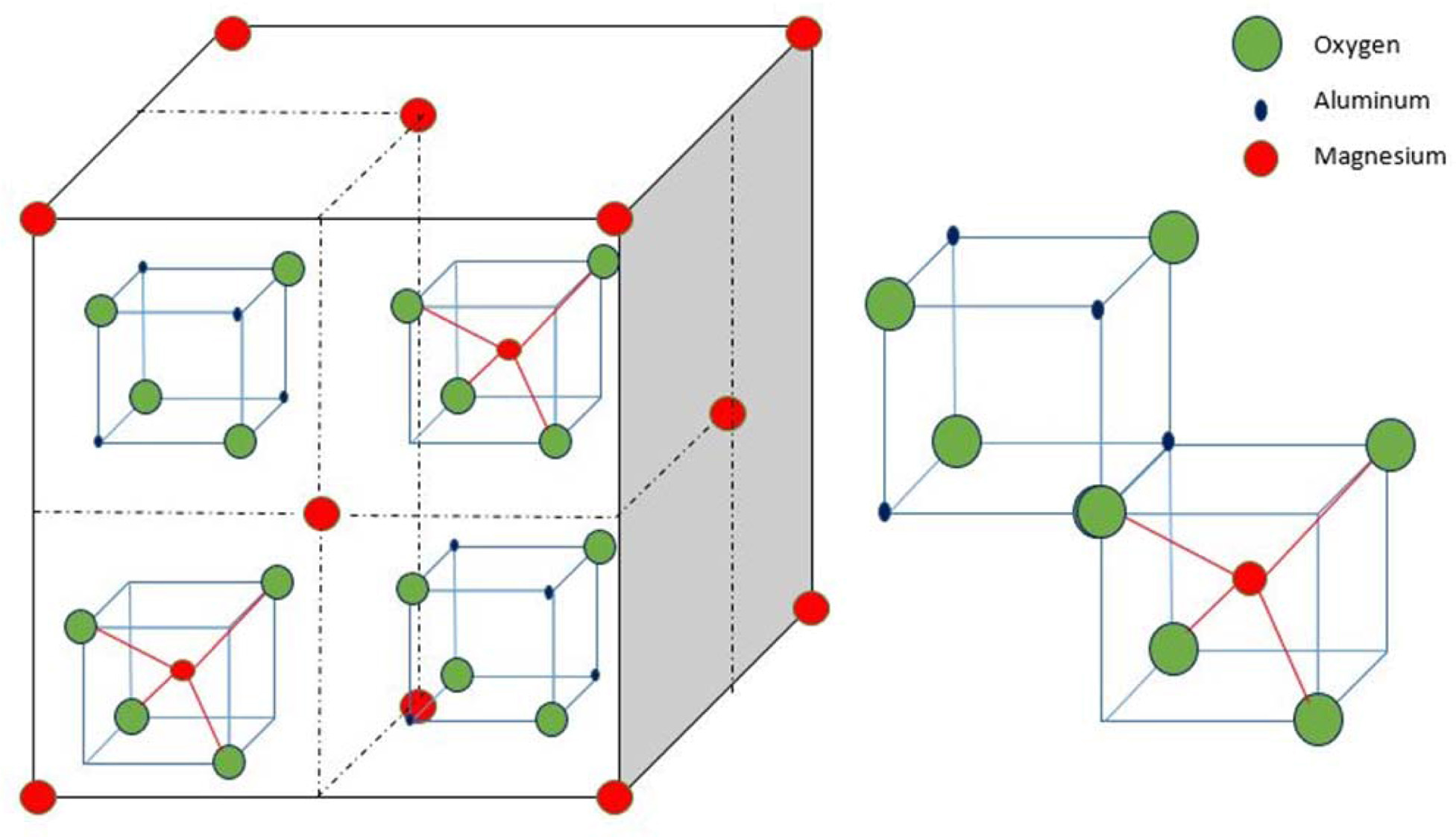
|
Fig. 1 Crystallographic structure of MgAl2O4 spinel (Redrawn from Kingrey [14]). |
Commercial Alumina (Al2O3 with 94%
purity) and Magnesium Oxide (MgO with 99% purity) were used as raw materials of
the MgAl2O4 spinel. Titanium oxide (TiO2 with
99% purity) and Zirconium oxide (ZrO2 with 99% purity) were used as
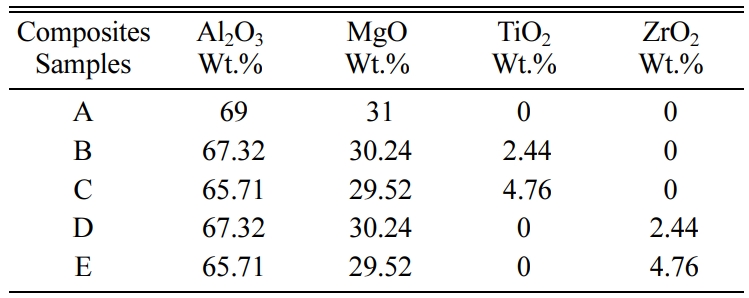
additives. Five samples were prepared for this study, sample A is a mixture of
Al2O3 and MgO without any additives, sample B is a
mixture of the MgAl2O4 spinel adding 2.44% Titanium oxide
of the mixture weight, sample C is a mixture of the MgAl2O4
spinel adding 4.76% Titanium oxide of the mixture weight, sample D is a mixture
of the MgAl2O4 spinel adding 2.44% Zirconium
oxide of the mixture weight, and sample E is a mixture of the MgAl2O4
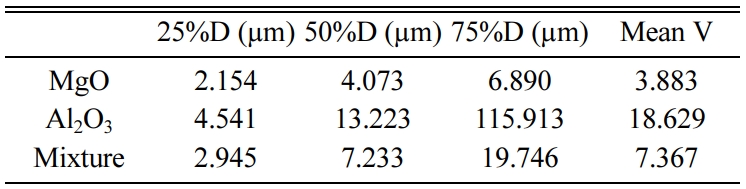
spinel adding 4.76% Zirconium oxide of the mixture weight. Table 1 shows the
particle size analysis for MgO, Al2O3 and the mixture
after milling for 1 h. Table 2 shows all samples with their specific weights.
The five batches of 250 gm powder was prepared by milling of five different
combinations using 10 small ZrO2 balls for one hour each. Then, the
batches were pressed by Cold Isostatic Press (CIP) at 200 MPa.
All prepared samples then were slowly heated with the rate
of 1 oC per minute to 100 oC with holding time
60 minutes, then to 1,750 oC with rate of 5 oC
per minute and holding time 60 min.
For particle size analysis, MgO and Al2O3
powders were analyzed before and after milling the mixtures for one hour using
“Shimadzu SALD-2300”. The porosity (ϕ) and density (ρ) of the
sintered samples was determined by the Archimedes method with water
as liquid media. The following equations used to calculate the porosity and the
permeability for the spinel:
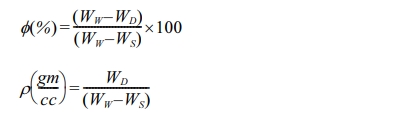
where:
WD = is the wt. of dried sample
(g).
Ws = is the wt. of sample measured in
water (g).
Ww= is the wt. of water soaked
after 24 h (g).
Thermal expansion was investigated using dilatometer
(DIL 402 PC, NETZSCH Geratebau GmbH, Germany) with temperature range
25-1,300 oC [17].
To calculate the Coefficient of Thermal Expansion (CTE)
the following equation were used [18]:

where:
αl =
thermal expansion coefficient for the parameter l, K-1
εl = dl/l is
the strain for the parameter l, and,
T = is the temperature, K.
The effect of adding TiO2 and ZrO2
to the spinel mixture on the coefficient of thermal expansion can be calculated
using equation (X) by setting sample A (when we have MgAl2O4
spinel without any additives) as a baseline to compare it with the other
samples.
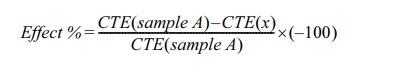
For thermal shock resistance study, the samples were exposed
to a temperature of 1,000 oC for 15 min, each thermal shock
cycle involved heating of the sample to 1,000 oC for 15 min in
an electric furnace, the first two cycles quenched at room temperature for 15
min, and the third cycle quenched in cold water [19].
Finally, for the X-ray analysis, the sintered samples were
ground into powder using milling. The powder was placed on a sample holder and
was irradiated by a monochromatic X-ray beam from an X-ray tube. The samples
were examined using scanning electron microscopy
(SEM) (MiniFlex 600, Rigaku, Japan) operated at 20 KV. Cu-Kα radiation passed
through nickel filter was used. The range of scanning angle (2θ) used was 0o-70o.
XRD of 1,750 oC for one hour sintered sample was done to see
the spinel phase.
The results of XRD, porosity and density, thermal shock
resistance, scanning electron microscopy (SEM), and the thermal expansion of
the spinel mixtures are presented in this section. All the
analysis and integration of the results obtained previously
discussed also in this section.
Phase
composition by X-Ray Diffraction (XRD)
Fig. 2 shows the XRD patterns of the samples after
sintering at 1,750 oC for 1 h and milling for 1 h. In the first
combination, Al2O3 was transformed completely to the
magnesium aluminate spinel but there are still small patterns of MgO, and the
same happened in the second combination when TiO2 added but the MgO
patterns were smaller than what happened in the first combination.
X-Ray Diffraction (XRD) patterns of non-additive and
additive contained batches are shown in Fig. 2. The XRD pattern of 1,750 oC
sintered compositions shows the presence of spinel in both non-additive and
additive contained batches. As the percentage of TiO2 was increased,
amount of spinel formation was also increased in all batches,
compared to that of no additives and ZrO2 contained
batches. The highest spinel peak intensity was observed in TiO2
containing compositions. This clearly indicates a higher
rate of spinel formation occurring in these compositions. The presence of only
spinel phase was observed in 5% TiO2 containing sample, this
observation of complete solid solubility of free MgO in spinel phase with TiO2
finds similarity with the work of Sarkar and Bannerjee [20]. However, small
peaks of unreacted phases were detected in all the other samples, indicating
incompletion of spinel formation reaction in the batch.
This is supported by comparing with the work of Quénard et
al. [21]. In addition to the spinel and ZrO2 phases, the presence of
a small amount of MgO. The relative intensity of the (200) MgO peak is similar
whatever the ZrO2 content.
When TiO2 was increased in the third
combination, MgO and Al2O3 were completely transformed to
magnesium aluminate spinel and there was small pattern of TiO2. When
ZrO2 was added, Al2O3 was transformed
completely to the magnesium aluminate spinel but there are still small patterns
of MgO and ZrO2, and when the ZrO2 percentage was
increased in the fifth combination the patterns of MgO and ZrO2 were
even bigger than what happened in the fourth combination.
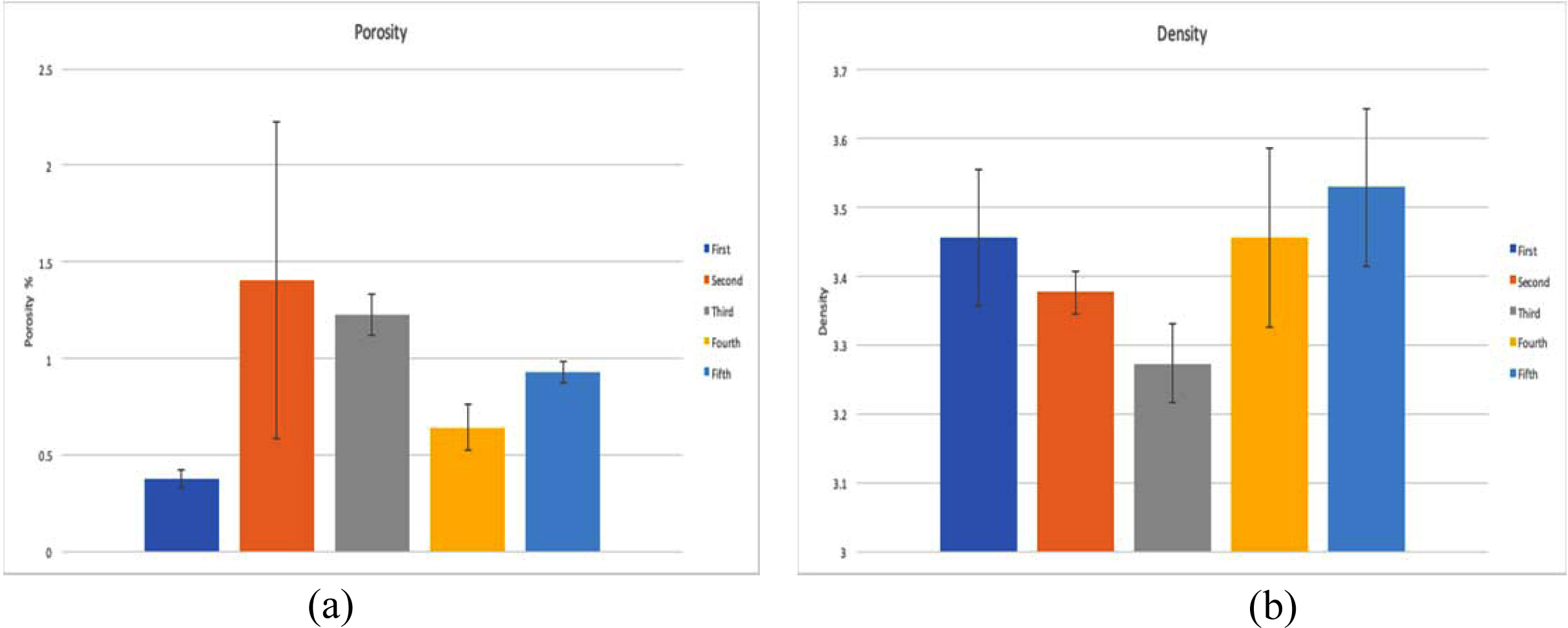
Porosity
and density
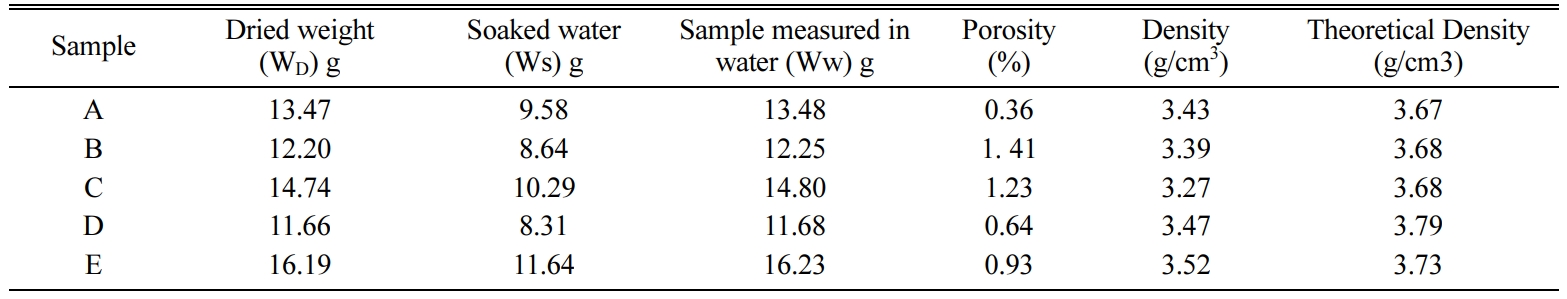
Porosity and Density of all the sintered samples at
1,750 oC for 1 h and 200 MPa are given in Table 3 and shown in
Fig. 3. In first combination, which is without any additives, the porosity has
the lowest value (0.36%). When TiO2 added to the mixture, the
porosity increased (2.30%), but when the percentage of TiO2
increased the porosity decreased (1.23%). For ZrO2, the porosity
increases with higher percentage of ZrO2 (0.66 at 2.5% ZrO2
and 0.92% at 5% ZrO2), but overall ZrO2 gives less
porosity than TiO2 as additives.
For Density, there isn’t much different with additives at
different percentages, but using TiO2 gives a lower value of density
than using ZrO2.
Thermal
shock resistance and thermal expansion
No visible cracks or damaged surface of the samples were
found as a results of thermal shock resistance test. Thermal expansion
coefficient depends mainly on its component materials. Spinel compositions
(Table 4) shows no significant spinalization reaction up to 1,000 oC.
Only a small, gradual increase in expansion values is observed with increasing
temperature. Above 1,000 oC, expansion values improve sharply, which
marks the starting of spinel formation reaction.
The addition of TiO2 and ZrO2 above
1,000 oC gives a better homogeneous spinel. On increase in its
growth, spherical shape particles are formed which help to achieve the
crystallization of those compositions at temperatures lower than that of model
spinel without adding these oxides. The fluctuation in the thermal expansion
coefficient value, when the temperature is below 1,000 oC, it
believes to be a result of temperature variation and rearrangement of grains.
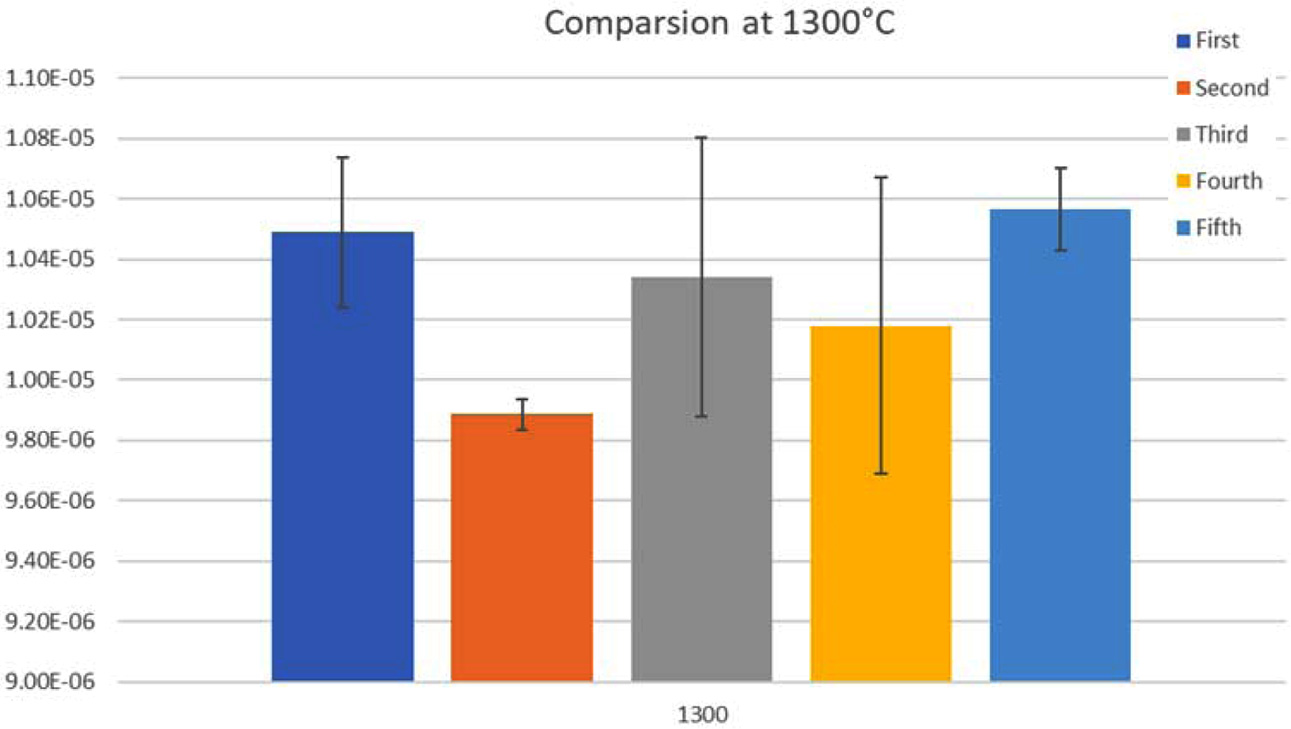
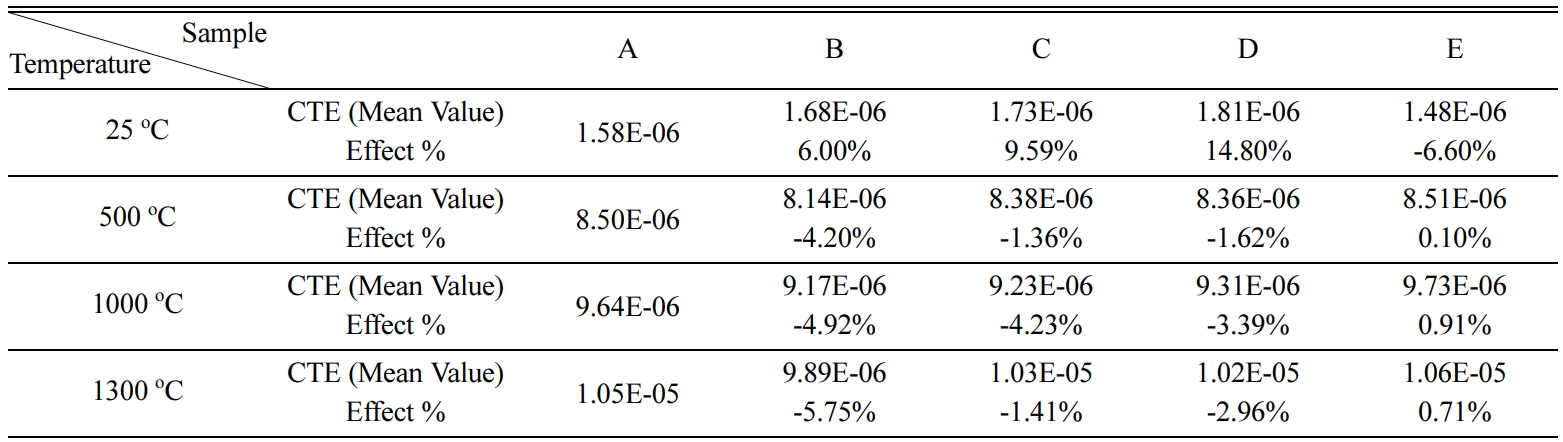
Fig. 4 shows the comparison between the five different
sample combinations at 1,300 oC, adding TiO2 or ZrO2
by small percentage gives the lowest CTE (9.89E-06, 1.02E-05) respectively, but
increasing ZrO2 increases the CTE.
Scanning
Electron Microscopy (SEM) results
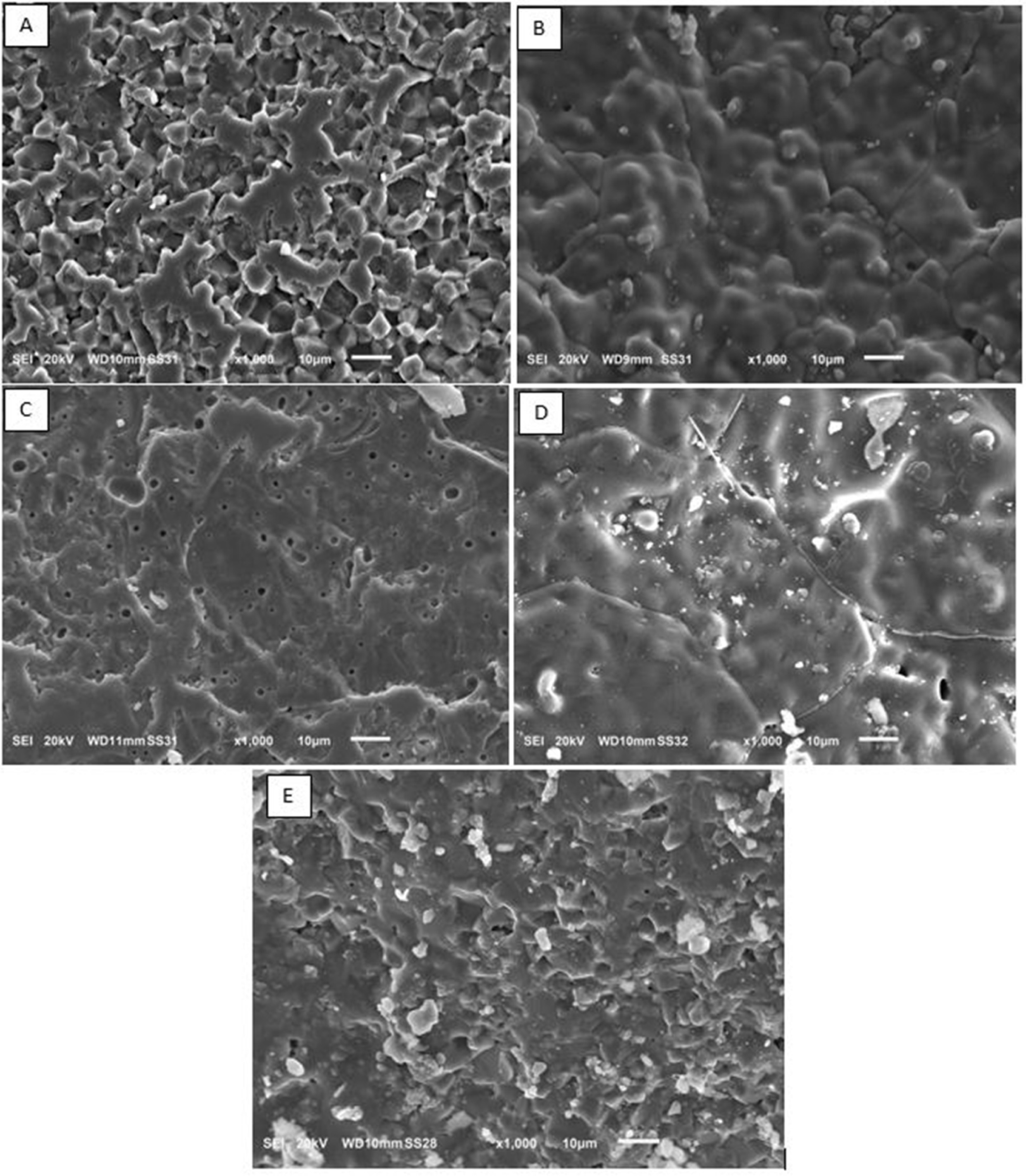
The morphology of the surface of the magnesium aluminate
spinel - with and without additives -are analyzed by SEM observations as shown
in Fig. 5. As can be seen from this figure, the baseline sample which is the
MgAl2O4 spinel without additives have smallest crystals
particles (see Fig. 5(a)) that maybe contributed to that
milling grind the particles and made homogenous small size
particle. With adding a TiO2 to the spinel (see Fig. 5(b)), the
porosity increased because of the excessing of the fractures that induces which
led to more connected accessible channels that led the fluid to occupied that
space. In Fig. 5(c), the amount of TiO2 has been doubled and the
porosity has been increased due to the induced fractured and air bubbles that
is generated as a results of liberated gases from adding the TiO2 to
the mixture similar behavior has been reported by Saleh and Hassen [22]. Not
that far from adding TiO2, ZrO2 has been added to the
spinel mixture to form the sample D as we can see in Fig. 5(d). It shows an
increase in porosity and fractures was clearly presented in the structure. By
doubling the amount of ZrO2 (Fig. 5(e)), the porosity has been
increased allowing more isolation property and more fluid can be stored in this
pours. Furthermore, it is clear that the adding TiO2 provided more
homogenous dispersion and distribution of the metal oxide particle than ZrO2.
This homogeneity agrees with thermal expansion values
where the reduction has been noticed in the case of adding TiO2. At
2.44%, however, farther addition slightly improve the thermal expansion this
could be due to agglomeration of the particle inside the spinel. In the other
hand, this mechanism and behavior of agglomeration has been noticed in the case
of ZrO2 even at 2.44% weigh. These results indicate the advantage of
adding TiO2 and ZrO2 for improve the thermal properties
of MgAl2O4 spinel. The optimal loading of TiO2
is 2.44% in this study.
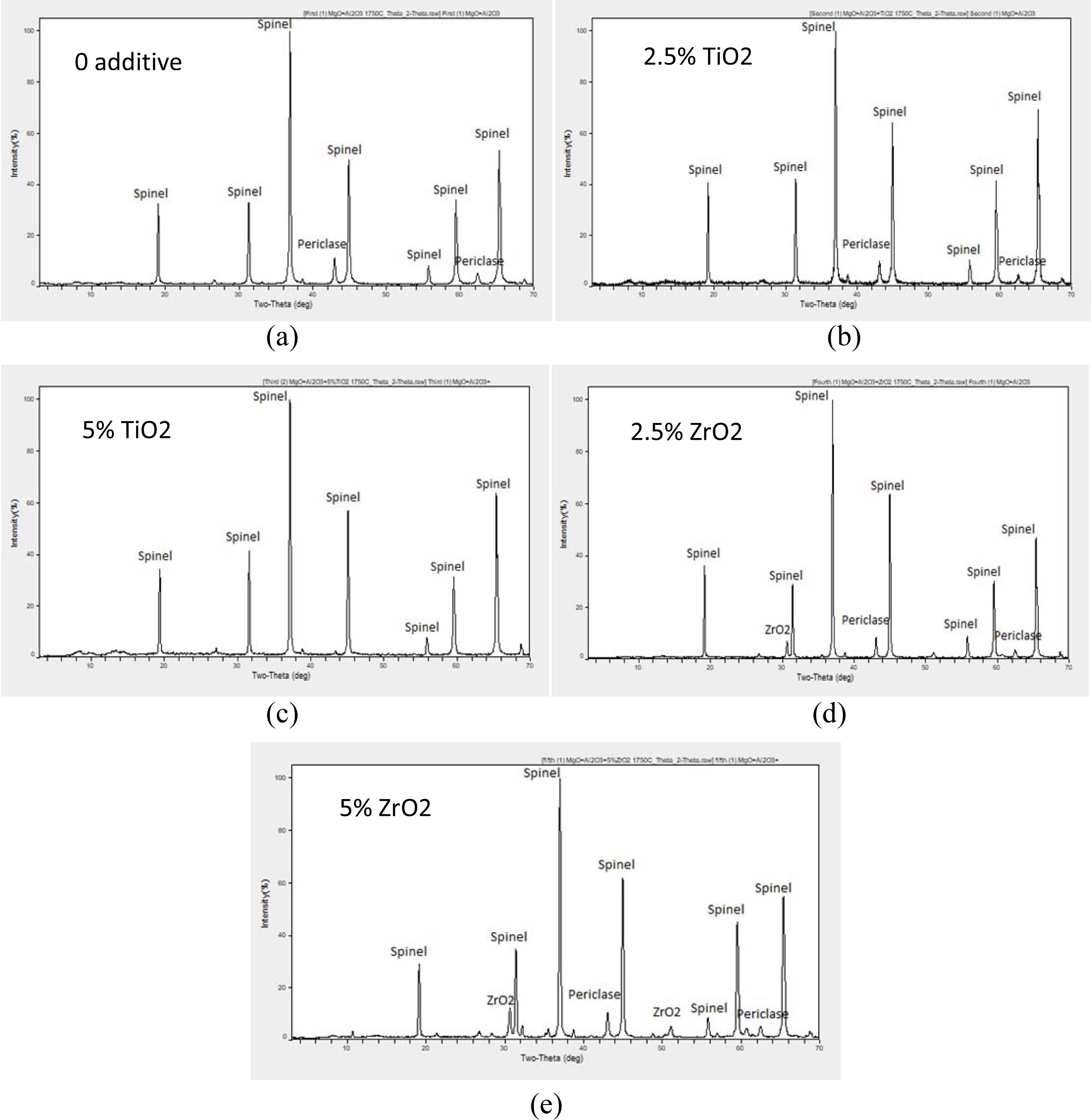
|
Fig. 2 Phase analysis study (XRD) of (200 MPa, 1,750 oC-1 h) sintered samples: (a) First Combination: Sample A, (b) Second Combination:
Sample B, (c) Third Combination: Sample C, (d) Fourth Combination: Sample D, (e) Fifth Combination: Sample E. |
|
Fig. 3 Comparison between different combinations: (a) Porosity (b) Density. |
|
Fig. 4 Comparison of CTE for the five combinations at 1,300 oC. |
|
Fig. 5 SEM photograph of MgAl2O4 samples: (A) MgAl2O4 without additives (baseline sample); (B) MgAl2O4 with 2.44% TiO2; (C) MgAl2O4 with 4.76% TiO2; (D) MgAl2O4 with 2.44% ZrO2; (E) MgAl2O4 with 4.76% ZrO2. |
|
Table 3 Experimental values of Porosity and Density with the theoretical density values |
|
Table 4 Results of CTE at different temperatures and effect % of adding TiO2 and ZrO2 |
In this study, different loading titanium oxide (TiO2)
and zirconium oxide (ZrO2) were used as additives to fabricate
magnesium aluminate spinel composites. The thermal expansion coefficients of
these composites and porosity were analyzed. For pure spinel (without any
additives), the porosity has the lowest value. However, adding TiO2
to the mixture increases the porosity, but when the percentage of TiO2
increased the porosity appeared to be decreasing. In other hand, increasing
loading of ZrO2 leads to the increase in the porosity. Generally,
ZrO2 gives less porosity than TiO2 when they added to the
mixture.
In all compositions there are no significant spinalization
reaction up to 1,000 oC, but above 1,000 oC
expansion values improve sharply, which marks the starting of spinel formation
reaction. The addition of TiO2 and ZrO2
above 1,000 oC gives a better homogeneous spinel.
At 1,300 oC, adding TiO2 or ZrO2 by small
percentage gives the lowest CTE.
- 1. I. Ganesh, S. Bhattacharjee, B.P. Saha, R. Johnson, K. Rajeshwari, R. Sengupta, M.V. Ramana Rao, and Y.R. Mahajan, Ceramics International 28[3] (2002) 245-253.
-

- 2. A.F. Dericioglu and Y. Kagawa, J. Euro. Ceram. Soc. 23[6] (2003) 951-959.
-

- 3. M. Shimada, T. Endo, T. Saito, and T. Sato, Mater. Lett. 28[4-6] (1996) 413-415.
-

- 4. J. Guo, H. Lou, H. Zhao, D. Chai, and X. Zheng, Appl. Catal. A: Gen 273[1-2] (2004) 75-82.
-

- 5. J. Guo, H. Lou, H. Zhao, X. Wang, and X. Zheng, Mater. Lett. 58[12-13] (2004) 1920-1923.
-

- 6. M. Beauvy, C. Dalmasso, C. Thiriet-Dodane, D. Simeone, and D. Gosset, Nucl. Instrum. Meth. Phys. Res. B 242[1-2] (2006) 557-561.
-

- 7. G. Gusmano, G. Montesperelli, E. Traversa, A. Bearzotti, G. Petrocco, A. D’Amico, and C.D. Natale, Sens. Actuators B 7[1-3] (1992) 460-463.
-

- 8. S. Mukhopadhyay, S. Ghosh, M.K. Mahapatra, R. Mazumder, P. Barick, S. Gupta, and S. Chakraborty, Ceramics International 28[7] (2002) 719-729.
-

- 9. A. Ghosh, S.K. Das, J.R. Biswas, H.S. Tripathi, and G. Banerjee, Ceramics International 26[6] (2000) 605-608.
-

- 10. I. Ganesh, R. Johnson, G.V.N. Rao, Y.R. Mahajan, S.S. Madavendra, and B.M. Reddy, Ceramics International 31[1] (2005) 67-74.
-

- 11. R.D. Maschio, B. Fabbri, and C. Fiori, Inds. Ceramics 8[2] (1988) 121-126.
- 12. W.H. Bragg, Philosophical Magazine and J. of Sci. 30[170] (1915) 305-315.
-

- 13. C. Aksel and F.L. Riley, J. Euro. Ceram. Soc. 23[16] (2003) 3079-3087.
-

- 14. W.D. Kingrey, in “Introduction to Ceramics” (John Wiley and Sons, 1960) p. 1.
- 15. A.M. Pachpinde, in “Ferrite Catalysts” (Lulu Publication, 2017) p.1.
- 16. Y.H. Huang, in “Pavement analysis and design” (Engle- wood Cliffs, 1993) p. 1.
- 17. ASTM E228-17, ASTM International, West Conshohocken, PA, 2017
- 18. R.I Belousov and S.K Filatov, Glass Phys. Chem. 33[3] (2007) 271-275
-

- 19. ASTM C1525-18, ASTM International, West Conshohocken, PA, 2018
- 20. R. Sarkar and G. Bannerjee, Euro. Ceram. Soc. 20[12] (2000) 2133-2141
-

- 21. O. Quénard, C. Laurent, A. Peigney, and A. Rousset, Mater. Res. Bull. 35[12] (2000) 1967-1977
-

- 22. Q. Saleh and B. Hassen, Iraqi Journal of Physics. 15[34] (2017) 114-122.
-

 This Article
This Article
-
2020; 21(6): 683-689
Published on Dec 31, 2020
- 10.36410/jcpr.2020.21.6.683
- Received on Jul 8, 2020
- Revised on Aug 23, 2020
- Accepted on Sep 3, 2020
 Services
Services
- Abstract
introduction
materials and experiment method
results and discussion
conclusion
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Fahad Albanumay
-
Material Science Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh 11442, Saudi Arabia
Tel : +966114883555 ex. 2794 Fax: +966114813526 - E-mail: falbanumay@kacst.edu.sa






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.